Abstract
The mitogen-activated protein kinase (MAPK) signaling pathway is associated with tumor cell proliferation, differentiation, apoptosis, angiogenesis, invasion and metastasis. The present review assesses the involvement of the MAPK signaling pathway in oral cancer progression and invasion based on analysis of individual sub-pathways and their mechanisms of action. The regulation of this pathway for targeted oral cancer therapy is explored and the challenges confronting this, as well as corresponding potential solutions, are discussed. Exploring this pathway with an emphasis on its components, subfamilies, sub-pathways, interactions with other pathways and clinical practice modes may improve oral cancer treatment.
Keywords: mitogen-activated protein kinase signaling pathway, oral cancer, oral squamous cell carcinoma, kinase, therapeutic targets, inhibitor, treatment
1. Introduction
Head and neck malignant carcinoma is a commonly diagnosed cancer and its incidence has risen over the past decade (1). Oral squamous cell carcinoma (OSCC) is an important oral malignancy, accounting for >90% of head and neck malignant carcinoma cases (2). It has a 5-year survival rate of ~50% (3), due to delayed diagnosis, disease recurrence, distant metastasis and therapeutic resistance. At present, multiple attempts are being made to develop efficient targeted therapies, which often involve the use of various inhibitors for improving treatment due to their special advantages, such as low cytotoxicity, strong specificity and long duration of action.
Targeted therapies aim at controlling the signaling pathways through which cell survival and death are regulated. Multiple signaling pathways may function in oncogenesis and in the growth of oral malignancies (4,5). Among these is the mitogen activated protein kinase (MAPK) signaling pathway, which regulates the expression of a large number of proteins involved in the control of cell proliferation, differentiation and apoptosis (6,7), and remains a focus of investigation for therapeutic targeting, along with the development of appropriate inhibitors (8). These inhibitors have been demonstrated to be effective for the treatment of oral cancer and other malignancies, including human colon cancer, breast cancer and lung cancer (9–12). Certain inhibitors, which have been examined in various clinical trials, are being used to treat patients with oral cancer and are eliciting promising results. The purpose of the present review is to provide an update on the involvement of the MAPK pathway in oral cancer progression and invasion, and to review the progress made in the development of pharmacologic inhibitors to regulate the MAPK signaling pathway, in order to improve oral cancer treatment.
2. MAPK signaling sub-pathways
The MAPK signaling pathway is comprised of four sub-pathways, namely the extracellular signal-regulated kinase (ERK)1/2) sub-pathway, the c-Jun N-terminal kinase (JNK) sub-pathway, the p38 sub-pathway and the ERK5 sub-pathway (13). These sub-pathways correspond to the ERK1/2, JNK1/2/3, p38α/β/γ/δ and ERK5 subfamilies of MAPK, respectively, and are a chain of proteins that transduce various extracellular signals to the nucleus, controlling gene expression through transcriptional factors (14). By regulating protein activities through signaling cascades that consist of MAPK kinase kinase (MAPKKK), MAPK kinase and MAPK (15), these sub-pathways impact tumor cell proliferation, differentiation and survival (16,17), as presented in Fig. 1.
Figure 1.
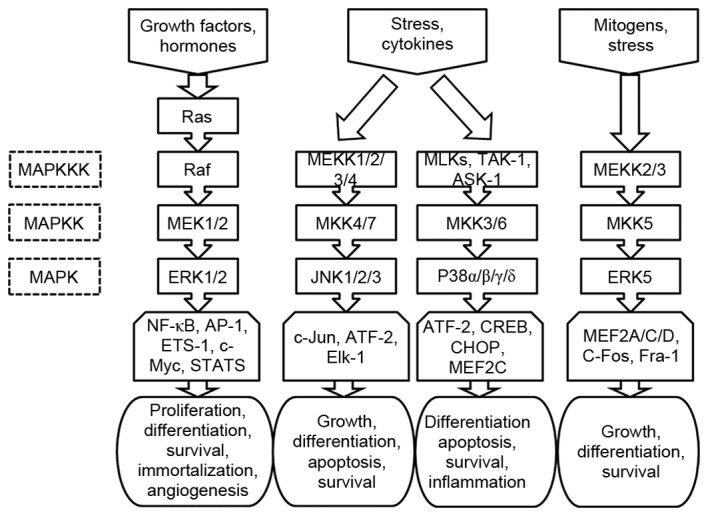
Schematic illustration of MAPK signaling pathway, where each MAPK phosphorylates several transcriptional factors, regulating protein expression.
The ERK1/2 sub-pathway has received the most attention thus far (18–20). It has 3 important components: Ras, Raf and MEK. As shown in Fig. 1, in response to the binding of growth factors and hormones to cell surface receptors (21), the level of Ras-guanosine triphosphate (GTP) increases in cells, which in turn promotes kinase activation. The GTP-bound forms of Ras directly bind and thus recruit cytosolic dimers of the Raf kinases to the plasma membrane (22). Once localized at the membrane, Raf is activated through phosphorylation by other kinases or autophosphorylation. It then phosphorylates and activates MEK, which subsequently induces the phosphorylation and activation of ERK. The activated ERK1/2 dissociates from the Ras-Raf-MEK-ERK1/2 complex and phosphorylates a number of cytoskeletal proteins, kinases, and transcription factors, including nuclear factor (NF)-κB, AP-1, ETS-1, c-Myc, and members of the signal transducer and activator of transcription family (23,24). The functional consequences of substrate-level phosphorylation by ERK1/2 include changes in cellular motility and gene expression that promote proliferation, differentiation, cellular survival, immortalization and angiogenesis (25).
The JNK sub-pathway is associated with activation of MAPKKK [mitogen activated protein kinase kinase kinase (MEKK)1/2/3/4], a response induced by a number of environmental stresses (including osmotic stress, UV light, heat shock and growth factor withdrawal) and cytokines [including tumor necrosis factor (TNF)-α and interleukin (IL)-β] (26). The activated MEKK1/2/3/4 in the sub-pathway phosphorylates a series of proteins in the following order: Mitogen activated protein kinase kinase (MKK) 4/7→JNK1/2/3→c-Jun, activating transcription factor (ATF)-2, and ELK1, ETS transcription factor. The sub-pathway contributes to cell growth, differentiation, apoptosis and survival through stress/cytokine-sensitive responses (27).
Similar to the JNK sub-pathway, the p38 sub-pathway is associated with the response of MAPKKK [mixed lineage kinases (MLKs), mitogen activated protein kinase kinase kinase 7 (TAK-1) and mitogen activated protein kinase kinase kinase 5 (ASK-1)] caused by stress and pro-inflammatory cytokines, including IL-1 and TNF-α (28). The activated MLKs, TAK-1 and ASK-1 in this sub-pathway phosphorylate and activate various kinases sequentially, from MLKs, TAK-1, ASK-1 to MKK3/6 to the four p38 isoforms (p38α/β/γ/δ). The activated p38 isoforms then activate the substrates ATF-2, cAMP response element-binding protein, DNA damage inducible transcript 3 and myocyte enhancer factor (MEF)2C. Cell differentiation, apoptosis, survival and inflammation are induced by this pathway (29).
In comparison with the other sub-pathways, the ERK5 sub-pathway has been less intensively investigated (30). It is associated with a variety of stimuli, including mitogens (for example, epithermal growth factor) and cellular stresses (including oxidation and osmotic stress) (31). Its activation is consistent with the cascade MEKK2/3→MKK5→ERK5→MEF2A/C/D AP-1 transcription factor subunit (C-Fos) and Fos related antigen-1, and the ERK5 sub-pathway regulates cell growth, differentiation and survival (32,33).
3. Action mechanisms of the MAPK signaling pathway in oral cancer
The MAPK signaling pathway is involved in various cellular responses, including cell proliferation, apoptosis, angiogenesis, cell migration and metastasis. The deregulation of MAPK signaling leads to inappropriate responses, induced through abnormal gene expression. Further elucidation of the mechanisms underlying the action of proteins that regulate MAPK signaling and of their expression patterns in oral cancer may provide novel insights into the signal transduction mechanisms in cell cycle progression and malignant transformation. A large number of proteins serve as targets that act on the oncogenesis and growth of oral tumor cells. These targets include angiopoietin-like 3, annexin A10, SH3 domain containing kinase binding protein 1, quaking 5, epidermal growth factor receptor, fibroblast activation protein, parathyroid hormone-related protein and 70-kDa ribosomal S6 kinase (34–41). Their functions and molecular mechanisms in the MAPK signaling pathway that concern oral cancer are summarized in Table I.
Table I.
Therapeutic targets of the MAPK signaling pathway for oral cancer treatment.
| Author, year | Classification | Target name | Target description | Target functions | Molecular mechanisms in MAPK signaling pathway | Effects | (Refs.) |
|---|---|---|---|---|---|---|---|
| Koyama et al, 2015 | Promoting cell proliferation | ANGPTL3 | A secretory glycoprotein | Regulating cell proliferation and promoting blood vessel formation | The proliferation significantly decreases in ANGPTL3 knockdown cells due to inactivated ERK and cell-cycle arrest at the G1 phase resulting from upregulation of CDK inhibitors, including p21Cip1 and p27Kip1. There is a marked reduction in the growth of ANGPTL3 knockdown-cell xenografts, with decreased levels of phosphorylated ERK relative to control-cell xenografts | A potentially useful diagnostic and therapeutic target for controlling proliferation of oral cancer | (34) |
| Shimizu et al, 2012 | ANXA10 | A calcium and phospholipid binding protein | Impacting endocytosis and exocytosis; having anticoagulant activity; interacting with cytoskeleton; differentiation; cellular proliferation | The ANXA10-knockdown cell proliferation is decreased due to inactivation of ERK and cell-cycle arrest at the G1 phase, associated with upregulation of CDK inhibitors | Serving as an indicator of cellular proliferation and a potential therapeutic target for developing new OSCC treatment regimens | (35) | |
| Wakasaki et al, 2010 | CIN85 | A widely expressed, multifunctional adaptor protein consisting of three N-terminal SH3 domains | Promoting the EGFR-mediated cancer cell growth pathway | CIN85 promotes TGF-α-induced activation of Ras and phosphorylation of downstream molecules including c-Raf, MEK and ERK, upregulating expression of c-Myc that is critical for sustained proliferation of OSCC | Serving as an attractive therapeutic target for controlling proliferation of OSCC | (36) | |
| Fu and Feng, 2015 | QKI-5 | An RNA-binding protein | Suppressing tumor proliferation | The underexpression of tumor suppressor QKI-5 activates the MAPK signaling pathway and contributes to uncontrolled cyclin D1 expression, resulting in increased proliferation of oral cancer cells | Serving as a diagnostic and therapeutic target for controlling proliferation of oral cancer cells | (37) | |
| Williams, 2010 | Promoting tumor | EGFR | A member of the tyrosine angiogenesis kinase family of receptors | Enhancing cell motility, altering cell adhesion and promoting angiogenesis | The downstream signaling activated by EGFR includes STAT1/3 and PI3K which activates Akt, Ras-Raf-MEK-ERK1/2 signaling and PLC-γ, contributing to cell survival, proliferation, and angiogenesis in OSCC | Serving as a potential therapeutic target for controlling cell proliferation and angiogenesis in OSCC | (38) |
| Wang et al, 2014 | Promoting cell migration and metastasis | FAP | A homodimeric integral membrane gelatinase belonging to the serine protease family | Regulating cell proliferation, adhesion, migration, invasion, and metastasis | The knocking down endogenous FAP suppresses cell proliferation, adhesion, migration, invasion, and metastasis by inactivating PTEN/PI3K/Akt and Ras-Raf-MEK-ERK1/2 signaling. This in turn inhibits GSK-3β and its downstream signals including cell-cycle regulators, EMT and MMPs in OSCC | Serving as a potential therapeutic target for controlling proliferation, invasion and metastasis of OSCC | (39) |
| Yamada et al, 2008 | PTHrP | A stimulator of osteoclastic bone resorption | Increasing cell proliferation, survival, adhesion, migration, and invasion | PTHrP is upregulated by EGF stimulation via ERK1/2 and p38 sub-pathways. The PTHrP silencing by EGFR inhibitor AG1478 treatment suppresses cell proliferation, migration, and invasiveness; the combined treatment with AG1478 and PTHrP knockdown achieves synergistic inhibition of malignant phenotypes | Serving as a target for the suppression of proliferation, migration, and invasion of oral malignancies | (40) | |
| Wu et al, 2016 | p70S6K | A serine/threonine kinase, belonging to the AGC-kinase family | Promoting cell growth and metastasis | The IL-6-induced p70S6K activation is attenuated by inhibitors of the PI3K/Akt/mTOR, MAPK, and JAK/STAT3 signaling pathways, suggesting that it is located downstream of these pathways. p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of OSCC | Serving as a target for inhibition of oral tumor growth and metastasis | (41) |
MAPK, mitogen-activated protein kinase; ANGPTL3, angiopoietin-like 3; ERK, extracellular signal-regulated kinase; CDK, cyclin-dependent kinase; ANXA10, annexin A10; OSCC, oral squamous cell carcinoma; CIN85, Sc-Cbl-interacting protein of 85 kDa; EGFR, epidermal growth factor receptor; TGF-α, transforming growth factor- α; MEK, MAPK/ERK kinase; QKI-5, quaking 5; STAT, signal transducer and activator of transcription; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; PLC-γ, phosphoinositide phospholipase C; FAP, fibroblast activation protein; PTEN, phosphatase and tensin homolog; GSK-3β, glycogen synthase kinase-3β; EMT, epithelial-mesenchymal transition; MMP, matrix metalloproteinase; PTHrP, parathyroid hormone-related protein; EGF, epidermal growth factor; IL, interleukin; p70S6K, 70-kDa ribosomal S6 kinase; mTOR, mammalian target of rapamycin; JAK, Janus kinase.
Promoting tumor cell proliferation
Within the MAPK signaling pathway, two main mechanisms are responsible for promoting tumor cell proliferation. The primary mechanism is associated with early gene-encoded c-Jun and C-Fos proteins, which constitute transcription factor AP-1 in the form of a heterodimer or homodimer following MAPK phosphorylation; for example, ERK phosphorylation (18). These factors then combine with corresponding promoter regions of DNA and upregulate the expression of cyclin D1, which promotes the progression of the cell cycle from the G1 phase to the S phase and accelerates cell proliferation. The other mechanism is attributed to the function of activated MAPK, which suppresses expression of cell cycle inhibitory proteins including p27KIP, attenuating the inhibition of the cell cycle (42).
Inhibiting tumor cell apoptosis
There are three main mechanisms of the MAPK signaling pathway that inhibit tumor cell apoptosis. Firstly, the signaling pathway may directly suppress the activity of the apoptosis end effector, caspase-3, which inhibits hydrolysis of tubulin to maintain the integrity of its spindle body. This function prevents cell apoptosis from being induced by various stimuli. Secondly, the pathway may indirectly suppress the activity of caspase-3 by activating the inhibitors of apoptosis molecules, including inhibitor of apoptosis and B cell lymphoma-2 family proteins (43,44). Thirdly, the pathway may block the release of mitochondrial cytochrome C and interfere with the activation process of upstream caspase-9, indirectly decreasing the activity of downstream caspase-3 (45).
Promoting tumor angiogenesis
Tumor angiogenesis not only provides nutrition for increasingly active tumor cell division, but is also involved in the outward expansion of the tumor. A number of vascular growth factors upregulate the expression level of vascular endothelial growth factor via activation of the MAPK signaling pathway, resulting in angiogenesis (38).
Inducing tumor invasion and metastasis
The process of tumor invasion relies on the precise coordination of extracellular matrix adhesion and hydrolysis in association with two members of the matrix metalloproteinase (MMP) family; namely MMP-2 and MMP-9. The expression of MMPs is primarily controlled by MAPK and other signaling pathways, including the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (46). This has been validated by a number of studies in which the inhibitory functions of anticancer drugs on tumor invasion and metastasis were demonstrated to occur by downregulating the MAPK signaling pathway, which resulted in reduced expressions of MMPs (47,48).
4. Regulation of the MAPK signaling pathway for targeted oral cancer therapy
The expression levels of the component proteins of the MAPK signaling pathway tend to increase in oral cancer (49). Activation of the MAPK signaling pathway is known to cause the transformation of normal cells to tumor phenotype (6). Thus, inhibition of this pathway may enable restoration of the tumor cells to the untransformed phenotype in vitro, thereby inhibiting the growth of the tumor in the body (50,51). Theoretically, intervention targeting any component in the MAPK signaling pathway has the potential to arrest tumor growth. For this reason, multiple inhibitors have been developed to target different components of the pathway (52–55). These inhibitors serve as promising, effective antineoplastic agents for the treatment of malignant oral tumors, and are characterized by low cytotoxicity, strong specificity, a long duration of action, high solubility and metabolic stability, all of which contribute to their anti-tumor effects. At present, certain inhibitors have been used in phase I or phase II clinical trials and achieved promising effects (55).
These inhibitors may inhibit oral tumor cell proliferation by blocking the activation of corresponding target molecules of the MAPK signaling pathway. They include epigallocatechin-3-gallate, aliphatic acetogenin, galanin receptor 1, and S-allylcysteine (56–59). Certain inhibitors, including pterostilbene, astaxanthin and sulfasalazine (SSZ) (60–62), may also induce tumor cell apoptosis. Other inhibitors include those that have the potential to inhibit either oral tumor angiogenesis (for example, cetuximab) (63) or tumor invasion and metastasis (for example, vinculin, phenethyl isothiocyanate, cardiotoxin III and resveratrol) (64–67). Their characteristics are listed in Table II.
Table II.
Target inhibitors of the MAPK signaling pathway for oral cancer treatment.
| Author, year | Classification | Target inhibitor | Inhibitor description | Inhibitor functions | Molecular mechanisms in MAPK/ERK pathway | Effects | (Refs.) |
|---|---|---|---|---|---|---|---|
| Lee et al, 2015 | Inhibiting cell proliferation | EGCG | A biologically active polyphenol in green tea | Inhibiting cell growth and inducing cell apoptosis | EGCG attenuates cell proliferation and arrests cell cycle at the G1 phase by upregulating BTG2 expression via p38 and ERK1/2 sub-pathways in OSCC cells | Serving as a new preventive agent for patients with OSCC | (56) |
| D'Ambrosio et al, 2011 | Aliphatic acetogenin | A class of compounds, mainly including: Compound 1 [(2S,4S)-2,4-dihydroxyheptadec-16-enyl acetate] and Compound 2 [(2S,4S)-2,4-dihydroxyheptadec-16-ynyl acetate] | Inhibiting cancer cell proliferation and growth | The Compounds 1 and 2 synergistically inhibit phosphorylation of c-RAF (Ser338) and ERK1/2 (Thr202/Tyr204). Only Compound 2 prevents EGF-induced activation of EGFR (Tyr1173) via the ERK1/2 sub-pathway | The molecular mechanism of aliphatic acetogenin indicates that targeting multiple molecules in the ERK1/2 sub-pathway simultaneously may offer more effective prevention and treatment of oral cancer compared with using specific inhibitors that target only one of the molecules in the pathway | (57) | |
| Henson et al, 2005 | GALR1 | A G-protein-coupled receptor | Regulating cell proliferation | GALR1 inhibits proliferation in immortalized and malignant keratinocytes by inactivating the MAPK signaling pathway. The inhibitory effects on proliferation in epithelial cells raise the the possibility that its inactivation or dysregulation may lead to uncontrolled proliferation and neoplastic transformation | Serving as a prognostic biomarker and a treatment option | (58) | |
| Tang et al, 2009 | SAC | A water soluble garlic extract | Having anticarcinogenic effects that inhibit tumor cell growth | SAC effectively inhibits the proliferation, upregulates the expression of E-cadherin and stabilizes the E-cadherin/β-catenin adherent junction complex in OSCC cells, partially through the suppression of MAPK signaling pathway and downregulation of the SLUG repressor protein | Serving as a potential anticancer agent with improved selectivity toward OSCC cells | (59) | |
| Ko et al, 2015 | Inducing apoptosis | Pterostilbene | A naturally occurring phytoalexin | Having antioxidant activity, cancer prevention activity, and cytotoxicity | Pterostilbene suppresses cell growth and induces apoptosis in SAS and OECM-1 cell lines by inhibiting Akt, p38 and ERK1/2 and activating the JNK sub-pathway | Serving as a novel and promising agent for treating oral cancer | (60) |
| Kavitha et al, 2013 | Astaxanthin | An antioxidant carotenoid | Having anti-inflammatory and anticancer properties; modulating immune response and oxidative stress | Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling via inactivation of the MAPK and PI3K/Akt signaling pathways to induce intrinsic apoptosis | Serving as a promising agent for prevention and therapy of oral cancer | (61) | |
| Han et al, 2014 | SSZ | A drug composed of sulfapyridine and mesalazine | Having anti-inflammatory properties; inducing apoptosis | SSZ induces autophagic cell death in HSC-4 cells by inhibiting Akt and activating the MAPK signaling pathway | Serving as an effective chemotherapeutic agent for the treatment of OSCC | (62) | |
| Psyrri et al, 2014 | Inhibiting angiogenesis | Cetuximab | A chimeric IgG1-human monoclonal antibody against the extracellular domain of EGFR apoptosis | Inhibiting cell cycle progression, angiogenesis and metastasis; inducing apoptosis | Cetuximab inhibits angiogenesis of OSCC by inactivating the ERK1/2 sub-pathway and/or PI3K/Akt signaling pathway | Serving as a potential therapeutic agent for the treatment of OSCC | (63) |
| Yoshimoto et al, 2014 | Inhibiting cell migration and metastasis | Vinculin | A cellular adhesion protein | Inhibiting cell migration | The upregulation of MT1-MMP transcription by vinculin knockdown is abrogated by ERK inhibition. MT1-MMP may facilitate further HSC-4 cell survival, induced by vinculin knockdown | Serving as a key agent for controlling OSCC cell migration through the MAPK signaling pathway | (64) |
| Chen et al, 2013 | PEITC | A member of the isothiocyanate family | Arresting cell cycle and stimulating apoptosis; inhibiting migration and invasion | PEITC is able to inhibit the invasion of EGF-stimulated SAS oral cancer cells by targeting EGFR and its downstream MAPK signaling molecules, reducing expression and enzymatic activities of MMP-2 and MMP-9 | Serving as a promising therapeutic agent for the treatment of oral cancer metastasis | (65) | |
| Yen et al, 2013 | CTXIII | A protein composed of 60 basic amino acid residues | Inhibiting cellular proliferation and inducing apoptosis | The CTXIII treatment leads to downregulated protein expression of MMP-2 and MMP-9, and the phosphorylation of JNK and p38 is increased independent of ERK phosphorylation | Serving as a potential agent for oral cancer therapy | (66) | |
| Lin et al, 2015 | Resveratrol | A polyphenolic compound | Having antioxidative, cardioprotective, and neuroprotective properties | Resveratrol inhibits TPA-induced MMP-9 gelatinolytic activity and protein expression, as well as phosphorylation of JNK1/2 and ERK1/2, involved in downregulating protein expression and the transcription of MMP-9 | Serving as a promising antimetastatic agent against oral cancer | (67) |
MAPK, mitogen-activated protein kinase; EGCG, epigallocatechin-3-gallate; BTG2, BTG family member 2; ERK, extracellular signal-regulated kinase; OSCC, oral squamous cell carcinoma; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; GALR1, galanin Receptor 1; SAC, S-allylcysteine; E-cadherin, epithelial cadherin; SLUG, snail family transcriptional repressor 2; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; SSZ, sulfasalazine; IgG, immunoglobulin G; MT1, membrane type 1; MMP, matrix metalloproteinase; PEITC, phenethyl isothiocyanate; CTXIII, Cardiotoxin III; TPA, 12-O-tetradecanoylphorbol-13-acetate.
Besides the aforementioned inhibitors, it is worth noting that certain inhibitors may induce autophagic cell death via activation of the MAPK signaling pathway. For example, high-concentration SSZ was revealed to initially trigger autophagy, which then induced apoptosis in HSC-4 cells by activating the ERK1/2 sub-pathway. Therefore, SSZ may have the potential to treat oral cancer (62).
5. Discussion
Although a number of genetic and experimental observations have validated that inhibitors of MAPK cascades would act as effective antineoplastic agents for the treatment of oral cancer with approved performance, there are a few of remaining challenges and questions to be resolved.
First, mutated members of the cascade, including Ras and Raf, are expected to serve as inhibitory targets in the MAPK pathway (68,69), but their functions have not been sufficiently illustrated. The most commonly observed mutations are those that arise in the Ras genes, including H-Ras, N-Ras and K-Ras, with corresponding mutations being detected in >30% of patients with OSCC in Southeast Asia, primarily caused by chewing tobacco (70). These Ras genes have the potential to regulate the MAPK and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathways, and a Ras mutation may therefore activate both of them. As for Raf, its activity in certain cell types is negatively regulated by Akt, revealing the presence of cross-talk between the two pathways (71). Activating mutations in the B-Raf gene (one of the three Raf genes, which include A-Raf, B-Raf and C-Raf) were also identified in oral cancer (19,72). Unlike mutation of Ras, mutation of B-Raf only causes activation of the MAPK signaling pathway. It functions independently in promoting the engagement of the MAPK cascade (73). These findings confirm the complicated functions of the targets in the MAPK pathway, indicating that different inhibitors of the same target and drugs for different targets within the same pathway may demonstrate marked differences in effectiveness, depending on tumor type and mutational status. Hence, improved understanding of their biochemical properties or modes of action, along with improvements in their pharmacologic profiles, will enable more optimal treatment of oral cancers through the development of more effective therapies.
Secondly, inhibiting only a single subfamily or sub-pathway has been revealed to be insufficient to promote apoptosis or arrest growth to control oral cancer, due to a potential compensation effect of alternative subfamilies or sub-pathways (13). The occurrence of most oral cancer is associated with dysregulation of multiple subfamilies and sub-pathways. Furthermore, certain sub-pathways, including the ERK5 sub-pathway, have not been sufficiently explored and their cross-functions remain unclear. From this perspective, clearly identifying the functions and interactions of these signaling transduction sub-pathways is critical to inducing tumor cell apoptosis and overcoming tumor resistance. This is the clinical need that must be satisfied in order to improve treatment for oral cancer, and therefore warrants additional attention.
Thirdly, delivering inhibitors to specific cancer cells is difficult in certain cases (74), and regulating the MAPK signaling pathway will have direct or indirect impact on the expression of multiple downstream targets. Because the MAPK signaling pathway may cross-talk with other pathways, including the PI3K/Akt pathway, and because these other pathways are crucial for multiple aspects of cellular growth and differentiation (for example, epithelial mesenchymal transition) (75,76), inhibiting the MAPK signaling pathway may result in dysregulation of the components of other pathways. The cross regulation that exists between the pathways has partially been revealed by the control of Raf activity. As aforementioned, the activity of Raf is regulated by mutations of Ras in the MAPK signaling pathway, and/or by mutations of genes in in the PI3K/Akt pathway (including PI3K, phosphatase and tensin homolog and Akt), which may synergistically lead to abnormal activation of the MAPK pathway in oral cancer. This discovery indicates the presence of an intimate link between the MAPK and the PI3K/Akt pathways. In addition, these two pathways were reported to not only cause phosphorylation of downstream targets, but also to regulate a variety of pathways, including JAK/STAT, NF-κB and TGF-β pathways, via ERK and Akt phosphorylation (76). In this regard, complete exploration of these pathways and their interactions should be considered in order to improve oral cancer treatment.
In addition, current clinical practice for oral cancer often discontinues a given therapy following cancer progression. However, continuous clinical practice, including use of a certain inhibitor, is desirable in certain cases because an inhibitor of one component in the MAPK signaling pathway (for example, Ras) may partially suppress another component (for example, Raf) in the same pathway, which will contribute to improved oral cancer treatment. For instance, it is helpful to add a Ras inhibitor while keeping the patient on the Raf inhibitor with which they were already being treated. This was partially confirmed when the clinical benefits of continued ERK1/2 sub-pathway inhibition following cancer progression were demonstrated (77). However, several preclinical models revealed that discontinuation of a drug based on the inhibitors or intermittent scheduling may also slow tumor growth (78). Therefore, it is necessary to carry out further clinical research to elucidate whether continuous or intermittent dosing is optimal. Proper clinical design and scheduling are important and will enable further development of effective therapies for oral cancer patients.
Finally, inhibitors that target the MAPK signaling pathway may be cytostatic and not cytotoxic (75). There would be substantial toxicity problems for cytotoxic inhibitors. To address these problems, the inhibitors should be combined with cytotoxic chemotherapeutic drugs or radiation therapy, as well as other techniques which inhibit the growth of oral cancer (75,79). Such combinations are expected to improve oral cancer treatment.
In brief, the MAPK signaling pathway is complex due to the existence of multiple components, subfamilies and sub-pathways, which regulate different cellular functions, and to its interactions with other pathways which may elicit synergetic effects on normal and malignant cell growth. By further exploring the components, subfamilies and sub-pathways of the MAPK signaling pathway, as well as its interactions with other pathways and clinical practice modes, it will be possible to develop improved oral cancer treatments.
6. Conclusions
The present review detailed the important function of the MAPK signaling pathway in oral cancer, due to its involvement in tumor cell proliferation, differentiation, apoptosis, angiogenesis, invasion and metastasis. Based on the analysis of the individual sub-pathways and their mechanisms of action, the potential for regulation of the MAPK signaling pathway for targeted oral cancer therapy was reviewed. The challenges faced by the field and future research directions were also expanded upon. Further elucidation of the molecular components of the pathway, subfamilies, sub-pathways, interactions with other pathways and clinical practice modes will enable the development of improved oral cancer treatments.
Acknowledgements
The present study was partially supported by the National Natural Science Foundation of China (grant no. 81300841), the Health and Family Planning Commission of Hunan Province (grant no. C2016092), the Fundamental Research Funds for the Central Universities of Central South University (grant no. 2016zzts484), and the Xiangya Distinguished Doctors Program of Central South University. The authors would like to thank Dr Zhiwei Peng of the School of Mineral Processing and Bioengineering, Central South University (Changsha, China) for helpful discussion.
References
- 1.Grsic K, Opacic IL, Sitic S, Milkovic Perisa M, Suton P, Sarcevic B. The prognostic significance of estrogen receptor β in head and neck squamous cell carcinoma. Oncol Lett. 2016;12:3861–3865. doi: 10.3892/ol.2016.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng Q, Wang Y, Quan H, Li Y, Tang Z. Oral verrucous carcinoma: From multifactorial etiology to diverse treatment regimens (Review) Int J Oncol. 2016;49:59–73. doi: 10.3892/ijo.2016.3501. [DOI] [PubMed] [Google Scholar]
- 3.Rogers SJ, Harrington KJ, Rhys-Evans P, O-Charoenrat P, Eccles SA. Biological significance of c-erbB family oncogenes in head and neck cancer. Cancer Metastasis Rev. 2005;24:47–69. doi: 10.1007/s10555-005-5047-1. [DOI] [PubMed] [Google Scholar]
- 4.Smolensky D, Rathore K, Bourn J, Cekanova M. Inhibition of the PI3K/AKT pathway sensitizes oral squamous cell carcinoma cells to anthracycline-based chemotherapy in vitro. J Cell Biochem. 2017;118:2615–2624. doi: 10.1002/jcb.25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pramanik KK, Singh AK, Alam M, Kashyap T, Mishra P, Panda AK, Dey RK, Rana A, Nagini S, Mishra R. Reversion-inducing cysteine-rich protein with Kazal motifs and its regulation by glycogen synthase kinase 3 signaling in oral cancer. Tumour Biol. 2016;37:15253–15264. doi: 10.1007/s13277-016-5362-x. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 7.Chiba T, Soeno Y, Shirako Y, Sudo H, Yagishita H, Taya Y, Kawashiri S, Okada Y, Imai K. MALT1 Inhibition of oral carcinoma cell invasion and ERK/MAPK activation. J Dent Res. 2016;95:446–452. doi: 10.1177/0022034515621740. [DOI] [PubMed] [Google Scholar]
- 8.Affolter A, Muller MF, Sommer K, Stenzinger A, Zaoui K, Lorenz K, Wolf T, Sharma S, Wolf J, Perner S, et al. Targeting irradiation-induced mitogen-activated protein kinase activation in vitro and in an ex vivo model for human head and neck cancer. Head Neck. 2016;38(Suppl 1):E2049–E2061. doi: 10.1002/hed.24376. [DOI] [PubMed] [Google Scholar]
- 9.Kim GT, Lee SH, Kim YM. Torilis japonica extract-generated intracellular ROS induces apoptosis by reducing the mitochondrial membrane potential via regulation of the AMPK-p38 MAPK signaling pathway in HCT116 colon cancer. Int J Oncol. 2016;49:1088–1098. doi: 10.3892/ijo.2016.3578. [DOI] [PubMed] [Google Scholar]
- 10.Song X, Wei Z, Shaikh ZA. Requirement of ERα and basal activities of EGFR and Src kinase in Cd-induced activation of MAPK/ERK pathway in human breast cancer MCF-7 cells. Toxicol Appl Pharmacol. 2015;287:26–34. doi: 10.1016/j.taap.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng F, Tang Q, Wu J, Zhao S, Liang Z, Li L, Wu W, Hann S. p38α MAPK-mediated induction and interaction of FOXO3a and p53 contribute to the inhibited-growth and induced-apoptosis of human lung adenocarcinoma cells by berberine. J Exp Clin Cancer Res. 2014;33:36. doi: 10.1186/1756-9966-33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguzzi A, Maggioni D, Nicolini G, Tredici G, Gaini RM, Garavello W. MAP kinase modulation in squamous cell carcinoma of the oral cavity. Anticancer Res. 2009;29:303–308. [PubMed] [Google Scholar]
- 13.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: A new perspective. Cancer. 2014;120:3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signaling cascades and transcriptional regulation. Gene. 2013;513:1–13. doi: 10.1016/j.gene.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65:3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/A:1023781114568. [DOI] [PubMed] [Google Scholar]
- 17.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 19.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 20.Crane EK, Wong KK. The therapeutic promise of anti-cancer drugs against the Ras/Raf/MEK/ERK pathway. Topics Anti-Cancer Res. 2013;2:63–94. doi: 10.2174/9781608051366113020006. [DOI] [Google Scholar]
- 21.Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, Kolch W. Raf family kinases: Old dogs have learned new tricks. Genes Cancer. 2011;2:232–260. doi: 10.1177/1947601911407323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: Post-translational modification of RAS. Nat Rev Mol Cell Biol. 2011;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: Physiological feedback and drug response. Clin Cancer Res. 2010;16:3329–3334. doi: 10.1158/1078-0432.CCR-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wortzel I, Seger R. The ERK cascade: Distinct functions within various subcellular organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gough NR. Focus issue: Recruiting players for a game of ERK. Sci Signal. 2011;4:eg9. doi: 10.1126/scisignal.2002601. [DOI] [PubMed] [Google Scholar]
- 26.Ku NO, Azhar S, Omary MB. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: Modulation by a keratin 1-like disease causing mutation. J Biol Chem. 2002;277:10775–10782. doi: 10.1074/jbc.M107623200. [DOI] [PubMed] [Google Scholar]
- 27.Gkouveris I, Nikitakis N, Karanikou M, Rassidakis G, Sklavounou A. JNK1/2 expression and modulation of STAT3 signaling in oral cancer. Oncol Lett. 2016;12:699–706. doi: 10.3892/ol.2016.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park MK, Lee HJ, Shin J, Noh M, Kim SY, Lee CH. Novel participation of transglutaminase-2 through c-Jun N-terminal kinase activation in sphingosylphosphorylcholine-induced keratin reorganization of PANC-1 cells. Biochim Biophys Acta. 2011;1811:1021–1029. doi: 10.1016/j.bbalip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Mishima K, Inoue K, Hayashi Y. Overexpression of extracellular-signal regulated kinases on oral squamous cell carcinoma. Oral Oncol. 2002;38:468–474. doi: 10.1016/S1368-8375(01)00104-X. [DOI] [PubMed] [Google Scholar]
- 30.Simões AE, Rodrigues CM, Borralho PM. The MEK5/ERK5 signalling pathway in cancer: A promising novel therapeutic target. Drug Discov Today. 2016;21:1654–1663. doi: 10.1016/j.drudis.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 32.Raviv Z, Kalie E, Seger R. MEK5 and ERK5 are localized in the nuclei of resting as well as stimulated cells, while MEKK2 translocates from the cytosol to the nucleus upon stimulation. J Cell Sci. 2004;117:1773–1784. doi: 10.1242/jcs.01040. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Pan YW, Wang W, Abel G, Zou J, Xu L, Storm DR, Xia Z. Targeted deletion of the ERK5 MAP kinase impairs neuronal differentiation, migration, and survival during adult neurogenesis in the olfactory bulb. PLoS One. 2013;8:e61948. doi: 10.1371/journal.pone.0061948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyama T, Ogawara K, Kasamatsu A, Okamoto A, Kasama H, Minakawa Y, Shimada K, Yokoe H, Shiiba M, Tanzawa H, Uzawa K. ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer. Cancer Med. 2015;4:759–769. doi: 10.1002/cam4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu T, Kasamatsu A, Yamamoto A, Koike K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. Annexin A10 in human oral cancer: Biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways. PLoS One. 2012;7:e45510. doi: 10.1371/journal.pone.0045510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakasaki T, Masuda M, Niiro H, Jabbarzadeh-Tabrizi S, Noda K, Taniyama T, Komune S, Akashi K. A Critical role of c-Cbl-Interacting protein of 85 kDa in the development and progression of head and neck squamous cell carcinomas through the Ras-ERK pathway. Neoplasia. 2010;12:789–796. doi: 10.1593/neo.10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu X, Feng Y. QKI-5 suppresses cyclin D1 expression and proliferation of oral squamous cell carcinoma cells via MAPK signalling pathway. Int J Oral Maxillofac Surg. 2015;44:562–567. doi: 10.1016/j.ijom.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Williams MD. Integration of biomarkers including molecular targeted therapies in head and neck cancer. Head Neck Pathol. 2010;4:62–69. doi: 10.1007/s12105-010-0166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y, Zhang Y, Hua S, Fu Q, Zhao M, et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis. 2014;5:e1155. doi: 10.1038/cddis.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada T, Tsuda M, Ohba Y, Kawaguchi H, Totsuka Y, Shindoh M. PTHrP promotes malignancy of human oral cancer cell downstream of the EGFR signaling. Biochem Biophys Res Commun. 2008;368:575–581. doi: 10.1016/j.bbrc.2008.01.121. [DOI] [PubMed] [Google Scholar]
- 41.Wu D, Cheng J, Sun G, Wu S, Li M, Gao Z, Zhai S, Li P, Su D, Wang X. p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma. Oncotarget. 2016;7:36539–36550. doi: 10.18632/oncotarget.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang RW, Zeng YY, Wei WT, Cui YM, Sun HY, Cai YL, Nian XX, Hu YT, Quan YP, Jiang SL, et al. TLE3 represses colorectal cancer proliferation by inhibiting MAPK and AKT signaling pathways. J Exp Clin Cancer Res. 2016;35:152. doi: 10.1186/s13046-016-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Huang RH, Quan YJ, Chen JH, Wang TF, Xu M, Ye M, Yuan H, Zhang CJ, Liu XJ, Min ZJ. Osteopontin promotes cell migration and invasion, and inhibits apoptosis and autophagy in colorectal cancer by activating the p38 MAPK signaling pathway. Cell Physiol Biochem. 2017;41:1851–1864. doi: 10.1159/000471933. [DOI] [PubMed] [Google Scholar]
- 44.Lv D, Wu H, Xing R, Shu F, Lei B, Lei C, Zhou X, Wan B, Yang Y, Zhong L, et al. HnRNP-L mediates bladder cancer progression by inhibiting apoptotic signaling and enhancing MAPK signaling pathways. Oncotarget. 2017;8:13586–13599. doi: 10.18632/oncotarget.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Jin ZL, Xu H. MEK/ERK signaling pathway in apoptosis of SW620 cell line and inhibition effect of resveratrol. Asian Pac J Trop Med. 2016;9:49–53. doi: 10.1016/j.apjtm.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Liao Y, Sun Q, Tang H, Wang G, Zhao F, Jin Y. Upregulation of matrix metalloproteinase-9 in primary cultured rat astrocytes induced by 2-chloroethanol Via MAPK signal pathways. Front Cell Neurosci. 2017;11:218. doi: 10.3389/fncel.2017.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schafer JM, Peters DE, Morley T, Liu S, Molinolo AA, Leppla SH, Bugge TH. Efficient targeting of head and neck squamous cell carcinoma by systemic administration of a dual uPA and MMP-activated engineered anthrax toxin. PLoS One. 2011;6:e20532. doi: 10.1371/journal.pone.0020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munshi HG, Wu YI, Mukhopadhyay S, Ottaviano AJ, Sassano A, Koblinski JE, Platanias LC, Stack MS. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-beta1-induced pericellular collagenolysis. J Biol Chem. 2004;279:39042–39050. doi: 10.1074/jbc.M404958200. [DOI] [PubMed] [Google Scholar]
- 49.So KY, Kim SH, Jung KT, Lee HY, Oh SH. MAPK/JNK1 activation protects cells against cadmium-induced autophagic cell death via differential regulation of catalase and heme oxygenase-1 in oral cancer cells. Toxicol Appl Pharmacol. 2017;332:81–91. doi: 10.1016/j.taap.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Cossa G, Gatti L, Cassinelli G, Lanzi C, Zaffaroni N, Perego P. Modulation of sensitivity to antitumor agents by targeting the MAPK survival pathway. Curr Pharm Des. 2013;19:883–894. doi: 10.2174/138161213804547187. [DOI] [PubMed] [Google Scholar]
- 51.De Luca A, Maiello MR, D'Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signaling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16(Suppl 2):S17–S27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Liu J, Cui F, Xing L, Wang J, Yan X, Zhang X. ERK and p38 MAPK signaling pathways are involved in ochratoxin A-induced G2 phase arrest in human gastric epithelium cells. Toxicol Lett. 2012;209:186–192. doi: 10.1016/j.toxlet.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Ahn HJ, Kim G, Park KS. Ell3 stimulates proliferation, drug resistance, and cancer stem cell properties of breast cancer cells via a MEK/ERK-dependent signaling pathway. Biochem Biophys Res Commun. 2013;437:557–564. doi: 10.1016/j.bbrc.2013.06.114. [DOI] [PubMed] [Google Scholar]
- 54.Neuzillet C, Hammel P, Tijeras-Raballand A, Couvelard A, Raymond E. Targeting the Ras-ERK pathway in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013;32:147–162. doi: 10.1007/s10555-012-9396-2. [DOI] [PubMed] [Google Scholar]
- 55.Ahmeda TA, Hayslip J, Leggas M. Simvastatin interacts synergistically with tipifarnib to induce apoptosis in leukemia cells through the disruption of RAS membrane localization and ERK pathway inhibition. Leuk Res. 2014;38:1350–1357. doi: 10.1016/j.leukres.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JC, Chung LC, Chen YJ, Feng TH, Chen WT, Juang HH. Upregulation of B-cell translocation gene 2 by epigallocatechin-3-gallate via p38 and ERK signaling blocks cell proliferation in human oral squamous cell carcinoma cells. Cancer Lett. 2015;360:310–318. doi: 10.1016/j.canlet.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 57.D'Ambrosio SM, Han C, Pan L, Kinghorn AD, Ding H. Aliphatic acetogenin constituents of avocado fruits inhibit human oral cancer cell proliferation by targeting the EGFR/RAS/RAF/MEK/ERK1/2 pathway. Biochem Biophys Res Commun. 2011;409:465–469. doi: 10.1016/j.bbrc.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henson BS, Neubig RR, Jang I, Ogawa T, Zhang Z, Carey TE, D'Silva NJ. Galanin receptor 1 has anti-proliferative effects in oral squamous cell carcinoma. J Biol Chem. 2005;280:22564–22571. doi: 10.1074/jbc.M414589200. [DOI] [PubMed] [Google Scholar]
- 59.Tang FY, Chiang EP, Chung JG, Lee HZ, Hsu CY. S-Allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J Nutr Biochem. 2009;20:1013–1020. doi: 10.1016/j.jnutbio.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Ko CP, Lin CW, Chen MK, Yang SF, Chiou HL, Hsieh MJ. Pterostilbene induce autophagy on human oral cancer cells through modulation of Akt and mitogen-activated protein kinase pathway. Oral Oncol. 2015;51:593–601. doi: 10.1016/j.oraloncology.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Kavitha K, Kowshik J, Kishore TK, Baba AB, Nagini S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim Biophys Acta. 2013;1830:4433–4444. doi: 10.1016/j.bbagen.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 62.Han HY, Kim H, Jeong SH, Lim DS, Ryu MH. Sulfasalazine induces autophagic cell death in oral cancer cells via Akt and ERK pathways. Asian Pac J Cancer Prev. 2014;15:6939–6944. doi: 10.7314/APJCP.2014.15.16.6939. [DOI] [PubMed] [Google Scholar]
- 63.Psyrri A, Lee JW, Pectasides E, Vassilakopoulou M, Kosmidis EK, Burtness BA, Rimm DL, Wanebo HJ, Forastiere AA. Prognostic biomarkers in phase II trial of cetuximab-containing induction and chemoradiation in resectable HNSCC: Eastern cooperative oncology group E2303. Clin Cancer Res. 2014;20:3023–3032. doi: 10.1158/1078-0432.CCR-14-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshimoto T, Takino T, Li Z, Domoto T, Sato H. Vinculin negatively regulates transcription of MT1-MMP through MEK/ERK pathway. Biochem Biophys Res Commun. 2014;455:251–255. doi: 10.1016/j.bbrc.2014.10.154. [DOI] [PubMed] [Google Scholar]
- 65.Chen HJ, Lin CM, Lee CY, Shih NC, Amagaya S, Lin YC, Yang JS. Phenethyl isothiocyanate suppresses EGF-stimulated SAS human oral squamous carcinoma cell invasion by targeting EGF receptor signaling. Int J Oncol. 2013;43:629–637. doi: 10.3892/ijo.2013.1977. [DOI] [PubMed] [Google Scholar]
- 66.Yen CY, Liang SS, Han LY, Chou HL, Chou CK, Lin SR, Chiu CC. Cardiotoxin III inhibits proliferation and migration of oral cancer cells through MAPK and MMP signaling. Scientific World Journal. 2013;2013:650946. doi: 10.1155/2013/650946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin FY, Hsieh YH, Yang SF, Chen CT, Tang CH, Chou MY, Chuang YT, Lin CW, Chen MK. Resveratrol suppresses TPA-induced matrix metalloproteinase-9 expression through the inhibition of MAPK pathways in oral cancer cells. J Oral Pathol Med. 2015;44:699–706. doi: 10.1111/jop.12288. [DOI] [PubMed] [Google Scholar]
- 68.Jänne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, Franke FA, Grinsted L, Zazulina V, Smith P, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: A randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 69.Grisham RN, Iyer G, Garg K, DeLair D, Hyman DM, Zhou Q, Iasonos A, Berger MF, Dao F, Spriggs DR, et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119:548–554. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das N, Majumder J, DasGupta UB. Ras gene mutations in oral cancer in eastern India. Oral Oncol. 2000;36:76–80. doi: 10.1016/S1368-8375(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 71.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: An updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 72.Collisson EA, De A, Suzuki H, Gambhir SS, Kolodney MS. Treatment of metastatic melanoma with an orally available inhibitor of the Ras-Raf-MAPK cascade. Cancer Res. 2003;63:5669–5673. [PubMed] [Google Scholar]
- 73.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, et al. Mechanism of activation of the RAF-ERK signalingpathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 74.Antipina MN, Kiryukhin MV, Skirtach AG, Sukhorukov GB. Micropackaging via layer-by-layer assembly: Microcapsulesand microchamber arrays. Int Mater Rev. 2014;59:224–244. doi: 10.1179/1743280414Y.0000000030. [DOI] [Google Scholar]
- 75.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and JAK/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 77.Carlino MS, Gowrishankar K, Saunders CA, Pupo GM, Snoyman S, Zhang XD, Saw R, Becker TM, Kefford RF, Long GV, Rizos H. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther. 2013;12:1332–1342. doi: 10.1158/1535-7163.MCT-13-0011. [DOI] [PubMed] [Google Scholar]
- 78.Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP, Dummer R, McMahon M, Stuart DD. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imparato G, Urciuolo F, Netti PA. In vitro three-dimensional models in cancer research: A review. Int Mater Rev. 2015;60:297–311. doi: 10.1179/1743280415Y.0000000003. [DOI] [Google Scholar]


