SUMMARY
SETTING
Limited data exist on the prevalence and correlates, including stigma, of mental health conditions, including depressive symptoms and alcohol use, among patients co-infected with tuberculosis (TB) and the human immunodeficiency virus (HIV) in sub-Saharan Africa, despite their negative impact on health outcomes.
OBJECTIVE
To assess the prevalence and correlates of depressive symptoms and hazardous/harmful alcohol use among TB-HIV patients in the Start TB patients on ART and Retain on Treatment (START) study.
DESIGN
START, a mixed-methods cluster-randomized trial, evaluated a combination intervention package vs. standard of care (SOC) to improve treatment outcomes in TB-HIV co-infected patients in Lesotho. Moderate/ severe depressive symptoms and hazardous/harmful alcohol use were measured using baseline questionnaire data collected from April 2013 to March 2015. Demographic, psychosocial, and TB- and HIV-related knowledge and attitudes, including stigma, were assessed for association with both conditions using generalized linear mixed models.
RESULTS
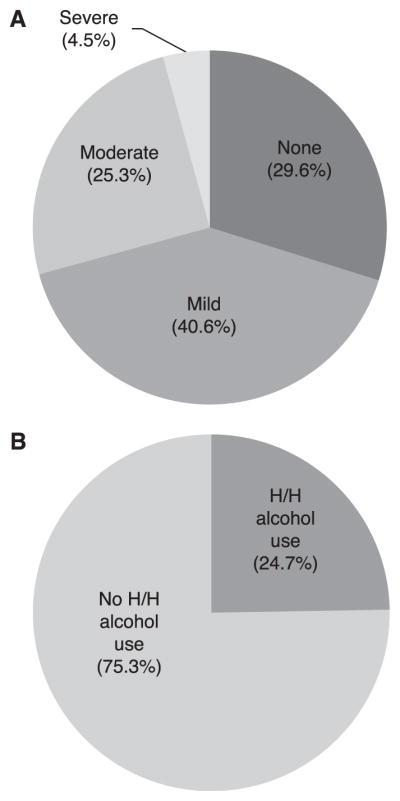
Among 371 participants, 29.8% reported moderate/severe depressive symptoms, and 24.7% reported hazardous/harmful alcohol use; 7% reported both. Depressive symptoms were significantly associated with less education, more difficulty understanding written medical information, non-disclosure of TB, greater TB stigma, and the SOC study arm. Hazardous/harmful alcohol use was significantly associated with male sex, as well as greater TB and external HIV stigma.
CONCLUSION
Prevalence of depressive symptoms and hazardous/harmful alcohol use were high, suggesting a need for routine screening for, and treatment of, mental health disorders in TB-HIV patients.
Keywords: TB-HIV co-infection, depression, alcohol, mental health, stigma
Although infectious diseases such as tuberculosis (TB) and the human immunodeficiency virus (HIV) remain among the most important causes of lives lost in sub-Saharan Africa, improved prevention and treatment for infectious diseases have resulted in increasing prominence of morbidity and mortality due to non-communicable diseases.1 It is to be noted that mental health disorders contributed an estimated 25 million disability-adjusted life-years in sub-Saharan Africa in 2012, with projected increases of 130% by 2050.1,2 In countries with a high TB and HIV burden, understanding the relationship between mental health and TB and HIV is critical in the response to these epidemics.
TB, HIV infection and mental health have a complex and bidirectional relationship. For example, alcohol use and alcohol-use disorders are associated with an increased risk of TB; alcohol use has also been shown to increase risky sexual behavior, which can lead to HIV transmission.3,4 Conversely, both TB and HIV are heavily stigmatized diseases,5 and diagnoses may lead to poor mental health outcomes.6 Mental health conditions may further negatively affect TB and HIV outcomes, including delayed access to care, poor adherence, greater loss to follow-up, and treatment failure.7–12
While the relationships between depressive symptoms or hazardous/harmful alcohol use with TB and HIV have been studied separately, as described above, there are limited data on TB-HIV co-infected patients. Some studies have suggested that co-infected patients bear an even larger burden of mental health conditions than their counterparts living with either HIV or TB,13,14 but the affected population has not been well characterized.
We sought to add to the limited literature on mental health among TB-HIV patients by using data from co-infected patients initiating antiretroviral therapy (ART) in Lesotho, which bears a high burden of both TB and HIV.15–17 Our study aims were to characterize the prevalence of depressive symptoms and hazardous/harmful alcohol use, and to explore correlates of these conditions.
STUDY POPULATION AND METHODS
Study design and participants
The scientific basis, design and methodology of the Start TB patients on ART and Retain on Treatment (START) study, including participant sampling, have been described elsewhere.18 Briefly, START was a mixed-methods cluster-randomized implementation trial conducted in 12 health facilities in Berea District, Lesotho, to evaluate the effectiveness, cost-effectiveness and acceptability of a combination intervention package (CIP) vs. standard of care (SOC) among TB-HIV patients. The CIP aimed to improve early ART initiation, retention, and TB treatment success using programmatic, structural, and psychosocial interventions.
The START population comprised all TB-HIV patients who initiated anti-tuberculosis treatment at study sites between April 2013 and March 2015 (n = 1233). Additional data were collected from a measurement cohort enrolled at the time of ART initiation,18 including 191 participants from CIP and 177 participants from SOC; this was the population used for the current analysis. Eligibility criteria for the measurement cohort included those who 1) were HIV-positive, 2) were on anti-tuberculosis treatment, 3) had initiated ART ≤2 months after anti-tuberculosis treatment initiation, 4) were aged ≥18 years, 5) were English- or Sesotho-speaking, and 6) were capable of providing written informed consent. Patients with multidrug-resistant TB were excluded.
The study protocol was approved by the institutional review board at the Columbia University Medical Center, New York, NY, USA (IRB-AAAK7103) and Lesotho’s National Health Institutional Review Board and Ethics Research Committee, Maseru, Lesotho (ID68-2012). Written informed consent was provided by all measurement cohort participants. The study was registered at Clinical-Trials.gov (NCT01872390).
Data sources and measures
In-person interviews in English or Sesotho were conducted at enrollment. Data on demographic characteristics, psychosocial variables, and TB- and HIV-related variables were collected. Detailed information on instruments and data management have been reported elsewhere.18
The primary outcomes of this analysis were depressive symptoms and hazardous/harmful alcohol use. Depressive symptoms were measured using the Patient Health Questionnaire-9 item (PHQ-9, Cronbach’s α 0.7),19 which assesses symptoms in the 2 weeks before the interview. Responses from the nine items were summed, and the following established thresholds were used to categorize participants’ depressive symptoms: <5, none; 5 to <10, mild; 10 to <15, moderate; ≥15, severe.19 For the analysis of correlates, symptoms were dichotomized to ‘none or mild’ and ‘moderate to severe’. Alcohol use was measured by the Alcohol Use Disorders Identification Test (AUDIT, Cronbach’s α 0.9),20 which assesses alcohol-use behaviors in the year prior to administration. The established threshold of score ≥8 was used to define hazardous/harmful alcohol use in the previous year.20
Correlates of interest included sociodemographic characteristics such as age, sex, marital status, education, head-of-household status, and household amenities, including electricity and improved water sources,21 as indicators of socio-economic status. To measure social support, participants were asked ‘how many people have provided help or encouragement to you in the past month?’ Understanding of written health information was measured by the question ‘how often do you have problems learning about your medical condition because of difficulty understanding written information?’ using a five-point Likert scale (1 = always, 5 = never) that was dichotomized with the middle category included as a negative response.
We had several measures of TB- and HIV-related stigma. To assess TB stigma, we used the 11-item community perspective TB stigma scale validated by van Rie et al. in 200822 (Cronbach’s α 0.9), which captures perceived community-based stigma. We also used three items related to internalized stigma and social isolation, as well as planned TB disclosure (see Appendix).* HIV stigma was assessed with the item ‘people taking antiretroviral medicines need to hide this from others’, as well as by disclosure status, which is often a proxy for stigma.23
Other TB- and HIV-related correlates were also included. Seven true/false items related to knowledge about TB and HIV assessed participants’ broad level of information on transmission, symptoms, and treatment (e.g., ‘people are cured of TB as soon as their symptoms stop’ and ‘antiretroviral [ARV] medicines will help [me/my partner] have a baby without HIV’); these were included in the analysis as two scores ranging from 0 to 7 (TB and HIV knowledge considered separately) based on the total number of items answered correctly, with ‘don’t know’ and ‘missing’ coded as ‘incorrect’. Attitudes about the prognosis (‘persons with HIV can live a long and healthy life if they take ARV medicines’) were administered as a four-point Likert scale (1 = strongly disagree, 4 = strongly agree) and dichotomized to agree/disagree. Clinical variables, including the time since HIV diagnosis and CD4 count at ART initiation, were collected from medical records.
As reporting of sensitive data may be affected by social desirability bias, the Marlowe-Crowne social desirability scale24 (Cronbach’s α 0.8) was administered to participants. Because of possible exposure to the CIP interventions in the time between TB treatment initiation and ART initiation, the CIP study arm was also included as a correlate.
Statistical analysis
All analyses were conducted using SAS® 9.4 (Statistical Analysis System, Cary, NC, USA). Participant characteristics and prevalence of outcomes were summarized with descriptive statistics. Odds ratios (ORs) for the associations between the correlates of interest and outcomes were assessed using generalized linear mixed models with a random effect for study site to account for the clustering of participants within health facilities in the study design.
RESULTS
Sample characteristics
Of 371 participants, the median age was 35 years (interquartile range [IQR] 30–44); 57% were male (Table 1). About half (54%) were married or cohabiting. Most participants (69%) had primary school education or less; a substantial majority (85%) had access to an improved water source, but less than half (48%) had any electricity in their homes. The median time since HIV diagnosis was 33 days (IQR 20–83); among 212 participants with available data, the median CD4 count at ART initiation was 151 cells/μl (IQR 59–307).
Table 1.
Characteristics of TB-HIV co-infected measurement cohort participants enrolled in the START study*
| Demographic variables | (n = 371) n (%) |
|---|---|
| Age, years, median [IQR] | 35 [30–44] |
| Female | 160 (43.1) |
| Marital status | |
| Married/cohabiting | 202 (54.4) |
| Divorced/widowed | 98 (26.4) |
| Never married/cohabited | 71 (19.1) |
| Head of household (sole/shared) | 168 (46.0) |
| Education | |
| None | 29 (7.8) |
| Primary | 228 (61.5) |
| Secondary or higher | 114 (30.7) |
| Home amenities | |
| Improved drinking water | 314 (84.6) |
| Any electricity | 178 (48.0) |
| Grid electricity | 108 (29.6) |
| Solar electricity | 51 (13.7) |
| Battery/generator | 38 (10.2) |
| Clinical variables | |
| Time since HIV diagnosis, days, median [IQR] | 33 [20–83] |
| CD4 cell count at ART initiation, /μl, median [IQR] | 151 [59–307] |
| CIP study arm | 191 (51.5) |
Column numbers may not sum to totals due to missing data. Percentages are provided among non-missing data; missing data: age (n = 1); head of household (n = 6); time since HIV diagnosis (n = 23); CD4 count (n = 159).
TB=tuberculosis; HIV=human immunodeficiency virus; START=Start TB patients on ARTand Retain on Treatment; IQR = interquartile range; ART =antiretroviral therapy; CIP = combination intervention package.
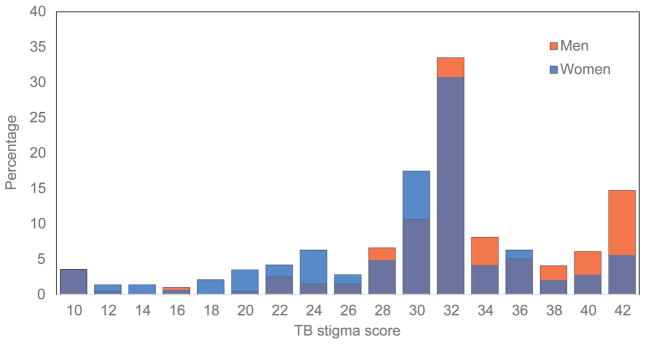
The prevalence of both depressive symptoms and hazardous/harmful alcohol use are shown in the Figure. Overall, 30% (95% confidence interval [CI] 25–35) of participants reported moderate or severe depressive symptoms. A quarter of the sample (25%, 95%CI 20–29) reported hazardous/harmful alcohol use. However, only 24 (7%) participants reported both moderate/severe depressive symptoms and hazardous/harmful alcohol use; these individuals were predominantly men (n = 20, 83%) who commonly endorsed needing to hide ARV medicines (n = 18, 75%; data not shown).
Figure.
Prevalence of depressive symptoms and H/H alcohol use among START measurement cohort participants. A) Prevalence and severity of depressive symptoms in the previous 2 weeks and B) prevalence of H/H alcohol use in the previous year. H/H = hazardous/harmful.
Correlates of depressive symptoms
Correlates of depressive symptoms among the 355 participants with complete data on depressive symptoms are shown in Table 2. Participants without education were substantially more likely to report moderate/severe depressive symptoms than those with at least some secondary education (OR 2.60, 95%CI 1.10–6.15); those with little trouble understanding written medical information were less likely to report moderate/severe depressive symptoms (OR 0.35, 95%CI 0.21–0.57). Those who felt ashamed of having TB (OR 2.39, 95%CI 1.28–4.46), and participants who believed that others would avoid them because of their TB (OR 1.74, 95%CI 1.08–2.81), or that some people feel hurt because of how others treat them due to their TB (OR 2.85, 95%CI 1.22–6.64), were also more likely to report moderate/ severe depressive symptoms than those who did not endorse those items. Those who planned to disclose their TB were less likely to report moderate/severe depressive symptoms than those who did not (OR 0.41, 95%CI 0.17–0.98). Fewer participants in CIP reported moderate/severe depressive symptoms compared to SOC (OR 0.56, 95%CI 0.35–0.89). Greater socially desirable response tendencies were associated with less reporting of moderate/severe depressive symptoms (OR 0.94 per point, 95%CI 0.90–0.98).
Table 2.
Factors associated with moderate-to-severe depressive symptoms among START measurement cohort participants*
| Moderate-to-severe depressive symptoms (n = 106) n (%) |
No moderate-to-severe depressive symptoms (n = 249) n (%) |
OR (95%CI) | |
|---|---|---|---|
| Demographic variables | |||
| Age, years, median [IQR] | 37 [31–44] | 35 [30–43] | 1.01 (0.99–1.03) |
| Male | 61 (57.6) | 143 (57.4) | 1.03 (0.65–1.64) |
| Marital status | |||
| Married/cohabiting | 59 (55.7) | 134 (53.8) | 1.17 (0.64–2.17) |
| Divorced/widowed | 28 (26.4) | 65 (26.1) | 1.15 (0.57–2.29) |
| Never married/cohabited | 19 (17.9) | 50 (20.1) | Reference |
| Head of household (sole/shared) | 48 (46.2) | 112 (45.7) | 1.04 (0.65–1.66) |
| Education | |||
| None | 13 (12.3) | 16 (6.4) | 2.60 (1.10–6.15)† |
| Primary | 67 (63.2) | 148 (59.4) | 1.49 (0.88–2.54) |
| Secondary or higher | 26 (24.5) | 85 (34.1) | Reference |
| Home amenities | |||
| Improved drinking water | 93 (87.7) | 208 (83.5) | 1.34 (0.71–2.75) |
| Any electricity | 49 (46.2) | 122 (49.0) | 0.90 (0.57–1.43) |
| Psychosocial variables | |||
| Hazardous or harmful alcohol use | 24 (22.6) | 64 (25.8) | 0.88 (0.51–1.51) |
| Size of support network, median [IQR] | 4 [3–10] | 5 [3–9] | 1.01 (0.98–1.04) |
| Little trouble understanding written medical information | 61 (57.5) | 196 (79.4) | 0.35 (0.21–0.57)† |
| Social desirability score, median [IQR] | 36 [34–43] | 39 [35–46] | 0.94 (0.90–0.98)† |
| TB- and HIV-related stigma variables | |||
| TB stigma scale score, median [IQR] | 33 [32–35] | 33 [30–37] | 1.00 (1.00–1.01) |
| You feel ashamed to have TB | 23 (21.9) | 26 (10.5) | 2.39 (1.28–4.46)† |
| People close to you would avoid you because of TB | 49 (48.5) | 89 (36.0) | 1.74 (1.08–2.81)† |
| Some people with TB feel hurt because of how they are treated by others | 98 (93.3) | 207 (83.8) | 2.85 (1.22–6.64)† |
| Plan to disclose TB | 94 (89.5) | 238 (95.6) | 0.41 (0.17–0.98)† |
| Believe that people need to hide ARVs | 60 (57.7) | 131 (52.8) | 1.22 (0.77–1.93) |
| Disclosed HIV status | 99 (93.4) | 243 (97.6) | 0.37 (0.12–1.15) |
| Other TB- and HIV-related variables | |||
| Time since HIV diagnosis, days, median [IQR] | 35 [21–62] | 30 [19–126] | 1.00 (1.00–1.00) |
| CD4 cell count at ART initiation, /μl, median [IQR] | 104 [54–192] | 181 [69–331] | 1.00 (1.00–1.00) |
| Other person in home with HIV | 39 (39.8) | 86 (36.0) | 1.22 (0.75–1.98) |
| HIV knowledge, median [IQR] | 5 [4–6] | 5 [4–6] | 0.94 (0.78–1.12) |
| Believe that ART can provide a long, healthy life | 98 (95.1) | 245 (98.4) | 0.32 (0.08–1.20) |
| TB knowledge, median [IQR] | 6 [5–6] | 6 [5–6] | 0.96 (0.79–1.16) |
| CIP study arm | 44 (41.5) | 139 (55.8) | 0.56 (0.35–0.89)† |
Analysis was limited to 355 participants with complete data on depressive symptoms. Column numbers may not sum to totals due to missing data. Percentages are provided among non-missing data; missing data: age (n =1); head of household (n =6); alcohol use (n =1); support network size (n =2); medical information literacy (n=2); social desirability (n=8); TB stigma (n=28); ashamed of having TB (n=2); TB avoid (n=7); hurt feelings due to TB (n=3); plan to disclose TB (n=1); need to hide ARVs (n = 3); time since HIV diagnosis, (n = 21); CD4 count (n = 150); other person with HIV in home (n = 18); ARVs provide long life (n = 3).
Statistically significant.
START =Start TB patients on ART and Retain on Treatment; OR =odds ratio; CI =confidence interval; IQR =interquartile range; TB =tuberculosis; HIV =human immunodeficiency virus; ART =antiretroviral therapy; CIP = combination intervention package; ARV =antiretroviral drug.
Correlates of hazardous/harmful alcohol use
Table 3 presents correlates of hazardous/harmful alcohol use among the 364 participants with complete data on alcohol use. Men were significantly more likely than women to report hazardous/harmful alcohol use (OR 9.17, 95%CI 4.54–18.50). Other significant correlates included believing that people on ARVs need to hide their medication (OR 1.93, 95%CI 1.17–3.19), that they have greater TB stigma (OR 1.04 per point on the van Rie scale, 95%CI 1.00–1.08), and that they would be avoided because they had TB (OR 1.87, 95%CI 1.14–3.07). Participants with greater socially desirable response tendencies were less likely to report hazardous/harmful alcohol use (OR 0.94 per point, 95%CI 0.91–0.99).
Table 3.
Factors associated with hazardous/harmful alcohol use among START measurement cohort participants*
| Hazardous/harmful alcohol use (n = 90) n (%) |
No hazardous/harmful alcohol use (n = 274) n (%) |
OR (95%CI) | |
|---|---|---|---|
| Demographic variables | |||
| Age, years, median [IQR] | 34 [30–42] | 36 [30–44] | 0.99 (0.97–1.01) |
| Male | 80 (88.9) | 127 (46.4) | 9.17 (4.54–18.50)† |
| Marital status | |||
| Married/cohabiting | 47 (52.2) | 150 (54.7) | 0.73 (0.39–1.34) |
| Divorced/widowed | 22 (24.4) | 74 (27.0) | 0.68 (0.34–1.38) |
| Never married/cohabited | 21 (23.3) | 50 (18.2) | Reference |
| Head of household (sole/shared) | 43 (48.3) | 122 (45.4) | 1.09 (0.67–1.79) |
| Education | |||
| None | 7 (7.8) | 22 (8.0) | 1.05 (0.40–2.78) |
| Primary | 56 (62.2) | 166 (60.6) | 1.08 (0.63–1.86) |
| Secondary or higher | 27 (30.0) | 86 (31.4) | Reference |
| Home amenities | |||
| Improved drinking water | 78 (86.7) | 230 (83.9) | 1.28 (0.64–2.56) |
| Any electricity | 43 (47.8) | 134 (48.9) | 0.93 (0.57–1.50) |
| Psychosocial variables | |||
| Depressive symptoms | |||
| None | 23 (26.1) | 81 (30.5) | Reference |
| Mild | 41(46.6) | 103 (38.7) | 1.39 (0.77–2.51) |
| Moderate to severe | 24 (27.3) | 82 (30.8) | 1.08 (0.56–2.08) |
| Size of support network, median [IQR] | 5 [3–12] | 4 [3–8] | 1.02 (0.99–1.05) |
| Little trouble understanding written medical information | 61 (67.8) | 203 (74.4) | 0.72 (0.42–1.23) |
| Social desirability score, median [IQR] | 36 [33–43] | 39 [35–46] | 0.94 (0.91–0.99)† |
| TB- and HIV-related stigma variables | |||
| TB stigma scale score, median [IQR] | 33 [33–37] | 33 [30–35] | 1.04 (1.00–1.08)† |
| You feel ashamed to have TB | 14 (15.7) | 36 (13.4) | 1.23 (0.62–2.44) |
| People close to you would avoid you because of TB | 47 (52.8) | 95 (36.1) | 1.87 (1.14–3.07)† |
| Some people with TB feel hurt because of how they are treated by others | 80 (89.9) | 229 (85.8) | 1.43 (0.66–3.12) |
| Plan to disclose TB status | 83 (93.3) | 253 (94.1) | 0.83 (0.31–2.23) |
| Believe that people need to hide ARVs | 60 (66.7) | 138 (50.9) | 1.93 (1.17–3.19)† |
| Disclosed HIV status | 86 (95.6) | 263 (96.0) | 0.82 (0.25–2.70) |
| Other TB- and HIV-related variables | |||
| Time since HIV diagnosis, days, median [IQR] | 31 [17–59] | 32 [20–99] | 1.00 (1.00–1.00) |
| CD4 cell count at ART initiation, /μl, median [IQR] | 156 [88–354] | 151 [55–301] | 1.00 (1.00–1.00) |
| Other person in home with HIV | 28 (31.8) | 98 (38.1) | 0.70 (0.41–1.19) |
| HIV knowledge, median [IQR] | 5 [4–6] | 5 [4–6] | 1.11 (0.91–1.36) |
| Believe that ART can provide long, healthy life | 87 (97.8) | 265 (97.4) | 1.12 (0.23–5.84) |
| TB knowledge, median [IQR] | 5 [5–6] | 6 [5–6] | 0.94 (0.76–1.16) |
| CIP study arm | 56 (62.2) | 132 (48.2) | 1.71 (0.97–3.01) |
Analysis was limited to participants with complete data on alcohol use. Column numbers may not sum to totals due to missing data. Percentages are provided among non-missing data; missing data: age (n =1); head of household (n =6); depressive symptoms (n =10); support network size (n =1); medical information literacy (n=1); social desirability (n=12); TB stigma (n=33); ashamed of having TB (n=7); TB avoid (n=12); hurt feelings due to TB (n=8); plan to disclose TB (n= 6); need to hide ARVs (n = 3); time since HIV diagnosis (n = 23); CD4 count (n = 156); other person with HIV in home (n = 19); ARVs provide long life (n = 3).
START =Start TB patients on ART and Retain on Treatment; OR =odds ratio; CI =confidence interval; IQR =interquartile range; TB =tuberculosis; HIV =human immunodeficiency virus; ART antiretroviral therapy; CIP = combination intervention package; ARV =antiretroviral drug.
DISCUSSION
The study sought to characterize the prevalence of moderate/severe depressive symptoms and hazardous/harmful alcohol use in TB-HIV co-infected patients in Lesotho, and to describe the correlates of these conditions. We found a high prevalence of both, a result that is consistent with the limited literature on mental health among individuals with HIV and TB in sub-Saharan Africa.11,25–27 Depressive symptoms and hazardous/harmful alcohol use appeared to primarily affect distinct groups. Due to the small sample size, we did not formally explore correlates, but individuals who reported both were predominantly men who endorsed higher external stigma about HIV.
In general, moderate/severe depressive symptoms were correlated with more disadvantage and vulnerability, and greater stigma. In agreement with previous studies, those with less education and those who had trouble understanding written medical information were more likely to report moderate/ severe depressive symptoms,10,28 which may be due to a lack of understanding of the diagnoses, treatment, and prognosis. Furthermore, our findings confirm that both internalized and external stigma, as measured by items related to shame, social isolation, and plans to disclose TB diagnosis, are important correlates of depressive symptoms in TB-HIV patients.10 The TB stigma scale was not significantly correlated with depressive symptoms; this may have been due to a greater relative importance of internalized stigma (as different types of stigma may operate in different ways), or because participants had only recently been diagnosed with TB and therefore the community-based stigma had not yet affected mental health outcomes.
The finding of fewer depressive symptoms in the CIP arm of the trial may be a reflection of exposure to the CIP interventions in the time between initiation of TB treatment and the interview at ART initiation; the PHQ-9 measures symptoms in the preceding 2 weeks, which was approximately the average time from TB treatment initiation to ART initiation in our sample. Further work is needed to assess the effect of the CIP interventions on depressive symptoms over time.
In our study, a very strong correlate of hazardous/ harmful alcohol use was male sex. This observation is consistent with other literature from the region,14,25 which suggests an ongoing need for outreach to men. There was also substantial evidence that measures of stigma related to both TB and HIV were correlated with hazardous/harmful alcohol use. In particular, external HIV stigma and perceived social isolation due to TB were both strongly related. Perceived community-based TB stigma was also a significant correlate; the small effect size could (at least in part) have been due to the fact that the OR represents a ‘per point’ increase in the odds of hazardous/harmful alcohol use, but also because the AUDIT measures alcohol use over the previous year,20 and participants had generally been diagnosed with TB in the previous 2 weeks. Because both hazardous/harmful alcohol use and stigma are associated with non-adherence to TB medication and ART,9,10,29 these individuals may be at particularly high risk for poor treatment outcomes. In addition, because alcohol-use behaviors likely predated TB diagnosis, counseling about behavior change may be needed before or at treatment initiation.
A key limitation of this study was its cross-sectional nature; some correlates, such as stigma, may become more important later after the diagnosis of TB and/or HIV. We did not have clinical diagnoses of depression and alcohol-use disorders, and although our measures have been validated in sub-Saharan Africa,20,30–32 some have suggested that alternative thresholds should be used.33 Nonetheless, the scales had good reliability in our sample and, in the absence of validated alternative thresholds, we chose to use standards to enhance comparability. Finally, more socially desirable Marlow-Crowne response tendencies were correlated with a lower likelihood of reporting both depressive symptoms and hazardous/ harmful alcohol use; this suggests that our high prevalence estimates are likely to be underestimates. Our results demonstrate the need for more research using culturally adapted clinical diagnoses of depression and alcohol-use disorders, which may be less subject to these limitations.
The high prevalence of symptomatology we found, coupled with the extensive evidence about poor treatment outcomes among TB and HIV patients with mental health conditions,7–11 suggest there is a need to increase and improve screening and treatment for depressive symptoms and hazardous/harmful alcohol use in Lesotho. Although our study is among the first to quantitatively assess these conditions among TB-HIV patients, and to investigate TB- and HIV-related stigma, the data were collected for another primary aim. Studies focusing on alcohol-use disorders and depression in this vulnerable population using culturally adapted clinical diagnoses would provide stronger evidence for the burden of this syndemic. In addition, more work is needed to determine the best approach for integrating mental health care given the limited resources available.33 The World Health Organization African Region has, on average, only 1.4 mental health workers per 100 000 population.34 Innovative approaches, such as the use of generalist or lay workers, have shown promise,35 and merit further research to meet the substantial need for mental health services among TB-HIV patients in high-burden settings such as Lesotho.
In conclusion, depressive symptoms and hazardous/harmful alcohol use were highly prevalent in our sample of TB-HIV patients in Lesotho. Future studies should use culturally adapted clinical diagnoses of alcohol-use disorders and depression to better characterize this syndemic. Nonetheless, hazardous/harmful alcohol use and depressive symptoms may have an important clinical impact, and integrating routine screening and treatment may help to achieve and sustain positive treatment outcomes for TB-HIV patients in resource-limited settings.
Acknowledgments
The authors thank the study participants for partaking in the study; the study team, including M Shale, L Lebelo, M Ntoane, staff at the study sites, village health workers in the surrounding communities, the Berea District Health Management Team and the Lesotho Ministry of Health, Maseru, Lesotho, for their invaluable roles in conducting this study.
This work was supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through the United States Agency for International Development (Washington DC, USA) under the terms of Award Number USAID-OAA-A-12-00022. EHL and YHM were supported by the National Institute of Allergy & Infectious Diseases of the National Institutes of Health, Bethesda, MD, USA, under award numbers T32AI114398 and 1K01A104351, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Government.
Conflicts of interest: none declared.
APPENDIX
TB stigma measures
Fourteen items assessed TB-related stigma: the 11 community-perspective stigma items reported by van Rie22 (listed in Table A.1), and three additional items (‘you are ashamed to have TB’, ‘people close to you would avoid you if they thought you had TB’ and ‘some people who have TB feel hurt because of how others react to knowing they have TB’). We conducted exploratory factor analysis of the 14 items, which were all administered as four-point Likert scale items (1 = strongly disagree, 4 = strongly agree), using parallel analysis, followed by principal axis factoring with oblique rotation.
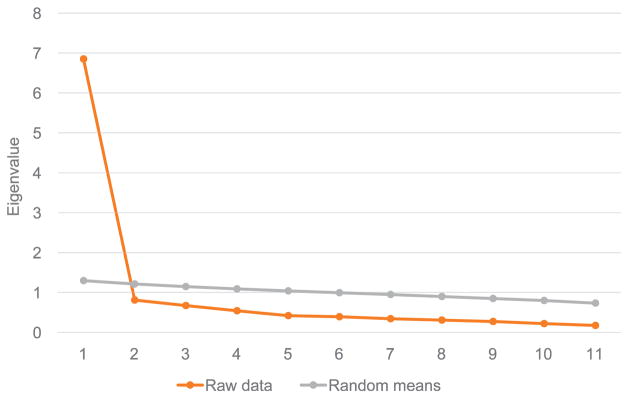
Exploratory factor analysis of the 14 items together suggested two factors, with two of the three additional items loading on a separate factor (data not shown). As a result, we chose to separate the van Rie scale from the additional TB stigma items. Parallel analysis of the van Rie scale suggested only one factor, where all 11 items loaded onto the factor; this single factor explained 62.3% of the variance in the 11 items (Figure A.1, Tables A.1 and A.2). The scale was created by summing the 11 items. Because no threshold was provided in the original validation by van Rie,1 the scale was treated as a continuous variable. The three additional items were dichotomized to agree/ disagree.
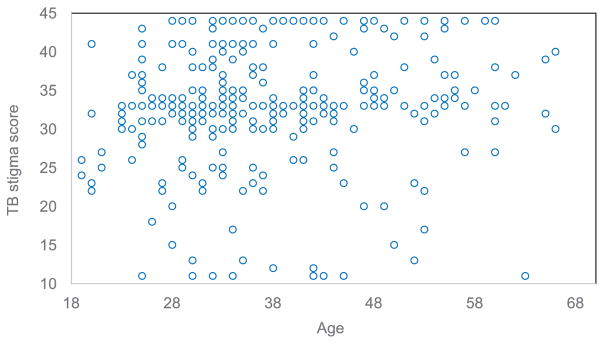
Overall, the distribution of stigma scores was shifted towards higher scores in men than in women (Figure A.2). This may have been due to men experiencing greater stigma at work than women who work within the home. Men in Lesotho commonly work in mines in South Africa, where they may be laid off if diagnosed with TB; risk of losing one’s job because of TB may result in the greater community-perceived stigma reported among men. No clear pattern in the distribution of community perspective TB stigma was observed based on age (Figure A.3).
Figure A.1.
Parallel analysis of the van Rie TB stigma scale.22 TB = tuberculosis.
Figure A.2.
Distribution of the van Rie TB stigma scale by sex.22 TB = tuberculosis.
Figure A.3.
Scatter plot of the van Rie TB stigma scale score by age.22 TB = tuberculosis.
Table A.1.
Items and factor loadings for one-factor solution for the van Rie TB stigma scale22
| Item | Factor loading |
|---|---|
| Some people try not to touch others with TB | 0.850 |
| Some people are afraid of those with TB | 0.843 |
| Some people keep their distance from people with TB | 0.836 |
| Some people do not want those with TB playing with their children | 0.823 |
| Some people think that those with TB are disgusting | 0.803 |
| Some people prefer not to have those with TB living in their community | 0.797 |
| Some people do not want to talk to others with TB | 0.795 |
| Some people feel uncomfortable about being near those with TB | 0.722 |
| Some people may not want to eat or drink with relatives who have TB | 0.717 |
| If a person has TB, some community members will behave differently towards that person for the rest of his or her life | 0.605 |
| Some people may not want to eat or drink with friends who have TB | 0.586 |
TB = tuberculosis.
Table A.2.
Eigenvalues for the van Rie TB stigma scale22
| Factor | Initial Eigenvalues | ||
|---|---|---|---|
|
| |||
| Eigenvalue | % of variance explained | Cumulative % of variance explained | |
| 1 | 6.852 | 62.288 | 62.288 |
| 2 | 0.810 | 7.366 | 69.653 |
| 3 | 0.670 | 6.089 | 75.743 |
| 4 | 0.543 | 4.932 | 80.675 |
| 5 | 0.419 | 3.805 | 84.480 |
| 6 | 0.392 | 3.560 | 88.040 |
| 7 | 0.343 | 3.120 | 91.160 |
| 8 | 0.306 | 2.786 | 93.946 |
| 9 | 0.271 | 2.468 | 96.414 |
| 10 | 0.220 | 2.001 | 98.415 |
| 11 | 0.174 | 1.585 | 100.000 |
TB = tuberculosis.
Footnotes
The appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2016/00000021/a00111s1/art00007
References
- 1.Charlson FJ, Diminic S, Lund C, Degenhardt L, Whiteford HA. Mental and substance use disorders in Sub-Saharan Africa: predictions of epidemiological changes and mental health workforce requirements for the next 40 years. PLoS ONE. 2014;9:e110208. doi: 10.1371/journal.pone.0110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2014 summary tables: DALY by cause, age and sex, by WHO region, 2000–2012. Geneva, Switzerland: WHO; 2014. Global health estimates. [Google Scholar]
- 3.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf-King SE, Steinmaus CM, Reingold AL, Hahn JA. An update on alcohol use and risk of HIV infection in sub-Saharan Africa: meta-analysis and future research directions. Int J Alcohol Drug Res. 2013;2:99–110. [Google Scholar]
- 5.Daftary A. HIV and tuberculosis: the construction and management of double stigma. Soc Sci Med. 2012;74:1512–1519. doi: 10.1016/j.socscimed.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Orza L, Bewley S, Logie CH, et al. How does living with HIV impact on women’s mental health? Voices from a global survey. J Int AIDS Soc. 2015;18(Suppl 5):20289. doi: 10.7448/IAS.18.6.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otwombe KN, Variava E, Holmes CB, Chaisson RE, Martinson N. Predictors of delay in the diagnosis and treatment of suspected tuberculosis in HIV co-infected patients in South Africa. Int J Tuberc Lung Dis. 2013;17:1199–1205. doi: 10.5588/ijtld.12.0891. [DOI] [PubMed] [Google Scholar]
- 8.Tachfouti N, Nejjari C, Benjelloun MC, et al. Association between smoking status, other factors and tuberculosis treatment failure in Morocco. Int J Tuberc Lung Dis. 2011;15:838–843. doi: 10.5588/ijtld.10.0437. [DOI] [PubMed] [Google Scholar]
- 9.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review: alcohol and the HIV continuum of care. Curr HIV/AIDS Rep. 2015;12:421–436. doi: 10.1007/s11904-015-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farley J, Miller E, Zamani A, et al. Screening for hazardous alcohol use and depressive symptomatology among HIV-infected patients in Nigeria: prevalence, predictors, and association with adherence. J Int Assoc Physicians AIDS Care (Chic) 2010;9:218–226. doi: 10.1177/1545109710371133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kader R, Seedat S, Govender R, Koch JR, Parry CD. Hazardous and harmful use of alcohol and/or other drugs and health status among South African patients attending HIV clinics. AIDS Behav. 2014;18:525–534. doi: 10.1007/s10461-013-0587-9. [DOI] [PubMed] [Google Scholar]
- 12.Sweetland A, Oquendo M, Wickramaratne P, Weissman M, Wainberg M. Depression: a silent driver of the global tuberculosis epidemic. World Psychiatry. 2014;13:325–326. doi: 10.1002/wps.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deribew A, Tesfaye M, Hailmichael Y, et al. Common mental disorders in TB-HIV co-infected patients in Ethiopia. BMC Infect Dis. 2010;10:201. doi: 10.1186/1471-2334-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louw J, Peltzer K, Naidoo P, Matseke G, McHunu G, Tutshana B. Quality of life among tuberculosis (TB), TB retreatment and/ or TB-HIV co-infected primary public health care patients in three districts in South Africa. Health Qual Life Outcomes. 2012;10:77. doi: 10.1186/1477-7525-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Lesotho Tuberculosis Profile. Geneva, Switzerland: WHO; 2015. [Accessed May 2017]. http://www.who.int/tb/country/data/profiles/en/ [Google Scholar]
- 16.Lesotho Ministry of Health and Social Welfare and ICF Macro. Lesotho Demographic and Health Survey 2014. Maseru, Lesotho: Lesotho Ministry of Health and Social Welfare; 2015. [Google Scholar]
- 17.World Health Organization. Global tuberculosis report, 2016. Geneva, Switzerland: WHO; 2016. WHO/HTM/TB/2016.13. [Google Scholar]
- 18.Howard A, Hirsch-Moverman Y, Frederix K, et al. The START Study to evaluate the effectiveness of a combination intervention package to enhance antiretroviral therapy uptake and retention during TB treatment among TB-HIV patients in Lesotho: rationale and design of a cluster randomized trial. Glob Health Action. 2016;9:31543. doi: 10.3402/gha.v9.31543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Water sanitation health. Geneva, Switzerland: WHO; 2012. [Accessed May 2017]. http://www.who.int/water_sanitation_health/monitoring/jmp2012/key_terms/en/ [Google Scholar]
- 22.Van Rie A, Sengupta S, Pungrassami P, et al. Measuring stigma associated with tuberculosis and HIV/AIDS in southern Thailand: exploratory and confirmatory factor analyses of two new scales. Trop Med Int Health. 2008;13:21–30. doi: 10.1111/j.1365-3156.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 23.Obermeyer CM, Baijal P, Pegurri E. Facilitating HIV disclosure across diverse settings: a review. Am J Public Health. 2011;101:1011–1023. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds WM. Development of reliable and valid short forms of the marlowe-crowne social desirability scale. J Clin Psychol. 1982;38:119–125. [Google Scholar]
- 25.O’Connell R, Chishinga N, Kinyanda E, et al. Prevalence and correlates of alcohol dependence disorder among TB and HIV infected patients in Zambia. PLoS ONE. 2013;8:e74406. doi: 10.1371/journal.pone.0074406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myer L, Smit J, Roux LL, Parker S, Stein DJ, Seedat S. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDS. 2008;22:147–158. doi: 10.1089/apc.2007.0102. [DOI] [PubMed] [Google Scholar]
- 27.Duko B, Gebeyehu A, Ayano G. Prevalence and correlates of depression and anxiety among patients with tuberculosis at WolaitaSodo University Hospital and Sodo Health Center, WolaitaSodo, South Ethiopia: cross sectional study. BMC Psychiatry. 2015;15:214. doi: 10.1186/s12888-015-0598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Heuvel L, Chishinga N, Kinyanda E, et al. Frequency and correlates of anxiety and mood disorders among TB- and HIV-infected Zambians. AIDS Care. 2013;25:1527–1535. doi: 10.1080/09540121.2013.793263. [DOI] [PubMed] [Google Scholar]
- 29.Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep. 2010;125(Suppl 4):34–42. doi: 10.1177/00333549101250S407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhana A, Rathod SD, Selohilwe O, Kathree T, Petersen I. The validity of the Patient Health Questionnaire for screening depression in chronic care patients in primary health care in South Africa. BMC Psychiatry. 2015;15:118. doi: 10.1186/s12888-015-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chishinga N, Kinyanda E, Weiss HA, Patel V, Ayles H, Seedat S. Validation of brief screening tools for depressive and alcohol use disorders among TB and HIV patients in primary care in Zambia. BMC Psychiatry. 2011;11:75. doi: 10.1186/1471-244X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med. 2009;24:189–197. doi: 10.1007/s11606-008-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweetland AC, Belkin GS, Verdeli H. Measuring depression and anxiety in sub-saharan Africa. Depress Anxiety. 2014;31:223–232. doi: 10.1002/da.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Mental health atlas 2014. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 35.van Ginneken N, Tharyan P, Lewin S, et al. Non-specialist health worker interventions for the care of mental, neurological and substance-abuse disorders in low- and middle-income countries. Cochrane Database Syst Rev. 2013;(11):CD009149. doi: 10.1002/14651858.CD009149.pub2. [DOI] [PubMed] [Google Scholar]