Abstract
AIM
To evaluate the effect of Lactobacillus rhamnosus GG supernatant (LGG-s) on the expression of serotonin transporter (SERT) in rats with post-infectious irritable bowel syndrome (PI-IBS).
METHODS
Campylobacter jejuni 81-176 (1010 CFU/mL) was used to induce intestinal infection to develop a PI-IBS model. After evaluation of the post-infectious phase by biochemical tests, DNA agarose gel electrophoresis, abdominal withdrawal reflex (AWR) test, and the intestinal motility test, four PI-IBS groups received different concentrations of LGG-s for 4 wk. The treatments were maintained for 1.0, 2.0, 3.0 or 4.0 wk during the experiment, and the colons and brains were removed for later use each week. SERT mRNA and protein levels were detected by real-time PCR and Western blot, respectively.
RESULTS
The levels of SERT mRNA and protein in intestinal tissue were higher in rats treated with LGG-s than in control rats and PI-IBS rats gavaged with PBS during the whole study. Undiluted LGG-s up-regulated SERT mRNA level by 2.67 times compared with the control group by week 2, and SERT mRNA expression kept increasing later. Double-diluted LGG-s was similar to undiluted-LGG-s, resulting in high levels of SERT mRNA. Triple-diluted LGG-s up-regulated SERT mRNA expression level by 6.9-times compared with the control group, but SERT mRNA expression decreased rapidly at the end of the second week. At the first week, SERT protein levels were basically comparable in rats treated with undiluted LGG-s, double-diluted LGG-s, and triple-diluted LGG-s, which were higher than those in the control group and PBS-treated PI-IBS group. SERT protein levels in the intestine were also comparable in rats treated with undiluted LGG-s, double-diluted LGG-s, and triple-diluted LGG-s by the second and third weeks. SERT mRNA and protein levels in the brain had no statistical difference in the groups during the experiment.
CONCLUSION
LGG-s can up-regulate SERT mRNA and protein levels in intestinal tissue but has no influence in brain tissue in rats with PI-IBS.
Keywords: Serotonin transporter, Intestinal infection, Lactobacillus rhamnosus supernatant, Irritable bowel syndrome
Core tip: There are few reports on the effect of the supernatant of Lactobacillus rhamnosus GG (LGG) on serotonin transporter (SERT) expression in rats with post-infectious irritable bowel syndrome (PI-IBS). An experimental rat model of PI-IBS was developed by Campylobacter jejuni infection. SERT levels in intestinal and brain tissues were detected to evaluate the effect of LGG-s.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGD), annually affecting 12% to 30% of the population worldwide[1-5]. IBS can be divided into four subtypes, namely, IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed-type IBS (IBS-M), and untyped IBS (IBS-U)[6-8]. Acute infectious gastroenteritis (IGE) is an important risk factor for developing IBS, with 5% to 31% of the patients developing post-infectious IBS (PI-IBS)[9-12]. Recent reports indicate that abnormalities in serotonergic signaling systems are involved in the development of PI-IBS, particularly those affecting serotonin (5-HT) levels in the gastrointestinal tract[13-15].
As a signal transducer and neurotransmitter, 5-HT modulates intestinal fluid secretion, gut motility, and gastrointestinal sensation[16]. Serotonin transporter (SERT) is a universally existing transmembrane transport protein that plays a key role in 5-HT reuptake[15,17]. SERT has two important polymorphic areas. The first is named 5-HT-transportergene-linked polymorphic region (5-HTTLPR), which is located in the regulatory region of its gene (SLC6A4; chromosome 17q11.1-q12)[18]. The most frequently studied variant is subdivided into long (L) and short (S) alleles[19,20]. The transcriptional efficiency of the L/L genotype is significantly higher than that of the L/S and S/S genotypes[21]. Furthermore, the frequency of the L/L genotype in C-IBS was significantly higher than that in D-IBS, and the S/S genotype is higher in D-IBS[21-24]. In other words, the expression of SERT is higher in C-IBS, and lower in D-IBS. The second is named STin2, which is also called variable number tandem repeats (VNTR). Wang et al[25] have found a higher ratio of STin2.12/10 and a lower ratio of STin2.12/12 in IBS patients, and there were no significant differences between different subtypes. However, a small-scale study on SERT in PI-IBS, conducted by Wheatcroft et al[26], showed that SERT expression was reduced in PI-IBS patients.
Lactobacillus rhamnosus GG (LGG) is the best studied member of the lactic acid bacteria and is known to have positive effects on human health[27]. Probiotics such as LGG are potential treatment options in patients with IBS[28]. LGG could exclude pathogens, promote mucosal immunity against Salmonella infection[29], and reduce the rotavirus-related diarrhea by increasing the levels of interferon-γ (IFN-γ)[30]. The ESPGHAN Working Group recommends using LGG for preventing nosocomial diarrhea[31]. Furthermore, LGG reduces the frequency and severity of abdominal pain in children with IBS[32], along with improving disease severity, especially in IBS-D and IBS-A subtypes[33].
Our previous study had confirmed that Lactobacillus rhamnosus GG supernatant (LGG-s) could up-regulate SERT mRNA and protein levels in intestinal epithelial cells and mouse intestinal tissues[34]. The aim of this study was to investigate the effects of LGG-s on the expression of SERT mRNA and SERT protein (SERT-P) in the colon and brain in a rat model of PI-IBS.
MATERIALS AND METHODS
Bacterial culture, LGG-s, and Campylobacter jejuni
LGG [53103, American Type Culture Collection (ATCC), United States] was incubated in Lactobacillus MRS broth (Oxoid CM0359) at 37 °C for 24 h, according to ATCC guidelines, diluted in MRS broth, and cultured again at 37 °C to reach log phase with the density determined as 0.5 at A600[34]. The culture suspension was centrifuged at 4000 g for 15 min, then the supernatant was collected and filtered through 0.20-μm filters[35].
C. jejuni 81-176 (BAA-2151, ATCC, United States) was grown on Skirrow’s selective medium (Columbia Agar Base, Oxoid CM0331, supplemented with 5% sheep blood and Campylobacter selective supplement, Oxoid SR0117) at 42 °C under micro-aerobic conditions for 24 h. The bacterial colony was obtained with an inoculating loop and diluted in phosphate buffered solution (PBS), until the concentration reached 1.0 × 1010 CFU/mL. The preliminary experiments showed that the concentration of 1010 CFU/mL achieved a higher diarrhea rate, visceral hypersensitivity, and serious clinical symptoms. Bacterial concentrations were measured with a spectrophotometer (TECAN infinite M200 PRO, Switzerland)[36].
Animal studies
The study was performed on male Sprague-Dawley rats (aged between 7 and 8 wk) obtained from Laboratory Animal Center of Chinese People’s Liberation Army General Hospital (Beijing, China). The rats were kept under the following conditions: the temperature of 23 °C ± 1 °C, a 12h/12 h light/dark cycle (lighting from 08:00 to 20:00), and free access to a sterile diet. The rats were then divided into two groups. The first group of rats were designated as the control group (n = 25, normal and healthy), which was given PBS (2 mL/d per rat), and another group as the model group of PI-IBS (n = 85), which was given C. jejuni (1010 CFU/mL, 2 mL/d per rat) through an intra-gastric needle (Thermo Fisher Scientific, Hampton, United States). The gavage continued for 7 d. Then, the stool culture, body weight of the rats, and relative content of stool water were tested to evaluate the phase of infection.
If the rats get rid of infection with a higher Bristol score of faeces, faster intestinal transit, and visceral hypersensitivity, they were considered to enter the post-infectious phase as PI-IBS.
After the model evaluation, the rats were regrouped; the control group was designated as M (n = 20) and given PBS, and the PI-IBS model group was divided into four groups and given PBS, undiluted LGG-s, double diluted LGG-s, and triple diluted LGG-s, respectively (A, B, C and D, respectively, n = 20) through a gavage needle[34]. PBS or LGG-s was administered at 2 mL/d/per rat, and the treatments were maintained for 1.0, 2.0, 3.0 or 4.0 wk during the experiment. Five rats of each group were sacrificed; the colons and brains were removed for later use each week.
During all experiments, housing and diet conditions were the same for all groups. Rats infected with C. jejuni were housed in another room to prevent cross-contamination from infected to uninfected. The protocol was approved by the Animal Use and Care Committee of Tianjin Medical University (Figure 1).
Figure 1.
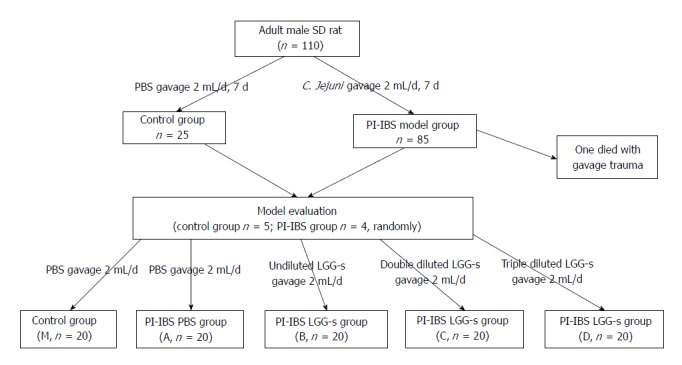
Experimental flow chart.
Campylobacter gavage
Before the infection, all rats received 1 mL of 5% (w/v) bicarbonate solution via a ball-tipped inoculating needle to neutralize the gastric acid. Thirty minutes later, C. jejuni in 2 mL of PBS was given to the PI-IBS model group and 2 mL of sterile PBS to the control group[37].
Assessment of acute colonization by C. jejuni
The fresh stool specimens were cultured for the presence of C. jejuni on Campylobacter selective agar plates using biochemical tests and DNA agarose gel electrophoresis, and the general condition, body weight, and relative content of stool water were observed or tested on the 3rd, 7th, 14th, 28th, 42nd, 56th, and 70th days after gavage. Successful intestinal colonization was defined by the detection of C. jejuni in stool at least once, and clearance of infection was defined by two consecutive tests with negative culture.
Biochemical tests contain catalase test, oxidase test, hippurate hydrolysis test, and 3-indoylacetate hydrolysis test (GB 4789.9-2014, China). Total DNA was extracted using a Campylobacter Nucleic Acid Test Kit (ZC-CAMPY-003, Kangda Zhongchuang Biotechnology Co. LTD, China) and tested by agarose gel electrophoresis.
For measuring the relative content of stool water, fresh stools were obtained from rats under manual restraint by spontaneous or perianal-stimulated defecation, weighed, and then dried at 50 °C for 72 h followed by room temperature for 48 h. Dry stools were weighed again to determine the percent wet weight of stool.
Determination of PI-IBS
In the case of two consecutive tests with negative culture, rats were considered to get rid of infection. Rats without infection were kept separately from infected rats. After all of the rats no longer had detectable C. jejuni in the stool, they were considered to be in the post-infectious time period. Fresh stool was collected for 3 consecutive days from all rats and graded by a modified Bristol Stool score[38]. Normal stool was graded as 1, soft and poorly formed stool graded as 2, and watery stool as 3. Five rats from the control group and four from the model group were randomly chosen to perform the tests. Visceral hypersensitivity was evaluated by abdominal withdrawal reflex (AWR) test, and the intestinal motility was detected.
AWR experiment was done as previously reported[39]. Rats were fasted for 18 h before the test was performed. The night before AWR, balloons (7-8 mm diameter) were inflated overnight to stretch the latex, then the balloons became compliant. Following anesthesia by ether inhalation, a balloon coated with paraffin oil was inserted into the rectum with the tail of the balloon, 1 cm from the anus, fixed at the base of the tail. The balloon was connected via a double barreled cannula, with one joint connected to the air pump and another connected to the pressure gauge. Rats were given 30 min to accommodate the environment. Then, the balloon was distended at the pressures of 20, 40, 60 and 80 mmHg. Distention was sustained for 15 s, at intervals of 10 min. The distention was performed three times at each pressure, and the AWR scores were recorded. AWR was done by researchers who had no understanding of the experiment.
Intestinal motility was detected by activated carbon solution gavage. Before the experiment, rats were fasted for 24 h and then given 2 mL of 10% activated carbon solution through an inoculating needle. After 50 min, rats were sacrificed by cervical dislocation. Then, a laparotomy was performed, and the whole bowel was taken out and moistened with PBS. The bowel was freely flattened on the table. The length of the bowel along which it contains the activated carbon and the length of the whole bowel were measured, and the ratio of these two lengths was taken as the intestinal transit rate (ITR)[40].
Real-time polymerase chain reaction
To evaluate the levels of SERT mRNA after treatment with LGG-s, colon and brain samples were harvested. Rats were sacrificed by cervical dislocation, and total RNA was prepared from 50 mg of tissue from each rat with Trizol, according to the manufacturer’s instructions (Life, Hilden, Germany). An iScriptcDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, United States) was used to synthesize the cDNA. The PCR were set up in a volume of 20 μL containing 1.0 μL cDNA, 10 μL 2 × iQSYBR Green Supermix (Roche Applied Science), and 0.6 μL both forward and reverse primers, replenished with DEPC treated ddH2O. Quantitative RT-PCR was performed on an ABI One plus setup PCR thermocycler. The sequences of primers are given in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was measured as an internal control. Relative mRNA expression was calculated using the 2-ΔΔCt method.
Table 1.
Primer sequences for RT-PCR
| Gene | Sequence (5’-3’) |
| rGAPDH | Forward: 5’-CCATCAACGACCCCTTCATT-3’ |
| Reverse: 5’-GACCAGCTTCCCATTCTCAG-3’ | |
| rSERT | Forward: 5’-ACTGTTACCAAGATGCCCTG-3’ |
| Reverse: 5’-ATCTTCATTCCTCATCTCCGC-3’ |
rGAPDH: rat glyceraldehyde-3-phosphate dehydrogenase; rSERT: rat serotonin transporter.
Western blot analysis
Proteins were extracted from colonic and cerebral tissues, and protein levels were quantified using a BCA kit (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China), according to the manufacturer’s instructions. The protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat milk, incubated with a primary antibody (SERT antibody: dilution 1:5000, EPR12735, Abcam Biotech Company, Cambridge, United Kingdom; β-actin: dilution 1:1000, 8457S, Cell Signaling Technology, Boston, United States) overnight at 4 °C, and then incubated with a secondary antibody (dilution 1:10000, BA1054, Boster Biological Technology Co., Ltd, Wuhan, China) for 1 h at room temperature. The immunoreactive bands were visualized using an ECL Western Blotting Substrate (Solarbio Life Sciences Co., Ltd, Beijing, China). β-actin was used as an internal control.
Statistical analysis
Statistical analyses were carried out using SPSS 22.0 (SPSS, Chicago, IL, United States). Quantitative data are expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) and post hoc tests (LSD and Dunnett’s T3) were used to compare the values of quantitative RT-PCR. For all analyses, P < 0.05 was defined as statistical significance.
RESULTS
Campylobacter colonization phase
A total of 110 rats were used in the study, none showed C. jejuni infection prior to the first gavage (as determined by stool culture), and all rats inoculated with C. jejuni exhibited C. jejuni colonization within 3 d after gavage (1 rat died from severe gavage trauma). Thus, a total of 109 rats (25 control rats and 84 PI-IBS model rats) were included before the model evaluation.
Three to fourteen days after infection, rats in the control group had good spirits, normal activity, and glossy hair. However, rats in the PI-IBS model group were decadent, indolent, and lackluster. The general conditions were returned to normal in about 42 d.
The rat weight in the model group was significantly lower than that in the control group at the 3rd, 7th, 14th and 28th days (P < 0.05). After that, the weight in the model group was still lower than that in the control group with no statistical significance (P > 0.05).
The relative content of water in the fresh stool in the model group was significantly higher than that of the control group at the 3rd, 7th, 14th, 28th and 70th days (P < 0.05). At the 42th and 56th days, the model group was a little higher than the control group (Figure 2).
Figure 2.
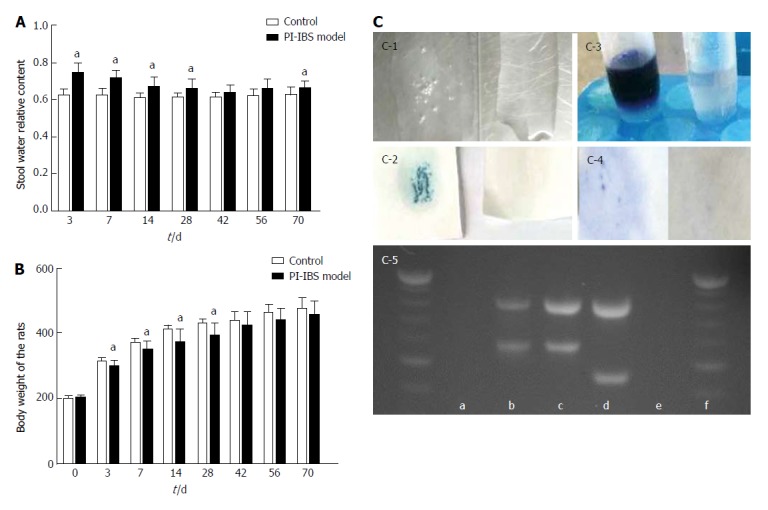
Assessment of Campylobacter colonization phase. A: Relative content of stool water during the observation period, aP < 0.05 vs control group; B: Body weight of rats during the observation period, aP < 0.05 vs control group; C: C-1, catalase test; C-2, 3-indoylacetate hydrolysis test; C-3, hippurate hydrolysis test; C-4, oxidase test; C-5, DNA agarose gel electrophoresis (a: negative sample; b: positive sample; c: positive quality control of Campylobacter jejuni; d: positive quality control of Campylobacter coli; e: negative quality control; f: DNA ladder. Control group, n = 25; PI-IBS group, n = 84.
Post-infectious phase
As two consecutive tests resulted in negative culture, rats were considered to be rid of infection. At the 42th day, 95% of the C. jejuni rats were no longer infected. At the 56th and 70th days, all C. jejuni rats tested negatively. At the 70th day, tests were done to evaluate the PI-IBS. The 3-d average Bristol score of stool and the intestinal transit rate were higher in the model group than in the control group (P < 0.05). AWR score of rats in the model group was also higher than that of the control group. However, there was no statistical difference between the two groups at the pressures of 20 and 80 mmHg (P = 0.12 and 0.06, respectively) (Figure 3).
Figure 3.
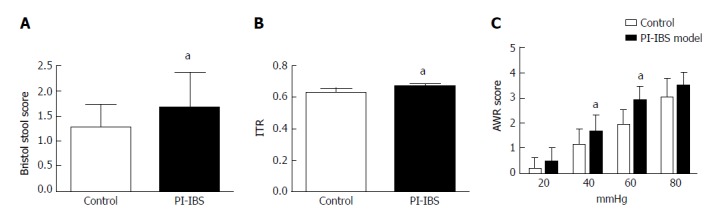
Assessment of PI-IBS phase. A: Bristol stool score; B: Intestinal transit rate (ITR). ITR = length of the activated carbon moving in the bowel (cm)/length of the whole bowel (cm); C: AWR scores at different pressures. aP < 0.05 vs control group. Control group, n = 5; PI-IBS group, n = 4.
Effects of LGG-s on SERT mRNA and SERT-P expression in rat intestinal tissues
For the PI-IBS PBS gavage group (A), the levels of SERT mRNA expression were lower than those of the control group (M) throughout the experiment. However, only at the 3rd week, SERT mRNA expression in group A was significantly lower than that in group M by 0.7-fold (P = 0.023, P < 0.05).
Undiluted LGG-s (B) up-regulated SERT mRNA expression level by 1.8-fold compared with group A by the 1st week (P = 0.002), and the level was slightly higher than that of group M (P > 0.05). By the end of the 2nd week, the level of SERT mRNA in group B was 2.67 times and 3.7 times higher than those in groups M and A, respectively (P = 0.018 and 0.012, respectively). SERT mRNA expression in group B increased continuously, and at the 3rd week, it was 4.4-fold, 6.1-fold, and 1.7-fold more than those in groups M, A, and D (triple diluted LGG-s), respectively (P = 0.003, 0.003 and 0.028, respectively), although there was no significant difference between groups B and C (double diluted LGG-s). By the last week, the level of SERT mRNA in group B was only 2.1-fold higher than that in group A (P = 0.016).
Double diluted LGG-s (C) moderately increased SERT mRNA levels by 1.8-fold compared with group A by the 1st week (P = 0.027). The level of SERT mRNA in group C was 2.3-fold higher than that of group A at the 2nd week (P = 0.012), and 4.6-and 6.4-fold at the 3rd week, and 1.9-and 2.6-fold at the last week compared to groups M and A (P = 0.012, 0.009, 0.032, and 0.011, respectively). However, there was no statistical significance between groups C and D at the 2nd week (P > 0.05).
Triple diluted LGG-s (D) significantly up-regulated SERT mRNA expression levels by 6.9-, 9.4-, 5.3- and 5.1-fold compared to groups M, A, B, and C at the end of the first week (P = 0.02, 0.01, 0.002, and 0.002, respectively), but the level decreased rapidly at the end of the 2nd week. The SERT expression levels were similar between the 2nd week and the 3rd week, which were 2.3- and 2.5-fold higher compared to group M (P = 0.015 and 0.001, respectively) and 3.1- and 3.5-fold higher compared to group A (P = 0.009 and 0.028, respectively).
The variation tendency of SERT-P level was similar to that of SERT mRNA. SERT-P level was lower in group A than in group M during the whole experiment, and there was no significant difference only at the 3rd week (P = 0.177). SERT-P level was similar between groups B and A, although it was lower in group B than in all other groups, at the 1st week. SERT-P level was comparable in groups B, C, and D by the 2nd and 3rd weeks. SERT-P level was slightly higher in groups B, C, and D than in group M at the 4th week, but there was no statistical significance (Figure 4).
Figure 4.
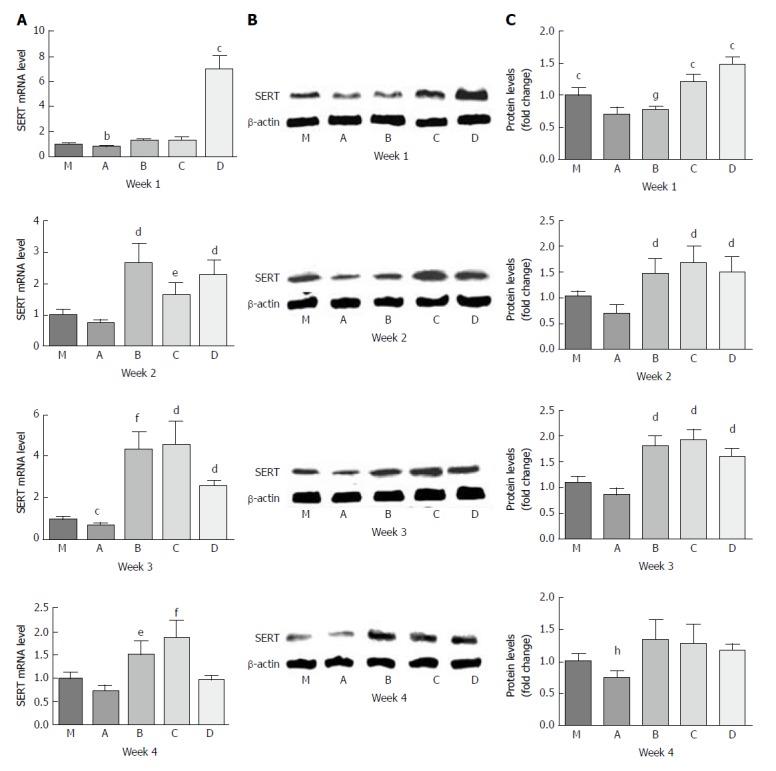
Effect of LGG-s on SERT mRNA and SERT-P expression in rat intestinal tissues. A: SERT mRNA levels at the first, second, third, and fourth weeks; B: SERT-P levels at the first, second, third, and fourth weeks analyzed by Western blot; C: Quantitative analysis of SERT-P levels at the first, second, third, and fourth weeks analyzed by Western blot. bP < 0.05 vs B or C; cP < 0.05 vs all others; dP < 0.05 vs M or A; eP < 0.05 vs A; fP < 0.05 vs M, A or D; gP < 0.05 vs M, C or D; hP < 0.05 vs M or D. Control group, n = 5; PI-IBS group, n = 5, each week.
Effect of LGG-s on SERT mRNA and SERT-P expression in rat brain tissues
There were no statistical differences in SERT mRNA or SERT-P expression between the different dilution concentrations. The levels of SERT mRNA in groups B, C, and D had a little increase compared to groups M and A at the 1st week, but turned lower at the last week. Double diluted LGG-s resulted in a minuscule increase in SERT-P at the 3rd week, which declined at the end of the study (Figure 5).
Figure 5.
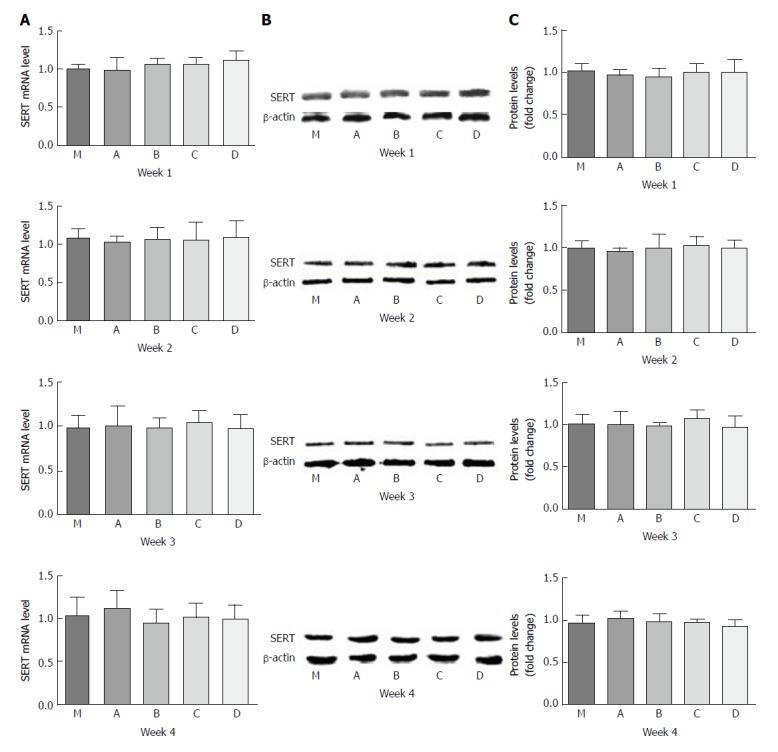
Effect of LGG-s on SERT mRNA and SERT-P expression in rat brain tissues. A: SERT mRNA levels at the first, second, third, and fourth weeks; B: SERT-P levels at the first, second, third, and fourth weeks analyzed by Western blot; C: Quantitative analysis of SERT-P levels at the first, second, third, and fourth weeks analyzed by Western blot. Control group, n = 5; PI-IBS group, n = 5, each week.
DISCUSSION
According to the Rome IV criteria, IBS is one of the lower gastrointestinal tract disorders, which identify about two thirds of suffers, along with requiring that the pain either be relieved by defecation or associated with changes in stool frequency or/and consistency[8,41,42]. PI-IBS often exhibits characteristics of diarrhea, and patients often have a history of acute gastrointestinal infection[9-12]. Many studies showed that infection with C. jejuni strain correlates with the development of PI-IBS[43-45]. C. jejuni produces a range of toxins including cytolethal distending toxin, which first produces a secretory diarrhea in the small intestine, subsequently invading the distal ileum and colon to produce an inflammatory ileocolitis[46]. Therefore, C. jejuni was used to build a model of PI-IBS in this study. The mechanisms that underlie chronic disturbance of gut function are thought to involve chronic microscopic mucosal inflammation, visceral hypersensitivity, dysregulation of gut microbiota, and abnormal neuromuscular function[47-50].
5-HT was found in the gastrointestinal tract and central nervous system (CNS). It functions both as a neurotransmitter and as a local hormone in the peripheral vascular system in the gut[51]. About 95% of body 5-HT is found in the gastrointestinal tract, with 90% in enterochromaffin cells (EC cell) and 10% in serotonergic neurons of myenteric plexus[51,52]. As a signal transducer and neurotransmitter, 5-HT is a key molecule, regulating visceral perception and intestinal motility[53]. 5-HT exerts its action by binding to its receptors (5-HT1 to 5-HT7) present in both intrinsic and extrinsic primary afferent neurons[54]. Serotonin receptors that are known to affect gut motor functions are those belonging to the 5-HT1, 2, 3, 4 and 7 subtypes[55-57]. 5-HT receptors contract effector cells when bound by 5-HT2A and relax cells by 5-HT4 and 5-HT7 subtypes[58]. Neuronal 5-HT3 leads to increased release of acetylcholine from cholinergic neurons[52]. The release of 5-HT acting on effector cells leads to secretory reflexes, peristaltic reflexes, and if superfluous, diarrhea, abdominal pain, or visceral hypersensitivity[59]. Clinical trials have shown an increased level of 5-HT in IBS[60-62]. Serotonin reuptake transporter (SERT) plays an irreplaceable role in 5-HT inactivation by decreasing the content of 5-HT in the synaptic cleft[63]. SERT on the cell membrane of enterocytes is vital to transport 5-HT into the cell, with 5-HT metabolized by monoamine oxidase[64]. SERT was expressed by nearly all of the intestinal epithelial cells on the surface of the lumen[65]. Biochemical abnormalities in PI-IBS patients including hyperplasia of the EC cells and depressed 5-hydroxyindole acetic acid/5-HT ratio suggested impaired SERT function[44]. Many researchers have demonstrated that IBS patients have a remarkably lower level of SERT expression in the intestine[66]. Coates et al[17] first demonstrated a significantly decreased level of SERT in IBS.
SERT expression can be regulated by a series of factors, such as gene polymorphisms, microRNAs, immunity, inflammation, gut microbiota, and growth factors[67]. The SERT gene, SLC6A4, containing 5-HTTLPR[19], VNTR STin2[68], and functional single nucleotide polymorphisms (SNPs), has a positive association with etiology of IBS[69]. 5-HTTLPR is the most frequently studied, which is subdivided into long (L) and short (S) alleles[19]. Previous studies found that the frequency of L/L genotype is significantly higher in C-IBS than those of L/S and S/S genotypes[21]. VNTR showed a higher ratio of STin2.12/10 and a lower ratio of STin2.12/12 in IBS[25].
Immune activation of the gut mucosa plays a critical role in EC cell hyperplasia and reduced SERT activity in PI-IBS[70,71]. Foley et al[72] found that SERT mRNA had a lower level in D-IBS, which was correlated with increased numbers of mucosal intraepithelial lymphocytes and mast cells. Pro-inflammatory mediators, such as IFN-γ and tumor necrosis factor-α, reduce the expression of SERT mRNA, SERT-P, and SERT function in Caco-2 cells[72]. However, a protective cytokine, transforming growth factor-β1, could rapidly activate SERT activity and inhibit intestinal inflammation via PI3K and syntaxin 3[73]. The acute infection of C. jejuni produces diarrhea in the small intestine, and leads to the inflammatory response, which increases the ratio of pro-/anti-inflammatory factors. In other words, the SERT mRNA and SERT-P levels in intestinal tissues were significantly decreased in the PI-IBS PBS group than in controls. The highest concentration of LGG-s did not induce the highest expression of SERT, which is inconsistent with a previous study which found a dose-dependent up-regulation of expression of SERT[34]. This may due to the differences in the contents of various substances (proteins, fatty acids, inorganic salts, etc.) in the supernatant, which, combined with previous C. jejuni infection, could lead to different immune activation.
In addition, gut host-microbial interactions are important factors in IBS. Studies have found that Lactobacillus, Bifidobacterium, Actinobacteria, and Bacteroidetes were decreased[74-76], while Proteobacteria, Firmicutes, and Firmicutes/Bacteroidetes ratios were increased in fecal samples of IBS-D patients[77]. Enteropathogenic E. coli and E. coli Nissle 1917 could decrease SERT mRNA and increase 5-HT bioavailability[78,79]. Our previous study proved that LGG-s could up-regulate the SERT mRNA and SERT-P levels in enterocytes and mouse intestinal tissues[34]. In this study, we also proved that LGG-s had a positive effect on SERT expression in colon tissues in PI-IBS rats. It may provide a novel solution for the treatment of IBS. Further studies are needed to find whether LGG-s has any impact on the composition of rat gut microbiota.
Growth factors, such as epidermal growth factor, basic fibroblast growth factor, and nerve growth factor, may be involved in the up-regulation of SERT expression[80-82]. A protein, known as p40, expressed by LGG, activates epidermal growth factor receptor (EGFR)[35], which might activate the mechanism of LGG-s induced up-regulation of SERT expression.
Gender may be another factor. Although IBS is an universally disease, women are more likely to suffer from this illness than men[83]. It has been found that SERT mRNA levels in the rectal mucosa of women with IBS-D were higher than those in men. Female SERT knockout rats showed remarkable visceral hypersensitivity than male rats[84]. However, male animals were also commonly used in PI-IBS research[85,86]. Ibeakanma et al[87] found that brain-gut interactions could exaggerate peripheral nociceptive signaling in male mice with PI-IBS. An experimental model of male mouse induced by stress or Giardia also showed prominent visceral hypersensitivity[88]. As for this study, male rats were selected to perform the research.
Alterations in the bidirectional interactions between the gut and the nervous system play an important role in IBS pathophysiology and symptom generation. Communication between the gut and the CNA, both in the ascending (gut-to-brain) and descending (brain-to-gut) directions, is called the gut-brain axis[89]. Gut microbes may communicate with the gut-brain axis via production of neuro-active and neuroendocrine molecules such as serotonin, aminobutyric acid (GABA), histamine, noradrenaline, and adrenaline[90]. Lactobacilli can convert glutamate into GABA[91], and administration of L. rhamnosus JB-1 to mice altered the patterns of GABA receptors in the brain[92]. There is very little study about the changes of SERT in the CNS. However, no significant differences were found in SERT expression between the LGG-s treated group and the control group in this study. It is possible that the signal might be prevented from entering the brain by the blood-brain barrier (BBB)[93]. The connections between cells in the BBB are tighter, which greatly limits the endothelial permeability via para-cellular and trans-cellular transport pathways[94]. The exchange of substances in the blood and brain is mainly accomplished by various transporters expressed in vascular endothelial cells[95]. Regulators or neurotransmitters, secreted by LGG, may lack specific transporters. Because of the BBB, it is very difficult for 5-HT in the blood to enter the CNS, whereas 5-HT in the central and peripheral nerves is a separate system with different functions. Therefore, as a reuptake transporter, SERT may also be regulated in different ways. The effect of LGG injected directly into the brain is still need to be studied.
In conclusion, we found that LGG-s up-regulated SERT expression in intestinal tissues, but had no statistical effect in brain tissues in PI-IBS rats. By decreasing 5-HT levels, LGG-s may be a potential strategy for helping improve clinical symptoms of IBS.
ARTICLE HIGHLIGHTS
Research background
Probiotics have been approved to be used to relieve irritable bowel syndrome (IBS), and Lactobacillus rhamnosus GG (LGG) is the best studied member of lactic acid bacteria and has supportive therapeutic efficacy in IBS. However, the mechanism remains a significant challenge to researchers. This study developed a PI-IBS model to evaluate the effect of LGG supernatant on serotonin transporter expression.
Research motivation
This study is a part of a National Natural Science Foundation of China project. On the basis of developing an experimental model of PI-IBS, this research explored the effect of LGG-s on SERT levels in intestinal and brain tissues.
Research objectives
This study detected the expression levels of SERT mRNA and SERT-P to evaluate the effect of LGG-s in PI-IBS rats, which were infected with C. jejuni. LGG-s could up-regulate SERT mRNA and SERT-P levels in rat intestinal tissues but had no influence in rat brain tissues. The more detailed research on LGG-s will contribute to more accurate treatment of IBS.
Research methods
The model group of PI-IBS (n = 85) was given C. jejuni (1010 CFU/mL, 2 mL/d per rat) for 7 d, then the body weight of the rats and the relative content of stool water were measured to evaluate the phase of infection, and the fresh stool specimens were cultured for the presence of C. jejuni on Campylobacter selective agar plates. After the model evaluation, the rats were regrouped, and each group was gavaged with different concentrations of LGG-s. The treatments were maintained for 1.0, 2.0, 3.0 or 4.0 wk during the experiment. Then, SERT expression was detected by RT-PCR and Western blot to evaluate the effect of LGG-s.
Research results
The levels of SERT mRNA and SERT-P in intestinal tissues were up-regulated by treatment with LGG-s of different concentrations. Triple-diluted LGG-s showed a more significant difference within a short term, while, in the long run, undiluted and double-diluted LGG-s proved better. However, there were no significant differences in SERT mRNA and SERT-P in the brain tissues between each group, with or without treatment with LGG-s. Some factors and differences in the contents of various substances (proteins, fatty acids, inorganic salts, etc.) in the supernatant may induce a different increase in SERT levels. More detailed research about LGG-s is needed.
Research conclusions
This study demonstrates that LGG-s up-regulates SERT expression in intestinal tissues, but has no statistical effect in brain tissues in PI-IBS rats. The previous study has proved that LGG-s could up-regulate the SERT levels in intestinal tissues in healthy mice. Moreover, LGG-s led to dose-dependent expression of SERT. The contents of substances in the supernatant, combined with their different concentrations, molecular mass, and previous C. jejuni infection, may result in this phenomenon. Therefore, more detailed research about LGG-s and relief of clinical symptoms with the treatment of LGG-s would be done in our next work.
Research perspectives
The infection with C. jejuni could help to build a PI-IBS model with lower expression of SERT. For the future accurate treatment of IBS, proteomics analysis of LGG-s is important and urgent.
ACKNOWLEDGMENTS
We thank our laboratory counselors, Jing-Wen Zhao and Wei-Qiang Wang, for their valuable support and guidance in the research work.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by the National Natural Science Foundation of China, No. 81570489.
Institutional review board statement: This study was approved by the Tianjin Medical University General Hospital.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Animal Ethics and Welfare Committee of Tianjin Medical University.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: Readers can get the data of this paper by contacting us via E-mail: ywang12@tmu.edu.cn.
Peer-review started: November 9, 2017
First decision: November 30, 2017
Article in press: December 12, 2017
P- Reviewer: Touil-Boukoffa C, Yu LCH S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Li D
Contributor Information
Ya-Nan Cao, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin 300052, China.
Li-Juan Feng, Department of Functional Division, Xingtai People’s Hospital, Xingtai 054031, Hebei Province, China.
Yuan-Yuan Liu, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin 300052, China.
Kui Jiang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin 300052, China.
Mao-Jun Zhang, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China.
Yi-Xin Gu, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China.
Bang-Mao Wang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin 300052, China.
Jia Gao, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin 300052, China.
Ze-Lan Wang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin 300052, China.
Yu-Ming Wang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin 300052, China. ywang12@tmu.edu.cn.
References
- 1.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 4.Porter CK, Faix DJ, Shiau D, Espiritu J, Espinosa BJ, Riddle MS. Postinfectious gastrointestinal disorders following norovirus outbreaks. Clin Infect Dis. 2012;55:915–922. doi: 10.1093/cid/cis576. [DOI] [PubMed] [Google Scholar]
- 5.Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment Pharmacol Ther. 2004;19:1217–1224. doi: 10.1111/j.1365-2036.2004.01939.x. [DOI] [PubMed] [Google Scholar]
- 6.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350–359. doi: 10.1111/j.1365-2036.2011.04948.x. [DOI] [PubMed] [Google Scholar]
- 8.Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology. 2013;145:1262–70.e1. doi: 10.1053/j.gastro.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM; Walkerton Health Study Investigators. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605–611. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 10.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM; Walkerton Health Study Investigators. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450; quiz 660. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 11.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 12.Andresen V, Löwe B, Broicher W, Riegel B, Fraedrich K, von Wulffen M, Gappmayer K, Wegscheider K, Treszl A, Rose M, et al. Post-infectious irritable bowel syndrome (PI-IBS) after infection with Shiga-like toxin-producing Escherichia coli (STEC) O104:H4: A cohort study with prospective follow-up. United European Gastroenterol J. 2016;4:121–131. doi: 10.1177/2050640615581113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L, Yi-Xuan L. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz J Med Biol Res. 2012;45:948–954. doi: 10.1590/S0100-879X2012007500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zang KH, Shao YY, Zuo X, Rao Z, Qin HY. Oridonin Alleviates Visceral Hyperalgesia in a Rat Model of Postinflammatory Irritable Bowel Syndrome: Role of Colonic Enterochromaffin Cell and Serotonin Availability. J Med Food. 2016;19:586–592. doi: 10.1089/jmf.2015.3595. [DOI] [PubMed] [Google Scholar]
- 15.Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–891. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 16.Barbara G, Cremon C. Serine proteases: new players in diarrhoea-predominant irritable bowel syndrome. Gut. 2008;57:1035–1037. doi: 10.1136/gut.2008.150821. [DOI] [PubMed] [Google Scholar]
- 17.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–162. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 19.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 20.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YM, Chang Y, Chang YY, Cheng J, Li J, Wang T, Zhang QY, Liang DC, Sun B, Wang BM. Serotonin transporter gene promoter region polymorphisms and serotonin transporter expression in the colonic mucosa of irritable bowel syndrome patients. Neurogastroenterol Motil. 2012;24:560–565, e254-e255. doi: 10.1111/j.1365-2982.2012.01902.x. [DOI] [PubMed] [Google Scholar]
- 22.Choi YJ, Hwang SW, Kim N, Park JH, Oh JC, Lee DH. Association Between SLC6A4 Serotonin Transporter Gene Lainked Polymorphic Region and ADRA2A -1291C>G and Irritable Bowel Syndrome in Korea. J Neurogastroenterol Motil. 2014;20:388–399. doi: 10.5056/jnm14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang ZF, Duan ZJ, Wang LX, Yang D, Zhao G, Zhang L. The serotonin transporter gene polymorphism (5-HTTLPR) and irritable bowel syndrome: a meta-analysis of 25 studies. BMC Gastroenterol. 2014;14:23. doi: 10.1186/1471-230X-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colucci R, Gambaccini D, Ghisu N, Rossi G, Costa F, Tuccori M, De Bortoli N, Fornai M, Antonioli L, Ricchiuti A, et al. Influence of the serotonin transporter 5HTTLPR polymorphism on symptom severity in irritable bowel syndrome. PLoS One. 2013;8:e54831. doi: 10.1371/journal.pone.0054831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang BM, Wang YM, Zhang WM, Zhang QY, Liu WT, Jiang K, Zhang J. [Serotonin transporter gene polymorphism in irritable bowel syndrome] Zhonghua Neike Zazhi. 2004;43:439–441. [PubMed] [Google Scholar]
- 26.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 27.Floch MH. Recommendations for probiotic use in humans-a 2014 update. Pharmaceuticals (Basel) 2014;7:999–1007. doi: 10.3390/ph7100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650–2661. doi: 10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang GY, Yu J, Su JH, Jiao LG, Liu X, Zhu YH. Oral Administration of Lactobacillus rhamnosus GG Ameliorates Salmonella Infantis-Induced Inflammation in a Pig Model via Activation of the IL-22BP/IL-22/STAT3 Pathway. Front Cell Infect Microbiol. 2017;7:323. doi: 10.3389/fcimb.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y, Ye L, Cui Y, Yang G, Yang W, Wang J, Hu J, Gu W, Shi C, Huang H, et al. Effects of Lactobacillus rhamnosus GG on the maturation and differentiation of dendritic cells in rotavirus-infected mice. Benef Microbes. 2017;8:645–656. doi: 10.3920/BM2016.0157. [DOI] [PubMed] [Google Scholar]
- 31.Hojsak I, Szajewska H, Canani RB, Guarino A, Indrio F, Kolacek S, Orel R, Shamir R, Vandenplas Y, van Goudoever JB, et al. Probiotics for the Prevention of Nosocomial Diarrhea in Children. J Pediatr Gastroenterol Nutr. 2018;66:3–9. doi: 10.1097/MPG.0000000000001637. [DOI] [PubMed] [Google Scholar]
- 32.Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, Lionetti E, Castellaneta S, Polimeno L, Peccarisi L, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126:e1445–e1452. doi: 10.1542/peds.2010-0467. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen N, Andersen NN, Végh Z, Jensen L, Ankersen DV, Felding M, Simonsen MH, Burisch J, Munkholm P. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20:16215–16226. doi: 10.3748/wjg.v20.i43.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YM, Ge XZ, Wang WQ, Wang T, Cao HL, Wang BL, Wang BM. Lactobacillus rhamnosus GG supernatant upregulates serotonin transporter expression in intestinal epithelial cells and mice intestinal tissues. Neurogastroenterol Motil. 2015;27:1239–1248. doi: 10.1111/nmo.12615. [DOI] [PubMed] [Google Scholar]
- 35.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pimentel M, Chatterjee S, Chang C, Low K, Song Y, Liu C, Morales W, Ali L, Lezcano S, Conklin J, et al. A new rat model links two contemporary theories in irritable bowel syndrome. Dig Dis Sci. 2008;53:982–989. doi: 10.1007/s10620-007-9977-z. [DOI] [PubMed] [Google Scholar]
- 37.Jee SR, Morales W, Low K, Chang C, Zhu A, Pokkunuri V, Chatterjee S, Soffer E, Conklin JL, Pimentel M. ICC density predicts bacterial overgrowth in a rat model of post-infectious IBS. World J Gastroenterol. 2010;16:3680–3686. doi: 10.3748/wjg.v16.i29.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pokkunuri V, Pimentel M, Morales W, Jee SR, Alpern J, Weitsman S, Marsh Z, Low K, Hwang L, Khoshini R, et al. Role of Cytolethal Distending Toxin in Altered Stool Form and Bowel Phenotypes in a Rat Model of Post-infectious Irritable Bowel Syndrome. J Neurogastroenterol Motil. 2012;18:434–442. doi: 10.5056/jnm.2012.18.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Xin H, Fang X, Dou H, Liu F, Huang D, Han S, Fei G, Zhu L, Zha S, et al. Isomalto-oligosaccharides ameliorate visceral hyperalgesia with repair damage of ileal epithelial ultrastructure in rats. PLoS One. 2017;12:e0175276. doi: 10.1371/journal.pone.0175276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. 2017;23:151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiller R. Clinical update: irritable bowel syndrome. Lancet. 2007;369:1586–1588. doi: 10.1016/S0140-6736(07)60726-0. [DOI] [PubMed] [Google Scholar]
- 43.Thornley JP, Jenkins D, Neal K, Wright T, Brough J, Spiller RC. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J Infect Dis. 2001;184:606–609. doi: 10.1086/322845. [DOI] [PubMed] [Google Scholar]
- 44.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Swan C, Duroudier NP, Campbell E, Zaitoun A, Hastings M, Dukes GE, Cox J, Kelly FM, Wilde J, Lennon MG, et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut. 2013;62:985–994. doi: 10.1136/gutjnl-2011-301213. [DOI] [PubMed] [Google Scholar]
- 46.Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–431. doi: 10.1007/s00535-011-0379-9. [DOI] [PubMed] [Google Scholar]
- 48.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 49.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–1791. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 51.Gershon MD, Drakontides AB, Ross LL. Serotonin: synthesis and release from the myenteric plexus of the mouse intestine. Science. 1965;149:197–199. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- 52.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 53.Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De Ponti F, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 54.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 55.Read NW, Gwee KA. The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol Ther. 1994;62:159–173. doi: 10.1016/0163-7258(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 56.Galligan JJ. Electrophysiological studies of 5-hydroxytryptamine receptors on enteric neurons. Behav Brain Res. 1996;73:199–201. doi: 10.1016/0166-4328(96)00096-4. [DOI] [PubMed] [Google Scholar]
- 57.Prins NH, Briejer MR, Van Bergen PJ, Akkermans LM, Schuurkes JA. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br J Pharmacol. 1999;128:849–852. doi: 10.1038/sj.bjp.0702762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuemmerle JF, Murthy KS, Grider JR, Martin DC, Makhlouf GM. Coexpression of 5-HT2A and 5-HT4 receptors coupled to distinct signaling pathways in human intestinal muscle cells. Gastroenterology. 1995;109:1791–1800. doi: 10.1016/0016-5085(95)90745-9. [DOI] [PubMed] [Google Scholar]
- 59.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Keszthelyi D, Troost FJ, Jonkers DM, van Eijk HM, Dekker J, Buurman WA, Masclee AA. Visceral hypersensitivity in irritable bowel syndrome: evidence for involvement of serotonin metabolism--a preliminary study. Neurogastroenterol Motil. 2015;27:1127–1137. doi: 10.1111/nmo.12600. [DOI] [PubMed] [Google Scholar]
- 61.Zhao JM, Lu JH, Yin XJ, Chen XK, Chen YH, Tang WJ, Jin XM, Wu LY, Bao CH, Wu HG, et al. Comparison of electroacupuncture and moxibustion on brain-gut function in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled trial. Chin J Integr Med. 2015;21:855–865. doi: 10.1007/s11655-015-2049-x. [DOI] [PubMed] [Google Scholar]
- 62.Keszthelyi D, Troost FJ, Jonkers DM, van Eijk HM, Lindsey PJ, Dekker J, Buurman WA, Masclee AA. Serotonergic reinforcement of intestinal barrier function is impaired in irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:392–402. doi: 10.1111/apt.12842. [DOI] [PubMed] [Google Scholar]
- 63.Bjerregaard H, Severinsen K, Said S, Wiborg O, Sinning S. A dualistic conformational response to substrate binding in the human serotonin transporter reveals a high affinity state for serotonin. J Biol Chem. 2015;290:7747–7755. doi: 10.1074/jbc.M114.573477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keating C, Beyak M, Foley S, Singh G, Marsden C, Spiller R, Grundy D. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J Physiol. 2008;586:4517–4530. doi: 10.1113/jphysiol.2008.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin DC, Cao HL, Xu MQ, Wang SN, Wang YM, Yan F, Wang BM. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J Gastroenterol. 2016;22:8137–8148. doi: 10.3748/wjg.v22.i36.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacKenzie A, Quinn J. A serotonin transporter gene intron 2 polymorphic region, correlated with affective disorders, has allele-dependent differential enhancer-like properties in the mouse embryo. Proc Natl Acad Sci USA. 1999;96:15251–15255. doi: 10.1073/pnas.96.26.15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan J, Kang C, Wang M, Wang Q, Li P, Liu H, Hou Y, Su P, Yang F, Wei Y, et al. Association study of serotonin transporter SLC6A4 gene with Chinese Han irritable bowel syndrome. PLoS One. 2014;9:e84414. doi: 10.1371/journal.pone.0084414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18:258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 72.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 73.Nazir S, Kumar A, Chatterjee I, Anbazhagan AN, Gujral T, Priyamvada S, Saksena S, Alrefai WA, Dudeja PK, Gill RK. Mechanisms of Intestinal Serotonin Transporter (SERT) Upregulation by TGF-β1 Induced Non-Smad Pathways. PLoS One. 2015;10:e0120447. doi: 10.1371/journal.pone.0120447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG; Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 78.Esmaili A, Nazir SF, Borthakur A, Yu D, Turner JR, Saksena S, Singla A, Hecht GA, Alrefai WA, Gill RK. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology. 2009;137:2074–2083. doi: 10.1053/j.gastro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA, Jones BV. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci Rep. 2015;5:17324. doi: 10.1038/srep17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kekuda R, Torres-Zamorano V, Leibach FH, Ganapathy V. Human serotonin transporter: regulation by the neuroprotective agent aurintricarboxylic acid and by epidermal growth factor. J Neurochem. 1997;68:1443–1450. doi: 10.1046/j.1471-4159.1997.68041443.x. [DOI] [PubMed] [Google Scholar]
- 81.Kubota N, Kiuchi Y, Nemoto M, Oyamada H, Ohno M, Funahashi H, Shioda S, Oguchi K. Regulation of serotonin transporter gene expression in human glial cells by growth factors. Eur J Pharmacol. 2001;417:69–76. doi: 10.1016/s0014-2999(01)00906-2. [DOI] [PubMed] [Google Scholar]
- 82.Gil C, Najib A, Aguilera J. Serotonin transport is modulated differently by tetanus toxin and growth factors. Neurochem Int. 2003;42:535–542. doi: 10.1016/s0197-0186(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 83.Katsumata R, Shiotani A, Murao T, Ishii M, Fujita M, Matsumoto H, Haruma K. Gender Differences in Serotonin Signaling in Patients with Diarrhea-predominant Irritable Bowel Syndrome. Intern Med. 2017;56:993–999. doi: 10.2169/internalmedicine.56.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galligan JJ, Patel BA, Schneider SP, Wang H, Zhao H, Novotny M, Bian X, Kabeer R, Fried D, Swain GM. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterol Motil. 2013;25:e373–e381. doi: 10.1111/nmo.12133. [DOI] [PubMed] [Google Scholar]
- 85.Morales W, Pimentel M, Hwang L, Kunkel D, Pokkunuri V, Basseri B, Low K, Wang H, Conklin JL, Chang C. Acute and chronic histological changes of the small bowel secondary to C. jejuni infection in a rat model for post-infectious IBS. Dig Dis Sci. 2011;56:2575–2584. doi: 10.1007/s10620-011-1662-6. [DOI] [PubMed] [Google Scholar]
- 86.Shao YY, Huang J, Ma YR, Han M, Ma K, Qin HY, Rao Z, Wu XA. Serum serotonin reduced the expression of hepatic transporter Mrp2 and P-gp via regulating nuclear receptor CAR in PI-IBS rats. Can J Physiol Pharmacol. 2015;93:633–639. doi: 10.1139/cjpp-2015-0039. [DOI] [PubMed] [Google Scholar]
- 87.Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, McDonald T, Spreadbury I, Cenac N, Cattaruzza F, Hurlbut D, Vanner S, Bunnett N, et al. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098–2108.e5. doi: 10.1053/j.gastro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Hsu LT, Hung KY, Wu HW, Liu WW, She MP, Lee TC, Sun CH, Yu WH, Buret AG, Yu LC. Gut-derived cholecystokinin contributes to visceral hypersensitivity via nerve growth factor-dependent neurite outgrowth. J Gastroenterol Hepatol. 2016;31:1594–1603. doi: 10.1111/jgh.13296. [DOI] [PubMed] [Google Scholar]
- 89.Sharma A, Lelic D, Brock C, Paine P, Aziz Q. New technologies to investigate the brain-gut axis. World J Gastroenterol. 2009;15:182–191. doi: 10.3748/wjg.15.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 91.Li H, Cao Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids. 2010;39:1107–1116. doi: 10.1007/s00726-010-0582-7. [DOI] [PubMed] [Google Scholar]
- 92.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 95.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]


