Abstract
AIM
To investigate the prevalence and clinical significance of autoimmune liver disease (ALD)-related autoantibodies in patients with biliary atresia (BA).
METHODS
Sera of 124 BA patients and 140 age-matched non-BA controls were assayed for detection of the following autoantibodies: ALD profile and specific anti-nuclear antibodies (ANAs), by line-blot assay; ANA and anti-neutrophil cytoplasmic antibody (ANCA), by indirect immunofluorescence assay; specific ANCAs and anti-M2-3E, by enzyme linked immunosorbent assay. Associations of these autoantibodies with the clinical features of BA (i.e., cytomegalovirus infection, degree of liver fibrosis, and short-term prognosis of Kasai procedure) were evaluated by Spearman’s correlation coefficient.
RESULTS
The overall positive rate of serum autoantibodies in preoperative BA patients was 56.5%. ALD profile assay showed that the positive reaction to primary biliary cholangitis-related autoantibodies in BA patients was higher than that to autoimmune hepatitis-related autoantibodies. Among these autoantibodies, anti-BPO was detected more frequently in the BA patients than in the controls (14.8% vs 2.2%, P < 0.05). Accordingly, 32 (25.8%) of the 124 BA patients also showed a high positive reaction for anti-M2-3E. By comparison, the controls had a remarkably lower frequency of anti-M2-3E (P < 0.05), with 6/92 (8.6%) of patients with other liver diseases and 2/48 (4.2%) of healthy controls. The prevalence of ANA in BA patients was 11.3%, which was higher than that in disease controls (3.3%, P < 0.05), but the reactivity to specific ANAs was only 8.2%. The prevalence of ANCAs (ANCA or specific ANCAs) in BA patients was also remarkably higher than that in the healthy controls (37.9% vs 6.3%, P < 0.05), but showed no difference from that in patients with other cholestasis. ANCA positivity was closely associated with the occurrence of postoperative cholangitis (r = 0.61, P < 0.05), whereas none of the autoantibodies showed a correlation to cytomegalovirus infection or the stages of liver fibrosis.
CONCLUSION
High prevalence of autoantibodies in the BA developmental process strongly reveals the autoimmune-mediated pathogenesis. Serological ANCA positivity may be a useful predictive biomarker of postoperative cholangitis.
Keywords: Biliary atresia, Anti-nuclear antibody, Anti-neutrophilic cytoplasmic antibody, Autoimmune liver diseases, Autoantibodies
Core tip: The autoimmune-mediated pathogenesis of biliary atresia (BA) is not fully understood, and non-invasive diagnostic methods cannot clearly discriminate BA from other causes of neonatal cholestasis. We investigated the prevalence and clinical significance of autoimmune liver disease-related autoantibodies in BA patients. The overall positive rate of autoantibodies in BA was 56.5%. The data showed that frequent detection of autoantibodies in BA may strongly support the autoimmune-mediated pathogenesis. Interestingly, preoperative anti-neutrophil cytoplasmic antibody positivity was closely associated with prediction of cholangitis occurrence after Kasai portoenterostomy.
INTRODUCTION
Biliary atresia (BA) is a severe neonatal disease, characterized by progressive inflammatory fibrosis and obliteration of both the intra-hepatic and extra-hepatic bile ducts[1,2]. Early Kasai portoenterostomy (KP), the first-line treatment for BA, may re-establish bile flow to alleviate liver injury caused by cholestasis and to prolong survival with the native liver[3]. However, in the majority of BA patients, the continued existence of the bile duct injury may eventually lead to cirrhosis and need for liver transplantation[4,5]. Thus, gaining a better understanding of the pathogenic mechanisms underlying BA may facilitate early diagnosis or development of clinical therapies to halt the injury of hepatic bile ducts and to preserve liver function.
Although the etiology of BA is not fully understood, accumulated evidence in the literature supports the theory that a primary perinatal viral infection triggers an aberrant autoimmune-mediated attack on bile duct epithelia by molecular mimicry, with both the cellular and humoral immunity playing important roles in the BA autoimmune injury mechanism[6-9]. Several viruses have been proposed as the infectious agents, and perinatal infection with the cytomegalovirus (CMV) has been demonstrated as an important etiological factor for BA in China[10]. Periductal immunoglobulin (Ig) and circulating autoantibodies which might be used in the classification of autoimmune diseases have been described in both patients with BA and animal models of the disease[11,12]; unfortunately, the specificity of these autoantibodies for BA has been far less satisfying.
BA and autoimmune liver disease (ALD) have some similar clinical manifestations and pathological features. However, ALD-related autoantibodies have not yet been comprehensively investigated in BA patients, to the best of our knowledge. Here, we describe our investigation into the prevalence of the ALD profile and the extent of positivity of anti-nuclear antibodies (ANAs) and anti-neutrophilic cytoplasmic antibodies (ANCAs) in the sera of BA patients. The associations of these autoantibodies with the clinical features of BA were also assessed statistically.
MATERIALS AND METHODS
Case enrollment
A total of 124 preoperative BA patients [mean age: 2.9 mo (interquartile range, IQR: 1.9-3.0)], 92 controls with other liver diseases [mean age: 2.8 mo (IQR: 2.0-3.2); including 42 with choledochal cysts, 35 with transient cholestasis of unknown origin, and 15 with neonatal intrahepatic cholestasis caused by citrin deficiency], and 48 healthy controls [mean age: 3.4 mo (IQR: 2.0-4.0)] were enrolled in this study. Table 1 shows the demographic and clinical features, and biochemical parameters of the study population. All study participants originated from Guangzhou Women and Children’s Medical Center (Guangzhou, China) between January 2015 and December 2016. Enrollment was proposed to all consecutive infants with diagnosed BA that had been confirmed by surgical exploration, cholangiography and histology. Diagnosis of all controls was based on the criteria published in our previous report[13]. Clinical information was collected when available, including CMV infection, biochemical indexes, histological liver fibrosis stages, and short-term outcomes. Histological liver fibrosis in BA was assessed by METAVIR fibrosis scores (F0-F4)[14,15]. Follow-up data which could evaluate persistence of jaundice (total bilirubin, TB: > 34 μmol/L), acute liver injury (alanine aminotransferase, ALT: >35 U/L), and occurrence of cholangitis within 3-10 mo after KP were collected for analysis of short-term outcomes[16].
Table 1.
Demographic and clinical features, and biochemical parameters1 of biliary atresia patients and non-biliary atresia controls n (%)
| Variable | BA, n = 124 |
Non-BA, n = 140 |
|
| Other liver diseases2, n = 92 | Healthy, n = 48 | ||
| Age, mo | 2.9 (1.9-3.0) | 2.8 (2.0-3.2) | 3.4 (2.0-4.0) |
| Sex, male/female | 60/64 | 50/42 | 25/23 |
| METAVIR score | |||
| F0 | 4 (3.2) | NA | NA |
| F1 | 37 (29.8) | NA | NA |
| F2 | 24 (19.4) | NA | NA |
| F3 | 50 (40.3) | NA | NA |
| F4 | 9 (7.3) | NA | NA |
| ALT, U/L | 162.4 (104.3-204.6)a | 132.0 (70.5-247.5) | 28.1 (22.4-40.8) |
| AST, U/L | 190.5 (151.4-257.4)a | 195.9 (117.0-292.5) | 38.0 (26.0-52.3) |
| γ-GT, U/L | 716.0 (411.0-1142.5)a | 598.4 (189.5-1043.2) | 32.7 (23.4-46.3) |
| ALP, U/L | 406.8 (316.5-522.2)a | 517.0 (409.7-660.3) | 275.8 (189.0-345.6) |
| TBIL, μmol/L | 157.0 (126.5-184.0)a | 145.7 (107.1-196.5) | 6.5 (2.8-14.8) |
| DBIL, μmol/L | 130.5 (103.5-152.7)a | 110.1 (88.4-137.8) | 3.1 (1.0-4.9) |
Data are described as median (interquartile range: 25th-75th percentile). 1Reference intervals: ALT, 3-35 U/L; AST, 5-60 U/L; γ-GT, 13-57 U/L; ALP, 118-390 U/L; TBIL, 2-17 μmol/L; DBIL, 0-7 μmol/L;
Choledochal cysts, transient cholestasis of unknown origin, and neonatal intrahepatic cholestasis caused by citrin deficiency were included as disease controls.
P < 0.05 vs healthy controls. ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BA: Biliary atresia; DBIL: Direct bilirubin; FO-F4: Fibrosis scores 0-4; γ-GT: Gamma-glutamyl transpeptidase; NA: Not applicable; TBIL: Total bilirubin.
ALD profile
The line-blot ALD profile contains the primary biliary cholangitis (PBC)-related antibodies [anti-mitochondrial antibody, AMA-M2 (pyruvate dehydrogenase complex, PDC), anti-BPO (recombinant fusion proteins of the E2 subunits derived from the 2-oxo-acid dehydrogenase complex targeted by the inner mitochondrial membrane), anti-Sp100, anti-promyelocytic leukemia protein (PML), and anti-gp210], autoimmune hepatitis (AIH)-related antibodies [anti-liver-kidney microsomal type 1 (LKM-1), anti-liver cytosolic antigen type 1 (LC-1), and anti-soluble liver antigen/liver-pancreas (SLA/LP)], and anti-Ro-52 antibodies. A commercially available kit (EUROIMMUN AG, Lübeck, Germany) was used according to the manufacturer’s instructions. Signal strengths of > 10 arbitrary units (AUs) were considered positive.
AMA reactivity was confirmed, with enlarged sample size by an enhanced anti-M2-3E enzyme linked immunosorbent assay (ELISA), which mixed enveloped recombinant fusion protein BPO and natively purified PDC from bovine heart mitochondria as antigenic targets (EUROIMMUN AG).
ANA and specific ANAs
ANA was detected by indirect immunofluorescence (IIF) using the antigen substrate panel of Hep-2 cells and primate liver. A serum titer ≥ 1:100 was considered positive. ANA positivity was subgrouped based upon the specific fluorescence patterns. Accordingly, line-blot immunoassay was used to determine the IgG autoantibody panel for 12 specific ANAs, which consisted of anti-nRNP/Sm, anti-Sm, anti-SS-A, anti-Ro-52, anti-SS-B, anti-Scl-70, anti-Jo-1, anti-CENP B, anti-dsDNA, anti-nucleosomes, anti-histone, and anti-ribosomal phosphoprotein. Experiments were performed following the manufacturer’s instructions (EUROIMMUN AG).
ANCA and specific ANCAs
A commercially available IIF assay was used for determination of ANCA on ethanol- and formaldehyde-fixed human neutrophils (EUROIMMUN AG). A positive ANCA finding was defined as a titer of antibodies > 1:10. The ANCA findings were subgrouped into cytoplasmic (c)-ANCA, perinuclear (p)-ANCA, and atypical (a)-ANCA according to the fluorescence patterns. Specific ANCAs of myeloperoxidase (MPO) and proteinase 3 (PR3) were further assayed by ELISA (EUROIMMUN AG).
Association of autoantibodies with clinical features
To determine whether the presence of autoantibodies in BA patients was associated with worse disease progression, we compared the clinical features of the BA patients who presented with and without autoantibodies. The clinical features of 124 BA patients (mainly composed of those with CMV infection) and degree of liver fibrosis were retrospectively analyzed for the period prior to the KP; in addition, the information of short-term outcomes in 52 BA patients who were followed postoperatively for > 3 mo was collected, and 24 of those 52 cases were re-assessed for preoperative and postoperative serum autoantibodies to compare the change of autoantibodies over time.
Statistical analysis
Normally distributed variables are represented as mean ± SD, and non-normally distributed variables as median (IQR). Categorical data are described as frequencies and/or percentages. For continuous variables, between-group differences were compared using the Student’s t-test or the Mann-Whitney U test. For categorical variables, the χ 2 test or Fisher’s exact test was used to compare the prevalence between groups when appropriate. Correlation was evaluated by the Spearman’s correlation coefficient. SPSS 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Armonk, NY, United States) was used to perform all statistical analyses. P values < 0.05 were considered statistically significant.
RESULTS
ALD profile in patients with BA compared to controls
Sera of 81 postoperative BA patients and 88 non-BA controls were evaluated for ALD profile (Table 2). The 88 non-BA controls consisted of 40 healthy controls and 48 disease controls, including 22 with choledochal cysts, 14 with neonatal intrahepatic cholestasis caused by citrin deficiency, and 12 with transient cholestasis of unknown origin. One or more of the PBC-related antibodies was detected in 18.5% (15/81) of the patients with BA. For the PBC-related antibodies, a positive reaction to AMA-M2, anti-BPO, anti-Sp100, anti-gp210, and anti-PML in BA patients was found in 1.2%, 14.8%, 1.2%, 2.5% and 3.7%, respectively. Among these autoantibodies, anti-BPO was detected more frequently in the BA patients than in the non-BA controls (14.8% vs 2.2%, P < 0.05) or the healthy controls (14.8% vs 0%, P < 0.05). The prevalence of any other antibodies was not different between the BA and non-BA groups. Only 1 (1.2%) in 81 of the BA patients showed positivity for both AMA-M2 and anti-BPO (Figure 1). For the AIH-related antibodies, low positivity was found in both the BA patients and non-BA controls (7.4% and 6.8%, respectively, P > 0.05). Among the total 81 postoperative BA patients, 5 (6.2%) and 1 (1.2%) showed positivity for anti-LC-1 and anti-SLA/LP, respectively.
Table 2.
Prevalence profile of autoimmune liver disease in biliary atresia patients and non-biliary atresia controls n (%)
| Variable | BA, n = 81 |
Non-BA, n = 88 |
||
| Other liver diseases1, n = 44 | Healthy, n = 40 | Total | ||
| PBC-related antibodies | 15 (18.5)c | 4 (9.1) | 1 (2.5) | 5 (5.7) |
| AMA-M2 | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) |
| Anti- BPO | 12 (14.8)ac | 2 (4.5) | 0 (0) | 2 (2.2) |
| Anti-Sp100 | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) |
| Anti-gp210 | 2 (2.5) | 1 (2.3) | 1 (2.5) | 2 (2.2) |
| Anti-PML | 3 (3.7) | 1 (2.3) | 0 (0) | 1 (1.1) |
| AMA-M2 + anti-BPO | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) |
| AMA-M2 + anti-BPO + anti-Sp100 + anti-PML | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) |
| AIH-related antibodies | 6 (7.4) | 3 (6.8) | 3 (7.5) | 6 (6.8) |
| Anti-LKM-1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Anti-LC-1 | 5 (6.2) | 3 (6.8) | 3 (7.5) | 6 (6.8) |
| Anti-SLA/LP | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) |
| Anti-Ro-52 | 5 (6.2) | 2 (4.5) | 2 (5) | 4 (4.5) |
Choledochal cysts, transient cholestasis of unknown origin, and neonatal intrahepatic cholestasis caused by citrin deficiency were included as disease controls. AMA-M2 + M2-3E: combined the positivity to AMA-M2 and BPO; AMA-M2 + BPO + Sp100 + PML: Combined the positivity to AMA-M2, BPO, Sp100, and PML.
P < 0.05 vs non-BA;
P < 0.05 vs healthy controls. AIH: Autoimmune hepatitis; BA: Biliary atresia; PBC: Primary biliary cholangitis; LKM-1: Liver-kidney microsomal type 1; LC-1: Liver cytosolic antigen type 1.
Figure 1.
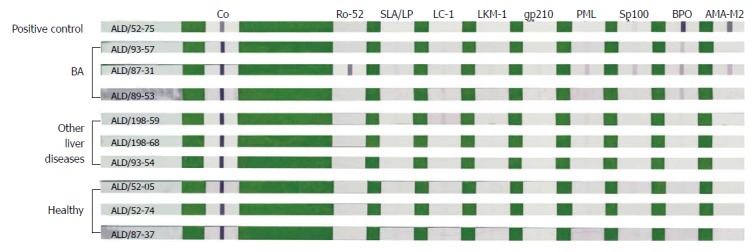
Representative strips after color development by line-blot immunoassay. The line-blot immunoassay strips had been coated with nine autoimmune liver disease-related antigens, including Ro-52, SLA/LP, LC-1, LKM-1, gp210, PML, Sp100, BPO and AMA-M2 (from left to right). BA group: ALD/93-57 with anti-BPO +; ALD/87-31 with anti-Ro-52 +++, anti-PML +, anti-Sp100 +, anti-BPO ++, and AMA-M2 +; ALD/89-53 with anti-BPO +; Other liver diseases group: Only ALD/198-59 with anti-LC-1 ±; Healthy group: All autoantibodies were negative. Positive control (ALD/52-75) showed anti-BPO +++ and AMA-M2 +++. Other liver diseases include choledochal cysts, transient cholestasis of unknown origin, and neonatal intrahepatic cholestasis caused by citrin deficiency. ALD: Autoimmune liver disease; BA: Biliary atresia; LC-1: Liver cytosolic antigen type 1.
With enlarged sample size, AMA reactivity was confirmed by an enhanced anti-M2-3E ELISA. As presented in Figure 2, 32 of the 124 BA patients showed a higher positive reaction to anti-M2-3E compared to the disease controls and the healthy controls (25.8% vs 8.6%, P < 0.05 and 25.8% vs 4.2%, P < 0.05, respectively).
Figure 2.
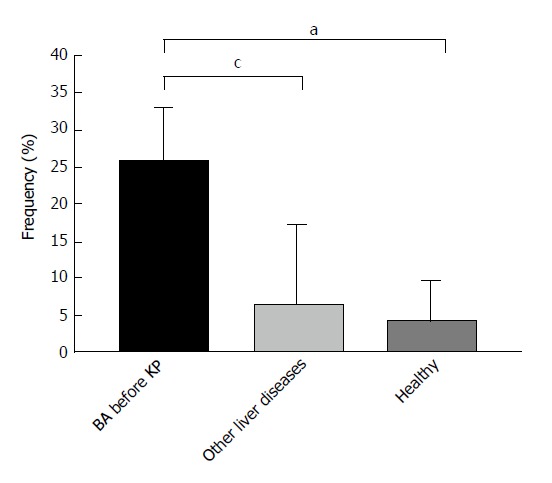
Positivity of anti-M2-3E detected by enzyme linked immunosorbent assay in biliary atresia patients and controls. aP < 0.05 vs healthy controls; cP < 0.05 vs other liver diseases. Other liver diseases include choledochal cysts, transient cholestasis of unknown origin, and neonatal intrahepatic cholestasis caused by citrin deficiency. BA: Biliary atresia; ELISA: Enzyme linked immunosorbent assay.
ANA and specific ANAs in patients with BA compared to controls
ANA exhibited a higher prevalence in 124 BA patients compared to 92 patients with other liver diseases (11.3% vs 3.3%, P < 0.05), but showed no difference from that in 48 healthy controls (4.2%). Nuclear homogeneous, speckled, and nucleolar types were the main fluorescence patterns of ANA positivity in BA (Table 3). Patterns involving nuclear dots, centrosome, cytoplasm, ring or rod Golgi, and centromere were present occasionally.
Table 3.
Prevalence of anti-nuclear antibodies in biliary atresia patients and non-biliary atresia controls n (%)
| Variable | BA, n = 124 | Other liver diseases1, n = 92 | Healthy, n = 48 |
| ANA, by IIF | 14 (11.3)c | 3 (3.3) | 2 (4.2) |
| Fluorescence patterns | |||
| Homogeneous | 3 (2.4) | 0 (0) | 0 (0) |
| Speckled | 3 (2.4) | 0 (0) | 1 (2.1) |
| Nucleolar | 3 (2.4) | 0 (0) | 1 (2.1) |
| Nuclear dots | 1 (0.8) | 0 (0) | 0 (0) |
| Centrosome | 1 (0.8) | 0 (0) | 0 (0) |
| Cytoplasm | 1 (0.8) | 0 (0) | 0 (0) |
| Ring or rod Golgi | 1 (0.8) | 2 (2.2) | 0 (0) |
| Centromere | 1 (0.8) | 0 (0) | 0 (0) |
| Spindle apparatus | 0 (0) | 1 (1.1) | 0 (0) |
| Specific ANA2, by line-blot | |||
| SSA | 1 (0.8) | 0 (0) | 0 (0) |
| Ro-52 | 5 (4.0) | 2 (2.2) | 2 (4.2) |
| CENP B | 4 (3.2) | 1 (1.1) | 0 (0) |
| dsDNA | 3 (2.4) | 0 (0) | 0 (0) |
Choledochal cysts, transient cholestasis of unknown origin, and neonatal intrahepatic cholestasis caused by citrin deficiency were included as disease controls;
Specific ANAs included 12 different antibodies, with only SSA, Ro-52, CENP B, and dsDNA positive in the study.
P < 0.05 vs other liver diseases. ANA: Anti-nuclear antibody; BA: Biliary atresia; IIF: Indirect immunofluorescence.
The specific ANA findings in the BA patients evaluated in this study were as follows (Table 3): positivity for anti-SSA in 1, for anti-Ro-52 in 5, for anti-CENP B in 4, and for anti-dsDNA in 3. At least one autoantibody was present in 8.9% (11/124) of the BA patients, which was a higher rate compared to the non-BA controls, but the difference did not reach the threshold for statistical significance. No other specific ANAs were found in the sera of BA patients.
ANCA and specific ANCAs in patients with BA compared to controls
Twenty-nine percent (36/124) of BA patients were positive for ANCA. The prevalence was similar to that of the patients with other liver diseases (25.0%, P > 0.05), and was higher than that of the healthy controls (4.2%, P < 0.05) (Table 4). As shown in Figure 3, p-ANCA and c-ANCA were present more frequently than a-ANCA in BA patients (20.2% and 8.1% compared to 0.8%, respectively)
Table 4.
Prevalence of anti-neutrophil cytoplasmic antibodies in biliary atresia patients and non-biliary atresia controls n (%)
| Variable | BA, n = 124 | Other liver diseases1, n = 92 | Healthy, n = 48 |
| ANCA, by IIF | 36 (29.0)a | 23 (25.0) | 2 (4.2) |
| c-ANCA | 10 (8.1) | 1 (1.1) | 0 (0) |
| p-ANCA | 25 (20.2) | 21 (22.8) | 2 (4.2) |
| a-ANCA | 1 (0.8) | 1 (1.1) | 0 (0) |
| Specific ANCA, by ELISA | |||
| Anti-MPO | 11 (8.9) | 6 (6.5) | 2 (4.2) |
| Anti-PR3 | 19 (15.3) | 13 (14.2) | 0 (0) |
| Anti-MPO and/or anti-PR3 | 23 (18.0)a | 13 (14.2) | 2 (4.2) |
| ANCAs | 47 (37.9)a | 31 (33.7) | 3 (6.3) |
Choledochal cysts, transient cholestasis of unknown origin, and neonatal intrahepatic cholestasis caused by citrin deficiency were included as disease controls.
P < 0.05 vs healthy controls. Anti-MPO and/or anti-PR3: Any positivity of specific ANCAs; ANCAs: Any positivity of ANCAs by IIF or specific ANCA by ELISA. ANCA: Anti-neutrophil cytoplasmic antibody; BA: Biliary atresia; ELISA: Enzyme linked immunosorbent assay; IIF: Indirect immunofluorescence.
Figure 3.
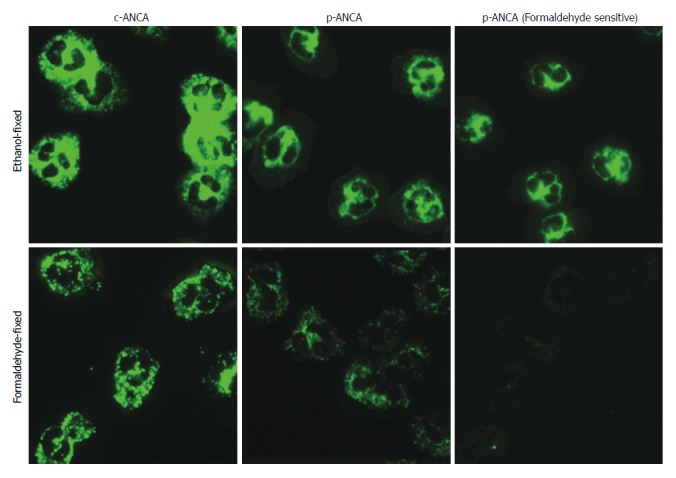
The main fluorescence patterns of anti-neutrophil cytoplasmic antibodies in biliary atresia patients. ANCA detection by indirect immunofluorescence assays was performed on ethanol-fixed (upper panel) and formaldehyde-fixed (lower panel) human neutrophils. Depending on whether reactivities of formaldehyde-fixed human neutrophils were positive or not, p-ANCA was divided into p-ANCA with formaldehyde resistance and p-ANCA with formaldehyde sensitivity. ANCA: Anti-neutrophil cytoplasmic antibody; BA: Biliary atresia; (c)-ANCA: Cytoplasmic-ANCA; (p)-ANCA: Perinuclear-ANCA.
Anti-MPO and anti-PR3, alone or associated, were found in 23/124 (18.0%) of BA patients, whose positive rates were higher than those in the healthy controls (P < 0.05). Among the 124 BA patients, 7 showed positive reactions for both anti-MPO and anti-PR3. The prevalence of ANCAs (ANCA, anti-MPO, or anti-PR3) in BA patients was higher than that in healthy controls (37.9% vs 6.3%, P < 0.05).
Association of autoantibodies with clinical features
In this study, anti-M2-3E, ANA, and ANCA had higher prevalence in 124 BA patients than in 140 non-BA controls. Among the BA patients, 56.5% showed positivity for at least one type of the autoantibodies (anti-M2-3E, ANA, and ANCA). Among the 80 BA patients, 45% had a perinatal infection with CMV (detected by combined analysis of anti-CMV IgG, IgM, and CMV DNA). The percentage of CMV infection in the anti-M2-3E-positive BA patients was similar to that in the anti-M2-3E-negative BA patients (51.9% vs 41.5%, P > 0.05). The prevalence of CMV infection in ANCA-positive BA patients was higher than that in the ANCA-negative BA patients (54.3% vs 37.8%, P > 0.05). There was no statistical relationship found between the presence of these autoantibodies and CMV infection.
Regardless of the severity of fibrosis, anti-M2-3E, ANA, or ANCA was present in the sera of BA patients. The positive rates of these autoantibodies showed no difference according to the extent of fibrosis (F0-F4) in the 124 BA patients (anti-M2-3E: 25.0%, 27.0%, 16.7%, 32.0%, and 22.2%, respectively, P > 0.05; ANA: 25.0%, 5.4%, 12.5%, 10.0%, and 22.2%, respectively, P > 0.05; ANCA: 50.0%, 30.0%, 41.7%, 32.0%, and 44.4%, respectively, P > 0.05).
Interestingly, as presented in Table 5, the prevalence of ANCA showed a positive correlation to the occurrence of postoperative cholangitis (r = 0.61, P < 0.05). Thus, the probability of ANCA negativity in postoperative cholangitis is very low. ANCA positivity did not correlate with the persistence of jaundice or acute liver injury, nor did anti-M2-3E positivity or ANA positivity show a statistical association with prognosis associated with the procedure.
Table 5.
Association of autoantibodies and prognosis of Kasai procedure with follow-up of 52 biliary atresia patients n (%)
| Parameter |
Anti-M2-3E |
ANA |
ANCA |
|||
| Positive, n = 17 | Negative, n = 35 | Positive, n = 8 | Negative, n = 44 | Positive, n = 29 | Negative, n = 23 | |
| TB, μmol/L | 105.5 ± 80.3 | 109.4 ± 106.8 | 41.6 ± 49.5 | 127.9 ± 135.0 | 133.0 ± 120.7 | 85.3 ± 66.2 |
| ALT, U/L | 184.1 ± 119.8 | 171.7 ± 121.4 | 127.9 ± 135.0 | 185.8 ± 115.7 | 171.46 ± 95.5 | 179.6 ± 140.3 |
| Occurrence of cholangitis | 9 (52.9) | 20 (57.1) | 5 (62.5) | 24 (54.5) | 24 (82.8)a | 5 (21.7)a |
Data are described as mean ± SD.
P < 0.05, r = 0.61; ANCA showed a positive correlation to the occurrence of postoperative cholangitis. TB is showed as a parameter of persistence of jaundice (TB > 34 μmol/L); ALT is showed as a parameter of acute liver injury (ALT > 35 U/L). ANCAs: Any positivity of ANCAs or specific ANCAs. ALT: Alanine aminotransferase; ANA: Anti-nuclear antibody; ANCA: Anti-neutrophil cytoplasmic antibody; BA: Biliary atresia; TB: Total bilirubin.
The longitudinal (follow-up) findings for autoantibodies showed that 24 BA patients exhibited slight variation from the first evaluation to re-assessment within an average of 6 mo, but without statistically significant differences between the paired samples.
DISCUSSION
In an earlier study, Hadchouel et al[11] found IgG deposits on the basement membranes of glandular formations in about one-third of biliary remnants studied. Accumulating evidence suggests that the outset of BA may be triggered by an initial perinatal hepatobiliary viral infection[7,17]. Studies have also demonstrated that CMV infection initiates the autoimmune process in BA by targeting intrahepatic biliary epithelial cells of the host, and that the only reactivity to the α-enolase antibody identified in BA was non-specific in human BA[10,18-20]. To date, serological specificity is too low for the diagnosis of BA, making it impossible to avoid damage from invasive surgery. Thus, the aim of this study was to seek a non-invasive biomarker which can discriminate BA from other causes of neonatal cholestasis or predict prognosis.
In the present study, 56.5% of BA patients were positive for at least one type of the autoantibodies. The presence of autoantibodies in the BA patients was not an epiphenomenon, and high prevalence of autoantibodies in BA provided evidence of an autoimmune-mediated pathogenesis.
For PBC-related autoantibodies, 18.5% of the BA patients showed positivity for one or more autoantibodies. Particularly, anti-BPO was present in 14.8% (12/81) of the BA patients, which was detected at a higher frequency than that in the non-BA controls or healthy subjects but at a lower frequency than that in the PBC patients (84.5%)[21]. To our knowledge, this was the first time that anti-BPO has been detected in BA infants, as it is often reported in adults but has been rarely reported in pediatric patients[22]. Compared with PBC patients, the sensitivity of both AMA-M2 and anti-BPO was relatively lower in BA patients, and the positivity of anti-BPO was higher than that of AMA-M2. This finding hinted that the corresponding antigen epitope of AMA in BA serum was different from that in PBC serum, whose reaction to E2 subunits of the oxo-glutarate dehydrogenase complex and branched-chain oxo-acid dehydrogenase complex was higher than that to PDC-E2. By enlarging the sample size and adopting ELISA to further confirm the detection of AMA in BA, we determined that 32 (25.8%) of 124 BA patients had a positive reaction for anti-M2-3E. Yet, the markedly increased anti-M2-3E positivity was not associated with CMV infection, severity of liver fibrosis, or prognosis of the Kasai procedure in BA.
It has been reported that anti-PML and anti-Sp100 are co-immunogenic in PBC patients, correlating with an unfavorable disease course and fast progression[23,24]. Our findings showed that the positivity for anti-Sp100, anti-gp210, and anti-PML in BA was rather low, and the co-existence of anti-Sp100 and anti-PML only occurred in a single BA patient, whose liver damage developed rapidly and seriously according to the diagnosis of pathological biopsy. Anti-gp210 is related to a severe disease course and poor prognosis of PBC. PBC patients who are positive for anti-gp210 will progress into liver failure more easily[25]. In our study, a total of four study participants displayed anti-gp210 positivity, including two with BA, one with choledochal cysts, and one healthy infant. The two patients with BA presented with biliary cirrhosis, while the one patient with choledochal cysts showed middle-stage liver damage. We were unsure whether the anti-gp210 in the healthy infant appeared earlier than the clinical manifestations, possibly as an alerting signal, similar to AMA-M2 in PBC. The AIH-related antibodies showed low positive rates in both BA and control groups. Among these autoantibodies, anti-LKM was not detected in any of the BA patients, which is consistent with the previous report by A-Kader et al[26].
For ANA, our study detected it at a higher frequency (11.3%) in BA patients than the previous report of 5%[26]. The prevalence was also significantly higher than that in patients with other liver diseases in our study. ANA, presenting mostly with a speckled pattern, is reportedly positive in approximately 6% of healthy children, which is similar to that found in the disease controls and healthy controls of our study[27,28]. As is known, ANA is fundamental for the diagnosis of a variety of autoimmune diseases, and the clinical value of ANA testing for autoimmune liver diseases is beyond doubt[29,30]. In our study, neither ANA nor specific ANAs exhibited any correlation to stages of liver fibrosis or prognosis of the KP procedure.
Both the positivity and titer of ANA are related to age of the autoimmune disease patient (being lower in childhood disease than in adulthood disease)[31,32]. Of note, the average age of BA patients in our study was 2.9 mo, a period in which nearly all of the IgG is maternally-derived. Thus, it is possible that maternally-derived IgG may cause development of the disease. This is in line with the findings of Hannam et al[33], who documented cases where placental transfer of maternal AMA was associated with neonatal liver disease. Based on these data, longitudinal analysis of ANA in BA patients was performed (comparison of detection at the first evaluation and at re-assessment within an average 6 mo of follow-up). There were no significant differences between the preoperative and the postoperative findings, suggesting that the autoantibodies that arose in the BA patients may not be transient.
In addition, the prevalence of ANCA (29.0%) was remarkably higher in sera from BA patients than in that of healthy controls, similar to the positivity rates detected for the special ANCAs, which included anti-MPO and anti-PR3. However, the detection of ANCA in BA did not correspond fully with other reports. In a preliminary observation[34], ANCA was detected in 91% of patients with BA, and was suggested as a promising biomarker for an immune-mediated process directed against specific hepatobiliary antigens; however, in another report by A-Kader et al[26], the ANCA was positive in only 1 of 20 patients. These differences in findings might reflect the differences in age of the patients studied or in the study methods applied.
In our study, we found that ANCA positivity in BA patients appeared more frequently with postoperative cholangitis than did ANCA negativity. As is known, ANCA is not only a helpful tool for establishing the diagnosis of Wegener’s granulomatosis and microscopic polyangiitis but also appears with non-vascular chronic inflammatory diseases. The presence of ANCA has also been associated with the occurrence of relapses in AIH, with decreased liver synthesis function in PBC and with increased cholestasis in primary sclerosing cholangitis[12,35]. One of the minor antigen targets of ANCA present in BA has been shown to be α-enolase, which is an enzyme involved in glycolysis and is ubiquitously expressed in a variety of cells, including biliary epithelial cells and hepatocytes[12]. It hinted that neutrophils may be activated in BA. The priming of neutrophils with tumor necrosis factor-α induces marked expression of ANCA antigens on the cell surface[36,37]. The expression of these target antigens facilitates the interaction with ANCA, resulting in subsequent polymorphonuclear leukocyte activation, which then induces production of reactive oxygen species, release of superoxide, and degranulation of neutrophils. ANCA was also reported to be involved in fibrosis of the lung and kidney[38,39]; however, we found that the rates of ANCA positivity were not significantly different in BA patients with fibrosis from stages F0 to F4, indicating that these antibodies might not be associated with the severity of liver fibrosis necessarily.
In summary, we found that the prevalence of ANCA increased prominently in BA, which showed a positive correlation to the occurrence of postoperative cholangitis. Although anti-M2-3E was not associated with prognosis of the KP procedure, its high prevalence provided evidence of autoimmune activity in the pathogenesis of BA. Thus, autoantibodies in sera of BA children were not an accidental epiphenomenon. Future research in multi-centers focusing on identifying potentially pathogenic, bile duct-specific autoantibodies and the titer and source of autoantibodies in BA should be pursued.
ARTICLE HIGHLIGHTS
Research background
Accumulating evidence supports the biliary atresia (BA) pathogenesis theory of a primary perinatal viral trigger that is followed by an aberrant autoimmune-mediated attack on bile duct epithelia, resulting in inflammatory and fibrosing obstruction of the bile ducts. Similarly, both primary biliary cholangitis and autoimmune hepatitis are characterized by autoimmune-mediated injury to liver cells and biliary ducts, and autoimmune liver disease (ALD)-related autoantibodies play a crucial role in the accurate classification for such. The authors hypothesized that ALD-related autoantibodies would be also associated with BA.
Research motivation
No previous study has comprehensively evaluated the prevalence of ALD-related autoantibodies and their roles in prognosis of BA. The authors attempted to explore the ALD-related autoantibodies in BA sera.
Research objectives
The authors tried to search appropriate non-invasive biomarker for diagnosis and prognosis of BA.
Research methods
The authors collected the sera of BA children and evaluated the prevalence of ALD-related autoantibodies using multiple methods, including the autoimmune liver diseases profile and specific anti-nuclear antibodies (ANAs) by line-blot assay; ANA and anti-neutrophil cytoplasmic antibody (ANCA) by indirect immunofluorescence assay; specific ANCAs and anti-M2-3E by enzyme linked immunosorbent assay. Simultaneously, associations of these autoantibodies with the clinical features of followed BA children were evaluated by Spearman’s correlation coefficient.
Research results
The overall positivity of serum ALD-related autoantibodies in BA patients was 56.5%. The prevalence of anti-M2-3E, ANA, and ANCA was significantly increased in a large cohort of infants with BA. In addition, the authors found that the ANCA showed a positive correlation to the occurrence of postoperative cholangitis.
Research conclusions
High prevalence of ALD-related autoantibodies in the BA developmental process strongly reveals the autoimmune-mediated pathogenesis. ANCA positivity may be a useful serum biomarker to predict postoperative cholangitis of BA.
Research perspectives
Our future research will focus on identifying potentially pathogenic, bile duct-specific autoantibodies and the titer and source of autoantibodies in BA.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Supported by the Guangdong Provincial Science and Technology Planning Project, No. 2014A020212520; and the Guangzhou Science and Technology Project, No. 201707010014.
Institutional review board statement: The study protocol was approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center, No. 2015090117.
Informed consent statement: Informed consent was obtained from the legal guardian of all patients prior to study enrolment.
Conflict-of-interest statement: The authors declare no conflict of interests related to this study.
Data sharing statement: No additional data are available.
Peer-review started: November 9, 2017
First decision: November 30, 2017
Article in press: December 20, 2017
P- Reviewer: Govindarajan GK, Watanabe T S- Editor: Chen K L- Editor: Wang TQ E- Editor: Li D
Contributor Information
Shu-Yin Pang, Clinical Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Yu-Mei Dai, Clinical Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Rui-Zhong Zhang, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Yi-Hao Chen, Clinical Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Xiao-Fang Peng, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Jie Fu, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Zheng-Rong Chen, Department of Pathology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Yun-Feng Liu, Clinical Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Li-Yuan Yang, Clinical Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Zhe Wen, Department of Neonatal Surgery, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Jia-Kang Yu, Department of Neonatal Surgery, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China.
Hai-Ying Liu, Clinical Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou 510623, Guangdong Province, China. xiangliuhaiying@aliyun.com.
References
- 1.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 2.Nizery L, Chardot C, Sissaoui S, Capito C, Henrion-Caude A, Debray D, Girard M. Biliary atresia: Clinical advances and perspectives. Clin Res Hepatol Gastroenterol. 2016;40:281–287. doi: 10.1016/j.clinre.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Pakarinen MP, Rintala RJ. Surgery of biliary atresia. Scand J Surg. 2011;100:49–53. doi: 10.1177/145749691110000109. [DOI] [PubMed] [Google Scholar]
- 4.Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology. 2005;41:366–371. doi: 10.1002/hep.20547. [DOI] [PubMed] [Google Scholar]
- 5.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Mack CL. What Causes Biliary Atresia? Unique Aspects of the Neonatal Immune System Provide Clues to Disease Pathogenesis. Cell Mol Gastroenterol Hepatol. 2015;1:267–274. doi: 10.1016/j.jcmgh.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada K. Sclerosing and obstructive cholangiopathy in biliary atresia: mechanisms and association with biliary innate immunity. Pediatr Surg Int. 2017;33:1243–1248. doi: 10.1007/s00383-017-4154-8. [DOI] [PubMed] [Google Scholar]
- 8.Mohanty SK, Donnelly B, Lobeck I, Walther A, Dupree P, Coots A, Meller J, McNeal M, Sestak K, Tiao G. The SRL peptide of rhesus rotavirus VP4 protein governs cholangiocyte infection and the murine model of biliary atresia. Hepatology. 2017;65:1278–1292. doi: 10.1002/hep.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimada T, Imaizumi T, Shirai K, Tatsuta T, Kimura T, Hayakari R, Yoshida H, Matsumiya T, Kijima H, Mizukami H, et al. CCL5 is induced by TLR 3 signaling in HuCCT1 human biliary epithelial cells: possible involvement in the pathogenesis of biliary atresia. Biomed Res. 2017;38:269–276. doi: 10.2220/biomedres.38.269. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Yu J, Zhang R, Yin Y, Ye J, Tan L, Xia H. The perinatal infection of cytomegalovirus is an important etiology for biliary atresia in China. Clin Pediatr (Phila) 2012;51:109–113. doi: 10.1177/0009922811406264. [DOI] [PubMed] [Google Scholar]
- 11.Hadchouel M, Hugon RN, Odievre M. Immunoglobulin deposits in the biliary remnants of extrahepatic biliary atresia: a study by immunoperoxidase staining in 128 infants. Histopathology. 1981;5:217–221. doi: 10.1111/j.1365-2559.1981.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 12.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. α-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology. 2010;139:1753–1761. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng X, Yang L, Liu H, Pang S, Chen Y, Fu J, Chen Y, Wen Z, Zhang R, Zhu B, et al. Identification of Circulating MicroRNAs in Biliary Atresia by Next-Generation Sequencing. J Pediatr Gastroenterol Nutr. 2016;63:518–523. doi: 10.1097/MPG.0000000000001194. [DOI] [PubMed] [Google Scholar]
- 14.Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Wang Z, Liao B, Liang JY, Zhou LY, Wang F, Li W, Liu JY, Xie XY, Lu MD, et al. Assessment of liver fibrosis in chronic hepatitis B using acoustic structure quantification: quantitative morphological ultrasound. Eur Radiol. 2016;26:2344–2351. doi: 10.1007/s00330-015-4056-x. [DOI] [PubMed] [Google Scholar]
- 16.Yang LY, Fu J, Peng XF, Pang SY, Gao KK, Chen ZR, He LJ, Wen Z, Wang H, Li L, et al. Validation of aspartate aminotransferase to platelet ratio for diagnosis of liver fibrosis and prediction of postoperative prognosis in infants with biliary atresia. World J Gastroenterol. 2015;21:5893–5900. doi: 10.3748/wjg.v21.i19.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. 2007;27:233–242. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen J, Xiao Y, Wang J, Pan W, Zhou Y, Zhang X, Guan W, Chen Y, Zhou K, Wang Y, et al. Low doses of CMV induce autoimmune-mediated and inflammatory responses in bile duct epithelia of regulatory T cell-depleted neonatal mice. Lab Invest. 2015;95:180–192. doi: 10.1038/labinvest.2014.148. [DOI] [PubMed] [Google Scholar]
- 19.Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L. Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmun Rev. 2007;6:176–182. doi: 10.1016/j.autrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Zani A, Quaglia A, Hadzić N, Zuckerman M, Davenport M. Cytomegalovirus-associated biliary atresia: An aetiological and prognostic subgroup. J Pediatr Surg. 2015;50:1739–1745. doi: 10.1016/j.jpedsurg.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Villalta D, Sorrentino MC, Girolami E, Tampoia M, Alessio MG, Brusca I, Daves M, Porcelli B, Barberio G, Bizzaro N; Study Group on Autoimmune Diseases of the Italian Society of Laboratory Medicine. Autoantibody profiling of patients with primary biliary cirrhosis using a multiplexed line-blot assay. Clin Chim Acta. 2015;438:135–138. doi: 10.1016/j.cca.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Gregorio GV, Portmann B, Mowat AP, Vergani D, Mieli-Vergani G. A 12-year-old girl with antimitochondrial antibody-positive autoimmune hepatitis. J Hepatol. 1997;27:751–754. doi: 10.1016/s0168-8278(97)80093-1. [DOI] [PubMed] [Google Scholar]
- 23.Sternsdorf T, Guldner HH, Szostecki C, Grötzinger T, Will H. Two nuclear dot-associated proteins, PML and Sp100, are often co-autoimmunogenic in patients with primary biliary cirrhosis. Scand J Immunol. 1995;42:257–268. doi: 10.1111/j.1365-3083.1995.tb03652.x. [DOI] [PubMed] [Google Scholar]
- 24.Muratori P, Muratori L, Ferrari R, Cassani F, Bianchi G, Lenzi M, Rodrigo L, Linares A, Fuentes D, Bianchi FB. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:431–437. doi: 10.1111/j.1572-0241.2003.07257.x. [DOI] [PubMed] [Google Scholar]
- 25.Gao L, Tian X, Liu B, Zhang F. The value of antinuclear antibodies in primary biliary cirrhosis. Clin Exp Med. 2008;8:9–15. doi: 10.1007/s10238-008-0150-6. [DOI] [PubMed] [Google Scholar]
- 26.A-Kader HH, Abdel-Hameed A, Al-Shabrawi M, Mohsen N, El-Karaksy H, Hassanein B, Elsayed B, Abdel-Khalik MK, Karjoo M. Is biliary atresia an autoimmune disease? Eur J Gastroenterol Hepatol. 2003;15:447. doi: 10.1097/00042737-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Wananukul S, Voramethkul W, Kaewopas Y, Hanvivatvong O. Prevalence of positive antinuclear antibodies in healthy children. Asian Pac J Allergy Immunol. 2005;23:153–157. [PubMed] [Google Scholar]
- 28.Hilário MO, Len CA, Roja SC, Terreri MT, Almeida G, Andrade LE. Frequency of antinuclear antibodies in healthy children and adolescents. Clin Pediatr (Phila) 2004;43:637–642. doi: 10.1177/000992280404300709. [DOI] [PubMed] [Google Scholar]
- 29.Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, Bossuyt X, Musset L, Cervera R, Plaza-Lopez A, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 30.Damoiseaux J, von Mühlen CA, Garcia-De La Torre I, Carballo OG, de Melo Cruvinel W, Francescantonio PL, Fritzler MJ, Herold M, Mimori T, Satoh M, et al. International consensus on ANA patterns (ICAP): the bumpy road towards a consensus on reporting ANA results. Auto Immun Highlights. 2016;7:1. doi: 10.1007/s13317-016-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg JN, Johnson GD, Holborow EJ, Bywaters EG. Eosinophil-specific and other granulocyte-specific antinuclear antibodies in juvenile chronic polyarthritis and adult rheumatoid arthritis. Ann Rheum Dis. 1975;34:350–353. doi: 10.1136/ard.34.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 33.Hannam S, Bogdanos DP, Davies ET, Hussain MJ, Portmann BC, Mieli-Vergani G, Vergani D. Neonatal liver disease associated with placental transfer of anti-mitochondrial antibodies. Autoimmunity. 2002;35:545–550. doi: 10.1080/0891693021000054057. [DOI] [PubMed] [Google Scholar]
- 34.Vasiliauskas EA, Cobb L, Vidrich A, Targan SR, Rosenthal P. Biliary atresia - an autoimmune disorder? Journal of Pediatric Gastroenterology and Nutrition. 1995;21:327. [Google Scholar]
- 35.Roozendaal C, de Jong MA, van den Berg AP, van Wijk RT, Limburg PC, Kallenberg CG. Clinical significance of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune liver diseases. J Hepatol. 2000;32:734–741. doi: 10.1016/s0168-8278(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 36.Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- 37.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosoda C, Baba T, Hagiwara E, Ito H, Matsuo N, Kitamura H, Iwasawa T, Okudela K, Takemura T, Ogura T. Clinical features of usual interstitial pneumonia with anti-neutrophil cytoplasmic antibody in comparison with idiopathic pulmonary fibrosis. Respirology. 2016;21:920–926. doi: 10.1111/resp.12763. [DOI] [PubMed] [Google Scholar]
- 39.Hervier B, Pagnoux C, Agard C, Haroche J, Amoura Z, Guillevin L, Hamidou MA; French Vasculitis Study Group. Pulmonary fibrosis associated with ANCA-positive vasculitides. Retrospective study of 12 cases and review of the literature. Ann Rheum Dis. 2009;68:404–407. doi: 10.1136/ard.2008.096131. [DOI] [PubMed] [Google Scholar]


