Abstract
The present case-control study was conducted on 110 children with acute lymphoblastic leukemia (ALL) and 120 healthy children to determine the impact of polymorphisms in paired-box gene 8 (PAX8) antisense RNA 1 (PAX8-AS1), namely rs4848320 C>T, rs6726151 T>G and rs1110839 G>T, on ALL risk. Genotyping was performed through the polymerase chain reaction-restriction fragment length polymorphism method. The findings indicated that the rs4848320 variant increased the risk of ALL in codominant [CT vs. CC: odds ratio (OR)=2.13, 95% confidence interval (CI)=1.16–3.90, P=0.014; and TT vs. CC: OR=2.21, 95% CI=1.03–4.74, P=0.041], dominant (CT+TT vs. CC: OR=2.15, 95% CI=1.22–3.81, P=0.009,) and allele (T vs. C: OR=1.55, 95% CI=1.07–2.25, P=0.024) inheritance models. The rs6726151 variant significantly increased the risk of ALL in codominant (GT vs. GG: OR=1.88, 95% CI=1.08–3.27, P=0.036) and overdominant (GT vs. GG+TT: OR=2.08, 95% CI=1.23–3.53, P=0.008) inheritance models. No significant relationship was identified between the rs1110839 G>T variant and disease risk/protection in childhood ALL. In conclusion, the findings of the present study indicated that rs4848320 and rs6726151 polymorphisms of PAX8-AS1 may be a risk factor for the development of childhood ALL. Further studies with larger sample sizes and different ethnicities are now required to confirm these findings.
Keywords: long non-coding RNA, paired-box gene 8 antisense RNA 1, polymorphism, acute lymphoblastic leukemia
Introduction
Acute lymphoblastic leukemia (ALL) is the most prevalent malignancy in children and constitutes approximately 75% of pediatric acute leukemias (1). While the etiology of ALL is not fully understood, previous reports have indicated that genetic factors serve a role in the development of childhood ALL (2–5).
Non-coding RNAs, comprising microRNAs and long non-coding RNAs (lncRNAs), do not encode protein sequences, yet are involved in various biological processes (6–8). In particular, lncRNAs, as transcripts of >200 nucleotides in length that lack protein-coding potential, regulate gene expression at various levels, including at the chromatin remodeling (9), transcription and post-transcriptional processing stages (10,11).
Paired-box gene 8 (PAX8) encodes a transcription factor required for cell growth and differentiation during embryonic development (12). Overexpression of PAX8 has been identified in various cancers (13–17). Though the precise role of PAX8 in cancer remains uncertain, it has been proposed that PAX8 contributes to the development and progression of specific cancers by maintaining tissue specific stem cells, by inhibiting terminal differentiation and apoptosis (18).
LncRNA PAX8 antisense RNA 1 (PAX8-AS1) is mapped to chromosome 2q13 in the upstream region of PAX8 (19). An expression quantitative trait loci (eQTL) is a locus containing a genetic variant that influences the expression level of a gene (20). PAX8-AS1, a potential regulator of PAX8, may contain polymorphisms that represent eQTLs for PAX8 (21). In particular, previous bioinformatics analyses have revealed that the polymorphisms rs4848320 C>T and rs1110839 G>T in PAX8-AS1 may be eQTLs for PAX8 (21). Furthermore, it has been suggested that rs4848320 and rs1110839 may affect the function or expression of PAX8-AS1, thereby influencing PAX8 expression (22,23). Few previous studies have evaluated the impact of PAX8-AS1 variants on cancer risk. Han et al (19) reported that rs4848320 and rs1110839 variants of PAX8-AS1 significantly decreased the risk of cervical cancer (19). Ma et al (24) identified that the two variants of PAX8-AS1 were significantly associated with the prognosis of hepatocellular carcinoma (HCC). However, to the best of our knowledge, no previous study has investigated the impact of PAX8-AS1 polymorphisms on childhood ALL. Based on the previous findings on PAX8-AS1 and cancer risk, it was hypothesized that polymorphisms of PAX8-AS1 may affect the risk of childhood ALL by disturbing the interaction between PAX8-AS1 and PAX8, to in turn influence PAX8 expression. PAX8 has been demonstrated to serve an important role in the pathogenesis of cancer by inhibiting cell differentiation and apoptosis (18). Therefore, the present study aimed to assess the possible association between the PAX8-AS1 polymorphisms rs4848320 C>T, rs1110839 G>T and rs6726151 T>G and the risk of childhood ALL in a Southeast Iranian population sample. In the analysis, the polymorphisms which have been implicated as potential risk factors for cancer were selected (19,24), while the rs6726151 T>G variant, with a minor allele frequency of 0.486 (25), was examined for the first time. The findings of the present study highlight the potential role of PAX8-AS1 variants in the pathogenesis of childhood ALL.
Materials and methods
Patients
A total of 230 subjects including 110 children diagnosed with ALL and 120 age- and sex-matched healthy children were enrolled in the present case-control study. The study design including the enrolled patients has been reported previously by our group (2,8). The local Ethics Committee of Zahedan University of Medical Sciences (Zahedan, Iran) approved the project (approval no. IR.ZAUMS.REC.1395.270) and informed consent was obtained from the parents of all participants. Extraction of genomic DNA from whole blood was performed using the salting out method as described previously (26).
Genotyping
Polymorphism genotyping was performed through the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The primer sequences and restriction enzymes are summarized in Table I. The primers were produced by Bioneer Corp., (Daejeon, Korea). Into a 0.20 ml PCR reaction tube, 1 µl genomic DNA (~100 ng/ml), 1 µl (10 µM each) forward and reverse primers, 10 µl 2X Prime Taq Premix, all from Genet Bio, Inc., (Daejeon, Korea), and 7 µl ddH2O were added. The PCR conditions were as follows: Preheating for 6 min at 95°C; 30 cycles of 95°C for 30 sec, 64°C for rs1110839 and rs4848320 for 30 sec or 62°C for rs6726151 for 30 sec, and 72°C for 30 sec; followed by a final extension step for 5 min at 72°C. Subsequently, 10 µl of amplified product was digested with the appropriate restriction enzyme (New England BioLabs, Inc., Ipswich, MA, USA), resolved on 2.5% agarose gel containing 0.5 µg/ml ethidium bromide, observed under a UV transilluminator (DigiDoc H101; UVP, LLC, Upland, CA, USA) and photographed. For quality control, 15% randomly selected samples were regenotyped and the outcome revealed 100% concordance.
Table I.
Primers and restriction enzymes used in the detection of paired-box gene 8 antisense RNA 1 polymorphisms.
| Polymorphism | Sequence, 5′-3′ | Restriction enzyme | Product size, bp |
|---|---|---|---|
| rs4848320 C>T | F: CTGCTTAGCATGTGCTTGGTGATG | PstI | T allele: 222; |
| R: GAAACACTGAGAACTAAGAGAAGCCTGCA | C allele: 195+27 | ||
| rs1110839 G>T | F: TCATCTCCCCAGGAGAGGTCCTCAGC | HhaI | T allele: 270; |
| R: ACAGTCCGGTTGGAGACTG C | G allele: 244+26 | ||
| rs6726151 T>G | F: CCCAAAGACCAGCACACA | MboI | G allele: 371; |
| R: AGACCCACCATTTCCATAACA | T allele: 211+160 |
F, forward; R, reverse.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software (IBM, Corp., Armonk, NY, USA). The categorical and continuous data were analyzed using χ2 and t-test, respectively. Individual single nucleotide polymorphism (SNP) associations with childhood ALL risk were assessed using unconditional logistic regression analyses, in which odds ratios (ORs) and 95% confidence intervals (CIs) were determined for codominant, dominant, recessive, overdominant and allele inheritance models. P<0.05 was considered to indicate a statistically significant difference. Haplotype and linkage disequilibrium analyses were conducted using SNPStats (https://www.snpstats.net/snpstats) (27) and Haploview 4.2 software both from Broad Institute (Cambridge, MA, USA) (28), respectively. Linkage disequilibrium between the PAX8-AS1 polymorphisms was estimated through calculation of D' (correlation coefficient between pairs of loci) and r2 (square of the correlation coefficient between two indicator variables) with Haploview 4.2.
Results
Patient characteristics
The demographic characteristics of the patients considered in the present study are reported previously (2,8).
Genotyping of the variants
Variants were genotyped by PCR-RFLP. When genotyping the rs4848320 variant, digestion of the PCR product (222 bp) yielded a fragment of 195 bp (and presumed 27 bp fragment not visible on agarose gel) for the C allele, and remained undigested for the T allele (Fig. 1). Regarding the rs1110839 variant, the T allele remained undigested (270 bp), while the G allele was digested and produced a fragment of 244 bp (and presumed 26 bp fragment not visible on agarose gel; Fig. 2). For rs6726151, the T allele was digested and produced 211 and 160 bp fragments while the G allele was undigested (371 bp; Fig. 3). The lengths of all fragments following restriction digestion are summarized in Table I.
Figure 1.
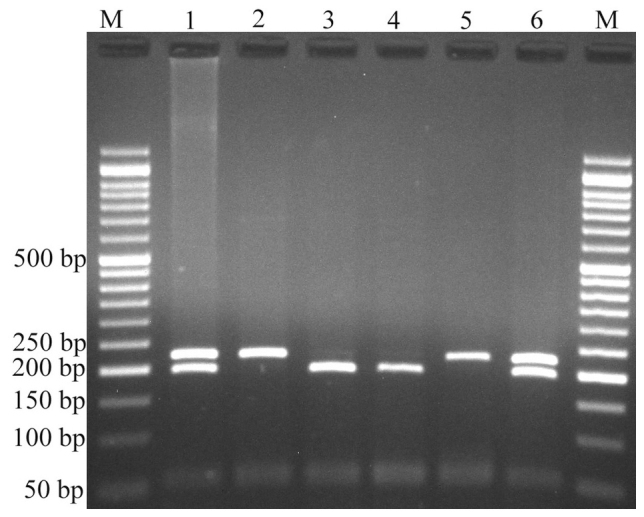
Electrophoresis of rs4848320 C>T polymorphism fragments. The C allele was digested by PstI and produced a 195 bp fragment and presumed 27 bp fragment not visible on agarose gel, while the T allele remained undigested (222 bp). M: DNA marker; lanes 1 and 6: CT; lanes 2 and 5: TT; lanes 3 and 4: CC.
Figure 2.
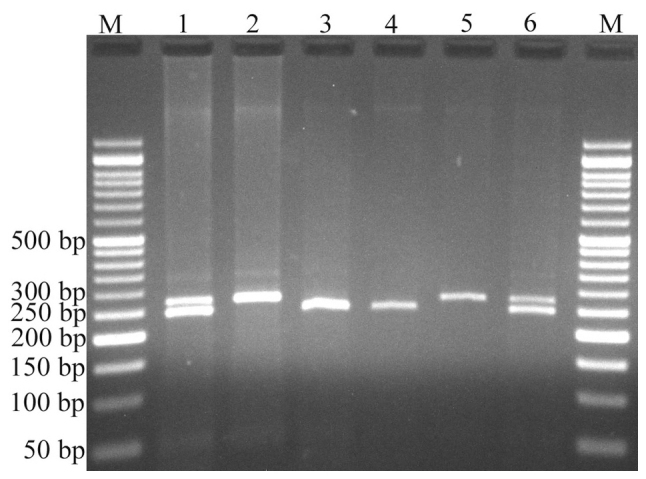
Electrophoresis of rs1110839 G>T polymorphism fragments. The G allele was digested by HhaI and produced 244 bp fragment and presumed 26 bp fragment not visible on agarose gel, while the T allele remained undigested (270 bp). M: DNA marker; lanes 1 and 6: GT; lanes 2 and 5: TT; lanes 3 and 4: GG.
Figure 3.
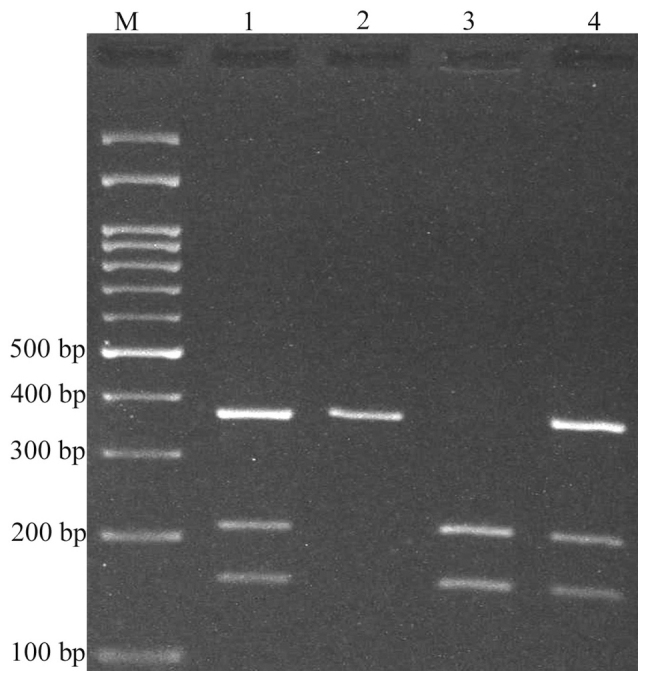
Electrophoresis of rs6726151 T>G polymorphism fragments. The T allele was digested by MboI and produced 211 and 160 bp fragments, while the G allele remained undigested (371 bp). M: DNA marker; lanes 1 and 4: GT; lane 2: GG; lane 3: TT.
Association between the variants and childhood ALL risk
The genotype and allele distributions of PAX8-AS1 polymorphisms in pediatric patients with ALL and healthy controls are presented in Table II. The findings suggested that the rs4848320 variant was associated with risk of ALL in codominant (CT vs. CC: OR=2.13, 95% CI=1.16–3.90, P=0.014; and TT vs. CC: OR=2.21, 95% CI=1.03–4.74, P=0.041), dominant (CT+TT vs. CC: OR=2.15, 95% CI=1.22–3.81, P=0.009,) and allele (T vs. C: OR=1.55, 95% CI=1.07–2.25, P=0.024,) inheritance models. For the rs6726151 variant, the findings indicated that this variant significantly increased the risk of ALL in codominant (GT vs. GG: OR=1.88, 95% CI=1.08–3.27, P=0.036) and overdominant (GT vs. GG+TT: OR=2.08, 95% CI=1.23–3.53, P=0.008) inheritance models. No significant association was observed between the rs1110839 G>T variant and disease risk/protection in childhood ALL.
Table II.
Association of paired-box gene 8 antisense RNA 1 polymorphisms and risk of acute lymphoblastic leukemia.
| Polymorphism | Cases, n (%) | Controls, n (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
| rs4848320 | ||||
| Codominant | ||||
| CC | 26 (23.6) | 48 (40.0) | 1.00 | – |
| CT | 60 (54.6) | 52 (43.3) | 2.13 (1.16–3.90) | 0.014 |
| TT | 24 (21.8) | 20 (16.7) | 2.21 (1.03–4.74) | 0.041 |
| Dominant | ||||
| CC | 26 (23.6) | 48 (40.0) | 1.00 | – |
| CT+TT | 84 (76.4) | 72 (60.0) | 2.15 (1.22–3.81) | 0.009 |
| Recessive | ||||
| CC+CT | 86 (78.2) | 100 (83.3) | 1.00 | – |
| TT | 24 (21.8) | 20 (16.7) | 1.39 (0.72–2.70) | 0.322 |
| Overdominant | ||||
| CC+TT | 50 (45.4) | 68 (56.7) | 1.00 | – |
| CT | 60 (54.6) | 52 (43.3) | 1.57 (0.93–2.64) | 0.090 |
| Allele | ||||
| C | 112 (50.9) | 148 (61.7) | 1.00 | – |
| T | 108 (49.1) | 92 (38.3) | 1.55 (1.07–2.25) | 0.024 |
| rs1110839 | ||||
| Codominant | ||||
| TT | 43 (39.1) | 54 (45.0) | 1.00 | – |
| TG | 43 (39.1) | 34 (28.3) | 1.59 (0.87–2.90) | 0.132 |
| GG | 24 (21.8) | 32 (26.7) | 0.94 (0.48–1.83) | 0.860 |
| Dominant | ||||
| TT | 43 (39.1) | 54 (45.0) | 1.00 | – |
| TG+GG | 67 (60.9) | 66 (55.0) | 1.27 (0.75–2.16) | 0.365 |
| Recessive | ||||
| TT+TG | 86 (78.2) | 88 (73.3) | 1.00 | – |
| GG | 24 (21.8) | 32 (26.7) | 0.77 (0.42–1.41) | 0.393 |
| Overdominant | ||||
| TT+GG | 67 (60.9) | 86 (71.7) | 1.00 | – |
| TG | 43 (39.1) | 34 (28.3) | 1.62 (0.93–2.82) | 0.085 |
| Allele | ||||
| T | 129 (58.6) | 142 (59.2) | 1.00 | – |
| G | 91 (60.4) | 98 (40.8) | 1.02 (0.70–1.48) | 0.924 |
| rs6726151 | ||||
| Codominant | ||||
| GG | 40 (36.3) | 56 (46.6) | 1.00 | – |
| GT | 63 (57.3) | 47 (39.2) | 1.88 (1.08–3.27) | 0.036 |
| TT | 7 (6.4) | 17 (14.2) | 0.58 (0.22–1.52) | 0.576 |
| Dominant | ||||
| GG | 40 (36.4) | 56 (46.6) | 1.00 | – |
| GT+TT | 70 (63.7) | 64 (53.4) | 1.53 (0.90–2.60) | 0.141 |
| Recessive | ||||
| GG+GT | 103 (93.6) | 103 (85.8) | 1.00 | – |
| TT | 7 (6.4) | 17 (14.2) | 0.41 (0.16–1.04) | 0.082 |
| Overdominant | ||||
| GG+TT | 47 (42.7) | 73 (60.8) | 1.00 | – |
| GT | 63 (57.3) | 47 (39.2) | 2.08 (1.23–3.53) | 0.008 |
| Allele | ||||
| G | 143 (65.0) | 159 (72.3) | 1.00 | – |
| T | 77 (35.0) | 81 (27.7) | 1.06 (0.72–1.55) | 0.844 |
OR, odds ratio; CI, confidence interval.
Results of the haplotype analysis of the three variants are presented in Table III. The findings did not support an association between haplotype and risk of childhood ALL. Associations between the PAX8-AS1 polymorphisms and patient clinical characteristics were also estimated. As depicted in Table IV, a significant association between rs4848320 and sex was observed [χ2=8.45, degrees of freedom (df)=2, P=0.015]. Notably, the CT genotype significantly decreased the risk of ALL in females (OR=0.32, 95% CI=0.13–0.84, P=0.0355; data not shown). For rs6726151, the findings indicated that this variant was associated with organomegaly (χ2=8.21, df=2, P=0.017) and lymphadenopathy (χ2=11.48, df=2, P=0.003; Table IV). No significant associations were identified between rs1110839 and patient clinical characteristics.
Table III.
Association of paired-box gene 8 antisense RNA 1 haplotypes and risk of acute lymphoblastic leukemia.
| Polymorphism | ||||||
|---|---|---|---|---|---|---|
| rs4848320 | rs1110839 | rs6726151 | Cases, frequency | Controls, frequency | OR (95% CI) | P-value |
| C | T | T | 0.2025 | 0.2216 | 1.00 [ref.] | – |
| T | G | G | 0.2010 | 0.1680 | 1.32 (0.71–2.47) | 0.390 |
| T | T | G | 0.2177 | 0.1472 | 1.63 (0.83–3.19) | 0.160 |
| C | T | G | 0.1402 | 0.2042 | 0.77 (0.38–1.58) | 0.480 |
| C | G | G | 0.0910 | 0.1431 | 0.68 (0.33–1.42) | 0.310 |
| C | G | T | 0.0754 | 0.0478 | 1.87 (0.55–6.43) | 0.320 |
| T | G | T | 0.0463 | 0.0495 | 1.09 (0.38–3.11) | 0.880 |
| T | T | T | 0.0259 | 0.0186 | 1.35 (0.20–9.20) | 0.760 |
OR, odds ratio; CI, confidence interval.
Table IV.
Association of paired-box gene 8 antisense RNA 1 polymorphisms with demographic and clinical features of patients.
| rs4848320 | rs1110839 | rs6726151 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | CC | CT | TT | P-value | TT | TG | GG | P-value | GG | GT | TT | P-value |
| Sex, n | 0.015 | 0.583 | 0.073 | |||||||||
| Male | 19 | 28 | 18 | 28 | 24 | 13 | 22 | 36 | 7 | |||
| Female | 7 | 32 | 6 | 15 | 19 | 11 | 18 | 27 | 0 | |||
| Age at diagnosis, years | 5.2±3.1 | 6.5±3.9 | 5.7±4.5 | 0.355 | 5.9±3.7 | 5.5±3.9 | 7.2±4.1 | 0.231 | 5.6±4.0 | 6.2±3.9 | 6.3±3.8 | 0.757 |
| WBC, ×106/ml, mean ± SD | 31.3±49.6 | 40.8±55.3 | 40.9±46.8 | 0.715 | 33.8±42.9 | 44.0±66.2 | 37.5±36.4 | 0.656 | 42.1±50.4 | 39.5±54.9 | 10.1±15.5 | 0.318 |
| Hemoglobin, g/dl, mean ± SD | 7.2±2.1 | 7.0±2.3 | 7.8±2.3 | 0.362 | 7.1±2.3 | 7.1±2.4 | 7.6±2.0 | 0.607 | 7.6±2. 3 | 7.9±2.2 | 8.0±1.6 | 0.172 |
| Platelet, ×106/ml, mean ± SD | 71.9±64.0 | 52.3±45.4 | 49.1±38.0 | 0.173 | 54.4±44.0 | 60.8±61.1 | 56.2±49.4 | 0.874 | 61.6±50.6 | 54.3±50.5 | 42.9±430.0 | 0.587 |
| Organomegaly | 0.152 | 0.163 | 0.017 | |||||||||
| Positive | 24 | 52 | 24 | 39 | 37 | 24 | 40 | 55 | 5 | |||
| Negative | 2 | 8 | 0 | 4 | 6 | 0 | 0 | 8 | 2 | |||
| Lymphadenopathy | 0.105 | 0.649 | 0.003 | |||||||||
| Positive | 14 | 44 | 19 | 31 | 28 | 18 | 31 | 45 | 1 | |||
| Negative | 12 | 16 | 5 | 12 | 15 | 6 | 9 | 18 | 6 | |||
| Cerebrospinal fluid involvement | 0.536 | 0.498 | 0.669 | |||||||||
| Positive | 1 | 5 | 3 | 5 | 2 | 2 | 4 | 5 | 0 | |||
| Negative | 25 | 55 | 21 | 38 | 41 | 22 | 36 | 58 | 7 | |||
Emboldened values indicate statistical significance (P<0.05). WBC, white blood cell; SD, standard deviation.
Furthermore, linkage disequilibrium was observed between rs4848320 and rs1110839 (D'=0.2242, r2=0.0455); rs4848320 and rs6726151 (D'=0.5268, r2=0.1117); and rs1110839 and rs6726151 (D'=0.2279, r2=0.0189; Fig. 4).
Figure 4.

Haploview linkage disequilibrium graph of the three polymorphisms analyzed in long non-coding paired-box gene 8 antisense RNA 1. Pairwise linkage disequilibrium coefficients (D' ×100) are indicated in each cell linkage.
Discussion
In the present study, the possible association between PAX8-AS1 polymorphisms and risk of childhood ALL in a Southeast Iranian population was investigated. The findings indicated that the rs4848320 and rs6726151 variants of PAX8-AS1 significantly increased the risk of developing childhood ALL, while there was no association between rs1110839 and disease risk/protection. By contrast, the haplotype analysis did not identify a significant association of any of the variants with risk of childhood ALL. However, stratification of the variants according to the clinical characteristics of patients indicated that the rs4848320 variant was associated with sex while the rs6726151 variant was associated with organomegaly and lymphadenopathy.
Previous results have indicated that non-coding transcripts in the human genome serve crucial and diverse biological roles (29). The findings of macromolecular interactions have revealed that tissue-specific lncRNAs form base-pairing interactions with numerous mRNAs associated with tissue-differentiation, indicating that tissue specificity is an critical factor in controlling human lncRNA-mRNA interactions (30). LncRNAs have tissue-specific expression and serve an important role in the human transcriptome by regulating normal tissue differentiation as well as cancer development (30). Notably, a number of previous studies have implicated a role of lncRNA dysregulation, of transcripts including leukemia-induced non-coding activator RNA, B-ALL-associated long RNA (BALR)-6 and −2, NOTCH1-associated lncRNA in T-ALL and CCDC26, in tumorigenicity in leukemias (31–34). SNPs may significantly influence gene expression and function. Altered expression of lncRNAs in various cancers indicates the potential tumor suppressor or oncogenic functions of the lncRNAs (35–39). Recently, an association between the rs2147578 polymorphism of lnc-LAMC2-1 and risk of childhood ALL has been demonstrated (6).
There is limited information on the impact of PAX8-AS1 polymorphisms on cancer risk (19,24). Han et al (19) demonstrated that PAX8-AS1 rs4848320 and rs1110839 polymorphisms decreased the risk of cervical cancer, while Ma et al (24) reported that rs4848320 and rs1110839 were associated with prognosis of HCC in a Chinese population. It has been proposed that the PAX genes act as oncogenes, and that PAX overexpression facilitates malignant development through effects on apoptotic resistance, tumor cell proliferation and migration, and repression of terminal differentiation (40). As PAX8-AS1 is a potential regulator of PAX8, polymorphisms in the PAX8-AS1 may affect its function and alter the expression of PAX8.
There are a number of limitations to the present study. First, a relatively small sample size was used. Second, there was a lack of data regarding the response of patients to treatment; therefore, it was not possible to analyze the association between the variants and response to treatment.
In conclusion, the present results suggested that PAX8-AS1 polymorphisms significantly increased the risk of childhood ALL in a Southeast Iranian population. As this, to the best of our knowledge, was the first study to examine the association of polymorphisms in PAX8-AS1 with risk of childhood ALL, future studies with larger sample sizes and different ethnicities are required to confirm the findings.
Acknowledgements
The present study was financially supported by a research grant from the Deputy for Research of Zahedan University of Medical Sciences, (Zahedan, Iran; grant no. 8224).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bahari G, Hashemi M, Naderi M, Taheri M. IKZF1 gene polymorphisms increased the risk of childhood acute lymphoblastic leukemia in an Iranian population. Tumour Biol. 2016;37:9579–9586. doi: 10.1007/s13277-016-4853-0. [DOI] [PubMed] [Google Scholar]
- 3.Bahari G, Hashemi M, Naderi M, Sadeghi-Bojd S, Taheri M. Association of SHMT1 gene polymorphisms with the risk of childhood acute lymphoblastic leukemia in a sample of Iranian population. Cell Mol Biol (Noisy-le-grand) 2016;62:45–51. [PubMed] [Google Scholar]
- 4.Hasani SS, Hashemi M, Eskandari-Nasab E, Naderi M, Omrani M, Sheybani-Nasab M. A functional polymorphism in the miR-146a gene is associated with the risk of childhood acute lymphoblastic leukemia: A preliminary report. Tumour Biol. 2014;35:219–225. doi: 10.1007/s13277-013-1027-1. [DOI] [PubMed] [Google Scholar]
- 5.Tong N, Chu H, Wang M, Xue Y, Du M, Lu L, Zhang H, Wang F, Fang Y, Li J, et al. Pri-miR-34b/c rs4938723 polymorphism contributes to acute lymphoblastic leukemia susceptibility in Chinese children. Leuk Lymphoma. 2016;57:1436–1441. doi: 10.3109/10428194.2015.1092528. [DOI] [PubMed] [Google Scholar]
- 6.Hashemi M, Bahari G, Naderi M, Bojd S Sadeghi, Taheri M. Association of lnc-LAMC2-1:1 rs2147578 and CASC8 rs10505477 polymorphisms with risk of childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev. 2016;17:4985–4989. doi: 10.22034/APJCP.2016.17.11.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashemi M, Bahari G, Naderi M, Sadeghi-Bojd S, Taheri M. Pri-miR-34b/c rs4938723 polymorphism is associated with the risk of childhood acute lymphoblastic leukemia. Cancer Genet. 2016;209:493–496. doi: 10.1016/j.cancergen.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155–165. doi: 10.1038/modpathol.2012.160. [DOI] [PubMed] [Google Scholar]
- 12.Poleev A, Fickenscher H, Mundlos S, Winterpacht A, Zabel B, Fidler A, Gruss P, Plachov D. PAX8, a human paired box gene: Isolation and expression in developing thyroid, kidney and Wilms' tumors. Development. 1992;116:611–623. doi: 10.1242/dev.116.3.611. [DOI] [PubMed] [Google Scholar]
- 13.Ordóñez NG. Value of PAX 8 immunostaining in tumor diagnosis: A review and update. Adv Anat Pathol. 2012;19:140–151. doi: 10.1097/PAP.0b013e318253465d. [DOI] [PubMed] [Google Scholar]
- 14.Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32:1566–1571. doi: 10.1097/PAS.0b013e31816d71ad. [DOI] [PubMed] [Google Scholar]
- 15.Tong GX, Weeden EM, Hamele-Bena D, Huan Y, Unger P, Memeo L, O'Toole K. Expression of PAX8 in nephrogenic adenoma and clear cell adenocarcinoma of the lower urinary tract: Evidence of related histogenesis? Am J Surg Pathol. 2008;32:1380–1387. doi: 10.1097/PAS.0b013e31816b1020. [DOI] [PubMed] [Google Scholar]
- 16.Tong GX, Yu WM, Beaubier NT, Weeden EM, Hamele-Bena D, Mansukhani MM, O'Toole KM. Expression of PAX8 in normal and neoplastic renal tissues: An immunohistochemical study. Mod Pathol. 2009;22:1218–1227. doi: 10.1038/modpathol.2009.88. [DOI] [PubMed] [Google Scholar]
- 17.Becker N, Chernock RD, Nussenbaum B, Lewis JS., Jr Prognostic significance of β-human chorionic gonadotropin and PAX8 expression in anaplastic thyroid carcinoma. Thyroid. 2014;24:319–326. doi: 10.1089/thy.2013.0117. [DOI] [PubMed] [Google Scholar]
- 18.Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: Roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Zhou W, Jia M, Wen J, Jiang J, Shi J, Zhang K, Ma H, Liu J, Ren J, et al. Expression quantitative trait loci in long non-coding RNA PAX8-AS1 are associated with decreased risk of cervical cancer. Mol Genet Genomics. 2016;291:1743–1748. doi: 10.1007/s00438-016-1217-9. [DOI] [PubMed] [Google Scholar]
- 20.Nica AC, Dermitzakis ET. Expression quantitative trait loci: Present and future. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120362. doi: 10.1098/rstb.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, Ingle CE, Dunning M, Flicek P, Koller D, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veyrieras JB, Kudaravalli S, Kim SY, Dermitzakis ET, Gilad Y, Stephens M, Pritchard JK. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 2008;4:e1000214. doi: 10.1371/journal.pgen.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma S, Yang J, Song C, Ge Z, Zhou J, Zhang G, Hu Z. Expression quantitative trait loci for PAX8 contributes to the prognosis of hepatocellular carcinoma. PLoS One. 2017;12:e0173700. doi: 10.1371/journal.pone.0173700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashemi M, Bojd H Hanafi, Nasab E Eskandari, Bahari A, Hashemzehi NA, Shafieipour S, Narouie B, Taheri M, Ghavami S. Association of adiponectin rs1501299 and rs266729 gene polymorphisms with nonalcoholic fatty kiver disease. Hepat Mon. 2013;13:e9527. doi: 10.5812/hepatmon.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solé X, Guinó E, Valls J, Iniesta R, Moreno V. SNPStats: A web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 28.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 29.Geisler S, Coller J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwakiri J, Terai G, Hamada M. Computational prediction of lncRNA-mRNA interactionsby integrating tissue specificity in human transcriptome. Biol Direct. 2017;12:15. doi: 10.1186/s13062-017-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Malavé NI, Fernando TR, Patel PC, Contreras JR, Palanichamy JK, Tran TM, Anguiano J, Davoren MJ, Alberti MO, Pioli KT, et al. BALR-6 regulates cell growth and cell survival in B-lymphoblastic leukemia. Mol Cancer. 2015;14:214. doi: 10.1186/s12943-015-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Wu P, Lin R, Rong L, Xue Y, Fang Y. LncRNA NALT interaction with NOTCH1 promoted cell proliferation in pediatric T cell acute lymphoblastic leukemia. Sci Rep. 2015;5:13749. doi: 10.1038/srep13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trimarchi T, Bilal E, Ntziachristos P, Fabbri G, Dalla-Favera R, Tsirigos A, Aifantis I. Genome-wide mapping and characterization of Notch-regulated long noncoding RNAs in acute leukemia. Cell. 2014;158:593–606. doi: 10.1016/j.cell.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano T, Yoshikawa R, Harada H, Harada Y, Ishida A, Yamazaki T. Long noncoding RNA CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol Cancer. 2015;14:90. doi: 10.1186/s12943-015-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun QL, Zhao CP, Wang TY, Hao XB, Wang XY, Zhang X, Li YC. Expression profile analysis of long non-coding RNA associated with vincristine resistance in colon cancer cells by next-generation sequencing. Gene. 2015;572:79–86. doi: 10.1016/j.gene.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Song JH, Cheng Y, Wu W, Bhagat T, Yu Y, Abraham JM, Ibrahim S, Ravich W, Roland BC, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–890. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing CY, Hu XQ, Xie FY, Yu ZJ, Li HY, Bin-Zhou, Wu JB, Tang LY, Gao SM. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015;589:1981–1987. doi: 10.1016/j.febslet.2015.04.061. [DOI] [PubMed] [Google Scholar]
- 38.Morlando M, Ballarino M, Fatica A. Long non-coding RNAs: New players in hematopoiesis and leukemia. Front Med (Lausanne) 2015;2:23. doi: 10.3389/fmed.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emmrich S, Streltsov A, Schmidt F, Thangapandi VR, Reinhardt D, Klusmann JH. LincRNAs MONC and MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol Cancer. 2014;13:171. doi: 10.1186/1476-4598-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–7997. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]


