Abstract
Objective(s):
Metformin (Met), an antidiabetic biguanide, reduces hyperglycemia via improving glucose utilization and reducing the gluconeogenesis. Met has been shown to exert neuroprotective, antioxidant and anti-inflammatory properties. The present study investigated the possible effect of Met on the D-galactose (D-gal)-induced aging in mice.
Materials and Methods:
Met (1 and 10 mg/kg/p.o.), was administrated daily in D-gal-received (500 mg/kg/p.o.) mice model of aging for six weeks. Anxiety-like behavior, cognitive function, and physical power were evaluated by the elevated plus-maze, novel object recognition task (NORT), and forced swimming capacity test, respectively. The brains were analyzed for the level of superoxide dismutase (SOD) and brain-derived neurotrophic factor (BDNF).
Results:
Met decreased the anxiety-like behavior in D-gal-treated mice. Also, Met treated mice showed significantly improved learning and memory ability in NORT compared to the D-gal-treated mice. Furthermore, Met increased the physical power as well as the activity of SOD and BDNF level in D-gal-treated mice.
Conclusion:
Our results suggest that the use of Met can be an effective strategy for prevention and treatment of D-gal-induced aging in animal models. This effect seems to be mediated by attenuation of oxidative stress and enhancement of the neurotrophic factors.
Keywords: Aging, D-galactose, Metformin, Mouse, Oxidative stress
Introduction
It is expected that by the year 2025, the elderly (over 65 years) population exceeds 800 million. The increasing number of the elderly will be associated with an increase in disability and illness (1). Therefore, studying the pathophysiology of aging and related diseases is a significant challenge for medical gerontology (2). Aging is a progressive multifactorial phenomenon exclusively leading to loss of cellular, molecular, and physiological functionality (1). Cognitive decline, anxiety, and sarcopenia are the main pathological conditions of aging (3). It is well established that overproduction of free radicals such as reactive oxygen species (ROS) leads to oxidative stress which results in senescence and aging-related disorders (4). Moreover, the brain neurons are more susceptible to oxidative stress due to the presence of high lipid content and higher oxygen consumption (2). The level and the activity of various antioxidant enzymes such as superoxide dismutase (SOD) are decreased during senescence (5). On the other hand, the level of the brain-derived neurotrophic factor (BDNF), which is an important mediator for neuronal proliferation and integrity, decreases by aging (6).
D-galactose (D-gal)-induced aging is an established model for pharmacological studies of age-dependent alterations. This model was reported by Xu in 1985, who found remarkable signs of aging in ICR (Institute of Cancer Research) mice after administration of D-gal for 8 weeks. At high levels, D-gal causes oxidative stress via the accumulation of ROS, stimulates free radical production and attenuates antioxidant enzyme activities (7). Oxidative damage and inflammation both play critical roles in mediating the age-related alterations in different organs such as the brain, muscle, and kidney (8).
Biguanides drugs such as metformin (Met) are antidiabetic drugs that reduce hyperglycemia via improving glucose utilization and reducing the gluconeogenesis. The most accepted mechanism of the glucose-lowering effect of Met is the activation of adenosine monophosphate (AMP)-activated protein kinase (a cellular energy sensor activated under metabolic stress) (9). In addition to this well-established property, Met exerts a variety of pleiotropic effects such as anti-inflammatory, antioxidant, and neuroprotective, which may extend its clinical indications (10). Met readily crosses the blood-brain barrier and is distributed within various regions of the brain. he neuroprotective effect of Met has been demonstrated in different neurological disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease (11), and seizures (12).
It has been shown that Met slows down aging and prolongs lifespan in naturally aged rats and mice (13, 14). In this study, we investigated the effect of antidiabetic biguanide Met on some aging-related changes such as anxiety, memory impairment, and decline in physical power and levels of some molecular parameters (BDNF and brain SOD) by using the D-gal-induced aging model in mice.
Materials and Methods
Animals
The experiments were performed on 50 male mice weighing 22–27 g. The animals were kept under standard laboratory conditions with food and water ad libitum and housed 10 per cage on a reversed 12 hr light: 12 hr dark cycle (lights on 08:00–20:00 hr). All efforts were made to minimize the number of animals used and their suffering. Behavioural tests and animal care were conducted in accordance with the standard ethical guidelines (NIH, publication no. 85-23, revised 1985; European Communities Directive 86/609/EEC) and approved by the Local Ethical Committee.
Drugs
Met (Glucophage™) was purchased from Merck Pharmaceuticals (France, Sante). D-gal was purchased from Sigma Aldrich (Germany). D-gal was dissolved in a measured quantity of mice drinking water. D-gal was given at the dose of 500 mg/kg in 10 ml drinking water for 6 weeks (7). Met was dissolved in standard drinking water and daily doses were given based on daily body weight measurements. The solution was freshly dissolved and administered orally in a volume of 10 ml/kg (15).
Experimental procedures and treatment
After 2 weeks of acclimatization, the mice (n=50) were randomly divided into five groups as follows: Control group served as healthy normal animals without any intervention; D-gal group received D-gal at the dose of 500 mg/kg for 6 weeks; Met 1+D-gal group received D-gal at the dose of 500 mg/kg plus Met 1 mg/kg/day orally for 6 weeks; Met 10+D-gal group received D-gal at the dose of 500 mg/kg plus Met 10 mg/kg/day orally for 6 weeks; Met 1 group received Met 1 mg/kg/day orally for 6 weeks. 24 hr after the last administration, the behavioral tests were done and all experiments were carried out at the same time of the day.
Anxiety-like behavior test
The elevated plus-maze (EPM) test is a standard method to assess the anxiety-like behaviors in rodents (16). The EPM apparatus was made of wood and consisted of two open and two closed arms with the same size (50 × 10 cm). The two closed arms were enclosed by 40 cm high walls. The four arms were linked by a central square platform (10 × 10 cm). The maze was elevated 50 cm above the floor. The mice were placed in the central square platform with its head facing an open arm for 5 min. The percent of time spent in the open arms [OAT% (the ratio of time spent in the open arms to total time spent in any arm × 100)] was used as a measure of anxiety. The percent of entries into the open arms was calculated as [OAE% (the ratio of entries into open arms to the total entries × 100)]. The number of total arm entries was used as a measure of spontaneous locomotor activity. Entry was defined as all four paws in the arm. The experiments were done in a testing room lit by a 60 W light bulb placed above the center of the EPM.
The novel object recognition task
Preference for novelty is an important component of working memory (17). The novel object recognition task (NORT) is one of the most widely used methods to assess this behavior (18). The test was carried out in a 50 × 50 × 50 cm Plexiglas white box. The day before the test, mice were placed in the chamber to get familiar with the environment for 5 min. During the training phases, two identical objects were placed in the chamber and the mouse was allowed to explore the objects freely for 5 min. Exploration was considered when the head of the mouse was oriented toward the objects with its nose within 2 cm of the object. The test phase was carried out 4 hr later. A novel object was replaced by a familiar object. The times spent with the two objects (novel and familiar) were recorded. Results were expressed as discrimination ratio (19). At the end of each session, the mouse was removed from the chamber and the experimental chamber was thoroughly cleaned with 50% ethanol and dried.
The forced swimming capacity test
The forced swimming capacity test is a method for the assessment of animals’ physical power and endurance. Briefly, the mice were dropped individually into a columnar swimming pool (45 cm tall and 20 cm in radius) filled with fresh water to a depth of 35 cm so that mice could not support themselves by touching the bottom with their tails. The temperature of the water was maintained at 34±1 °C. A weight (steel ring) equivalent to 5% of body weight was attached to the tail root of each mouse. The animal exhaustion time was recorded when they failed to rise to the surface of the water to breathe within 7 sec (20). The behavioral tests were done in the order of EPM, NORT, and forced swimming capacity test.
Evaluation of BDNF and SOD levels
24 hr after the last behavioral test, mice were sacrificed by decapitation and the brains were immediately removed under aseptic conditions. Brains were homogenized (1:10 w/v) in sterile phosphate-buffered saline (PBS) (15). The tissue homo-genates were centrifuged (Eppendorf, Germany) at 5000 × rpm, 4 °C for 20 min and the supernatants were obtained and stored at −80 °C for measurements of the biochemical analyses. BDNF and SOD levels were measured with the enzyme-linked immunosorbent assay (ELISA) kits (Zellbio, Germany). The samples were put in the BDNF and SOD kit wells. In the next step, the BDNF and SOD streptavidin-HRP conjugated antibodies were added to each kit plate and they were incubated for 45 min on the shaker at the room temperature. After incubation, free antibodies or antigens were washed by using a washer buffer (PBS, pH=7.4). Then according to the SOD and BDNF kit protocols, sufficient amount of a chromogenic substance (3,3’,5,5’-tetramethylbenzidine (TMB)) was added to each well and the incubation was done again in a 15 min time interval (37 °C). Then, the stop solution (sulfuric acid 2 N) was used for stopping the enzyme-substrate reaction. At the end, the light absorption of yielded yellow color was immediately read by the ELISA reader device (RT-2100C, Rayto, China) at the wavelength of 450 nm for each BDNF and SOD ELISA kits. Results were expressed as % changes compared to the control group.
Statistical analysis
Statistical analysis was performed via GraphPad Prism version 6.01 for Windows (GraphPad Software, USA). Data are expressed as mean±SEM and the differences between the groups were tested with the analysis of variance (one-way ANOVA) followed by Tukey’s post hoc test. Repeated measurement ANOVA (RMA) followed by Tukey’s post hoc test were used for comparison of weight changes in groups. A P<0.05 was considered as a statistically significant difference.
Results
The effect of Met on body weight
As shown in Figure 1, at the beginning of the experiment, we did not find any difference in mean body weight of different groups. Results from RMA showed a significant difference between groups (P<0.001). Tukey’s post hoc analysis showed that administration of D-gal decreases the body weight compared to the control group (P<0.001). Met at the doses of 0.1 and 1 mg/kg improved body weight of D-gal-treated mice (all P<0.001). In addition, administration of Met (1 mg/kg) in normal mice for 6 weeks did not change the body weight compared to the control mice.
Figure 1.
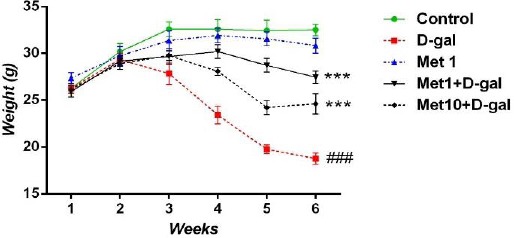
The effect of metformin (1 and 10 mg/kg for 6 weeks) on the body weight of D-galactose-induced aging mice. Values are expressed as mean±SEM (n=10 in each group). ### P<0.001 compared to the control group. *** P<0.001 compared to the D-gal group
The effect of Met on anxiety-like behaviors
The results of the EPM are shown in Table 1. These results showed that D-gal decreases %OAT and %OAE compared to the control group (all P<0.05) and Met at the doses of 1 and 10 mg/kg increases %OAT and %OAE in aging mice (all P<0.001) compared to the D-gal group. Administration of Met alone in healthy mice for 6 weeks increases %OAT and %OAE (all P<0.001) compared to the control group (Table 1). Statistical analysis revealed that the locomotor activity of different groups does not change significantly (Table 1).
Table 1.
The effect of metformin (1 and 10 mg/kg for 6 weeks) on anxiety-like behaviors and locomotor activity of D-galactose-induced aging mice
| Groups | |||||
|---|---|---|---|---|---|
| Control | Met 1 | D-gal | Met 1+D-gal | Met 10+D-gal | |
| Open arm time (%) | 28.33±2.87 | 57.39±2.11### | 15.02±3.53# | 55.28±3.49*** | 52.33±3.43*** |
| Open arm entries (%) | 30.65±2.47 | 54.19±1.82### | 17.83±3.81# | 47.37±3.31*** | 49.53±2.09*** |
| Total arm entries (n) | 25.29±1.86 | 25.57±1.13 | 21.00±2.42 | 23.00±0.53 | 23.57±1.39 |
Values are expressed as mean±SEM (n=10 in each group);
P<0.05 and
P<0.001 compared to the control group;
P<0.001 compared to the D-gal group
The effect of Met on novel object recognition task
The D-gal group mice showed impaired working memory and preference for novelty on the NORT (P<0.05). Met at the doses of 10 mg/kg prevented the D-gal-induced deficiency of working memory and preference for novelty, as evidenced by higher discrimination ratio in Met 10+D-gal mice compared to the D-gal model (P<0.01). Administration of Met alone to the healthy mice for 6 weeks did not change the preference for novelty in normal mice (Figure 2).
Figure 2.
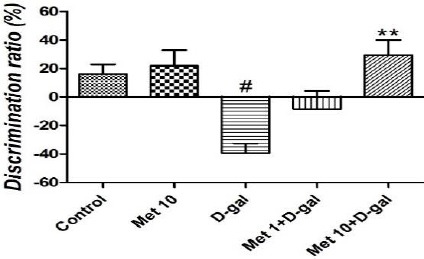
The effect of metformin (1 and 10 mg/kg for 6 weeks) on working memory and preference for novelty of D-galactose-induced aging mice. Values are expressed as mean±SEM (n=10 in each group). # P<0.05 compared to the control group. **P<0.01 compared to the D-gal group
The effect of Met on exhaustion swimming time
The exhaustion swimming time of mice treated with D-gal showed a significant decrease compared to the control group (P<0.05) (Table 2). The aging mice treated with Met at the doses of 1 and 10 mg/kg exhibited an increased exhaustion swimming time compared to the D-gal group (all P<0.001). Moreover, treatment of aging mice with 10 mg/kg Met increased the exhaustion swimming time significantly compared to the control group (P<0.001). Administration of Met alone in the normal mice for 6 weeks increased exhaustion swimming time compared to the control group (P<0.001) (Table 2).
Table 2.
The effect of metformin (1 and 10 mg/kg for 6 weeks) on swimming time to exhaustion of D-galactose-induced aging mice
| Groups | |||||
|---|---|---|---|---|---|
| Control | Met 1 | D-gal | Met 1+D-gal | Met 10+D-gal | |
| Exhaustive swimming time (min) | 60.50±4.72 | 123.3±3.94### | 26.70±10.13# | 88.10±5.80*** | 108.7±8.35###,*** |
Values are expressed as mean±SEM (n=10 in each group);
P<0.05 and
P<0.001 compared to the control group;
P<0.001 compared to the D-gal group
The effect of Met on BDNF and brain SOD levels
As shown in Figure 3A, the BDNF level was significantly decreased in D-gal group compared to the control group (P<0.01). In contrast, Met at the doses of 10 mg/kg increased the BDNF level in D-gal treated mice (P<0.05).
Figure 3.
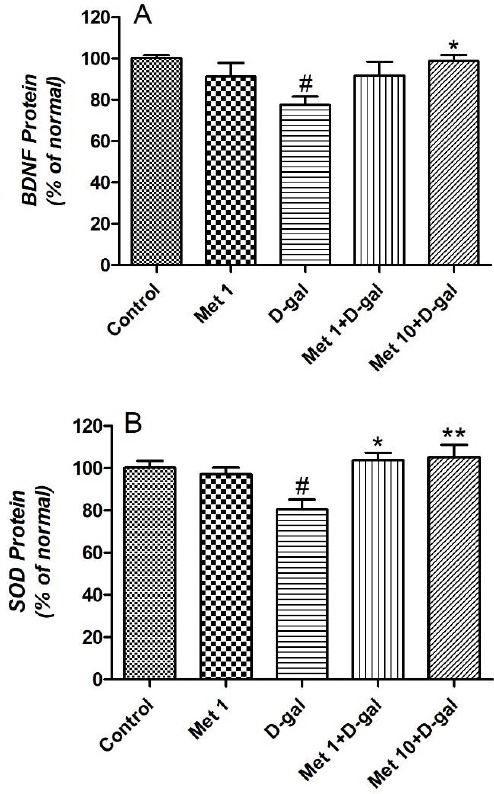
(A) The effect of metformin (1 and 10 mg/kg for 6 weeks) on BDNF and (B) brain SOD levels of D-galactose-induced aging mice. Values are expressed as mean±SEM (n=10 in each group). # P<0.05 compared to the control group. * P<0.05 and ** P<0.01 compared to the D-gal group
Our results demonstrated that the level of SOD in the brain of D-gal treated mice was significantly lower than that of the controls (P<0.05) (Figure 3B). Met administration at the doses of 1 and 10 mg/kg restored D-gal and induced a decrease in SOD level (P<0.05 and P<0.01, respectively).
Administration of Met alone (1 mg/kg) in normal mice for 6 weeks did not change the BDNF and brain SOD levels.
Discussion
It has been shown that long-term treatment with the antidiabetic biguanide Met significantly delays age-related pathological complications and extends lifespan in mammals (13, 14). Most studies on Met effects and aging have been performed in naturally aging animals. Therefore, we conducted an aging model with D-gal which is a well-established model for anti-aging pharmacological therapy (15). One of the main advantages of the D-gal aging model is that we can study only the aging development whereas in the natural aging model the comorbidities such as diabetes, hypertension, and malignancies are of confounding variables. The results of our study demonstrated that administration of D-gal (500 mg/kg per 10 ml drinking water for 6 weeks) causes severe aging-related changes including decrease in body weight, working memory, physical power, BDNF level, and SOD level and increase in expression of anxiety-like behaviors. Administration of Met, mostly at the dose of 10 mg/kg, could reverse these deteriorating effects in D-gal senescence model.
During aging, cognitive functions such as memory are adversely affected, which might be, at least partially, due to the occurrence of neuronal loss in specific brain regions. (21). In addition, previous studies showed that chronic administration of D-gal impairs learning and memory in rodents (22). Consistent with these studies, the results of our study showed that chronic administration of D-gal impairs the novelty-induced exploratory behaviors or working memory in mice. We also observed that Met (10 mg/kg) improves working memory and preference for novelty in aging mice. The reported findings about Met and cognitive function are controversial. Several lines of studies have reported that Met improves learning and memory. For example, one study (23) showed that Met attenuated cognitive impairment in Morris water maze after ischemia/reperfusion-induced brain damage in rats via survival of hippocampal CA1 pyramidal neurons. Another study (24) found that Met decreases neuro-inflammation and loss of neurons in the hippocampus of diabetic animals, which can subsequently improve the spatial memory scores of diabetic animals. Moreover, Ashabi et al. (25) declared that Met has a protective role against memory impairment in cerebral ischemia. Conversely, Thangthaeng et al. (26) examined the effect of chronic oral Met treatment on motor and cognitive function in young, middle-aged, or old male mice. They confirmed that Met treatment has a deleterious effect on spatial memory and reduces SOD activity in various brain regions. The mentioned discrepancies in reported findings may result from the difference in doses of Met (200–300 mg/kg vs. 1–10 mg/kg) as well as different aging models (natural vs. D-gal) used by the two studies. Met may decrease the cognitive impairments and improve object recognition perfor-mance in mice through the neuroprotective effects.
We found that level of BDNF is decreased in D-gal treated mice in comparison with control animals. Our results also indicated that Met (10 mg/kg) increases the BDNF level in aging mice, which is consistent with its improvement in cognitive function. BDNF is an important neurotrophin factor and it has been demonstrated that reduction of BDNF leads to neuronal atrophy and death (27). Also, BDNF has a critical role in the regulation of neurocognitive functions like learning, memory, synaptic transmission, and plasticity (28). Previous studies showed that level and expression of BDNF decreases in both accelerated and natural aging (6). On the other hand, various studies have consistently demonstrated that Met elevates the BDNF level. Researchers (29) reported that Met pre-treatment for 2 weeks increases the BDNF level and improves neurological scores following global cerebral ischemia in rats. Another study (30) investigated the effects of antioxidant and anti-senescence effects of Met on mouse olfactory ensheathing cells. They found that Met induces these effects via decreasing the level of reactive oxygen species and increasing mRNA expression of BDNF. Therefore, it is possible that the restorative effects of Met are mediated by increasing the BDNF level.
ROS production increases with age and this may lead to age-related degenerative processes via oxidative damage to DNA, lipids, and proteins (2). On the other hand, it has been reported that antioxidant enzymes such as SOD are decreased following the induction of senescence by D-gal (22). In our study, the SOD activity was measured in the brains of animals and we observed that D-gal decreases the level of brain SOD in mice. We also found that Met (1 and 10 mg/kg) significantly increases the level of brain SOD compared to D-gal treated mice. Many studies have indicated that Met has a potent antioxidant activity. One study (31) showed that Met prevents renal stone formation through an antioxidant mechanism. It found that Met enhances the activity of SOD and decreases MDA levels both in vivo and in vitro. Another study (32) showed that administration of Met significantly restores the GSH content, increases SOD activity and decreases MDA level in rats with non-alcoholic fatty liver. Moreover, Mansour et al. (33) investigated the modulatory effect of Met on cisplatin-induced hepatotoxicity in rats. They demonstrated that Met decreases lipid peroxidases while increasing SOD activity and glutathione content. Furthermore, it was shown (34) that Met mitigates the decrease in the antioxidant enzyme activities such as SOD in various brain regions in a rat model of type-2 diabetes mellitus undergone chronic stress. Therefore, this indicates that the anti-aging effect of Met might be possibly mediated by its antioxidative defense.
Around 15%–28% of the elderly show anxiety disorders which are associated with reduced life satisfaction and functional impairment (35). Chronic administration of D-gal increased anxiety-like behavior in animals which could mainly result in overproduction of ROS (36). In addition, previous studies have shown that administration of antioxidants such as resveratrol and ascorbic acid can attenuate the anxiety-like behavior in humans and animals (37). In line with these studies, the results of our study showed that anxiety-like behavior increases in D-gal group. We also found that Met, at the doses of 1 and 10 mg/kg, decreases this behavior in aged animals. The anxiolytic effect of Met was demonstrated in several studies. Met ameliorates anxiety-like behaviors in a rat model of ischemia/reperfusion-induced brain damage via exerting the antiapoptotic effects (23). In another study, Met exhibited anti-stress and anxiolytic-like activity in type-2 diabetes mellitus rat models undergone repeated stress via increased antioxidant defense such as SOD and catalase (34). It was shown (38) that Met improves anxiety-like behaviors following global cerebral ischemia via regulation of autophagy. For the first time, we showed that Met (10 mg/kg for 6 weeks) attenuates the anxiety-like behaviors in healthy mice. Accordingly, it seems that treatment with Met decreases anxiety-like behaviors in aging mice via antioxidative and neuroprotective effects.
Sarcopenia (a progressive loss of muscle mass and strength) is a typical characteristic of the elderly that lowers the quality of life and increases disability (39). The mitochondrial dysfunction and over-production of ROS have a critical role in the onset and progression of sarcopenia (40). Previous reports have indicated that D-gal model induces aging in the skeletal muscles like other organs and mitochondrial dysfunction is associated with poor skeletal muscle strength induced by D-gal (8, 39). Consistent with these studies, we observed that D-gal reduces the physical power in forced swimming capacity test compared to the control group. Our results also showed that the treatment of aged mice with Met improves the physical power even more than normal level. Interestingly, we demonstrated for the first time that Met increases the physical power in normal mice which has not been previously reported. The protective effects of Met on mitochondrial dysfunction has been well characterized. Met improves the cardiac function after myocardial infarction in mice via mitigating the damage to mitochondrial membrane and improving the respiratory function of mitochondria (41). A study (42) found that Met can efficiently counteract with alterations of mitochondrial dynamics in Down syndrome mouse models through enhancing oxygen consumption, ATP production, and overall mitochondrial activity. Accordingly, Met might attenuate the sarcopenia and increase the skeletal muscle strength through the enhancement of mitochondrial function.
Conclusion
The results of this study demonstrated that Met improves aging-related disorders such as cognitive impairment, anxiety, and sarcopenia. Taken together, the protective mechanism/s underlying Met effects on D-gal-induced aging might be mediated by increasing the activity of SOD and the level of BDNF. However, further studies are required to unveil the precise underlying mechanisms.
Acknowledgment
Results presented in this work were obtained from a master’s thesis. This study was financially supported by a grant from Research Deputy of Rafsanjan University of Medical Sciences, Rafsanjan, Iran (grant no. 20/295).
Conflict of interest
The authors declare no conflicts of interest relevant to this study.
References
- 1.Shin KR, Kim MY, Kim YH. Study on the lived experience of aging. Nurs Health Sci. 2003;5:245–252. doi: 10.1046/j.1442-2018.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy BK, Pennypacker JK. Drugs that modulate aging: the promising yet difficult path ahead. Transl Res. 2014;163:456–465. doi: 10.1016/j.trsl.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pak JW, Herbst A, Bua E, Gokey N, McKenzie D, Aiken JM. Mitochondrial DNA mutations as a fundamental mechanism in physiological declines associated with aging. Aging Cell. 2003;2:1–7. doi: 10.1046/j.1474-9728.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 5.van Velzen LS, Wijdeveld M, Black CN, van Tol MJ, van der Wee NJA, Veltman DJ, et al. Oxidative stress and brain morphology in individuals with depression, anxiety and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:140–144. doi: 10.1016/j.pnpbp.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Joseph J, Cole G, Head E, Ingram D. Nutrition, brain aging, and neurodegeneration. J Neurosci. 2009;29:12795–12801. doi: 10.1523/JNEUROSCI.3520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Wu T, Jin Y, Fu Z. Effects of age and jet lag on D-galactose induced aging process. Biogerontology. 2009;10:153–161. doi: 10.1007/s10522-008-9158-2. [DOI] [PubMed] [Google Scholar]
- 8.Wei H, Li L, Song Q, Ai H, Chu J, Li W. Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav Brain Res. 2005;157:245–251. doi: 10.1016/j.bbr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Kothari V, Galdo JA, Mathews ST. Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res. 2016;9:27–38. doi: 10.2147/JIR.S86917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Łabuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacological Reports. 2010;62:956–965. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 11.Markowicz-Piasecka M, Sikora J, Szydlowska A, Skupien A, Mikiciuk-Olasik E, Huttunen KM. Metformin –a future therapy for neurodegenerative diseases. Pharm Res. 2017;34:1–14. doi: 10.1007/s11095-017-2199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao R-r, Xu X-c, Xu F, Zhang W-l, Zhang W-l, Liu L-m, et al. Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem Biophys Res Commun. 2014;448:414–417. doi: 10.1016/j.bbrc.2014.04.130. [DOI] [PubMed] [Google Scholar]
- 13.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.McCarty MF. Chronic activation of AMP-activated kinase as a strategy for slowing aging. Med Hypotheses. 2004;63:334–339. doi: 10.1016/j.mehy.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Kaviani E, Rahmani M, Kaeidi A, Shamsizadeh A, Allahtavakoli M, Mozafari N, et al. Protective effect of atorvastatin on D-galactose-induced aging model in mice. Behav Brain Res. 2017;334:55–60. doi: 10.1016/j.bbr.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Pellow S, Chopin P, File SE, Briley M. Validation of open - closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 17.Fatemi I, Shamsizadeh A, Ayoobi F, Taghipour Z, Sanati MH, Roohbakhsh A, et al. Role of orexin-A in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2016;291:101–109. doi: 10.1016/j.jneuroim.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Roohbakhsh A, Shamsizadeh A, Arababadi MK, Ayoobi F, Fatemi I, Allahtavakoli M, et al. Tactile learning in rodents: neurobiology and neuropharmacology. Life Sci. 2016;147:1–8. doi: 10.1016/j.lfs.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Ayoobi F, Fatemi I, Roohbakhsh A, Shamsizadeh A. Tactile learning within the early phase of experimental autoimmune encephalomyelitis in mice. Neurophysiology. 2013;45:306–311. [Google Scholar]
- 20.Zamanian M, Hajizadeh MR, Nadimi AE, Shamsizadeh A, Allahtavakoli M. Anti-fatigue effects of troxerutin on exercise endurance capacity, oxidative stress and MMP-9 levels in trained male rats. Fundam Clin Pharmacol. 2017;31:447–455. doi: 10.1111/fcp.12280. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XL, An LJ, Bao YM, Wang JY, Jiang B. D-galactose administration induces memory loss and energy metabolism disturbance in mice: protective effects of catalpol. Food Chem Toxicol. 2008;46:2888–2894. doi: 10.1016/j.fct.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Pourmemar E, Majdi A, Haramshahi M, Talebi M, Karimi P, Sadigh-Eteghad S. Intranasal cerebrolysin attenuates learning and memory impairments in D-galactose-induced senescence in mice. Exp Gerontol. 2017;87:16–22. doi: 10.1016/j.exger.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Ge XH, Zhu GJ, Geng DQ, Zhang HZ, He JM, Guo AZ, et al. Metformin protects the brain against ischemia/reperfusion injury through PI3K/Akt1/JNK3 signaling pathways in rats. Physiol Behav. 2017;170:115–123. doi: 10.1016/j.physbeh.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira WH, Nunes AK, Franca ME, Santos LA, Los DB, Rocha SW, et al. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016;1644:149–160. doi: 10.1016/j.brainres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Ashabi G, Sarkaki A, Khodagholi F, Zareh Shahamati S, Goudarzvand M, Farbood Y, et al. Subchronic metformin pretreatment enhances novel object recognition memory task in forebrain ischemia: behavioural, molecular, and electrophysiological studies. Can J Physiol Pharmacol. 2016;95:388–395. doi: 10.1139/cjpp-2016-0260. [DOI] [PubMed] [Google Scholar]
- 26.Thangthaeng N, Rutledge M, Wong JM, Vann PH, Forster MJ, Sumien N. Metformin impairs spatial memory and visual acuity in old male mice. Aging Dis. 2017;8:17–30. doi: 10.14336/AD.2016.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda M, Takahashi M, Matsumoto S. Inflammation enhanced brain-derived neurotrophic factor-induced suppression of the voltage-gated potassium currents in small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone. Neuroscience. 2014;261:223–231. doi: 10.1016/j.neuroscience.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Pareek V, Faiq MA, Kumar P, Raza K, Prasoon P, et al. Regulatory role of NGFs in neurocognitive functions. Rev Neurosci. 2017;28:649–673. doi: 10.1515/revneuro-2016-0031. [DOI] [PubMed] [Google Scholar]
- 29.Ghadernezhad N, Khalaj L, Pazoki-Toroudi H, Mirmasoumi M, Ashabi G. Metformin pretreatment enhanced learning and memory in cerebral forebrain ischaemia: the role of the AMPK/BDNF/P70SK signalling pathway. Pharm Biol. 2016;54:2211–2219. doi: 10.3109/13880209.2016.1150306. [DOI] [PubMed] [Google Scholar]
- 30.Smieszek A, Strek Z, Kornicka K, Grzesiak J, Weiss C, Marycz K. Antioxidant and anti-senescence effect of metformin on mouse olfactory ensheathing cells (mOECs) may be associated with increased brain-derived neurotrophic factor levels-an ex vivo study. Int J Mol Sci. 2017;18:872–891. doi: 10.3390/ijms18040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Ding H, Qin Z, Zhang C, Qi S, Zhang H, et al. Metformin prevents renal stone formation through an antioxidant mechanism in vitro and in vivo. Oxid Med Cell Longev. 2016;2016:4156075–4156085. doi: 10.1155/2016/4156075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Lakkany NM, Seif El-Din SH, Sabra AA, Hammam OA, Ebeid FA. Co-administration of metformin and N-acetylcysteine with dietary control improves the biochemical and histological manifestations in rats with non-alcoholic fatty liver. Res Pharm Sci. 2016;11:374–382. doi: 10.4103/1735-5362.192487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansour HH, El Kiki SM, Galal SM. Metformin and low dose radiation modulates cisplatin-induced oxidative injury in rat via PPAR-gamma and MAPK pathways. Arch Biochem Biophys. 2017;616:13–19. doi: 10.1016/j.abb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Garabadu D, Krishnamurthy S. Diazepam potentiates the antidiabetic, antistress and anxiolytic activities of metformin in type-2 diabetes mellitus with cooccurring stress in experimental animals. Biomed Res Int. 2014;2014:693074–693089. doi: 10.1155/2014/693074. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Therrien Z, Hunsley J. Assessment of anxiety in older adults: a systematic review of commonly used measures. Aging Ment Health. 2012;16:1–16. doi: 10.1080/13607863.2011.602960. [DOI] [PubMed] [Google Scholar]
- 36.Haider S, Liaquat L, Shahzad S, Sadir S, Madiha S, Batool Z, et al. A high dose of short term exogenous D-galactose administration in young male rats produces symptoms simulating the natural aging process. Life Sci. 2015;124:110–119. doi: 10.1016/j.lfs.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Damian JP, Acosta V, Da Cuna M, Ramirez I, Oddone N, Zambrana A, et al. Effect of resveratrol on behavioral performance of streptozotocin-induced diabetic mice in anxiety tests. Exp Anim. 2014;63:277–287. doi: 10.1538/expanim.63.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkaki A, Farbood Y, Badavi M, Khalaj L, Khodagholi F, Ashabi G. Metformin improves anxiety-like behaviors through AMPK-dependent regulation of autophagy following transient forebrain ischemia. Metab Brain Dis. 2015;30:1139–1150. doi: 10.1007/s11011-015-9677-x. [DOI] [PubMed] [Google Scholar]
- 39.Chang L, Liu X, Liu J, Li H, Yang Y, Liu J, et al. D-galactose induces a mitochondrial complex I deficiency in mouse skeletal muscle: potential benefits of nutrient combination in ameliorating muscle impairment. J Med Food. 2014;17:357–364. doi: 10.1089/jmf.2013.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;8:349–369. doi: 10.1002/jcsm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun D, Yang F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem Biophys Res Commun. 2017;486:329–335. doi: 10.1016/j.bbrc.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 42.Izzo A, Nitti M, Mollo N, Paladino S, Procaccini C, Faicchia D, et al. Metformin restores the mitochondrial network and reverses mitochondrial dysfunction in Down syndrome cells. Hum Mol Genet. 2017;26:1056–1069. doi: 10.1093/hmg/ddx016. [DOI] [PubMed] [Google Scholar]


