Abstract
Objective(s):
Breast cancer is one of the most common cancers in the world and is on the increase. MUC1 and HER2 as tumor-associated antigens (TAAs) are abnormally expressed to some extent in 75–80% of breast cancers. In our present research, a novel chimeric MUC1-HER2 (HM) protein was designed and used to study whether an immune response can be generated against these TAAs. In vitro analysis of the HER2-MUC1 construct confirmed the co-expression of MUC1 and HER2.
Materials and Methods:
BALB/c mice were immunized with this novel chimeric protein. The humoral immune response was assessed by enzyme-linked immunosorbent assay (ELISA). Then, BALB/c mice were injected subcutaneously 2×105 4T1-MUC1-HER2 tumor cells. Subsequently, tumor size and tumor necrosis measurements, MTT, cytokines assay and survival test were performed.
Results:
The results implied a critical role of HER2 and MUC1 antibodies in vaccination against breast cancer. This engineered protein can be a good vaccine to stop breast cancer.
Conclusion:
The results implied a critical role of HER2 and MUC1 antibodies in vaccination against breast cancer. This engineered protein can be a good vaccine to stop breast cancer.
Keywords: Breast cancer, HER2, MUC1, Recombinant antigen, Vaccine
Introduction
Breast cancer is the second most regular growth in women after skin malignancy, but can happen in both men and women, however, it is extremely uncommon in men (1). In the most recent decades, a few endeavors have been made to create systems that could viably induce potent immune responses against different tumor types. Application of the immune system against disease cells is viewed as one of the advantageous strategies since the immune system can control different sorts of tumors (2). In this manner, active immunization is a promising technique to activate the immune system to attack cancerous cells that has low poisonous quality and wonderful specificity. Cancer immunizations change the way introductory patient reacts and reactions to consequent treatments post-vaccination is assessed (3). Using monoclonal antibodies and cytotoxic T lymphocytes (CTL) are two immunotherapeutic methodologies for the treatment of bosom disease (4, 5). Mucin (MUC1) and HER2/neu (HER2) are two tumor marker candidates for diagnosis and vaccination of breast cancer. Subsequently, vaccines give new insight into immunization to produce a powerful response to HER2 or MUC1 expressing carcinomas (5, 6). Human mucin (MUC1), as a transmembrane glycoprotein, has an extracellular domain consisting of a tandem repeat 20 amino acid polypeptide sequence with various O-glycosylation residues (7, 8). The number of these repeats is different based on population genetics. The broad glycosylation of MUC1 present in ordinary tissues may further interfere with immune system reaction. It has been proposed that the peptides of MUC1s are not completely glycosylated in cancer cells, bringing about the presentation to the insusceptible arrangement of peptide groupings that are not normally exposed (9, 10). The MUC1 protein is unusually expressed on an expected 75% of all human solid tumor diseases (11). There is preclinical information in rodents exhibiting that MUC1 based immunizations are immunogenic in both humoral and cell styles. For example, inhibition of tumor growth has been associated with antibody production and T cell activation (7, 10). HER2 is another source of TAAs that has been broadly examined in breast malignancy. HER2/neu is an individual from the epidermal development component receptor group of trans-membrane tyrosine kinases developing tumor progression and is found in about 75–80% of breast carcinoma (12). This overexpression can bring about a 100–200-fold HER2 protein in tumor versus typical tissue and is a set up for immunizer and vaccination (4, 6). These findings have suggested that immunization against HER2 and MUC1 may be possible and that this immunization might prevent tumor regrowth in patients with breast cancer.
For these reasons, we have tested this approach against two targets that are commonly co-expressed in breast cancer, namely MUC1 and HER2 (13).
Our aim in this study was to employ the recombinant HER2-MUC1 (rHM) as a chimeric protein vaccination in a mouse model to develop a more efficacious vaccine against breast cancer.
Materials and Methods
Construct design, culture condition and preparation of HM protein vaccine
Using in silico analyses, the antigenic sequence of the human HER2 extracellular domain (480-620 aa) and MUC1 (220-360 aa) were selected and linked together by a hydrophilic linker (5 repetitive sequences of EAAAK). The chimeric gene (Accession No. KF430636) was constructed, optimized, and synthesized as a clone into the pUC57 vector (Shine gene Molecular Biotech, Inc). Secondary structure consensus prediction was performed using SPOMA method (Self-Optimized Prediction Method with Alignment), and GOR (14). Structure prediction was performed by I-TASSER server and was uploaded to the Swiss-PdbViewer server to depict the tertiary structural illustrations (15).
pET28a-her2-muc1 (pET-hm) plasmid was prepared and confirmed as previously described. Hexahistidine-tagged HER2-MUC1 was purified by IMAC (Immobilized Metal Affinity Chromatography) using Ni–NTA agarose (Qiagen) under denaturing conditions and verified based on SDS-PAGE and Western blotting analysis and restored at -70°C for further analysis (16).
The mice breast cancer cell line 4T1, which expresses MUC1 and HER2, was purchased from the cell line bank (Pasture Institute of Iran). Cells were cultured in RPMI1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Invitrogen), penicillin (100 U/ml), and streptomycin (100 µg/ml) (Sigma) and incubated at 37 °C in 5% CO2 with appropriate humidity.
Immunization, vaccination and tumor challenge
In the prophylactic immunization experiment, twenty female inbred BALB/c mice (6 weeks old, 25–30 g, Pasteur Institute of Iran), as test group, received 10 µg rHM protein conjugated in Freund’s complete adjuvant (SIGMA) by subcutaneous injection behind the neck. Two and four weeks after injection, the animals were boosted with incomplete Freund’s adjuvant. While complete adjuvant was applied to induce both humoral and cellular immune responses, incomplete adjuvant was used to enhance humoral immune response after the first injection (17-19). As control group, 20 BALB/c mice were injected with complete and incomplete Freund’s adjuvant or PBS with the same procedure. Eye bleeding was performed after immunization. Antibody specific responses against rHM protein were evaluated using the ELISA method and then the mice sera were collected (16). One month after the last immunization, 2×105 4T1-MUC1- HER2 tumor cells in 100 µl of PBS were injected subcutaneously into the right flanks of mice to form tumors (20). Palpable tumors usually developed on day 7. Tumor growth and general condition of the mice were monitored every other day and measured using a caliper. Each tumor volume in mm3 was calculated by the following formula: V= 0.5 × D×d2 (V, volume; D, longitudinal diameter; d, latitudinal diameter) (21).
Cell proliferation assay (MTT assay)
Ten mice from each group (test and control) were sacrificed after final tumor size measurment and separation of the splenocytes. The proliferation response of splenocytes was determined using 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (22). Briefly, spleens from each mouse were collected under aseptic conditions. In order to per-form cell proliferation assay, splenocytes at the concentration of 1×105 cells/100 μl were cultured in RPMI1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), penicillin (100 U/ml) and streptomycin (100 μg/ml) (SIGMA) with 10 ng/μl of rHM, and incubated at 37 °C in 5% CO2 with appropriate humidity. Stimulation of mice lymphocytes was measured using MTT assay. After incubation, an aliquot of 100 μl of MTT reagent (0.5 mg/ml final concentration) was added to each well and incubated for another 4 hr and the plates were then centrifuged at 1500×g for 10 min. A total of 100 µl of culture supernatant was discarded from each well. Finally, 100 µl of 2.5% dimethyl sulfoxide (DMSO) was added to each well and mixed thoroughly to dissolve formazan crystals. Then the optical density (OD) of color intensity was read at 570 nm in a microplate reader (Bio-Rad). The stimulation index (SI) was calculated according to the following formula: mean OD of test cells / mean OD of control cells × 100 and the splenocytes were used without proximity to chimeric antigen HM. Each assay was repeated three times (23, 24).
Splenocytes activation assays
The spleen from each mouse was isolated, cut into small pieces, rinsed twice with PBS, and minced usin forceps and scalpel. The suspensions were passed through a 100 µm stainless steel mesh to obtain a g single cell suspension, and erythrocytes were lysed at room temperature using ACK lysis buffer (150 mM NH4Cl, 1 mM KHCO3, and 0.001 mM Na2-EDTA). The cells were washed and resuspended in RPMI1640 supplemented with 10% FBS. The cells at a concentration of 1×106/ml were cultured with rHM at 10 ng/well. After 3 days of incubation, the supernatants were collected and tested for cytokines (IFNγ, IL-17, and IL-4) by sandwich based ELISA kits from R&D (Minneapolis, MN, USA) according to the manufacturer’s instructions. Experiments were performed in triplicates (25, 26).
Histopathology experiments
The histopathological analysis was performed on tumors and organs like (lung, liver, sternum, and lymph nodes) fixed in formalin. Tumor tissues of approximately 0.5 cm ×0.5 cm in size were isolated, transferred into an automatic processing machine for 12 hr, and then embedded in paraffin. Five-μm-thick sections were prepared and stainings with hematoxylin and eosin (H&E) were made by the histology unit of Baqiyatallah hospital for histopathological examina-tions. Necrosis and metastases were also determined under light microscopy (26).
Statistical analysis
All experiments were performed three times. Data obtained from ELISA, cell proliferation assays and other experiments were analyzed using ANOVA and the statistical significance level was set at P ≤ 0.05. All statistical analysis was performed using SPSS software (version 16.0; SPSS, Inc.) and data analysis software (Microsoft Excel 2007).
Results
Construct design
Two fragments of proteins, 140 amino acids from HER2 and MUC1 were linked together using EAAAK repeats; the arrangements of fragment junctions and linker sites are shown in Figure 1a. By uploading several models to the I-TASSER server, the tertiary structure of the fusion protein was predicted (15). Tertiary predication result of the fusion protein construction showed a protein with two domains linked together with a linker (Figure 1b), furthermore, protein construction showed a helix pick located between positions 141–165 that corresponded to the linker fragment. The confidence score (C-score) for estimating the quality of predicted models by I-TASSER was -3.07. C-score is typically in the range of −5 to 2, where a C-score of higher value signifies the model with a high confidence. In addition, the expected TM-score for this model was 0.37±0.12. The expected RMSD was 13.6±4.0. The used amino acid sequences of HER2, linker, and MUC1 in the designed construct are shown in Figure 1c.
Figure 1.
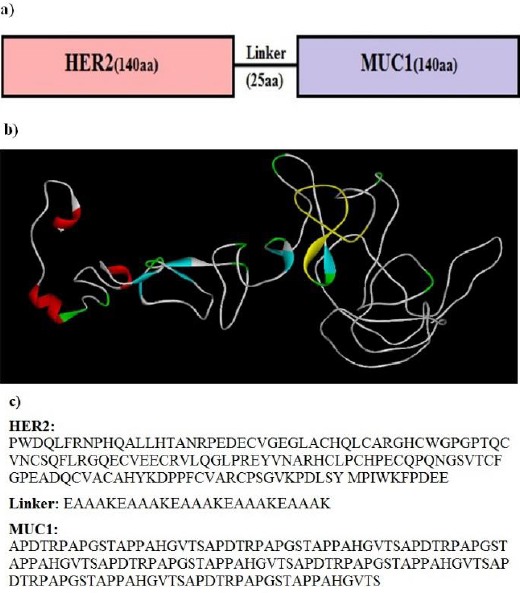
a) Schematic representation of chimeric antigenic construct consists of HER2 and MUC1 genes bound together by an appropriate linker. b) The models predicted by I-TASSER server and visualized by Discovery Studio viewer. c) The used amino acid sequences of HER2, linker, and MUC1 in designed construct.
The PET28a-HER2-MUC1 construct was approved by the double digestion method and recombinant HM was observed followed by induction in SDS-PAGE and confirmed by reaction with the anti-His-tag antibodies with Western blot (16).
Humoral immunity
HM specific antibody titers were induced in BALB/c mice after vaccination with recombinant protein vaccines. Mean IgG antibody titers generated in mice immunized with the rHM were significantly higher (P<0.05) than the control group after six weeks (Figure 2). Commercial anti-HER2 (DAKO) and MUC1 (Abnova) were as reference (16).
Figure 2.
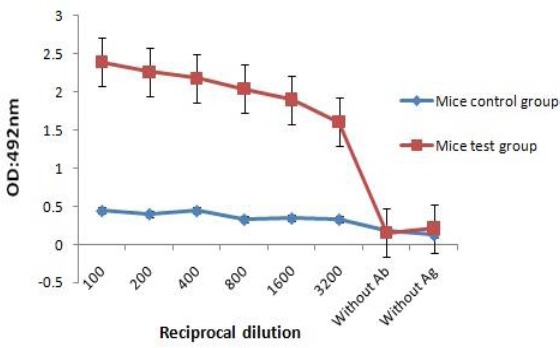
Optical density based on serial dilution of anti rHM-specific showed 1/100 dilution as an optimum density
Tumor size after the 4T1-HM tumor cells injection
4T1-HM tumor cells were challenged in the two groups of mice on day 1 and the changes in the tumor volumes were observed on day 14. To determine if the vaccine elicited immune response affecting tumor growth, we monitored the tumor size every 2 days up to 30 days (27).
The comparisons of tumor sizes are summarized as illustrated in Figure 3; the relative tumor volume in test and control groups were 653.54 and 901.42 mm3, respectively.
Figure 3.
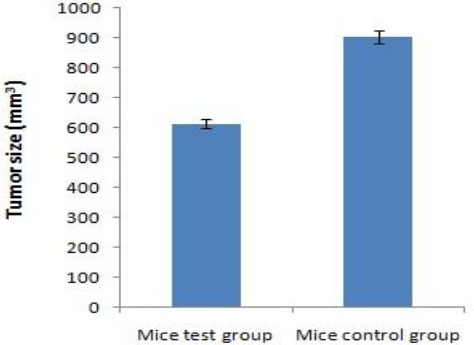
Tumor progression following vaccination. The reduction of tumor growth observed in BALB/c mice immunized with rHM as compared to the control group mice (P<0.05)
Cellular immune response induced by HM
To further investigate the cell-mediated immunity (CMI) response induced by the vaccine, we analyzed the proliferative splenocyte response. Splenocytes from BALB/c mice were cultured in RPMI1640 containing the HM antigen for 72 hr. Then the splenocytes proliferation was examined by MTT assay. As shown in Figure 4, vaccine induced significant and specific splenocyte proliferation in immunized BALB/c mice in response to recombinant protein HM compared with PBS or adjuvant immunization (P< 0.05).
Figure 4.
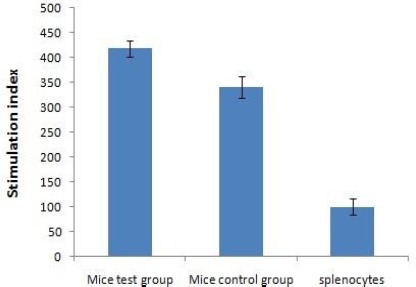
The proliferation rates of mouse lymphocyte cells (MTT Assay) after 72 hr exposure to HM expressed as stimulation index. Statistical analysis was performed using the two-tailed Mann Whitney nonparametric test, and a P-value of P<0.05 was considered as a statistically significant difference. Splenocytes were used without proximity to HM (Splenocytes). The mean ± SD of triplicate determinations are shown
Levels of IFNγ, IL-17, and IL-4 analysis
To elucidate the immune mechanism triggered by the recombinant HM, we assessed the proportion of splenocytes collected from BALB/c mice immunized either with vaccine, or control group mice with PBS, secreting IFNγ, IL-17, and IL-4 in response to in vitro stimulation with recombinant antigens.
The results are shown in Figure 5; a high proportion of splenocytes secreting IFNγ and IL-17 was demonstrated in mice immunized with rHM (P<0.05). We could also notice a slightly higher proportion of splenocytes secreting IL-4 in mice immunized with the combined preparation as compared to the PBS mice control group(P<0.05).
Figure 5.
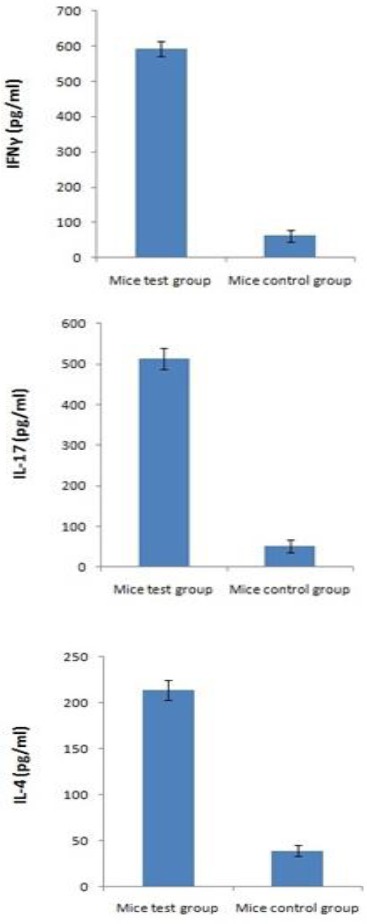
Increase of IFNγ, IL-17 and IL-4 secretion by splenocytes isolated from BALB/c mice immunized with vaccine. Splenocytes were cultured in vitro with HM. The supernatant of each group was harvested after 72 hr of culture and released IFNγ, IL-17, and IL-4 were measured by ELISA. These experiments were run in triplicate
These data demonstrate the induction of Th1 type response towards MUC1 and HER2 upon vaccination with rHM.
Histopathology, tumor necrosis, and survival test analysis
Histological examinations used H&E stained sections, the summary of which is illustrated in Figures 6a and b. A significantly increased induction of necrosis was evident in the mice test group (P<0.05). Furthermore, the size of metastases presented in the lungs of mice vaccinated with rHM was clearly smaller in comparison to the lung metastases in the control group mice. Also, when these mice were followed for survival determination 50% of HM immunized mice survived for over 10 days compared to control group mice.
Figure 6.
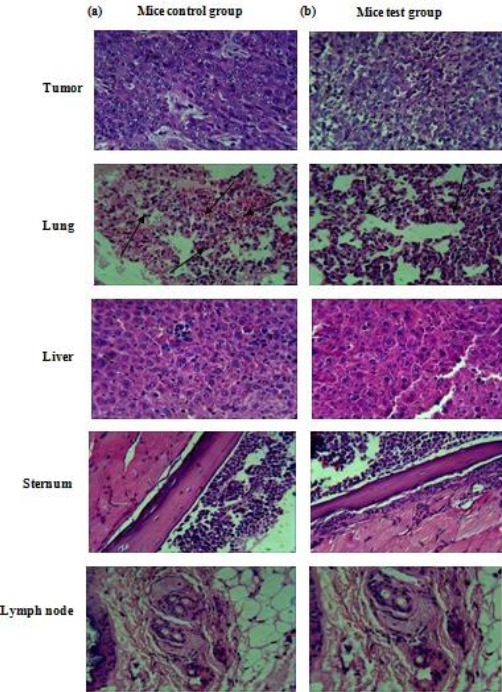
Results of the H&E staining of tumor, lung, liver, sternum, and lymph nodes from mice immunized with PBS or HM (a) Mice control group (b) Mice test group
Discussion
Tumors, treated by surgery, chemotherapy, and radiotherapy often relapse and they regularly metastasize. Initiation of the patients’ own immune system is one of a few promising vaccination techni-ques for controlling cancer development. On the other hand, cancerous cells are generally resistant to the anti-tumor activities including apoptosis, necrosis, and so on. Since tumor cells regularly do not display their own antigens to T cells, one of the significant objectives of tumor immunotherapy is producing tumor particular T cells (26, 28, 29). Immunotherapy against cancer is one of the new fields of research and creates active response against the tumor surface antigens. Cancer vaccine based on surface antigens can be a new way to treat tumors. The main goal of the vaccine is inducing specific response against the tumors, then it is considered nontoxic and can eventually give long immunity against the tumor, which prevents the tumor from returning (29, 30).
Human MUC1 is one of the few well-characterized tumor neoantigens. Polarized expression of MUC1 in a few epithelial tumors is lost and the ordinarily vigorously glycosylated protein is overexpressed in hypo and non-glycosylated forms. This irregular glycosylation leads to the expression of novel B and T cell epitopes inside the tandemly repeating sequence of 20 amino acids. Low recurrence CTL and low titer IgM responses against MUC1 are available in cancer patients, however, they don’t counteract malignancy development. In this way, boosting MUC1 specific immunity with immunizations could efficiently trigger cancer immunotherapy (7, 31). One of the key proto-oncogenes in breast cancer is HER2 (known as ErbB2). A few cytotoxic CD8+ T lymphocyte (CTL) peptide epitopes of HER2 have been characterized and their capacity to affect HER2 peptide particular CTLs could be shown in clinical trials (28). Without a doubt, HER2 is essential in proliferation and viability of tumor and numerous parts of tumor development are positively influenced through its activation. HER2 receptors and signaling from them and associated molecules lead to increased cell motility, decreased apoptosis, enhancing signaling interactions with the microenvironment, regulating adhesion, as well as a multitude of other functions (28, 32). These findings suggest that HER2 may offer significant promise as a potential target for antigen-specific immunotherapy of HER2 positive human cancers. In addition to TAAs, HER2 and MUC1 also can stimulate an expansive number of T cells, triggering cytokine secretion from T cells and monocytes; therefore, the antitumor activity of the immune system is promoted to inhibit tumor development and metastasis (30). Inducing a strong anti-tumor T cell response is one of the vital procedures to obtain an appropriate immunotherapy (10, 13, 29). Evidence is emerging that a successful cancer vaccine should be multimodal and activate several aspects of the immune system at once. Although cellular and humoral immune responses against MUC1 and HER2 have been observed in some cancer patients, it has been difficult to design cancer vaccine candidates that can elicit both of these responses (13, 30, 33). In this study, we demonstrated the immunization activity of a recombinant chimeric HM protein vaccine expressing epitopes of MUC1 and HER2 in E. coli BL21(DE3), fused together using a linker by which the domains efficiently lead to their appropriate folding on both tumor growth and metas-tases formation. Having evaluated the sequences of HER2 and MUC1 genes in both human and mouse, we found no considerable difference between related sequences in these organisms. Therefore, mice can be applied as a suitable immunological model to eluci-date the immunotherapeutic capability of our human-based HER2-MUC1 vaccine. Because of this, we employed a mouse model for mammary cancer to demonstrate that the bilateral vaccine can elicit IgG antibodies which can lyse MUC1 or HER2 expressing cancer cells, stimulate cytotoxicity of T lymphocytes, and activate innate immune responses, thereby reversing resistance and creating a remedial response. Because the pathways of murine T cell activation are similar to those of human T cells, experimental results in mice can be also generalized to humans (23). HM in our study stimulated the proliferation of splenocytes and produced cytotoxic cytokines (mainly INFγ) indicating its particular role in tumor cell lysis and necrosis. Although it is not well-known how necrosis is induced, it is assumed that the injuries are triggered by the induced CTLs as well as the produced cytokines.
We used the MTT assay in order to evaluate the proliferation or suppressive effects of above the mentioned recombinant protein. A greater proliferation of the cells obviously leads to the formation of more crystals and an increase in the SI. It has been clarified in this study that HM can cause stimulation and proliferation of mouse splenocytes better than the splenocytes without proximity with HM (Figure 4). According to the results obtained from Bioinformatics tools, measuring the tumor size (Figure 3), measuring the cytokines IFNγ, IL-17 and IL-4 (Figure 5) and measuring the magnitude of tumor necrosis (Figure 6), it was shown that tumor growth in the mice test group compared with the mice control group displayed significant reduction (P< 0.05; Figure 3). Measurement of IFNγ, IL-17, and IL-4 in this group showed that the amount of the produced IFNγ and IL-17 compared with the produced IL-4, in the mice control group was significantly different (P<0.05; Figure 5). In this study, we showed that the recombinant HM evoked a Th1 response toward the inserted epitopes, as evidenced by IFNγ secretion by splenocytes isolated in response to HER2 and MUC1 stimuli, whereas the Th2 response appeared weak. Therefore, the increase of IFNγ and IL-17 compared with IL-4 indicates that the HM protein enhances the antitumor activity. Our results showed that the magnitude of tumor necrosis in the mice test group compared with the mice control group was significantly different (P<0.05), and the magnitude of tumor necrosis caused by administering HM in comparison with the mice control group, just Freund’s adjuvant, or PBS was higher; this finding goes together with the results indicating reduction of tumor size. Our evaluation of HM vaccination may open a new approach for clinical management of patients with breast cancer. This in vivo investigation indicates that HM could be useful, but further investigations including detailed studies searching for side effects are warranted. Another dataset demonstrates that dual targeting can lead to the delivery of complementary signals that enhance splenocytes proliferation (13).
However, other evidence showed that polyvalent vaccines, including protein vaccines and DNA vaccines, can engender more effective protection than univalent vaccines in some cases (28, 34, 35).
Conclusion
The designed construct has the significant potential to induce both humoral and cellular immune responses effectively, and the results showed that the antigenicity of MUC1 and HER2 differs between malignant and normal tissue, and therefore, a HER2-MUC1 based cancer vaccine, if effective, could hold a great promise and would be highly valuable in medical oncology. However, results of this study demonstrated the necessity to continue the search for more effective immunization approaches.
Acknowledgment
This work was supported by Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran (no. 2582).
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Shayan R, Achen MG, Stacker SA. Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis. 2006;27:1729–1738. doi: 10.1093/carcin/bgl031. [DOI] [PubMed] [Google Scholar]
- 2.Emens L, Reilly R, Jaffee E. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocrine-Related Cancer. 2005;12:1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 3.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med. 2008;233:522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milani A, Sangiolo D, Montemurro F, Aglietta M, Valabrega G. Active immunotherapy in HER2 overexpressing breast cancer: current status and future perspectives. Ann Oncol. 2013;24:1740–1748. doi: 10.1093/annonc/mdt133. [DOI] [PubMed] [Google Scholar]
- 5.Pietersz GA, Li W, Osinski C, Apostolopoulos V, McKenzie IF. Definition of MHC-restricted CTL epitopes from non-variable number of tandem repeat sequence of MUC1. Vaccin. 2000;18:2059–2071. doi: 10.1016/s0264-410x(99)00515-0. [DOI] [PubMed] [Google Scholar]
- 6.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer immunol immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, et al. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 8.Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Wang L, Zhang P, Wei H, Gao R, Liu X, et al. Detection of circulating anti-mucin 1 (MUC1) antibodies in breast tumor patients by indirect enzyme-linked immunosorbent assay using a recombinant MUC1 protein containing six tandem repeats and expressed in Escherichia coli. Clin Vaccine Immunol. 2010;17:1903–1908. doi: 10.1128/CVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Graeber LA, Helling F, Ragupathi G, Adluri S, Lloyd KO, et al. Augmenting the immunogenicity of synthetic MUC1 peptide vaccines in mice. Cancer Res. 1996;56:3315–3319. [PubMed] [Google Scholar]
- 11.Hikita ST, Kosik KS, Clegg DO, Bamdad C. MUC1*mediates the growth of human pluripotent stem cells. PLoS One. 2008;3:e3312. doi: 10.1371/journal.pone.0003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna M., Jr Human vaccines & immunotherapeutics: news. Hum Vaccin Immunother. 2014;10:1773–1777. doi: 10.4161/hv.36241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 14.Geourjon C, Deleage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheybi E, Amani J, Salmanian AH, Mashayekhi F, Khodi S. Designing a recombinant chimeric construct contain MUC1 and HER2 extracellular domain for prediagnostic breast cancer. Tumour Biol. 2014;35:11489–11497. doi: 10.1007/s13277-014-2483-y. [DOI] [PubMed] [Google Scholar]
- 17.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castiglione F, Mantile F, De Berardinis P, Prisco A. How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput Math Methods Med. 2012:842329. doi: 10.1155/2012/842329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panthel K, Meinel KM, Sevil Domenech VE, Geginat G, Linkemann K, Busch DH, et al. Prophylactic anti-tumor immunity against a murine fibrosarcoma triggered by the Salmonella type III secretion system. Microbes Infect. 2006;8:2539–2546. doi: 10.1016/j.micinf.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Ma W, Yu H, Wang Q, Jin H, Solheim J, Labhasetwar V. A novel approach for cancer immunotherapy: tumor cells with anchored superantigen SEA generate effective antitumor immunity. J Clin Immunol. 2004;24:294–301. doi: 10.1023/B:JOCI.0000025451.41948.94. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Fooladi AA, Sattari M, Nourani MR. Study of T-cell stimulation and cytokine release induced by Staphylococcal enterotoxin type B and monophosphoryl lipid A. Arch Med Sci. 2009;5:335–341. [Google Scholar]
- 24.Singha H, Mallick AI, Jana C, Fatima N, Owais M, Chaudhuri P. Co-immunization with interlukin-18 enhances the protective efficacy of liposomes encapsulated recombinant Cu-Zn superoxide dismutase protein against Brucella abortus. Vaccine. 2011;29:4720–4727. doi: 10.1016/j.vaccine.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 25.Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, et al. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- 26.Fooladi AA, Sattari M, Hassan ZM, Mahdavi M, Azizi T, Horii A. In vivo induction of necrosis in mice fibrosarcoma via intravenous injection of type B staphylococcal enterotoxin. Biotechnol Lett. 2008;30:2053–2059. doi: 10.1007/s10529-008-9805-3. [DOI] [PubMed] [Google Scholar]
- 27.Kindy MS, Yu J, Zhu H, Smith MT, Gattoni-Celli S. A therapeutic cancer vaccine against GL261 murine glioma. J Transl Med. 2016;14:1. doi: 10.1186/s12967-015-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohanty K, Saha A, Pal S, Mallick P, Chatterjee SK, Foon KA, et al. Anti-tumor immunity induced by an anti-idiotype antibody mimicking human Her-2/neu. Breast Cancer Res Treat. 2007;104:1–11. doi: 10.1007/s10549-006-9391-9. [DOI] [PubMed] [Google Scholar]
- 29.Naz RK, Dabir P. Peptide vaccines against cancer, infectious diseases, and conception. Front Biosci. 2007;12:1833–1844. doi: 10.2741/2191. [DOI] [PubMed] [Google Scholar]
- 30.Soliman H. Developing an effective breast cancer vaccine. Cancer Control. 2010;17:183–190. doi: 10.1177/107327481001700307. [DOI] [PubMed] [Google Scholar]
- 31.Pejawar-Gaddy S, Rajawat Y, Hilioti Z, Xue J, Gaddy DF, Finn OJ, et al. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immunother. 2010;59:1685–1696. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87:1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, et al. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci U S A. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo D, Ni B, Li P, Shi W, Zhang S, Han Y, et al. Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucella abortus in BALB/c mice. Infect Immun. 2006;74:2734–2741. doi: 10.1128/IAI.74.5.2734-2741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piechocki MP, Ho YS, Pilon S, Wei WZ. Human ErbB-2 (Her-2) transgenic mice: a model system for testing Her-2 based vaccines. J Immunol. 2003;171:5787–5794. doi: 10.4049/jimmunol.171.11.5787. [DOI] [PubMed] [Google Scholar]


