Abstract
Klotho (KL) encodes a single-pass transmembrane protein and is predominantly expressed in the kidney, parathyroid glands, and choroid plexus. Genetic studies on the KL gene have revealed that DNA hypermethylation is one of the major risk factors for aging, diseases, and cancer. Besides, KL exerts anti-inflammatory and anti-tumor effects by regulating signaling pathways and the expression of target genes. KL participates in modulation of the insulin/insulin-like growth factor-1 (IGF-1) signaling, which induces the growth hormone (GH) secretion. Accordingly, KL mutant mice display multiple aging-like phenotypes, which are ameliorated by overexpression of KL. Therefore, KL is an important contributor to lifespan. KL is further identified as a regulator of calcium (Ca2+) channel-dependent cell physiological processes. KL has been also shown to induce cancer cell apoptosis, thus, it is considered as a potential tumor suppressor. Our recent studies have indicated that KL modulates an influx of Ca2+ from the extracellular space, leading to a change in CCL21-dependent migration in dendritic cells (DCs). Interestingly, the regulation of the expression of KL was mediated through a phosphoinositide 3-kinase (PI3K) pathway in DCs. Moreover, downregulating of KL expression by using siRNA knockdown technique, we observed that the expression of Ca2+ channels including Orai3, but not Orai1, Orai2, TRPV5 and TRPV6 was significantly reduced in KL-silenced as compared to control BMDCs. Clearly, additional research is required to define the role of KL in the regulation of organismic and cellular functions through the PI3K signaling and the expression of the Ca2+ channels.
Keywords: Aging, Calcium channel, Cancer, Dendritic cells, Klotho
Introduction
Klotho (KL) gene is a five-exon gene located at chromosome 13q12 in humans, encodes a single-pass transmembrane protein with 86% identity with the mouse counterpart and elicits anti-aging, anti-inflammatory, and anti-tumor effects by regulating signaling pathways and the expression of target genes. This protein is most abundant in the kidney, parathyroid glands, and choroid plexus and to a lesser extent in the brain, placenta, ovary, prostate gland, and the small intestine (1). KL exists in at least two forms including a membrane form and a soluble secreted form. These two forms of KL have distinct functions in the regulation of various physiological and pathophysiological processes. Membrane KL forms a complex with the fibroblast growth factor 23 (FGF-23) to regulate renal phosphate excretion (2). The extracellular portion rather than the potential alternative splicing of KL gene is cleaved into a soluble form, which is released into the circulation and tissue fluids (3) and acts as a circulating hormone. The secreted KL protein has a putative sialidase activity (3) that removes terminal sialic acids from N-linked glycans.
Thus, KL modulates the activities of multiple glycoproteins on the cell surface including ion channels (1), the insulin-like growth factor 1 (IGF-1)/insulin and Wnt (4). In humans, the secreted form of KL is predominantly expressed rather than the membrane form (2).
The present brief review lists the pathophysiological and genetic features of KL in defending against aging, diseases, and cancer and mechanisms involved in the regulation of cell physiological processes.
Importantly, our preliminary investigation in mouse dendritic cells (DCs) indicated that the presence of KL contributed to the expression level of a Ca2+ channel, Orai3.
Review
Regulation of physiological functions by KL
KL functions as a humoral factor to modulate the transcription of multiple genes involved in the regulation of multiple physiological processes such as maturation/differentiation, proliferation, phagocytosis, migration, lifespan and survival, all mediated through the regulation by intracellular signaling pathways. Firstly, KL attenuates the activation of insulin/insulin-like growth factor-1 (IGF-1) signaling to enhance the secretion of growth hormone (GH), a peptide hormone correlated to the stimulation of growth, cell reproduction, and regeneration in humans and animals (5, 6). Accordingly, downregulation of KL expression leads to reduced GH secretion (5) and KL mutant mice exhibit multiple aging-like phenotypes, suffer from severe growth retardation, and subsequently have a shortened lifespan and die within less than 5 months (7). In contrast, mice overexpressing KL have an increased lifespan (5). All effects have been shown to be mediated through activation of the IGF-1/GH axis (6).
Next, the effects of KL on cell maturation and differentiation have been revealed as KL contributes as a regulator of inflammatory cytokine release in β-cell (8) and B cells (9). Thus, KL could be considered as an anti-inflammatory factor in immune response. Other studies on neuropathology have shown that in oligodendrocytes, the myelinating cells of the central nervous system (CNS), KL participates in enhancing oligodendrocyte differentiation and myelination via the regulation of phosphoinositide 3-kinase (PI3K)/Akt and extracellular signal-regulated kinases (ERK) signaling pathways (10). In contrast, KL regulates negatively activation of the Wnt pathway as KL functions as a secreted Wnt antagonist to delay the development of myelinating oligodendrocytes (11). Therefore, mice lacking KL exhibit severe cognitive impairment (7).
In addition to its effects on cell maturation and differentiation, KL is further identified as a protective factor for the apoptotic cell death through production of ROS, endoplasmic reticulum stress, and oxidative stress (12, 13). Studies on human cells have shown that KL protects cells from the damage of oxidative stress (12) and/or mediated via pro-apoptotic protein p53 activity (14). Besides, investigations in KL mutant mice have indicated that ROS production and oxidative stress in KL-deficient hepatocytes and caspase activity in KL-deficient endothelial cells all are enhanced through activation of mitogen-activated protein kinases (MAPK) p38 pathway (13).
Furthermore, KL also participates in regulating other physiological functions such as the phagocytosis in retinal pigment epithelial cells (15) and the migration in human dermal microvascular endothelial cells (16). It is similar to our recent research in DCs, the most potent professional antigen-presenting cells for naive T cells (17) that DC migration is lacking following KL downregulation (18) and the antigen uptake of DCs was sensitive to the presence of KL (4).
Regulation of Ca2+ channels by KL
Recently, KL has been shown to participate in regulating intra- and extra-cellular calcium (Ca2+) levels (18) as well as renal Ca2+ handling (19). The Ca2+ channels are plasma membrane proteins containing calcium-selective pores, whose opening is dependent on membrane voltage (1). Investigations in mice lacking KL have shown the diminished effect of renal calcium reabsorption by KL, resulting in severe hypercalciuria (19), since renal Ca2+ excretion is crucial for total body calcium homeostasis. These mice exhibit inflammation-related aging-like phenotypes due to the excessive level of serum 1-a, 25-dihydroxyvitamin D3 (1,25(OH)2D3) resulting from a defect in calcium homeostasis, which contributes to immunosuppression in these knockout mice.
Next, cell studies have revealed that an increase in Ca2+ influx into cells results from their upstream signal transductions such as the PI3K signaling (20) leading to the transcriptions of specific target genes correlated with cell biological functions (1). Activation of Ca2+ entry pathway promotes the release of inflammatory cytokines in several immune cells (20, 21) and cell migration to draining lymph nodes (LNs) (18, 20). Importantly, our research in DCs indicated the association among the expression of KL, Ca2+ influx, and PI3K/Akt signaling pathway. Accordingly, the pharmacological inhibition of the PI3K pathway with LY294002 enhances the level of KL protein (4) and the increase in Ca2+ entry is abolished when KL expression is absent (18).
Furthermore, KL has been known to regulate a Ca2+ release-activated Ca2+ (CRAC) channel, the major plasma membrane Ca2+ channel participating in the modulation of Ca2+ influx pathway to replenish internal calcium stores in several cell types including platelets (22), DCs (18), and cancer cells (23). The CRAC channels are composed of Orai proteins Orai1, Orai2, and Orai3, which are identified as the pore-forming subunits of the CRAC channels (22). Several recent reports revealed that the inhibitory effect of KL on Orai1 expression is dependent on serum 1,25(OH)2D3 concentration (22, 23).
In addition to the CRAC channels, the transient receptor potential calcium (TRPV)5 and TRPV6 channels contributing as the gatekeepers of Ca2+ entry in plasma membrane have been additionally identified to be modulated by KL protein (24, 25). TRPV5 induces Ca2+ reabsorption from pro-urine, while TRPV6 plays an important role in intestinal Ca2+ uptake (25). KL participate in preventing the endocytosis of TRPV5 by modifying their N-linked glycans on the cell surface (3) leading to the diminished renal Ca2+ reabsorption, which causes severe hypercalciuria in mice lacking TRPV5 (26). The effects are similar to those observed in KL-deficient mice (19). Thus, further studies are needed to identify whether or not the precise Ca2+ channels are involved in the contribution of KL to changes in cell biological functions.
Since the promoting roles of KL in modifying Ca2+ dependent cellular functions have been revealed in several studies (18, 22, 23). We performed experiments on mouse bone marrow-derived DCs (BMDCs) to determine the contribution of KL on the expression of various Ca2+ channel proteins. As a result of preliminary findings, we indicated that the mRNA level of Orai3 was significantly reduced in KL-silenced as compared with control BMDCs, whereas mRNA levels of other Ca2+ channels including Orai1, Orai2, TRPV5, and TRPV6 were unaltered with KL silencing (Figure 1). The evidence suggested that KL participated in the modulation of the Orai3 expression in BMDCs.
Figure 1.
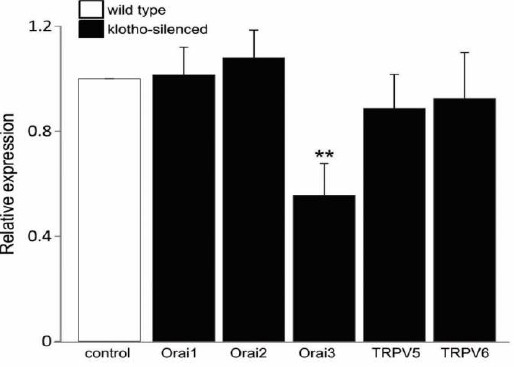
RT-PCR analysis of Orai1, Orai2, Orai3, TRPV5, and TRPV6 in control and KL-silenced BMDCs. Arithmetic means ± SEM (n = 5-7) of levels of Orai1, Orai2, Orai3, TRPV5, and TRPV6 transcripts in control (white bar) and KL-silenced (black bar) BMDCs. ** (P<0.01) represents significant difference from control BMDCs (ANOVA)
Factors affecting the regulation of KL expression
Genetic observations on this gene have shown that in cancer cells (27, 28) and in uremic toxin-injected mice (29), the downregulation of KL expression is caused by the DNA hypermethylation. The level of KL has been shown to decline with age due to the enhanced level of Ca2+ in sera of healthy volunteers and in animals (5, 30). Besides, internal and external environmental factors such as troglitazone (31), testosterone (32), and estrogen (33) also affect the expression level of KL.
Little is known about how the precise molecular mechanisms involved in the regulation of KL transcription. Its expression may be modulated by activation of the GH/IGF-1/PI3K pathway (4, 5) and/or an androgen receptor-mediated pathway as reported by Hsu et al (32).
Our recent observations in DCs have shown that serum deprivation and pharmacological inhibition of PI3K/Akt signaling resulted in increased KL mRNA level (4) and the regulatory role of DC maturation and differentiation was mediated through the presence of the KL gene (4, 34).
The evidence suggests that the regulation of cellular physiological functions by activation of the PI3K signaling may be correlated with the contribution of the KL protein in DCs.
Effects of KL on human pathologies and cancer
Since KL is identified as an anti-inflammatory and anti-apoptotic protein and KL plays a crucial role in the pathogenesis of many organs such as oxidative stress, inflammation, and tissue injury (35). Thereby, downregulation of KL expression results in various diseases including chronic kidney disease (CKD) (36), cardiovascular disease (CVD), osteopenia, hematopoietic development (37), rheumatoid arthritis (38), and Alzheimer’s disease (39). The renal KL gene expression is even decreased at a very early stage in patients with CKD (40). The higher level of KL contributes to lower risk of CVD (41) and inhibits the renin-angiotensin system in patients with type II diabetes mellitus with albuminuria (42). However, the excessive level of KL is a risk factor for several severe diseases including active acromegaly that is characterized by the excessive GH concentration due to the disturbed activation of the IGF-1/GH axis (43).
KL is also identified as an anti-tumor/cancer protein. Cancer progression is caused by genetic alterations or by defects in the mechanisms underlying cellular physiological processes such as proliferation, migration, invasion, and cell death. Genetic alteration in KL gene is considered as one of the major causes leading to the pathogenesis of several cancers such as cervical carcinoma (27), hepatocellular carcinoma (28), gastric (44), breast (45), and colorectal (46) cancers. Besides, KL exerts also the different physiological effects among tumors. KL acts as a circulating hormone to inhibit the cell growth, migration, and proliferation of cancer cells through the modulation of tumor-related signal transduction pathways, such as IGF-1/insulin, Wnt/β-catenin, and PI3K/Akt signaling pathways (28, 47, 48). The inhibitory effects of tumor cell migration in immune evasion, which in turn leads to the decreased aggressiveness of the tumor in several cancers such as hepatocellular carcinoma, cervical, and lung cancers all are regulated by KL protein (28, 49) since cancer is a disease of uncontrolled cell migration leading to cancer metastasis.
In contrast to the protective role of apoptotic cell death in normal cells, KL contributes as an inducer of apoptosis of cancer cells, since the signaling of suicidal death is different between normal and cancer cells, as cancer cells grow and divide in an uncontrolled manner whereas normal cells undergo apoptosis as a normal physiologic phenomenon. The promoting role of cancer cell death by KL is via the regulation by death receptor-triggered apoptotic pathways and the molecular mechanisms underlying the regulation of this process have been studied extensively. Accordingly, the expression of KL attenuates the enhanced activation of the IGF-1/insulin pathway (50) and subsequently reduces cell proliferation in several cancer types including epithelial ovarian cancer cells (51) and breast cancer MCF-7 cells (47). KL also exerts the pro-apoptotic effect in HEK293, L6, and HepG2 cells via the regulation of other signaling pathways such as Wnt, rather than insulin signaling (52). Therefore, the regulation of abnormal activation of Wnt/β-catenin signaling by KL results in the development of a variety of cancers (48), including hepatocellular carcinoma, colorectal carcinoma, lung cancer, malignant breast tumors, ovarian cancer, endometrial cancer, and esophageal cancer (53). In addition to the extrinsic pathway of apoptosis, the pro-apoptotic effects of KL on cancer cells is identified additionally through the regulation of mitochondrial-mediated intrinsic pathways involved in the expression of Bcl-2 family members in several cancer cells such as lung cancer (54). Accumulating evidence has suggested that KL may be a potential therapeutic target for the treatment of different diseases and cancers.
Genetic alteration in the KL gene and human diseases
A number of studies have shown that human aging and multiple diseases are caused by genetic variations in the single KL gene. Firstly, the alterations in this gene such as promoter DNA hypermethylation is identified as a major cause leading to down-regulation of KL expression, which results in the pathogenesis of several cancers such as cervical carcinoma (27), hepatocellular carcinoma (28), gastric (44), breast (45), and colorectal (46) cancers.
Secondly, a common functional mutation of KL (KL-VS) including two amino acid substitutions of the KL-VS (F325V and C370S) has been detected in various diseases. The mutation alters the protein function and influences the trafficking and catalytic activity of KL. Therefore, the heterozygous KL-VS carriers, who have an increased risk of breast and ovarian cancer (55), however, facilitate survival beyond the age of 75 (56). Differently, people exhibiting homozygous genotype of KL-VS are sensitive to cardiovascular disorder, including low high-density lipoprotein cholesterol, high systolic blood pressure, early-onset coronary artery disease (CAD) and CKD (57).
Next, other single nucleotide polymorphisms (SNPs) of KL gene including G395A in the promoter region and C1818T in exon 4 of KL gene have been also shown to contribute to increased risks of multiple diseases including cardiovascular disease, priapism, nephron- pathy, osteoarthritis, diabetes, and increased bone mineral density (49, 58). The effects of these SNPs in KL gene differ from one disease to another. Most recently, a study reported that the G395A, but not the C1818T polymorphism in the KL gene increases the risk of colorectal cancer (59), whereas the G395A SNP is a protective genetic factor for essential hypertension in a Chinese population (60). An investigation in Korean population indicated that G395A and C1818T variants but not KL-VS variants in the KL gene are associated with CAD in an age-dependent manner (61).
Furthermore, other SNPs in KL gene have been additionally determined to be sensitive to different diseases. Recent studies in American patients showed that both rs211234 and rs2249358 SNPs are associated with an increased risk of priapism and both rs516306 and rs685417 SNPs are linked to leg ulcers (62). Another study in peripheral blood leukocytes indicated that the associations between the rs211234 to priapism and the rs516306 to leg ulcers are not found in Brazilian patients (63). An investigation on a cohort of hemodialyzed patients showed that the CC genotype of a specific intronic KL variant (rs577912) is linked to higher risk of mortality as compared with the AA/AC genotypes (64).
Conclusion
In several cell types including platelets and DCs, KL participates in modulating the influx of Ca2+ from the extracellular space mediated through the regulation of the upstream molecular signaling pathways such as the PI3K. Thereby, the alteration of KL expression affects the cell biological functions associated with cell maturation/differentiation, proliferation, and apoptosis. Since, KL is identified as an anti-aging, anti-inflammatory, and anti-tumor protein, thus, it plays an important role in protecting against aging- and inflammation-related diseases and cancers. In this study, we performed the experiments in BMDCs to determine whether the precise signaling involved in the modulation of KL expression and the contribution of KL to the expression of the Ca2+ channels. We initially observed that the expression of Orai3 but not Orai1, Orai2, TRPV5, and TRPV6 channels was affected by the absence of KL in mouse DCs. Differently, another study indicated that KL promotes platelet activation through the expression of Orai1. Therefore, the regulatory role of KL mediated through the Ca2+ channels varies between different cell types. Further studies on the regulation of cellular and organismic biological functions by KL and mechanisms involved in the PI3K and/or Ca2+ signaling pathways are needed to determine whether KL contributes to increased success in the treatment of various cancers and other diseases.
Acknowledgment
This research was funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-YS.06-2013.21.
Conflict of interest
The authors of this paper declare that they have no financial/commercial conflicts of interest.
References
- 1.Sopjani M, Dermaku-Sopjani M. Klotho-dependent cellular transport regulation. Vitam Horm. 2016;101:59–84. doi: 10.1016/bs.vh.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xuan NT, Hoang NH, Nhung VP, Duong NT, Ha NH, Hai NV. Regulation of dendritic cell function by insulin/IGF-1/PI3K/Akt signaling through klotho expression. J Recept Signal Transduct Res. 2016;37:1–7. doi: 10.1080/10799893.2016.1247862. [DOI] [PubMed] [Google Scholar]
- 5.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinek T, Modan-Moses D. Klotho and the growth hormone/insulin-like growth factor 1 axis: novel insights into complex interactions. Vitam Horm. 2016;101:85–118. doi: 10.1016/bs.vh.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Zhang Q. Periodontitis aggravated pancreatic beta-cell dysfunction in diabetic mice through interleukin-12 regulation on Klotho. J Diabetes Investig. 2016;7:303–311. doi: 10.1111/jdi.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada S, Yoshida T, Hong Z, Ishii G, Hatano M, Kuro OM, et al. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int Immunol. 2000;12:861–871. doi: 10.1093/intimm/12.6.861. [DOI] [PubMed] [Google Scholar]
- 10.Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, et al. The antiaging protein klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci. 2013;33:1927–1939. doi: 10.1523/JNEUROSCI.2080-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 12.Carracedo J, Buendia P, Merino A, Madueno JA, Peralbo E, Ortiz A, et al. Klotho modulates the stress response in human senescent endothelial cells. Mech Ageing Dev. 2012;133:647–654. doi: 10.1016/j.mad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11:410–417. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Kokkinaki M, Abu-Asab M, Gunawardena N, Ahern G, Javidnia M, Young J, et al. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci. 2013;33:16346–1659. doi: 10.1523/JNEUROSCI.0402-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markiewicz M, Panneerselvam K, Marks N. Role of Klotho in migration and proliferation of human dermal microvascular endothelial cells. Microvasc Res. 2016;107:76–82. doi: 10.1016/j.mvr.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CY, Zhang ZH, Yang HF, Xu H, Cheng FF, Xu JZ. Effect of vitamin D3 on maturation and antigen-presenting function of dendritic cells treated with Mycobacterium tuberculosis. Asian Pac J Trop Med. 2016;9:54–57. doi: 10.1016/j.apjtm.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Shumilina E, Nurbaeva MK, Yang W, Schmid E, Szteyn K, Russo A, et al. Altered regulation of cytosolic Ca(2)(+) concentration in dendritic cells from klotho hypomorphic mice. Am J Physiol Cell Physiol. 2013;305:C70–77. doi: 10.1152/ajpcell.00355.2012. [DOI] [PubMed] [Google Scholar]
- 19.Leibrock CB, Voelkl J, Kuro OM, Lang F, Lang UE. 1,25(OH)2D3 dependent overt hyperactivity phenotype in klotho-hypomorphic mice. Sci Rep. 2016;6:24879. doi: 10.1038/srep24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzner N, Zemtsova IM, Nguyen TX, Duszenko M, Shumilina E, Lang F. Ion channels modulating mouse dendritic cell functions. J Immunol. 2008;181:6803–6809. doi: 10.4049/jimmunol.181.10.6803. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Yang W, Li J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-kappaB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-alpha production in lipopolysaccharide-stimulated rat peritoneal macrophages. J Biol Chem. 2006;281:31337–31347. doi: 10.1074/jbc.M602739200. [DOI] [PubMed] [Google Scholar]
- 22.Borst O, Munzer P, Schmid E, Schmidt EM, Russo A, Walker B, et al. 1,25(OH)2 vitamin D3-dependent inhibition of platelet Ca2+signaling and thrombus formation in klotho-deficient mice. FASEB J. 2014;28:2108–2119. doi: 10.1096/fj.13-239277. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Yan J, Umbach AT, Fakhri H, Fajol A, Schmidt S, et al. NFkappaB-sensitive Orai1 expression in the regulation of FGF23 release. J Mol Med (Berl) 2016;94:557–566. doi: 10.1007/s00109-015-1370-3. [DOI] [PubMed] [Google Scholar]
- 24.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeber JP, Hoenderop JG, Bindels RJ. Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochem Soc Trans. 2007;35:115–119. doi: 10.1042/BST0350115. [DOI] [PubMed] [Google Scholar]
- 26.Topala CN, Bindels RJ, Hoenderop JG. Regulation of the epithelial calcium channel TRPV5 by extracellular factors. Curr Opin Nephrol Hypertens. 2007;16:319–324. doi: 10.1097/MNH.0b013e3281c55f02. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Jeong DJ, Kim J, Lee S, Park JH, Chang B, et al. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu G, Xie B, Ren F, Liu DC, Zhou J, Li Q, et al. Restoration of klotho expression induces apoptosis and autophagy in hepatocellular carcinoma cells. Cell Oncol (Dordr) 2013;36:121–129. doi: 10.1007/s13402-012-0118-0. [DOI] [PubMed] [Google Scholar]
- 29.Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81:640–650. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagishi T, Saito Y, Nakamura T, Takeda S, Kanai H, Sumino H, et al. Troglitazone improves endothelial function and augments renal klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with multiple atherogenic risk factors. Hypertens Res. 2001;24:705–709. doi: 10.1291/hypres.24.705. [DOI] [PubMed] [Google Scholar]
- 32.Hsu SC, Huang SM, Lin SH, Ka SM, Chen A, Shih MF, et al. Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem J. 2014;464:221–229. doi: 10.1042/BJ20140739. [DOI] [PubMed] [Google Scholar]
- 33.Oz OK, Hajibeigi A, Howard K, Cummins CL, van Abel M, Bindels RJ, et al. Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice *. J Bone Miner Res. 2007;22:1893–1902. doi: 10.1359/jbmr.070808. [DOI] [PubMed] [Google Scholar]
- 34.Xuan NT, Trang PT, Van Phong N, Toan NL, Trung DM, Bac ND, et al. Klotho sensitive regulation of dendritic cell functions by vitamin E. Biol Res. 2016;49:45. doi: 10.1186/s40659-016-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai T, Yamada K, Kim HC, Noda Y, Nabeshima Y, Nabeshima T. [Cognition impairment in the klotho gene mutant mice and oxidative stress] Nihon Shinkei Seishin Yakurigaku Zasshi. 2003;23:211–217. [PubMed] [Google Scholar]
- 36.Kawamura Y, Matsuo H, Chiba T, Nagamori S, Nakayama A, Inoue H, et al. Pathogenic GLUT9 mutations causing renal hypouricemia type 2 (RHUC2) Nucleosides Nucleotides Nucleic Acids. 2011;30:1105–1111. doi: 10.1080/15257770.2011.623685. [DOI] [PubMed] [Google Scholar]
- 37.Vadakke Madathil S, Coe LM, Casu C, Sitara D. Klotho deficiency disrupts hematopoietic stem cell development and erythropoiesis. Am J Pathol. 2014;184:827–841. doi: 10.1016/j.ajpath.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinour D, Bahn A, Ganon L, Ron R, Geifman-Holtzman O, Knecht A, et al. URAT1 mutations cause renal hypouricemia type 1 in Iraqi Jews. Nephrol Dial Transplant. 2011;26:2175–2181. doi: 10.1093/ndt/gfq722. [DOI] [PubMed] [Google Scholar]
- 39.Doege H, Bocianski A, Joost HG, Schurmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 2000;350(3):771–776. [PMC free article] [PubMed] [Google Scholar]
- 40.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 41.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol. 2013;8:1899–1905. doi: 10.2215/CJN.02700313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmid C, Neidert MC, Tschopp O, Sze L, Bernays RL. Growth hormone and Klotho. J Endocrinol. 2013;219:R37–57. doi: 10.1530/JOE-13-0285. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Wang X, Jie P, Lu H, Zhang S, Lin X, et al. Klotho is silenced through promoter hypermethylation in gastric cancer. Am J Cancer Res. 2011;1:111–119. [PMC free article] [PubMed] [Google Scholar]
- 45.Rubinek T, Shulman M, Israeli S, Bose S, Avraham A, Zundelevich A, et al. Epigenetic silencing of the tumor suppressor klotho in human breast cancer. Breast Cancer Res Treat. 2012;133:649–657. doi: 10.1007/s10549-011-1824-4. [DOI] [PubMed] [Google Scholar]
- 46.Pan J, Zhong J, Gan LH, Chen SJ, Jin HC, Wang X, et al. Klotho, an anti-senescence related gene, is frequently inactivated through promoter hypermethylation in colorectal cancer. Tumour Biol. 2011;32:729–735. doi: 10.1007/s13277-011-0174-5. [DOI] [PubMed] [Google Scholar]
- 47.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimoyama Y, Taki K, Mitsuda Y, Tsuruta Y, Hamajima N, Niwa T. KLOTHO gene polymorphisms G-395A and C1818T are associated with low-density lipoprotein cholesterol and uric acid in Japanese hemodialysis patients. Am J Nephrol. 2009;30:383–388. doi: 10.1159/000235686. [DOI] [PubMed] [Google Scholar]
- 50.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 51.Lojkin I, Rubinek T, Orsulic S, Schwarzmann O, Karlan BY, Bose S, et al. Reduced expression and growth inhibitory activity of the aging suppressor klotho in epithelial ovarian cancer. Cancer Lett. 2015;362:149–157. doi: 10.1016/j.canlet.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 52.Lorenzi O, Veyrat-Durebex C, Wollheim CB, Villemin P, Rohner-Jeanrenaud F, Zanchi A, et al. Evidence against a direct role of klotho in insulin resistance. Pflugers Arch. 2010;459:465–473. doi: 10.1007/s00424-009-0735-2. [DOI] [PubMed] [Google Scholar]
- 53.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 54.Chen B, Wang X, Zhao W, Wu J. Klotho inhibits growth and promotes apoptosis in human lung cancer cell line A549. J Exp Clin Cancer Res. 2010;29:99. doi: 10.1186/1756-9966-29-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf I, Laitman Y, Rubinek T, Abramovitz L, Novikov I, Beeri R, et al. Functional variant of KLOTHO: a breast cancer risk modifier among BRCA1 mutation carriers of Ashkenazi origin. Oncogene. 2010;29:26–33. doi: 10.1038/onc.2009.301. [DOI] [PubMed] [Google Scholar]
- 56.Arking DE, Krebsova A, Macek M, Sr, Macek M, Jr, Arking A, Mian IS, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 58.Rhee EJ, Oh KW, Yun EJ, Jung CH, Lee WY, Kim SW, et al. Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the KLOTHO gene with glucose metabolism and cardiovascular risk factors in Korean women. J Endocrinol Invest. 2006;29:613–618. doi: 10.1007/BF03344160. [DOI] [PubMed] [Google Scholar]
- 59.Liu C, Cui W, Wang L, Yan L, Ruan X, Liu Y, et al. Klotho gene polymorphisms are related to colorectal cancer susceptibility. Int J Clin Exp Pathol. 2015;8:7446–7449. [PMC free article] [PubMed] [Google Scholar]
- 60.Wang HL, Xu Q, Wang Z, Zhang YH, Si LY, Li XJ, et al. A potential regulatory single nucleotide polymorphism in the promoter of the Klotho gene may be associated with essential hypertension in the Chinese Han population. Clin Chim Acta. 2010;411:386–390. doi: 10.1016/j.cca.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Rhee EJ, Oh KW, Lee WY, Kim SY, Jung CH, Kim BJ, et al. The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism. 2006;55:1344–1351. doi: 10.1016/j.metabol.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Nolan VG, Baldwin C, Ma Q, Wyszynski DF, Amirault Y, Farrell JJ, et al. Association of single nucleotide polymorphisms in klotho with priapism in sickle cell anaemia. Br J Haematol. 2005;128:266–272. doi: 10.1111/j.1365-2141.2004.05295.x. [DOI] [PubMed] [Google Scholar]
- 63.Lustosa Souza CR, Azevedo Shimmoto MM, Vicari P, Mecabo G, Arruda MM, Figueiredo MS. Klotho gene polymorphisms and their association with sickle cell disease phenotypes. Rev Bras Hematol Hemoter. 2015;37:275–276. doi: 10.1016/j.bjhh.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman DJ, Afkarian M, Tamez H, Bhan I, Isakova T, Wolf M, et al. Klotho variants and chronic hemodialysis mortality. J Bone Miner Res. 2009;24:1847–1855. doi: 10.1359/JBMR.090516. [DOI] [PMC free article] [PubMed] [Google Scholar]


