Abstract
Objective(s):
Protosappanin A (PrA) is an effective and major ingredient of Caesalpinia sappan L. The current study was aimed to explore the effect of PrA on atherosclerosis (AS).
Materials and Methods:
Firstly, the experimental model of AS was established in rabbits by two-month feeding of high fat diet. Then, the rabbits were randomly divided into five groups and treated with continuous high lipid diet (model control), high lipid diet containing rosuvastatin (positive control), 5 mg/kg PrA (low dose) or 25 mg/kg PrA (high dose).
Results:
Our results showed that PrA markedly alleviated AS as indicated by hematoxylin/eosin (HE) staining. PrA also reduced hyperlipidemia (as demonstrated by the serum levels of total blood cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL)) in a time and dose-dependent manner, and decreased inflammation (as indicated by the serum levels of matrix metalloproteinase-9 [MMP-9], interleukin-6 [IL-6] and tumor necrosis factor-α [TNF-α]). Moreover, PrA significantly inactivated nuclear factor kappa B (NF-κB) signaling as indicated by nuclear NF-κB p65 protein expression, as well as the mRNA expression and serum levels of downstream genes, interferon-γ (IFN-γ) and interferon-gamma-inducible protein 10 (IP10).
Conclusion:
This study proved that PrA might protect against atherosclerosis via anti-hyperlipidemia, anti-inflammation and NF-κB signaling pathways in hyperlipidemic rabbits.
Keywords: Anti-hyperlipidemic, Anti-inflammatory, Atherosclerosis, NF-Κb, Protosappanin A
Introduction
Chinese herb Caesalpinia sappan L., as a member of the leguminous plant family, exhibits therapeutic potential for burning sensations, leprosy, skin diseases, dysentery and diabetic complications in China and other Asian countries (1-4). Protosappanin A (PrA), is an important active ingredient isolated and identified from Caesalpinia sappan L. (1). PrA, as an immunosuppressive ingredient, plays an important role in prolonging graft heart survival (5). Moreover, it has been proved that PrA suppresses CD4+/CD8+ ratio of peripheral T cell, and inhibits expression of perforin and granzyme B (secreted by T cells to kill donor cells) (6).
Atherosclerosis (AS) mainly occurring in the large or medium arteries has been called the first killer of human health because of the significant increasing morbidity and mortality (7). AS is also known as an important risk factor for ischemic cardiac-cerebral vascular diseases (8). AS animal models have been established through various strategies, including high-fat-diet method, intimae-injury method, immunestimulation method, etc (9-11). The causes and mechanisms of AS have not been fully understood. Hyperlipidemia, which is characterized by the increased total blood cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and decreased high-density lipoprotein (HDL) (12), is a well-known cause for AS (13). Moreover, inflammation plays a vital role in the occurrence, development and complications of hyperlipidemia and AS (14). Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are key cytokines that mediates effector pathways in atherosclerotic vessels (15-18). Matrix metalloproteinase-9 (MMP-9) is critical for the formation and rupture of the unstable AS plaques (19-21). As an ubiquitous transcription factor, nuclear factor kappa B (NF-κB) has been implicated in survival of transplantation, lymphocyte activation and immunological processes (22). Activated NF-κB is observed in atheromatous areas of the AS lesion, indicating the involvement of NF-κB in the pathogenesis of AS (23). Interferon-γ (IFN-γ) and IFN-γ-inducible protein 10 (IP10), downstream effectors of NF-κB signaling (24), may promote the development of AS (18, 25). In this current study, we established AS rabbit models by high-fat-diet method and then PrA-administration experiments were performed. We proved that PrA significantly alleviated the occurrence of AS and hyperlipidemia, and decreased levels of several inflammatory cytokines. Notably, PrA significantly decreased NF-κB signaling. Taken together, PrA might protect against AS via anti-hyperlipidemia, anti-inflammation and through NF-κB signaling pathways in hyperlipidemic rabbits. The study suggested that PrA might be used for the treatment of AS.
Materials and Methods
Grouping of animals
Sixty healthy male New Zealand rabbits (2.5±0.4 kg) were obtained from Shanghai SLRC Experimental Animal Co LTD (Shanghai, China) and were housed in a room under controlled temperature (21±1°C) with free access to water. All the rabbits were fed with high fat diet containing 1% high cholesterol for 60 days (10), and the food intake was limited to 150 g/d. Rabbits were then randomly divided into five groups with each group containing 12 rabbits: Group 1 was healthy control and offered continued normal diet (Mock); Group 2 was model control and offered continued high fat diet; Group 3 was the positive control and fed with high fat diet containing 0.5 mg/kg rosuvastatin (26) once every day; Group 4 (PrA low dose) and Group 5 (PrA high dose) were fed with high fat diet containing 5 mg/kg PrA and 25 mg/kg PrA (5), also once every day, respectively. The serum levels of total blood cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were measured at 28 days and 42 days after treatment. After 42 days, the following assays were performed.
Hematoxylin/eosin (HE) staining of the large or medium arteries
Tissue blocks of large or medium arteries were resected, fixed in 10% formalin for 48 hr, embedded in paraffin, sectioned and stained with hematoxylin/eosin (HE). The degree of AS was analyzed under a light microscope (magnification, ×100).
Measurement of TC, TG, LDL, HDL and inflammatory cytokines in serum
At 28 days and 42 days after the rabbits were treated with the corresponding drugs, blood was collected from the ear vein after overnight fasting. The serum levels of TC and TG were detected by enzyme method using TC and TG Detection Kit (JRDun Biotechnology, Co, Ltd), respectively. The serum levels of LDL and HDL were detected by selective precipitation method using LDLC and HDLC Kit (JRDun Bio), respectively. The serum concentrations of MMP-9, IL-6, TNF-α, IFN-γ and IP10 were measured by Enzyme linked immunosorbent assay (ELISA) with the kits purchased from JRDun.
Quantitative real-time PCR (qRT-PCR) analysis
The mRNA levels of IFN-γ and IP10 from the arteries were quantified by qRT-PCR. Total RNA was isolated from arteries tissues using Trizol Reagent (Invitrogen, Japan). cDNA was synthesized from 5 μg of toal RNA using AMV reverse transcriptase (Fermentas, USA). qRT-PCR reactions were performed in a 25 μl total volume and on Roche Light Cycler® 480II System (Roche Diagnostics Ltd., Switzerland) using SYBR® Green 10 × Supermix (Thermo, #K0223), with GAPDH as an internal control. Primer pairs for indicated genes were designed using the Primer Express Software (Applied Biosystems, Shanghai, China) and listed in Table 1. The PCR procedure was: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 sec, 60 °C for 45 sec; one cycle of 95 °C for 15 sec, 60 °C for 1 min; one cycle of 95 °C for 15 sec, 60 °C for 15 sec. All PCR reactions were performed in triplicate and the relative expression levels of different groups were calculated by normalizing to the mRNA expression level of GAPDH using 2-ΔΔCT method (27).
Table 1.
Primers used in qRT-PCR analysis of IFN-γ and IP10
| Gene | Primer sequence | Species | Amplicon (bps) |
|---|---|---|---|
| IFN-γ | Forward: 5’-GACTCTCGTTTCAACTTCTTC-3’ Reverse: 5’-CCTTTAGGTGTTCTGTTTCTC-3’ | Oryctolagus | 148 |
| IP10 | Forward: 5’-TGCCACACTTCCCTTCTTC-3’ Reverse: 5’-ACGTAGCAGCTTGGTGTAG-3’ | Oryctolagus | 172 |
| GAPDH | Forward: 5’-CTCCTGCGACTTCAACAGTG-3’ Reverse: 5’-TGAGGGCTCTTACTCCTTGG-3’ | Oryctolagus | 172 |
Western blot analysis
Fresh arteries tissues was frozen in liquid nitrogen and the nuclear extract was prepared as previously described (28). The protein concentration was quantified using BCA kit (Thermo Fisher Scientific Inc, USA). A total of 80 μg protein for each sample was loaded on SDS-PAGE gel and transferred to PVDF membranes. The blots were blocked with 5% non-fat milk and incubated overnight with different primary antibodies, which were as follows: NF-κB, Abcam, Ab32360, 1:1500 dilution; H3, Abcam, Ab4729, 1:1000 dilution. Blots were then incubated for 1 hr at 37 °C with goat anti-mouse or anti-rabbit secondary antibody (Beyotime, Shanghai, China) and Intensities were measured using enhanced chemiluminescence (ECL, Thermo Scientific, Shanghai, China). H3 was used as a loading control. Quantitation of the results was performed by Image J software.
Statistical analysis
Experiments were performed with 12 rabbits per group. Values were expressed as means±standard errors (SD). Statistical analysis was determined by ANOVA using Graphpad Prism 6.0 software. Differences were considered statistically significant at a value of P<0.05.
Results
PrA obviously alleviated high-fat-diet-induced AS
In order to identify the experimental model of AS and explore the effects of PrA on AS, HE staining was performed. In the model group, the endarterium was thicker with accumulation of multiple foam cells, the medial layer was thinner, and multiple immersed cells were observed between endarteriuma and the medial layer. This suggested that the experimental model of AS was successfully established in rabbits through high fat diet feeding (10). In the Rosuvastatin treated group, as a positive control, the degree of AS was mitigated as indicated by the thinner endarterium and decreased foam. PrA alleviated the degree of AS and high dose of PrA had better effects than low dose of PrA (Figure 1).
Figure 1.
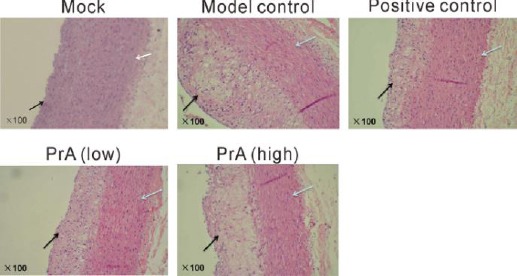
Histological analysis by hematoxylin/eosin staining. Rabbits were then randomly divided into five groups (n=12): Mock, rabbits were offered continued normal diet; Model control, rabbits were offered continued high fat diet; Positive control, rabbits were fed with high fat diet containing 0.5 mg/kg rosuvastatin once every day; PrA low, rabbits were fed with high fat diet containing 5 mg/kg PrA once every day; PrA high, rabbits were fed with high fat diet containing 25 mg/kg PrA once every day. After 42 days, large or medium arteries were collected and subjected to HE staining. The dark arrow represents end arterium, and the white arrows represents medial layer (magnification, ×100)
Effects of PrA on the serum levels of TC, TG, LDL and HDL
In order to determine the influence of PrA on hyperlipidemia, serum biochemical indices, TC, TG, LDL and HDL were detected (Figure 2). Compared to healthy control (Mock), the model group showed a significant increase in TC, TG and LDL and a notable decrease in HDL, suggesting the hyperlipidemia in model group. In Rosuvastatin group (positive control), the levels of TC, TG and LDL were decreased, and the levels of HDL were increased in a time-dependent manner. In the groups treated with PrA, the serum levels of the four indices were changed in a time and dose dependent manner (n=12). Effects of high dose PrA and rosuvastatin on these serum biochemical indices were almost equal. It suggested that PrA might alleviate
Figure 2.
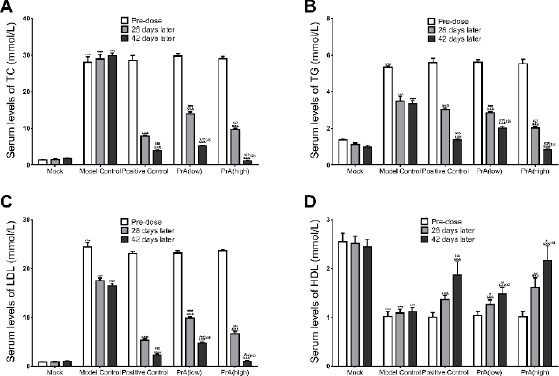
Protosappanin A markedly decreased serum levels of cholesterol (A), triglyceride (B) and low-density lipoprotein (C), and increased serum levels of high-density lipoprotein (D) in rabbits with hyperlipidemia in a time- and dose-dependent manner. n=12, mean ± SD. ***P<0.001 vs. Mock control in corresponding period; &&&P<0.001 vs. Model control in corresponding period; #P<0.05, ##P<0.01, ###P<0.001 vs. positive control (Rosuvastatin group) in corresponding period; $$P<0.05, $$$P<0.001 vs. 28 days later in the same medication group
AS by inhibiting hyperlipidemia like the positive control rosuvastatin (29).
PrA decreased the serum levels of inflammatory cytokines
In order to determine the influence of PrA on inflammatory cytokines, the serum levels of MMP-9, IL-6 and TNF-α were detected. Results showed that, compared with the healthy control (Mock), levels of all the three inflammatory cytokines were increased in the model group. Treatment with rosuvastatin and PrA for 42 days significantly decreased the levels of detected cytokines. The effects of PrA were in a dose dependent manner (n=12, P<0.001). It suggested that PrA might alleviate AS through the way of anti-inflammation by reducing the serum levels of MMP-9, IL-6 and TNF-α (Figure 3).
Figure 3.
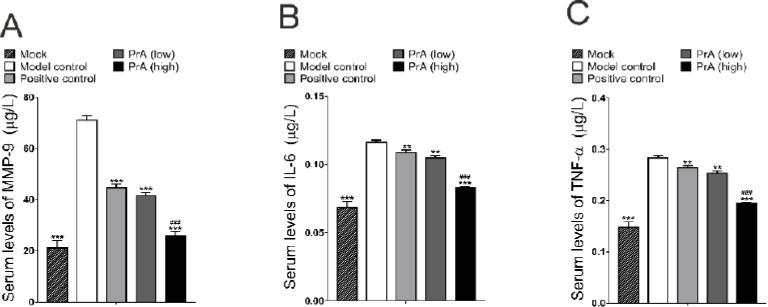
The serum levels of inflammatory cytokines were decreased by protosappanin A. Compared with the model control, levels of MMP-9 (A), IL-6 (B) and TNF-α (C) were decreased in rosuvastatin treatment groups (positive control), and were also decreased in PrA treatment groups in a dose dependent manner. n=12, mean±SD. **P<0.01, ***P<0.001 vs. model control; ###P<0.001 vs. positive control (Rosuvastatin group)
PrA inhibited the nuclear NF-κB p65 protein expression
To explore the possible molecular mechanisms how PrA alleviated AS, the nuclear NF-κB p65 protein expression was examined. Results showed that compared with healthy control (Mock), NF-κB was increased in the model group, and 42 days of treatment with PrA dose-dependently decreased NF-κB (Figure 4). The positive control rosuvastatin showed a more obvious effect than PrA. It suggested that PrA might alleviate AS by regulating NF-κB signaling pathway.
Figure 4.
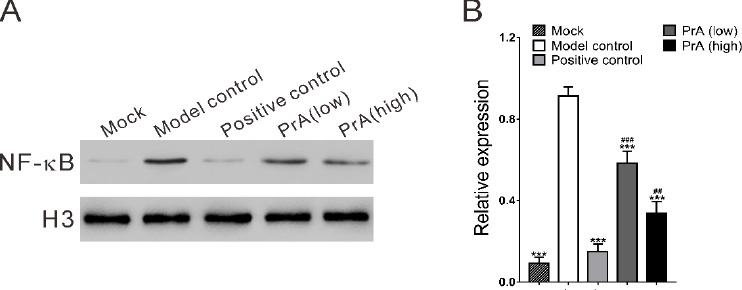
Effects of protosappanin A on the nuclear NF-κB p65 protein expression. A. The nuclear protein levels of NF-κB were down-regulated by PrA compared with the model and positive control. B. Quantitation of Western blot was performed by Image J software and shown. n=3, mean ± SD. ***P<0.001 vs. model control; #P<0.05, ###P<0.001 vs. positive control (Rosuvastatin group)
PrA suppressed the expression of IFN-γ and IP10
To get a further insight into the possible molecular mechanisms of PrA on AS, the expression levels of IFN-γ and IP10 were detected as subsequent downstream genes of NF-κB. Results showed that the expression levels of IFN-γ and IP10 were down-regulated by PrA with gene expression levels relative to the reference GAPDH (Figure 5A). Serum production of IFN-γ and IP10 was significantly decreased in PrA treatment group as indicated by ELISA assay (Figure 5B).
Figure 5.
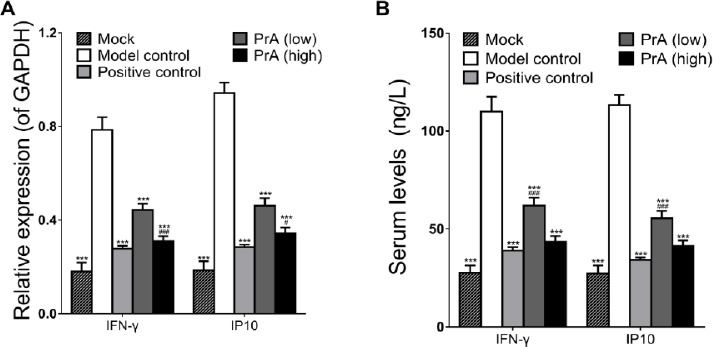
Protosappanin A suppressed the mRNA and serum levels of interferon-γ (IFN-γ) and interferon-gamma-inducible protein 10 (IP10). A. The mRNA levels of IFN-γ and IP10 were significantly decreased by PrA, compared with the model control. B. Serum production of IFN-γ and IP10 were inhibited in PrA treatment group. n=12, mean ± SD. ***P<0.001 vs. model control; #P<0.05, ###P<0.001 vs. positive control (Rosuvastatin group)
Discussion
Establishing the atherosclerosis (AS) model is the foundation of the study on measuring the effects of therapy. In this study, the AS model were successfully established in rabbits by high-fat-diet feeding according to the previous reports (10). Protosappanin A (PrA) is an active ingredient in the ethanol extract of Caesalpinia sappan L (5). The effects of PrA on AS have been not reported. In this current study, we showed that PrA inhibited high-fat-diet-induced AS. Hypertriglyceridemia were closely related with the pathologies of AS. Increase in blood lipids induces endothelial damage and lipid deposition in vascular wall, leading to the formation of plaques and AS (30). Therefore, we elucidated the effects of PrA on hyperlipidemia by measuring the serum levels of TC, TG, LDL and HDL. As expected, rabbits in model group were suffering from hyperlipidemia as compared to healthy control. Notably in treatment groups, the serum levels of TC, TG and LDL was significantly decreased in a time- and dose-dependent manner, while HDL levels were increased. Our data suggested that PrA might protect against AS through reducing hyperlipidemia (31).
In addition, studies have shown that AS is associated with the inflammation responses (32). For patients with hyperlipidemia, with the increase of blood lipid levels, the serum levels of inflammatory cytokines, such as TNF-α and IL-6 will be correspondingly increased (33-35). TNF-α and IL-6 play a critical role in fat metabolism, involving in the inflammatory response. Excess of TNF-α and IL-6 can cause direct damage to vascular endothelial cells (36, 37). Moreover, MMP-9 plays an important role in the formation and rupture of the unstable AS plaques by promoting the degradation of extracellular matrix in fiber membrane of AS plaques (19-21). Here, in PrA treatment groups, the serum levels of MMP-9, TNF-α and IL-6 was significantly decreased, indicating that PrA might protect against AS by decreasing inflamma-tion.
As Wu et al. reported, PrA can induce immunosuppression via NF-κB signaling pathway (6), we further detected nuclear NF-κB p65 protein expression. In our study, NF-κB was greatly reduced by PrA treatment. These results were in line with the study of Wu et al (6). IFN-γ and IP10, downstream genes of NF-κB signaling (24), may promote the development of AS (18, 25). Here, we indicated that mRNA expression and serum concentrations of IFN-γ and IP10 were reduced in PrA-treated rabbits. The results supported our presumption that NF-κB signaling pathway might involve in the immunosuppressive action of PrA, although it still needs to be explored further.
Conclusion
We established a rabbit model with AS. PrA alleviated AS, and markedly inhibited the serum levels of TC, TG and LDL in a time and dose-dependent manner with HDL being increased. PrA decreased the serum production of the inflammatory cytokines, MMP-9, IL-6 and TNF-α, and might exerted its functions through NF-κB signaling pathway. And it can be speculated that PrA might be a new drug during the treatment of AS.
Acknowledgment
This study was supported by Shanghai Pudong New Area Health System “Academic Leaders” Incidental issues (PWRd2012-08).
Conflict of interest
The authors declare that no conflict of interest exists.
References
- 1.Badami S, Moorkoth S, Rai SR, Kannan E, Bhojraj S. Antioxidant activity of Caesalpinia sappan heartwood. Biol Pharm Bull. 2003;26:1534–1537. doi: 10.1248/bpb.26.1534. [DOI] [PubMed] [Google Scholar]
- 2.Hikino H, Taguchi T, Fujimura H, Hiramatsu Y. Antiinflammatory principles of Caesalpinia sappan wood and of Haematoxylon campechianum wood. Planta Med. 1977 doi: 10.1055/s-0028-1097516. [DOI] [PubMed] [Google Scholar]
- 3.Kim E-C, Hwang Y-S, Lee H-J, Lee S-K, Park M-H, Jeon B-H, et al. Caesalpinia sappan induces cell death by increasing the expression of p53 and p21WAF1/CIP1 in head and neck cancer cells. Am J Chin Med. 2005;33:405–414. doi: 10.1142/S0192415X05003016. [DOI] [PubMed] [Google Scholar]
- 4.Sireeratawong S, Piyabhan P, Singhalak T, Wongkrajang Y, Temsiririrkkul R, Punsrirat J, et al. Toxicity evaluation of sappan wood extract in rats. J Med Assoc Thai. 2011;93:50. [PubMed] [Google Scholar]
- 5.Wu J, Hou J, Zhang M, Zou Y, Yu B. Transplantation proceedings. Elsevier; 2008. Protosappanin a, an immunosuppressive constituent from a Chinese herb, prolongs graft survival and attenuates acute rejection in rat heart allografts. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Zhang M, Jia H, Huang X, Zhang Q, Hou J, et al. Protosappanin A induces immunosuppression of rats heart transplantation targeting T cells in grafts via NF-κB pathway. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:83–92. doi: 10.1007/s00210-009-0461-5. [DOI] [PubMed] [Google Scholar]
- 7.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 8.Karpe F, Steiner G, Uffelman K, Olivecrona T, Hamsten A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis. 1994;106:83–97. doi: 10.1016/0021-9150(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 9.Friedman R, Moore S, Singal D. Repeated endothelial injury and induction of atherosclerosis in normolipemic rabbits by human serum. Lab Invest. 1975;32:404–415. [PubMed] [Google Scholar]
- 10.Hu M-Y, Li Y-L, Jiang C-H, Liu Z-Q, Qu S-L, Huang Y-M. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition. 2008;24:1030–1038. doi: 10.1016/j.nut.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo C-c. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E—deficient mice. J Infect Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- 12.Thirumalai T, Tamilselvan N, David E. Hypolipidemic activity of Piper betel in high fat diet induced hyperlipidemic rat. J Acute Disease. 2014;3:131–135. [Google Scholar]
- 13.ørgen Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease an eight-year follow-up in the Copenhagen male study. Circulation. 1998;97:1029–36. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- 14.Maseri A. Inflammation, atherosclerosis, and ischemic events—exploring the hidden side of the moon. N Engl J Med. 1997;336:1014–1016. doi: 10.1056/NEJM199704033361409. [DOI] [PubMed] [Google Scholar]
- 15.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6:410–417. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 16.Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, et al. IL-6, TNF-αand atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170:277–283. doi: 10.1016/s0021-9150(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 17.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 18.Klinghammer L, Urschel K, Cicha I, Lewczuk P, Raaz-Schrauder D, Achenbach S, et al. Impact of telmisartan on the inflammatory state in patients with coronary atherosclerosis–influence on IP-10, TNF-αand MCP-1. Cytokine. 2013;62:290–296. doi: 10.1016/j.cyto.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Loftus I, Naylor A, Bell P, Thompson M. Matrix metalloproteinases and atherosclerotic plaque instability. Br J Surg. 2002;89:680–694. doi: 10.1046/j.1365-2168.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 20.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 22.Cooper M, Lindholm P, Pieper G, Seibel R, Moore G, Nakanishi A, et al. Myocardial nuclear factor-κB activity and nitric oxide production in rejecting cardiac allorafts. Transplantation. 1998;66:838–844. doi: 10.1097/00007890-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Brand K, Page S, Walli AK, Neumeier D, Baeuerle PA. Role of nuclear factor-kappa B in atherogenesis. Exp Physiol. 1997;82:297–304. doi: 10.1113/expphysiol.1997.sp004025. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knockout mice. J Clin Invest. 1997;99:2752. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones SP, Gibson MF, Rimmer DM, Gibson TM, Sharp BR, Lefer DJ. Direct vascular and cardioprotective effects of rosuvastatin, a new HMG-CoA reductase inhibitor. J Am Coll Cardiol. 2002;40:1172–1178. doi: 10.1016/s0735-1097(02)02115-0. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Jessberger R, Podust V, Hubscher U, Berg P. A mammalian protein complex that repairs double-strand breaks and deletions by recombination. J Biol Chem. 1993;268:15070–15079. [PubMed] [Google Scholar]
- 29.Crouse JR, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–53. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 30.Laakso M, Sarlund H, Salonen R, Suhonen M, Pyörälä K, Salonen JT, et al. Asymptomatic atherosclerosis and insulin resistance. Arterioscler Thromb. 1991;11:1068–1076. doi: 10.1161/01.atv.11.4.1068. [DOI] [PubMed] [Google Scholar]
- 31.Soh J, Iqbal J, Queiroz J, Fernandez-Hernando C, Hussain MM. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med. 2013;19:892–900. doi: 10.1038/nm.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 33.Li S-L, Zhang Y, Lin W, Liu J, Chen S-Y, Meng S, et al. Relationship between markers of inflammaion and serum lipd levels in healthy persons and patients with pure hyperlipemia. Chin J Cardiovasc Rehabil Med. 2010;19:227. [Google Scholar]
- 34.Chen X, Xun K, Chen L, Wang Y. TNF-α, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- 35.Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41(Supplement 2):97–101. doi: 10.2337/diab.41.2.s97. [DOI] [PubMed] [Google Scholar]
- 36.Libby P, Ridker PM. Novel inflammatory markers of coronary risk theory versus practice. Circulation. 1999;100:1148–1150. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- 37.Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]


