Abstract
Objective:
Cancer has risen as the main cause of diseases with the highest rate of mortality in the world. Drugs used in cancer, usually demonstrate side effects on normal tissues. On the other hand, anticancer small peptides, effective on target tissues, should be safe on healthy organs, as being naturally originated compounds. In addition, they may have good pharmacokinetic properties. carnosine, a natural dipeptide, has shown many biological functions, including anti-oxidant, anti-senescence, anti-inflammatory and anticancer activities. This study, with the aim of introducing new anticancer agents with better properties, is focused on the synthesis and cytotoxic evaluation of some peptide analogues of carnosine.
Materials and Methods:
The cytotoxic activity of the synthesized peptides, prepared by the solid-phase peptide synthesis method, was evaluated against two cell lines of HepG2 and HT-29 using MTT assay, lactate dehydrogenase (LDH) assay and flow cytometry analysis.
Results:
Linear and cyclic analogues of carnosine peptide showed cytotoxicity, demonstrated by several experiments, against HepG2 and HT-29 cell lines with mean IC50 values ranging from 9.81 to 16.23 µg/ml. Among the peptides, compounds 1c, 3c and 6b (linear analogue of 3c) showed a considerable toxic activity on the cancerous cell lines.
Conclusion:
The cyclic peptide analogues of carnosine with His-β-Ala-Pro-β-Ala-His (1c) and β-Ala-His-Pro-His-β-Ala (3c) sequences showed cytotoxic activity on cancerous cells of HepG2 and HT-29, better than carnosine, and thus can be good candidates to develop new anticancer agents. The mechanism of cytotoxicity may be through cell apoptosis.
Keywords: Anticancer agents, Carnosine analogues, Cytotoxicity, Flow cytometry, Peptide synthesis, MTT assay
Introduction
Cancer has risen as the main cause of diseases with the highest rate of mortality in the world. It is estimated that the death toll from cancer reach to 15.5 million people in 2020 (1). Cancer treatments by conventional methods of chemotherapy result in wide-ranging cell killing without differentiating between tissues and so usually induce toxic effects on normal cells (2). Therefore, it is highly demanded to look for new therapies, which focus on treating cancerous cells with less or no toxicity on healthy tissues. Anticancer peptides, being effective on target tissues, may be safe and less harmful on healthy organs, as being naturally originated entities. In addition, small peptides with such activity have good physicochemical properties, extent tissue penetration and high solubility pattern, which make them accessible by target tissue while excrete from blood and other unwanted tissue rapidly (3). L-carnosine is a dipeptide composed of ß-alanine and L-histidine, found with high concentration in the skeletal muscles and central nervous system of vertebrates (4, 5).
This substance has a variety of physiological functions including anti-oxidative, anti-glycating, anti-aging activities, pH buffering capacity and metal chelating properties (6-9). It also acts as a scavenger of reactive oxygen species (10). In 1986, it was reported that carnosine could inhibit the growth of cancerous cells (11). Later, a study showed that carnosine has a selective effect on cultured transformed cells (12). In 2008, it was demonstrated that carnosine prevented glioblastoma cell growth, cultured in the medium (13). Other studies reported that carnosine could suppress tumor growth in animals (14). Also, it was proven that carnosine presents anticancer activity in vitro as well as in vivo (15). Several mechanisms for anticancer activity of carnosine were suggested as effect on: glycolytic enzymes, redox biology, apoptosis, gene expression and metastasis (16). However, the exact mechanism of anticancer activity of Carnosine is not known (17, 18) and still needs to be investigated. In this work, for studying this interesting aspect of Carnosine function, we examined the effects of carnosine and some synthesized peptide analogues of carnosine on human cancer cell lines including HepG2 (human liver cancer cell line) and HT-29 (human colorectal adenocarcinoma cell line). Analogues of carnosine peptide, designed and studied in the present work, were seven linear and four cyclic peptides.
Materials and Methods
Trifluoroacetic acid (TFA), Triisopropylsilane (TIS), Fmoc amino acids and coupling reagent O-(7-Azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate (HATU) and benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBop) were supplied by the Merck Co, Germany. Solvents, acetonitrile (MeCN), Piperazine, N, N-diisopropylethylamine (DIPEA), diethylether, Dichloromethane (DCM), N, N-dimethylformamide (DMF), and methanol (MeOH) were purchased from the Merck Co, Germany. 2-Chlorotritylchloride(2-CTC) resin (1% DVB, 200-400 mesh, 1 mmol/g) was from the Santa Cruz Biotechnology Co, USA. Commercially available chemicals were used as received unless otherwise stated. Fourier Transform Infrared spectra were obtained by the Shimadzu recording spectrometer, Japan. The mass spectral measurements were performed using a 6410 Agilent LCMS triple quadrupole mass spectrometer (LCMS) with an electrospray ionization (ESI) interface, USA. The cell lines were purchased from Iranian Biological Resource Center (IBRC), Tehran, Iran.
General procedure for the synthesis of protected carnosine and relevant linear peptides (scheme 1)
Scheme 1.

The solid-phase synthesis of carnosine analogue (1) via 2-chlorotrityl chloride resin
Synthesis was carried out using 2-chlorotritylchloride resin (1 mmol/g) following the standard Fmoc strategy. Fmoc-His (Trt)-OH (680 mg, 2 mmol) was attached to the 2-CTC resin with DIPEA (1 ml) in anhydrous DCM: DMF (30 ml, 1:1) at room temperature for 2 hr. After filtration, the remaining tritylchloride groups were capped by a solution of DCM/ MeOH/ DIPEA (2:2:1.5) for 30 min. Then, the resin-bound Fmoc-amino acid was filtered and washed thoroughly with DCM (1 × 10 ml), DMF (2 × 20 ml). The resin was treated with piperazine 10% in DMF (100 ml) for 30 min and then washed with DMF (4 × 20 ml).
Next, a solution of Fmoc-β-alanine-OH (780 mg, 2.01 mmol), HATU (650 mg, 1.7 mmol), and DIPEA (0.5 ml) in 10 ml DCM were added to the resin-bound amino acid with free amine and shaken for 2 hr at room temperature. After completion of coupling, resin was washed with DMF (2 × 10 ml). The resin-bound dipeptide was treated with piperazine 10% in DMF (100 ml) for 30 min and washed with DMF (4 × 20 ml). Then, the second Fmoc-β-alanine-OH and Fmoc-His (Trt)-OH were added to the resin, subsequently following the same procedure as mentioned for attaching the previous Fmoc-β-alanine-OH to the resin. Final Fmoc-deprotection was implemented for the last amino acid attached to the resin. In all cases for the presence or absence of free primary amino groups, Chloranil test was used. The resultant dimer of carnosine (tetrapeptide or pentapeptide analogue) was cleaved from the resin by treatment of TFA 1% in DCM followed by filtration and neutralization with pyridine 4% in MeOH. The solvent was removed under reduced pressure and the residue was precipitated in water. The precipitate was filtered and dried. Other analogues of carnosine peptide were synthesized in the same way.
General procedure for the synthesis of deprotected carnosine and relevant linear peptides
A mixture of trifluoroacetic acid/ dichlorome thane/ triisopropylsilane (TFA/ DCM/ TIS) (10:10:1) were added to the protected linear analogues of carnosine and stirred for 1 hr. Under such strong acidic condition, His (Trt) side chain deprotection (N-Trt) was carried out in one step. The solvent was removed under reduced pressure. The desired peptide was precipitated in cold diethyl ether and deprotected linear analogues of carnosine were obtained.
General procedure for the synthesis of cyclic carnosine analogues
The protected linear analogues of carnosine (1 meq) were dissolved in CH3CN (100 ml) and treated with PyBop (1 meq) and DIPEA (4 meq). The cyclic peptides were thus prepared and isolated by column chromatography (4:1, chloroform: methanol). Final deprotection of the cyclic (N-Trt) peptides was performed by treatment with TFA containing scavengers, phenol, anisole, distilled water and EDT (82.5: 5: 5: 5: 2.5). The solvent was removed under reduced pressure. The desired peptides were precipitated in cold diethyl ether.
MTT assay
To determine the cytotoxicity of the linear and cyclic analogues of inverse carnosine, two human tumor cell lines were used, HepG2 and HT-29. Fibroblast cell line was chosen as a safety control for normal cells. For the measurement of cell viability, toxicity was assessed using the MTT test after 6 hr incubation of the cells with analogues of inverse carnosine (1.25, 2.5, 5, 10, 20, 50 and 100 µg/ml). The above mentioned cells were cultured in RPMI 1640 medium at 37 °C under 5% CO2/95% air, supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were seeded into 96-well plates with 104 cells/well and allowed to grow for 24 hr and then incubated with 10 µg/ml concentration of the each peptide compound for 6 hr. Cell activity was analyzed using MTT method, which is based on the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) orange dye to purple formazan crystals by mitochondrial succinate dehydrogenase enzyme in living cells. At the end of each treatment period, MTT (10 μl, 5 mg/ml in phosphate-buffered saline) was added to each well and the microplate was incubated at 37 °C for 4 hr. The medium containing MTT was removed and DMSO (10 μl) was added to each well to dissolve the formazan crystals. The plate was incubated for 30 min at 37 °C and the absorbance was read at 570 nm using a spectrophotometer plate reader (Infinite® M200, TECAN) (19). 5-Fluorouracil was also used as a positive control and DMSO solvent as the blank for the test compounds. Data are presented as the mean of triplicate measurement of the number of living cells and their capacity to reduce MTT reagent. IC50 values were calculated by using Prism software.
Cell culture for LDH assay
For lactate dehydrogenase (LDH) assay, HepG2 and HT-29 cells were maintained in cell culture flasks (75 cm2) with RPMI 1640 cell culture medium containing 10% FBS in an incubator (37 °C, 5% CO2, 95% air) and were passaged every 4 or 5 days. To passage cells, the cell culture flasks were washed with phosphate-buffered saline (PBS) three times and trypsin was added to detach cells from the bottom of the flasks.
LDH assay
LDH catalyzes the conversion of lactate to pyruvate, the forward reaction and the conversion of pyruvate to lactate, the reverse reaction. Lactate and NAD+ are converted to pyruvate and NADH by the action of LDH. NADH strongly absorbs light at 340 nm, whereas NAD+ does not. The rate of increase in absorbance at 340 nm is directly proportional to the LDH activity in the sample (20). Cytotoxicity induced by peptides was assessed by LDH leakage into the culture medium. The activity of LDH in the medium was determined using a commercially available kit from Sigma-Aldrich. To prepare samples for the LDH assay, the cancerous cells of passage numbers 11-20 were used. The cell samples (1 ml, at a density of 107 cells/ml RPMI containing 10% FBS) were seeded in each well of 12-well plates and grown for 24 hr before exposure to linear and cyclic analogues of carnosine. The cells were washed with PBS three times. Cell samples were treated with 10 µg/ml concentration of peptides for 6 hr exposure in RPMI medium containing 1% FBS. The 12-well plates were shaken briefly to homogenize the released LDH in the cell culture medium and the medium was transferred to 1.5 ml-microcentrifuge tubes and centrifuged at 1,000 ×g at 4 °C for 15 min to remove any cell debris. The supernatant was separated and the absorbance at 340 nm was measured using ELISA reader (21, 22). The LDH activity assay of the samples was obtained by measuring optical density.
Flow cytometry analysis and identification of apoptosis by PI staining
Flow cytometry analysis was determined by analytical DNA flow cytometry. In this work, HepG2 and HT-29 cells were harvested and adjusted to 104 cells/ml (SPL, Korea) and then incubated for 6 hr with 10 µg/ml of peptide samples. The cells were centrifuged at high speed (12,000 rpm) for 20 sec. The pellet was washed with saline buffer, after repeating centrifugation, resuspended in 0.2 ml of lysis buffer (0.1% sodium citrate and 0.1% Triton X-100) containing 50 ?g/ml propidium iodide (PI), a highly water-soluble fluorescent compound, and stained with this reagent at 37 °C for 15 min in the dark. The cells were then evaluated for the DNA fragmentation analysis using a FACScalibur flow cytometry equipment (Becton Dickinson, CA, USA) supplied with the flowing software 2.5.1 (23).
Ethidium bromide (EB) staining
Ethidium bromide stains only cells that have lost membrane integrity. Apoptotic cells, stained with green color, contain bright green dots in the nuclei as a consequence of chromatin condensation and nuclear fragmentation. Cells are viewed under a fluorescence microscope and counted to quantify apoptosis. In this experiment, HepG2 and HT-29 cells were harvested and adjusted to 104 cells/ml (SPL, Korea) and then incubated for 6 hr with 10 µg/ml of peptide samples.
The cells were centrifuged at high speed (12,000 rpm) for 20 sec. The pellet was washed with saline buffer and resuspended in PBS as 0.5×106 to 2×106 cells/ml. Ethidium bromide (1 μl, 0.001 mg/ml in PBS) was added to the cell suspension (25 µl) in each well of a 96-well microplate, and the microplate was incubated at 37 °C. Then, the cell suspension (10 µl) was placed onto a microscopic cover slide and at least 300 cells were examined under a fluorescence microscope (24).
Statistical analysis
Analyses were performed using Graph Pad Prism 5 (Graph Pad Software, La Jolla, CA, USA). Statistical analysis among groups was performed using multiple comparisons by one way ANOVA followed by Tukey’s post hoc test. All data are presented as arithmetic mean ± SEM of at least triplicate determinations. Significance was accepted at P<0.05.
Results
Synthesis of His-β-Ala-β-Ala-His (1)
a) Synthesis of protected peptide
Yield: 75%; Yellow oily liquid; IR (KBr): ν (cm-1) 3477.52 (NH), 2161.95 and 2576.03 (C=N in amino acid Histidine), 1667.99 (C=O amide), 1546.81 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS electrospray ionization (ESI) m/z calculated for (1a) 918.42, Found m/z = 919.60000(M+H)+.
b) Synthesis of deprotected peptide
Yield: 78%; white solid; IR (KBr): ν (cm-1) 3434.17 (NH), 2165.21 and 2558.13 (C=N in amino acid Histidine), 1663.92 (C=O amide), 1545.38 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (1b) 434.215, Found m/z = 433.00000(M-H).
Synthesis of His-β-Ala-Pro-β-Ala-His (2)
a) Synthesis of protected peptide
Yield: 75%; Yellow oily liquid; IR (KBr): ν (cm-1) 3437.16 (NH), 2165.15 and 2559.93 (C=N in amino acid Histidine), 1665.85 (C=O amide), 1547.00 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (2a) 1015.48, Found m/z = 1016.60000(M+H)+.
b) Synthesis of deprotected peptide
Yield: 75%; white solid; IR (KBr): IR (KBr): ν (cm-1) 3441.96 (NH), 2165.88 and 2559.85 (C=N in amino acid Histidine), 1679.60 (C=O amide), 1548.23 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (2b) 531.268, Found m/z = 530.20000(M-H).
Synthesis of Pro-His-β-Ala-β-Ala-His (3)
a) Synthesis of protected peptide
Yield: 75%; Yellow oily liquid; IR (KBr): ν (cm-1) 3429.71 (NH), 2166.65 and 2557.15 (C=N in amino acid Histidine), 1665.90 (C=O amide), 1546.67 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (3a) 1015.48, Found m/z = 1016.60000(M+H)+.
b) Synthesis of deprotected peptide
Yield: 75%; white solid; IR (KBr): ν (cm-1) 3451.30 (NH), 2162.11 and 2587.56 (C=N in amino acid Histidine), 1670.61 (C=O amide), 1547.35 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (3b) 531.268, Found m/z = 530.20000(M-H).
Synthesis of His-β-Ala (4)
a) Synthesis of protected peptide
Yield: 80%; Yellow oily liquid; IR (KBr): ν (cm-1) 3467.47 (NH), 2163.48 and 2550.27 (C=N in amino acid Histidine), 1663.84 (C=O amide), 1546.37 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (4a) 468.228, Found m/z = 467.30000(M-H).
b) Synthesis of deprotected peptide
Yield: 80%; white solid; IR (KBr): ν (cm-1) 3438.64 (NH), 2163.43 and 2556.97 (C=N in amino acid Histidine), 1664.82 (C=O amide), 1546.27 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (4b) 226.119, Found m/z = 225.10000(M-H).
Synthesis of β-Ala-His-His-β-Ala (5)
a) Synthesis of protected peptide
Yield: 78%; Yellow oily liquid; IR (KBr): ν (cm-1) 3445.04 (NH), 2166.36 and 2555.13 (C=N in amino acid Histidine), 1666.35 (C=O amide), 1546.49 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (5a) 918.434, Found m/z = 917.90000(M-H).
b) Synthesis of deprotected peptide
Yield: 78%; white solid; IR (KBr): ν (cm-1) 3437.19 (NH), 2162.88 and 2567.21 (C=N in amino acid Histidine), 1667.21 (C=O amide), 1546.68 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (5b) 434.215, Found m/z = 433.90000(M-H).
Synthesis of β-Ala-His-Pro-His-β-Ala (6)
a) Synthesis of protected peptide
Yield: 78%; Yellow oily liquid; IR (KBr): ν (cm-1) 3445.49 (NH), 2162.20 and 2547.25 (C=N in amino acid Histidine), 1679.79 (C=O amide), 1592.31 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (6a) 1015.49, Found m/z = 1014.10000(M-H).
b) Synthesis of deprotected peptide
Yield: 78%; white solid; IR (KBr): ν (cm-1) 3425.83 (NH), 2165.03 and 2566.72 (C=N in amino acid Histidine), 1666.93 (C=O amide), 1546.88 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (6b) 531.468, Found m/z = 530.60000(M-H).
Synthesis of Pro-β-Ala-His-His-β-Ala (7)
a) Synthesis of protected peptide
Yield: 78%; Yellow oily liquid; IR (KBr): ν (cm-1) 3458.84 (NH), 2161.39 and 2629.39 (C=N in amino acid Histidine), 1677.66 (C=O amide), 1548.05 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (7a) 1015.29, Found m/z = 1014.00000(M-H).
b) Synthesis of deprotected peptide
Yield: 78%; white solid; IR (KBr): ν (cm-1) 3440.13 (NH), 2163.05 and 2573.18 (C=N in amino acid Histidine), 1669.65 (C=O amide), 1546.74 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (7b) 531.468, Found m/z = 530.90000(M-H).
Synthesis of β-Ala-His (carnosine) (8)
a) Synthesis of protected peptide
Yield: 78%; Yellow oily liquid; IR (KBr): ν (cm-1) 3438.64 (NH), 2165.88 and 2556.05 (C=N in amino acid Histidine), 1664.42 (C=O amide), 1546.45 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (8a) 468.228, Found m/z = 467.30000(M-H).
b) Synthesis of deprotected peptide
Yield: 78%; white solid; IR (KBr): ν (cm-1) 3434.17 (NH), 2164.07 and 2561.28 (C=N in amino acid Histidine), 1666.62 (C=O amide), 1547.02 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (8b) 226.219, Found m/z = 225.10000(M-H).
Synthesis of cyclo (His-β-Ala-Pro-β-Ala-His) (1c)
Yield: 75%; Yellow solid; IR (KBr): IR (KBr): ν (cm-1) 3447.80 (NH), 1673.09 (C=O amide), 1537.69 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (1c) 513.268, Found m/z = 514.30000(M+H)+.
Synthesis of cyclo (Pro-His-β-Ala-β-Ala-His) (2c)
Yield: 75%; Yellow solid; IR (KBr): ν (cm-1) 3415.11 (NH), 1673.93 (C=O amide), 1544.00 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (2c) 513.268, Found m/z = 514.30000(M+H)+.
Synthesis of cyclo (β-Ala-His-Pro-His-β-Ala) (3c)
Yield: 75%; Yellow solid; IR (KBr): IR (KBr): ν (cm-1) 3438.45 (NH), 1673.24 (C=O amide), 1537.69 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (3c) 513.268, Found m/z = 514.30000(M+H)+.
Synthesis of cyclo (Pro-β-Ala-His-His-β-Ala) (4c)
Yield: 75%; Yellow solid; IR (KBr): IR (KBr): ν (cm-1) 3448.45 (NH), 1675.32 (C=O amide), 1527.65 (C=C in amino acid Histidine), 600-800 (out of plane bending vibration C-H in amino acid Histidine); LC-MS (ESI) m/z calculated for (4c) 513.268, Found m/z = 514.30000(M+H)+.
MTT assay results
The deprotected linear and cyclic analogues of inverse carnosine peptides showed cytotoxicity to HepG2 and HT-29 cancer cell lines in varying degree (ranging IC50 values from 9.81 to 16.23 µg/ml). Table 1 shows the IC50 results for all the deprotected linear and cyclic analogues of inverse carnosine along with IC50 result for 5-flurouracil, chosen as a standard cytotoxic drug. Viability percentage of the HepG2 and HT-29 cells affected by the peptides are shown in Table 2.
Table 1.
IC50 values (μg/mL) for toxicity activity of peptides on HepG2 and HT-29 cells in MTT assay. Values are presented as mean ± SD of three independent experiments (n= 3). The stars show the values are significantly different from the corresponding control (***P-value <0.001).
| Linear | IC50 cell line HepG2 | IC50 cell line HT-29 |
|---|---|---|
| 1b | 10.88±0.32*** | 12.33±0.25*** |
| 2b | 10.52±0.10*** | 10.19±0.13*** |
| 3b | 11.12±0.32*** | 10.33±0.25*** |
| 4b | 11.23±0.12*** | 12.43±0.12*** |
| 5b | 11.09±0.26*** | 10.25±0.33*** |
| 6b | 11.67±0.13*** | 11.58±0.10*** |
| 7b | 10.33±0.37*** | 16.01±0.33*** |
| 8b | 10.01±0.0063*** | 16.23±0.0021*** |
| 1c | 10.67±0.17*** | 14.01±0.10*** |
| 2c | 10.28±0.26*** | 11.23±0.11*** |
| 3c | 10.91±0.13*** | 9.81±0.20*** |
| 4c | 11.76±0.64*** | 10.89±0.27*** |
| 5-Fluorouracil (standard drug) | 3.16 | 6.08 |
Table 2.
Viability percentage of HepG2 and HT-29 cells affected by linear and cyclic analogues of carnosine in MTT assay
| Entry | Viability percentage (HepG2) | Viability percentage (HT-29) |
|---|---|---|
| 1b | 16.95 | 26.52 |
| 2b | 5.74 | 14.08 |
| 3b | 17.01 | 26.47 |
| 4b | 6.11 | 13.55 |
| 5b | 13.8 | 35.02 |
| 6b | 7.03 | 11.49 |
| 7b | 18.3 | 34.86 |
| 8b | 8.01 | 27.79 |
| 1c | 9.36 | 10.75 |
| 2c | 13.88 | 11.07 |
| 3c | 7.01 | 21.3 |
| 4c | 34.23 | 29.27 |
| 5-Fu | 3.22 | 9.28 |
| Control | 100 | 100 |
LDH analysis results
The results of viability percentage peptides-treated HepG2 and HT-29 cells calculated by measuring optical density in the LDH assay are shown in Table 3.
Table 3.
LDH assay of linear and cyclic analogues of carnosine for cell lines HepG2 and HT-29
| Entry | Viability percentage (HepG2) | Viability percentage (HT-29) |
|---|---|---|
| 1b | 61.65 | 20.88 |
| 2b | 60.98 | 20.88 |
| 3b | 20.26 | 20.43 |
| 4b | 73.38 | 11.43 |
| 5b | 24.25 | 17.36 |
| 6b | 84.37 | 10.7 |
| 7b | 63.7 | 83.39 |
| 8b | 73.19 | 21.64 |
| 1c | 10.48 | 29.01 |
| 2c | 16.92 | 71.61 |
| 3c | 9.92 | 26.54 |
| 4c | 84.49 | 34.26 |
| 5-Fu | 7.78 | 6.19 |
| Control | 100 | 100 |
Flow cytometry analysis results
Table 4 and Figure 1 show flow cytometry results of carnosine and the relevant linear and cyclic peptides analogues. All the related histograms were included in the supplementary file. Figure 3 shows the histogram 1b as example.
Table 4.
Apoptosis percentage of linear and cyclic analogues of carnosine for cell lines HepG2 and HT-29
| Entry | Apoptosis percentage (HepG2) | Apoptosis percentage (HT-29) | Viability percentage (HepG2) | Viability percentage (HT-29) |
|---|---|---|---|---|
| 1b | 99.7 | 99.69 | 0.3 | 0.31 |
| 2b | 90.56 | 85.37 | 0.52 | 0.75 |
| 3b | 88.47 | 86.58 | 0.4 | 0.66 |
| 4b | 99.56 | 67.12 | 0.44 | 32.76 |
| 5b | 99.65 | 99.35 | 0.35 | 0.65 |
| 6b | 99.3 | 90.07 | 0.09 | 9.19 |
| 7b | 85.07 | 85 | 0.68 | 1.15 |
| 8b | 86.69 | 84.65 | 7.86 | 0.42 |
| 1c | 91.35 | 91.16 | 0.72 | 0.39 |
| 2c | 85.17 | 28.25 | 0.37 | 25.04 |
| 3c | 99.1 | 99.71 | 0.9 | 0.29 |
| 4c | 99.54 | 99.13 | 0.46 | 0.88 |
Figure 1.

Flow cytometry results obtained for peptide-treated HepG2 and HT-29 cells
Figure 3.
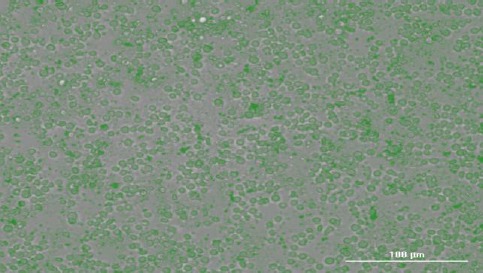
Apoptosis of HepG2 cells treated with peptide, which are stained with ethidium bromide
The left dot plots present the forward scatter (FSC) parameter in horizontal axis and side scatter (SSC) parameter in vertical axis, which can be correlated with the relative size and granularity of the cells, respectively. The histograms show the intensity of fluorescence of the samples in the FL-2 channel, which corresponds to PI emission wavelength (23).
Ethidium bromide (EB) staining
Figures 2 and 3 show untreated and peptide-treated HepG2 cells exposed to ethidium bromide.
Figure 2.

Apoptosis of Untreated HepG2 cells which are stained with ethidium bromide
Dilute solution preparation for peptide cyclization is obligatory in order to avoid side reactions such as dimerization or polymerization as a result of two or more linear peptide chain coupling reaction.
Discussion
The peptide analogues of carnosine were designed as dimer, with direct or inverse sequence of carnosine, with or without proline. Proline molecule, an amino acid existed in the cis-configuration within the peptide chain, facilitates cyclization of the linear peptides. One of the preferred methods for peptide synthesis is solid phase synthesis on resin. The resin employed for the synthesis of carnosine peptide and its analogues was 2-chlorotritylchloride resin. Trityl linker is commonly used in solid phase peptide synthesis, which allows the “protected” compound to be subjected to various chemical manipulations and consequently to afford pure compounds without numerous purification steps (25-28). At first, the cleavage step was completed with TFA 1% in dichloromethane owing to the high stability of trityl cations attached to the resin (29) and the desired protected analogues of carnosine peptide were obtained in good yields and high purities without producing byproducts due to the side reactions of protecting groups released in the solution. At the second step, deprotection of the side chains (N-Trt) of linear peptides was carried out with TFA 95% containing scavengers to produce the target molecules. On the other hand, for the cyclization of linear carnosine analogues, the linear (N-Trt) protected peptides were first employed in a dilute solution using coupling reagents, and then the final deprotection of the cyclic peptides, thus prepared, was achieved by treating with TFA 95% containing scavengers.
The synthesized linear and cyclic carnosine analogues exhibited considerable cytotoxic activity against the cell lines of HepG2 and HT-29 with mean IC50 values ranging from 9.81 to 16.23 μg/ml, comparable with the high potent drug 5-fluorouraci (5-FU), as a standard, in MTT assay All the synthesized linear and cyclic compounds had a high viability percentage on skin fibroblast. As shown in Table 2, compounds 2b, 4b, 6b, 8b, 1c, and 3c imposed low viability of HepG2 cells to reduce less than 10%. On the other hand, compounds 2b, 4b, 6b, 1c and 2c caused low viability of HT-29 cells less than 15 %. The LDH assay of the samples was obtained by measuring optical density. Increase in optical density, indicates greater LDH activity of cancerous cell lines HepG2 and HT-29, while decrease in optical density presents lower LDH activity of the cell lines. Compounds 3b, 5b, 1c, 2c and 3c resulted in low viability percentages of the cancerous cells of HepG2, less than 25%, and among them, compounds 1c and 3c were the most effective peptides. Compounds 1c and 3c were also effective peptides on HepG2 according to MTT assay. On the other hand, in LDH assay, compounds 1b, 2b, 3b, 4b, 5b, 6b, 8b, 1c and 3c caused low viability percentages of the cancerous cells of HT-29, under 30%, and among the peptides, compounds 4b and 6b gave the lowest viability percentages of HT-29. Compounds 4b and 6b were also effective peptides on HT-29 according to MTT assay. The flow cytometry analysis of the peptides-treated cancerous cell lines gave the relevant histograms. In the histograms, signals obtained in H-1 area mean that PI color was able to enter the cells and bind to DNA and so apoptosis has occurred, whereas, the signals appeared in H-2 area indicate that the cancerous cells treated with peptides remained intact and so no apoptosis has happened (23). The most effective peptide analogues caused apoptosis of the both cell lines upon the experiment conditions, demonstrated in flow cytometry analysis. Among them, compounds 1c, 3c, 4b and 6b showed apoptotic activities on HepG2 more than carnosine (compound 8b). Also, compounds 1c, 3c and 6b (except 4b) showed apoptotic activities on HT-29 more than carnosine. Compounds 3c and 6b that showed higher cytotoxic activity than Carnosine are the cyclic and linear analogue peptides of carnosine with the identical sequence of β-Ala-His-Pro-His-β-Ala. Compound 4b, with the sequence of His-β-Ala, is the inverse analogue of carnosine. The cytotoxic activity of this compound was lower than that of carnosine on HT-29. However, compound 1c, the cyclic peptide with the sequence of His-β-Ala-Pro-β-Ala-His, although contains the compound 4b structure in its sequence, showed improvement in cytotoxic activity on both cell lines. It should be noted that compound 1c also contains proline as an extra amino acid, compared with 1b, and is a cyclic peptide, versus the linear peptide 2b with the identical sequence. Moreover, compound 1c possesses Carnosine structure in its building block. Therefore, compound 1c with the three characteristic of having Pro, carnosine structure and a cyclic scaffold, could recover and improve the cytotoxic activity in comparison with 1b, 2b, 4b and carnosine. All these synthesized peptide analogues of carnosine, as well as carnosine itself, could impose their cytotoxic activity through apoptosis mechanism, as the flow cytometry analysis demonstrated. This finding is in accordance with the recently published paper (18).
Conclusion
The synthesized linear and cyclic analogues of carnosine peptide showed toxic effects on chosen cancerous cells of liver and colon with varying degrees. Among them, compounds 3c and 6b emerged as the best analogues, which showed higher cytotoxic activity than carnosine. These peptides are the cyclic and linear analogue peptides of carnosine with the identical sequence of β-Ala-His-Pro-His-β-Ala. In addition, compound 1c, a cyclic peptide with the sequence of His-β-Ala-Pro-β-Ala-His, with the three characters of being cyclic peptide, containing Pro and carnosine structure in its sequence, also showed a high cytotoxic activity, compared with 1b, 2b, 4b and carnosine. Thus, compound 1c was chosen as the other favorite sequence. Our results showed that these synthesized peptide analogues of carnosine, as well as carnosine itself, might impose their cytotoxic activity through apoptosis.
Acknowledgment
The authors are grateful to Shahid Beheshti University of Medical Sciences, Tehran, Iran, by grant number 8107 and the University of Kashan, Kashan, Iran, by grant number 159148/53 and for providing support to this work.
Conflict of interest
The authors declare that there is no conflict of interest on this research work.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:2011–2030. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang TH, Mao CP, He L, Tsai YC, Liu K, La V, et al. Tumor targeted delivery of IL-2 by NKG2D leads to accumulation of antigen-specific CD8+T cells in the tumor loci and enhanced anti-tumor effects. PLoS ONE. 2012;7:1–9. doi: 10.1371/journal.pone.0035141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dongdong W, Yanfeng G, Yuanming Q, Lixiang C, Yuanfang M, Yanzhang L. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014;351:13–22. doi: 10.1016/j.canlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Boldyrev AA. Carnosine and oxidative stress in cells and tissues. Nova Publishers. 2007:1–286. [Google Scholar]
- 5.Gaunitz F, Hipkiss AR. Carnosine and cancer: a perspective. Amino Acids. 2012;43:135–142. doi: 10.1007/s00726-012-1271-5. [DOI] [PubMed] [Google Scholar]
- 6.Alpsoy L, Akcayoglu G, Sahin H. Anti-oxidative and anti-genotoxic effects of carnosine on human lymphocyte culture. Hum Exp Toxicol. 2011;30:1979–1985. doi: 10.1177/0960327111404908. [DOI] [PubMed] [Google Scholar]
- 7.Sale C, Saunders B, Harris RC. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino acids. 2010;39:321–333. doi: 10.1007/s00726-009-0443-4. [DOI] [PubMed] [Google Scholar]
- 8.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosins. Physiol Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 9.Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 10.Chan KM, Decker EA, Feustman C. Endogenous skeletal muscle antioxidants. Crit Rev Food Sci Nutr. 1994;34:403–426. doi: 10.1080/10408399409527669. [DOI] [PubMed] [Google Scholar]
- 11.Nagai K, Suda T. Antineoplastic effects of carnosine and betaalanine–physiological considerations of its antineoplastic effects. Nihon Seirigaku Zasshi. 1986;48:741–747. [PubMed] [Google Scholar]
- 12.Holliday R, McFarland GA. Inhibition of the growth of transformed and neoplastic cells by the dipeptide carnosine. Br J Cancer. 1996;73:966–971. doi: 10.1038/bjc.1996.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renner C, Seyffarth A, de Arriba S, Meixensberger J, Gebhardt R, Gaunitz F. Carnosine inhibits growth of cells isolated from human glioblastoma multiforme. Int J Pept Res Ther. 2008;14:127–135. [Google Scholar]
- 14.Horii Y, Shen J, Fujisaki Y, Yoshida K, Nagai K. Effects of Lcarnosine on splenic sympathetic nerve activity and tumor proliferation. Neurosci Lett. 2012;510:1–5. doi: 10.1016/j.neulet.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 15.Iovine B, Oliviero G, Garofalo M, Orefice M, Nocella F, Borbone N, et al. The anti-proliferative effect of L-carnosine correlates with a decreased expression of hypoxia inducible factor 1 alpha in human colon cancer cells. PloS One. 2014;9:1–10. doi: 10.1371/journal.pone.0096755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hipkiss AR, Gaunitz F. Inhibition of tumour cell growth by carnosine: some possible mechanisms. Amino Acids. 2014;46:327–337. doi: 10.1007/s00726-013-1627-5. [DOI] [PubMed] [Google Scholar]
- 17.Gaunitz F, Hipkiss AR. Carnosine and cancer—a perspective. Amino Acids. 2012;43:135–142. doi: 10.1007/s00726-012-1271-5. [DOI] [PubMed] [Google Scholar]
- 18.Yun B, Saidan D, Jiaoyan C, Yuan L, Bingyu W, Huijuan X, et al. Carnosine inhibits the proliferation of human cervical gland carcinoma cells throughinhibiting both mitochondrial ioenergetics and glycolysis pathways and retarding cell cycle progression. Integr Cancer Ther. 2016:1–12. doi: 10.1177/1534735416684551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Amador E, Dorfman LE, Wacker WE. Serum lactic dehydrogenase activity: an analytical assessment of current assays. Clin Chem. 1963;9:391–399. [PubMed] [Google Scholar]
- 21.Han X, Gelein R, Corson N, Wade-Mercer P, Jiang J, Biswas P, et al. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology. 2011;287:99–104. doi: 10.1016/j.tox.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang M, Neuzil P, Volk T, Manz A, Kleber A. On-chip three-dimensional cell culture in phaseguides improves hepatocyte functions in vitro. Biomicrofluidics. 2015;9(034113):1–12. doi: 10.1063/1.4922863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 24.Farha AK, Geetha BS, Mangalam SN, Dhanya SR, Latha PG, Remani P. Apoptosis mediated cytotoxicity induced by isodeoxyelephantopin on nasopharyngeal carcinoma cells. Asian J Pharm Clin Res. 2013;6:51–56. [Google Scholar]
- 25.Park JG, Langenwalter KJ, Weinbaum CA, Casey PJ, Pang Y-P. Improved loading and cleavage methods for solid-phase synthesis using chlorotrityl resins: synthesis and testing of a library of 144 discrete chemicals as potential farnesyl transferase inhibitors. J Comb Chem. 2004;6:407–413. doi: 10.1021/cc0340729. [DOI] [PubMed] [Google Scholar]
- 26.Olsen CA, Witt M, Jaroszewski JW, Franzyk H. Solid-Phase Synthesis of Rigid Acylpolyamines Using Temporary N-4,¬4¬'-Dimethoxytrityl Protection in the Presence of Trityl Linkers. J Org Chem. 2004;69:6149–6152. doi: 10.1021/jo049278q. [DOI] [PubMed] [Google Scholar]
- 27.Lundquist JT, IV, Satterfield AD, Pelletier JC. Mild and adaptable silver triflate-assisted method for trityl protection of alcohols in solution with solid-phase loading applications. Org Lett. 2006;8:3915–3918. doi: 10.1021/ol0614018. [DOI] [PubMed] [Google Scholar]
- 28.Crestey F, Ottesen LK, Jaroszewski JW, Franzyk H. Efficient loading of primary alcohols onto a solid phase using a trityl bromide linker. Tetrahedron Lett. 2008;49:5890–5893. [Google Scholar]
- 29.Rothman DM, Vazquez ME, Vogel EM, Imperiali B. Caged phospho-amino acid building blocks for solid-phase peptide synthesis. J Org Chem. 2003;68:6795–6798. doi: 10.1021/jo0344891. [DOI] [PubMed] [Google Scholar]


