Abstract
Objective(s)::
Lipopolysaccharide (LPS)-induced endotoxemia is known to cause male infertility. This study was designed to explore the effects of bacterial LPS on histomorphometric changes of mice testicular tissues.
Materials and Methods:
In experiment 1, a pilot dose responsive study was performed with mice that were divided into five groups, receiving 36000, 18000, 9000, and 6750 µg/kg body weight (B.W) of LPS or only saline (control). White blood cells (WBC) were observed for 3 days after LPS inoculation. In experiment 2, two groups of mice were treated with 6750 µg/kg B.W of LPS or only saline (control). Five cases from each experimental group were sacrificed at 3, 30, and 60 days after LPS inoculation. Left testes were fixed in Bouin’s solution, and stained for morphometrical assays.
Results:
Time-course changes of WBC obtained from different doses of LPS-treated mice showed that inoculation of 6750 µg/kg B.W produced a reversible endotoxemia that lasts for 72 hr and so it was used in the second experiment. In experiment 2, during the first 3 days, no significant changes were observed in the evaluated parameters instead of seminiferous tubules diameter. Spermatogenesis, Johnsen’s score, meiotic index, and epithelial height were significantly affected at 30th day. However, complete recovery was only observed for the spermatogenesis at day 60. Interestingly, deleterious effects of LPS on spermatogonia were only seen at 60th day (P<0.05).
Conclusion:
Endotoxemia induced by LPS has long-term detrimental effects on spermatogonia and later stage germ cells, which are reversible at the next spermatogenic cycle.
Keywords: Endotoxemia, Lipopolysaccharide, Meiotic index, Spermatogenesis, Spermatogonia
Introduction
Inflammatory diseases, which often originate from bacterial contamination, are reputed to be responsible for male infertility (1). Bacterial lipopolysaccharide (LPS) is an active component of the outer membrane of the cell wall of Gram-negative bacteria, which modulates an acute inflammatory response in the host that is known as acute endotoxemia. Since inflammatory condition and endotoxemia can be mimicked by the administration of LPS, so LPS-induced animal models can open a useful way to investigate a relationship between mechanisms of inflammatory diseases and infertility (2). LPS is responsible for activating immune cells such as mast cells and neutrophils (3, 4). Indeed, LPS binds to receptors on leukocytes and induces the release of pro-inflammatory cytokines, including interleukin-6, interleukin-1β, tumor necrosis factor α, interferon-γ, transforming growth factor β (5). Cytokines are regulators of acute phase response such as fever, anorexia (6), renal failure (7), and endocrine changes (8), which disturb spermatogenesis and steroidogenesis (9, 10). Thus, LPS induces the production of reactive oxygen species (ROS) in testes, and ROS production is reported as a main factor for testicular damage (11).
A previous study has shown an increase in apoptosis of testes germ cells as well as remarkable effects on sperm concentration and motility in LPS-induced mice (12); in another report, administration of sub-lethal doses of LPS to mice has resulted in a rapid decrease in serum testosterone levels after 24 hr (13). However, short and long-term effects of LPS on spermatogenesis and testicular structure have been well examined in the other mammals such as rats and rabbits (9, 11, 14, 15). Unfortunately, few studies concerning the effects of bacterial LPS on mice testicular functions can be found. Therefore, the aim of the present study was to determine the short and long-term effects of bacterial LPS administration on histomorphometric changes in mice testicular tissues.
Materials and Methods
Animals
The mice were purchased from Razi Vaccine and Serum Research Institute in the Southeastern part of Iran, Kerman branch. The animals were kept in standard conditions (12 hr light: 12 hr dark and 22 ± 2°C), and underwent treatment at the Laboratory Animal House of the Veterinary Faculty of Shahid Bahonar University of Kerman, Iran, for at least one week before the treatment and during the experimental period. A total of 50 healthy adult male NMRI mice (aged 6–8 weeks, 27–31 g) were used in this study, which were fed standard commercial laboratory chow ((pellet form), Javaneh Khorasan Co., Mashhad, Iran) and water ad libitum. All the investigations were conducted in accordance with the Guiding Principles for the Care and Use of Research Animals. The experimental protocol was approved by the Ethics Committee of Faculty of Veterinary Medicine, Shahid Bahonar University of Kerman, Iran.
Experiment 1
A pilot dose-response study was performed to establish a suitable LPS (lipopolysaccharide from Escherichia coli O55:B5, L2880, Sigma-Aldrich) dosage for the induction of a reversible endotoxemia state. Twelve healthy NMRI male mice were randomly divided into five experimental groups and intraperitoneally injected with 36000, 18000, 9000, and 6750 µg/kg B.W of LPS (dissolved in 0.1 ml of sterile saline) or only sterile saline as a control group. The inflammatory response was determined by the number of the white blood cells (WBC) just before (at time zero) and up to 72 hr after LPS treatment.
Experiment 2
Thirty new male mice were randomly assigned to either control or treatment groups with fifteen mice in each group. To monitor the short and long-term effects of LPS on testicular tissue changes and based on the results of experiment 1, fifteen mice from the treatment group were intraperitoneally inoculated with 6750 µg/kg B.W of LPS. Saline-treated mice served as the control group (n=15).
Five cases of 15 mice from each experimental group were sacrificed at 3, 30, and 60 days following LPS inoculation and their left testes were removed and used for histopathological evaluations.
Histopathological procedures
All tissue specimens of testes were fixed in Bouin’s solution, embedded in paraffin wax, sectioned with 5 µm thicknesses, stained with hematoxylin and eosin (H&E) and studied by a light microscope (Nikon, Digital Sight DS-Fi2, Japan).
Morphometrical assays
Spermatogenesis was determined by the semi-quantitative method (Johnsen’s score) in 100 seminife- rous tubules of each cross-section at the same magnification and summed up as mean Johnsen’s score (JS) (16), and quantitative method in which 200 seminiferous tubules were examined under light microscopy. In the quantitative method, the presence of spermatozoa within the seminiferous tubule was considered as the evidence of spermatogenesis (SP). Lack of the spermatozoa even in the presence of orderly progression of primary and secondary spermatocytes was not considered as evidence of spermatogenesis for the purpose of the present experimental study (17). The seminiferous tubules diameter (STD) and epithelial height (EH) were measured in each testis for evaluation of morphometrical assays. The ten smallest, roundest tubules were selected for each animal per experimental group, and the epithelium height and diameter of tubules were measured with an ocular micrometer under light microscopy. The average diameter of spermatogonia cells nuclei (SND) was measured from 10 cells for each testis. The number of round spermatids for each pachytene primary spermatocytes was also calculated as meiotic index (MI) for determination of cell loss percentage during cell division (18).
Statistical analysis
The results were subjected to analysis by SPSS17.0 (SPSS Inc., Chicago, IL, USA) package. All data were tested for homogeneity of variances using Levene static test. Evaluation of significant difference between the experimental groups was performed using one-way analysis of variance (one-way ANOVA) followed by the least significant difference test (LSD) for multiple comparisons when the variances were homogenous, otherwise Tamhane’s test was used as post hoc. Values were expressed as mean ± SEM. The significance considered level was P<0.05.
Results
The effects of LPS induced endotoxemia on changes of testicular tissue structures were evaluated by planning two series of experiments including experiment 1 for exploring suitable LPS dose and experiment 2 for monitoring LPS effects based on the results obtained from experiment 1. The results of the above mentioned experiments are presented separately.
Experiment 1
Data relative to time-course changes in the number of white blood cells (WBC/mL) obtained from the different doses of LPS-treated male mice up to 24 hr has been shown in Figure 1. Blood leukocytes (WBC/ml) rapidly increased during 12 hr, so that all the mice in the experimental group with 36000 µg/kg B.W of LPS died. However, the death of mice was observed in the two other experimental groups following a decrease in WBC including experimental groups with 18000 and 9000 µg/kg B.W of LPS. Only, a dose of 6750 µg/kg B.W showed reversible endotoxemia without killing the animals. Overall, according to the obtained results a dose of 6750 µg/kg B.W of LPS was used in the second experiment. Detailed time-course changes in the number of WBC treated with 6750 µg/kg B.W of LPS and the control group up to 72 hr has been shown in Figure 2.
Figure 1.
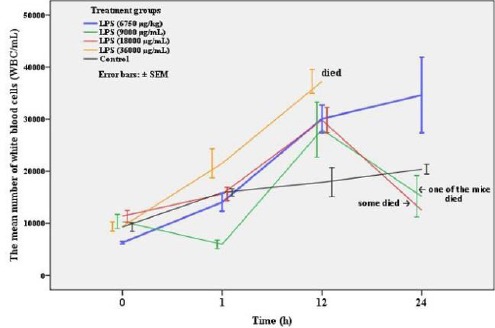
Time-course changes in white blood cells count (mean ± SEM) of mice treated with different doses of LPS including 36000, 18000, 9000, and 6750 µg/kg B.W and the control group
Figure 2.
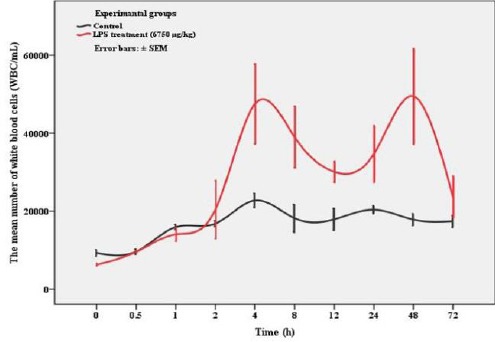
Variation in white blood cells count (mean ± SEM) of mice treated with 6750 µg/kg B.W of LPS and the control group up to recovery (72 hr)
Detailed data relative to the number of WBC obtained from the LPS treated mice with a dose of 6750 µg/kg B.W showed a marked increase 4 hr after the administration of LPS followed by a decrease during 12 hr and then, blood leukocytes reached a maximum value at 48 hr again. However, complete recovery was achieved after 72 hr, in which the data did not show any significant difference with the control group.
Experiment 2 (morphometrical evaluations)
In experiment 2, some histomorphometrical parameters including JS, SP, EH, MI, STD, and SND were evaluated after 3, 30, and 60 days of LPS treatment (Table 1 and Figure 3), which are explained below separately.
Table 1.
The mean ± SEM of different parameters including spermatogenesis, Johnsen’s score, meiotic index, epithelial height, seminiferous tubules diameter, and spermatogonia cell nucleus diameter following inoculation of LPS
| Parameters (scale) | Leven’s test P-value | Control (no. of mice) | LPS treatment groups | ||
|---|---|---|---|---|---|
| Day 3 (n=5) | Day 30 (n=5) | Day 60 (n=5) | |||
| SP (%) | 0.301 | 95.50 ± 0.41a (13) | 95.35 ± 0.91a | 90.86 ± 1.48b | 95.46 ± 0.56a |
| JS (count) | 0.000 | 9.11 ± 0.07a (14) | 8.70 ± 0.20abc | 8.12 ± 0.23bc | 8.00 ± 0.90c |
| MI (index) | 0.010 | 2.14 ± 0.02a (14) | 2.03 ± 0.04a | 1.64 ± 0.03b | 1.85 ± 0.04c |
| EH (µm) | 0.025 | 66.33 ± 0.59a (14) | 64.45 ± 0.79a | 61.05 ± 0.81b | 57.65 ± 0.86c |
| STD (µm) | 0.079 | 217.28 ± 1.55a (14) | 205.40 ± 2.14bc | 200.80 ± 2.22c | 212.40 ± 2.25a |
| SND (µm) | 0.000 | 5.12 ± 0.02a (14) | 5.20 ± 0.05a | 5.13 ± 0.01a | 5.02 ± 0.01b |
SP, Spermatogenesis; JS, Johnsen score (testicular biopsy score count); MI, meiotic index; EH, epithelial height; STD, seminiferous tubules diameter; SND, spermatogonia cell nucleus diameter
Different alphabetic letters in each row, show significant difference (P<0.05) between the control and treatment groups for each individual day (3, 30, and 60)
Figure 3.
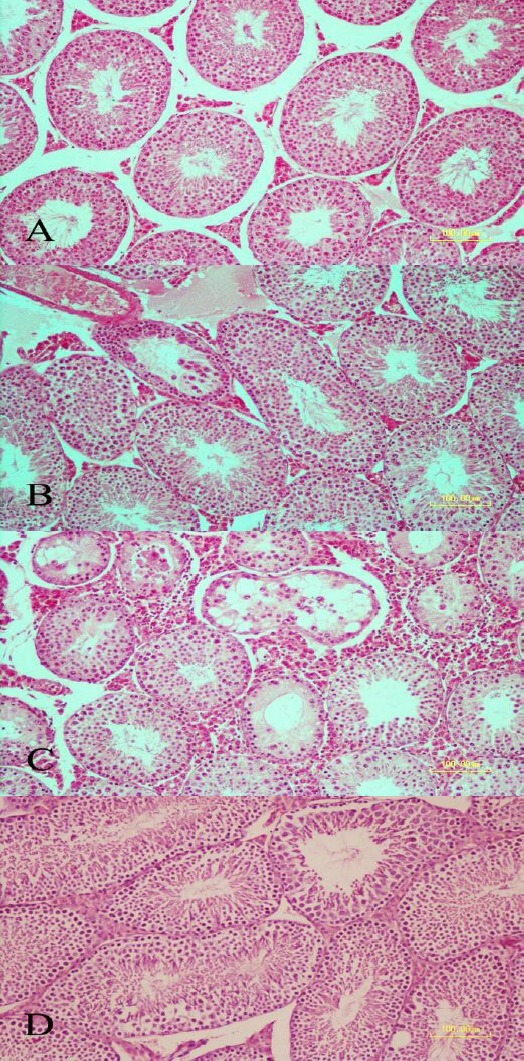
Testicular sections stained with hematoxylin and eosin (bar = 100 µm). A: Control group section showing normal seminiferous tubules morphology. B: A few seminiferous tubules revealing mild degenerative changes on day 3 after LPS administration. C: Photomicrograph showing seminiferous tubules at days 30 following LPS administration. Severe degenerative changes are observed. Depletion and vacuolation of epithelium with decreased number of germinal layers are seen in some of the seminiferous tubules. D: Photomicrograph showing seminiferous tubules at day 60 of LPS administration. Normal histomorphology of tubules is preserved
Spermatogenesis (semi-quantitative and quantitative methods)
The quantitative method of spermatogenesis evaluation revealed a significant decrease in the percentage of spermatogenesis at day 30 after LPS administration compared to the control group (90.86±1.48 vs. 95.50±0.41, P=0.000, respectively), although complete recovery was observed at day 60.
A similar result was observed in semi-quantitative method evaluated by JS in which significant impairment of spermatozoa was revealed until day 30 time-dependently in comparison with the control group (8.12±0.23 vs. 9.11±0.07, P=0.001, respectively).
Meiotic index (MI)
In spite of decreased MI during the first three days compared with the control group (P>0.05), significant impairment of MI was seen at day 30 compared with the control group following LPS administration (1.64±0.03 vs. 2.14±0.02, P=0.000, respectively) with a significant recovery at day 60.
Epithelial height (EH)
LPS administration caused a time-dependent reduction in EH observed from day 3 to 60. However, significant reduction of EH was after 30 days compared to the control group (61.05±0.81 vs. 66.33±0.59, P=0.000, respectively).
Seminiferous tubules diameter (STD)
Significant reduction of STD was observed from day 3 after LPS administration in comparison with the control group (205.40±2.14 vs. 217.28±1.55, P=0.000, respectively) and continued up to day 30, although there was not any significant difference between third and 30th days.
Thus, the mean of STDs at day 60 following LPS treatment was completely recovered in comparison with days 3 and 30 (P<0.05) in which no significant difference was observed between the control group and day 60.
Spermatogonia cell nucleus diameter (SND)
Detrimental effects of LPS administration were just observed at day 60 compared to the control group (5.02±0.01 vs. 5.12±0.02, P=0.019, respectively) and there was not a significant difference among the control, day 3, and day 30 groups (P>0.05).
Discussion
There are so many reports that reveal the impact of LPS-induced endotoxemia on animal testes (9, 11, 13-15, 19), but we could not find an answer for what is the long-term influence of LPS-induced endotoxemia on mice testicular structures? The present study revealed a permanent damage to the testicular structure following 30 days of LPS treatment except for spermatogonia, which rebounded at the end of the second spermatogenesis cycle (60 days of LPS treatment).
In the present study, the prominent point about spermatogonia was the observable damage that happened at the end of the second mice spermatogenesis cycle (Table 1). In other words, the first significant decrease in the spermatogonia cell nucleus diameter (SND) was seen at day 60 compared to day 30 after LPS administration (5.02±0.01 vs. 5.13±0.01, P=0.036), while there were not any significant differences among the control, day 3, and day 30 groups. Previous reports documented apoptosis in germ cells of rats (9) and mice (12), but resulting long-term effects on spermatogonia were not examined. A major pathway of apoptosis is controlled by the Fas/Fas Ligand (FasL) system. Fas including APO-1 and CD95 are a transmembrane receptor glycoprotein, which is classified as tumor necrosis factor (TNF) or nerve growth factor receptor family (20) and FasL is a type II transmembrane protein that is connected to TNF family (21). A previous paper reported an increase in the number of Fas-positive germ cells 24 hr after LPS treatment in coordination with testicular germ cell apoptosis (12). Indeed, germ cell apoptosis observed in the report of Kajihara et al. (12) after LPS treatment could be a result of direct effects of LPS on spermatogonia. Although in disagreement with the above-mentioned report, in the present study short-term effects of LPS on spermatogonia was not histopathologically observed. We know that Toll-like receptor 4 (TLR4) is the main LPS receptor that has been found to be expressed in epididymal epithelium and sperm (22, 23). LPS triggers common TLR signaling and so activates the nuclear factor-κB (NFκB), which is translocated to the nucleus. Finally, it controls transcription of the TLR-response cytokines such as TNFα (24). But, we know that TLR receptors are lesser extended in germ cells (24) and so possibly long-term effects of LPS, observed in this study, is indirect by the influence of its products such as interleukin (IL)-1α, IL-6, and IL-18 (19, 25).
The released cytokines may play a role in the impairment of Leydig cell steroidogenesis (26) and there have been reports that the inhibition of testosterone can induce male germ cell death by apoptosis (27). But new research showed that the localization of androgen receptors to germ cells are controversial (28, 29). Cell-specific knock out of androgen receptor in germ cells such that androgen receptor is not expressed during or after meiosis did not alter spermatogenesis or fertility indicating that androgen receptor is not required in later stage germ cells (30). This is in agreement with our results that showed another cell type especially spermatozoa rather than spermatogonia are more susceptible to damage induced by LPS. It means that optimal testosterone concentration is important for maintaining spermatogenesis (31). In the absence of testosterone or androgen receptors, spermatogenesis rarely progresses beyond meiosis (32).
So many studies have highlighted the role of reactive oxygen species (ROS) in the testis (33, 34). ROS is known as a mediator of testicular damage during inflammation, infection, testicular torsion, and cryptorchidism (17, 35). It is known that LPS-induced cytokines are a potent activator of macrophages and stimulate ROS production such as H2O2, NO (36), and superoxides (37). Spermatogonia are highly tolerant to ROS attack while advanced stage germ cells such as spermatozoa are much more susceptible (38) and this is in agreement with our findings. Some reports have shown that in mice exposed to mild heat stress, which can lead to oxidative stress, apoptotic spermatogonia were rarer than late-type germ cells (39, 40). A variety of antioxidant defense systems protect cells against ROS. Among them, superoxide dismutases (SOD) are well demonstrated (41). It seems that the decrease in the level of SOD activity and expression of Cu/Zn SOD, as well as of levels of Zn, as spermatogenesis progress are the reasons for the vulnerability of advanced stage germ cells to ROS attack. Thus, the high levels of Cu/Zn, SOD, and Zn in spermatogonia may render them less susceptible to ROS attack (38).
Conclusion
Our study clearly demonstrated that endotoxemia induced by LPS in mice has deleterious long-term effects on spermatogonia and later stage germ cells according to the histomorphometrical evaluations. So that instead of SND diameter the other evaluated parameters rebounded at the end of the second spermatogenesis cycle.
Acknowledgment
This study was financially supported by a grant for research works by Vice Chancellor of Research of Shahid Bahonar University of Kerman, Kerman, Iran.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Sarkar O, Bahrainwala J, Chandrasekaran S, Kothari S, Mathur PP, Agarwal A. Impact of inflammation on male fertility. Front Biosci (Elite Ed) 2011;3:89–95. doi: 10.2741/e223. [DOI] [PubMed] [Google Scholar]
- 2.Lynn WA, Cohen J. Adjunctive therapy for septic shock: a review of experimental approaches. Clin Infect Dis. 1995;20:143–158. doi: 10.1093/clinids/20.1.143. [DOI] [PubMed] [Google Scholar]
- 3.Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res. 2004;24:271–281. doi: 10.1089/107999004323065057. [DOI] [PubMed] [Google Scholar]
- 4.Hailman E, Vasselon T, Kelley M, Busse LA, Hu MC, Lichenstein HS, et al. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 5.Zhou J, Schmidt M, Johnston B, Wilfart F, Whynot S, Hung O, et al. Experimental endotoxemia induces leukocyte adherence and plasma extravasation within the rat pial microcirculation. Physiol Res. 2011;60:853–859. doi: 10.33549/physiolres.932054. [DOI] [PubMed] [Google Scholar]
- 6.Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- 7.Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, et al. Endotoxemic renal failure in mice: Role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int. 2001;59:2243–2249. doi: 10.1046/j.1523-1755.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull AV, Lee S, Rivier C. Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Ann N Y Acad Sci. 1998;840:434–43. doi: 10.1111/j.1749-6632.1998.tb09582.x. [DOI] [PubMed] [Google Scholar]
- 9.O'Bryan MK, Schlatt S, Phillips DJ, de Kretser DM, Hedger MP. Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. 2000;141:238–246. doi: 10.1210/endo.141.1.7240. [DOI] [PubMed] [Google Scholar]
- 10.Hales KH, Diemer T, Ginde S, Shankar BK, Roberts M, Bosmann HB, et al. Diametric effects of bacterial endotoxin lipopolysaccharide on adrenal and Leydig cell steroidogenic acute regulatory protein. Endocrinology. 2000;141:4000–4012. doi: 10.1210/endo.141.11.7780. [DOI] [PubMed] [Google Scholar]
- 11.Reddy MM, Mahipal SV, Subhashini J, Reddy MC, Roy KR, Reddy GV, et al. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod Toxicol. 2006;22:493–500. doi: 10.1016/j.reprotox.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Kajihara T, Okagaki R, Ishihara O. LPS-induced transient testicular dysfunction accompanied by apoptosis of testicular germ cells in mice. Med Mol Morphol. 2006;39:203–208. doi: 10.1007/s00795-006-0334-7. [DOI] [PubMed] [Google Scholar]
- 13.Bosmann HB, Hales KH, Li X, Liu Z, Stocco DM, Hales DB. Acute in vivo inhibition of testosterone by endotoxin parallels loss of steroidogenic acute regulatory (StAR) protein in Leydig cells. Endocrinology. 1996;137:4522–4525. doi: 10.1210/endo.137.10.8828518. [DOI] [PubMed] [Google Scholar]
- 14.Brecchia G, Cardinali R, Mourvaki E, Collodel G, Moretti E, Dal Bosco A, et al. Short- and long-term effects of lipopolysaccharide-induced inflammation on rabbit sperm quality. Anim Reprod Sci. 2010;118:310–316. doi: 10.1016/j.anireprosci.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Collodel G, Castellini C, del Vecchio MT, Cardinali R, Geminiani M, Rossi B, et al. Effect of a bacterial lipopolysaccharide treatment on rabbit testis and ejaculated sperm. Reprod Domest Anim. 2012;47:372–378. doi: 10.1111/j.1439-0531.2011.01882.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 17.Azizollahi S, Babaei H, Derakhshanfar A, Oloumi MM. Effects of co-administration of dopamine and vitamin C on ischaemia-reperfusion injury after experimental testicular torsion-detorsion in rats. Andrologia. 2011;43:100–105. doi: 10.1111/j.1439-0272.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- 18.Kheirandish R, Askari N, Babaei H. Zinc therapy improves deleterious effects of chronic copper administration on mice testes: histopathological evaluation. Andrologia. 2014;46:80–85. doi: 10.1111/and.12047. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Aoyama-Ishikawa M, Kamoshida S, Nishino S, Sasano M, Oka N, et al. Endogenous interleukin 18 regulates testicular germ cell apoptosis during endotoxemia. Reproduction. 2015;150:105–114. doi: 10.1530/REP-14-0427. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe-Fukunaga R, Brannan CI, Itoh N, Yonehara S, Copeland NG, Jenkins NA, et al. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992;148:1274–1279. [PubMed] [Google Scholar]
- 21.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 22.Palladino MA, Savarese MA, Chapman JL, Dughi MK, Plaska D. Localization of Toll-like receptors on epididymal epithelial cells and spermatozoa. Am J Reprod Immunol. 2008;60:541–555. doi: 10.1111/j.1600-0897.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 23.Sahnoun S, Sellami A, Chakroun N, Mseddi M, Attia H, Rebai T, et al. Human sperm Toll-like receptor 4 (TLR4) mediates acrosome reaction, oxidative stress markers, and sperm parameters in response to bacterial lipopolysaccharide in infertile men. J Assist Reprod Genet. 2017;34:1067–1077. doi: 10.1007/s10815-017-0957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhushan S, Schuppe HC, Fijak M, Meinhardt A. Testicular infection: microorganisms, clinical implications and host-pathogen interaction. J Reprod Immunol. 2009;83:164–167. doi: 10.1016/j.jri.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Jegou B, Cudicini C, Gomez E, Stephan JP. Interleukin-1, interleukin-6 and the germ cell-Sertoli cell cross-talk. Reprod Fertil Dev. 1995;7:723–730. doi: 10.1071/rd9950723. [DOI] [PubMed] [Google Scholar]
- 26.Lin T, Wang D, Stocco DM. Interleukin-1 inhibits Leydig cell steroidogenesis without affecting steroidogenic acute regulatory protein messenger ribonucleic acid or protein levels. J Endocrinol. 1998;156:461–467. doi: 10.1677/joe.0.1560461. [DOI] [PubMed] [Google Scholar]
- 27.Troiano L, Fustini MF, Lovato E, Frasoldati A, Malorni W, Capri M, et al. Apoptosis and spermatogenesis: evidence from an in vivo model of testosterone withdrawal in the adult rat. Biochem Biophys Res Commun. 1994;202:1315–1321. doi: 10.1006/bbrc.1994.2074. [DOI] [PubMed] [Google Scholar]
- 28.Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30:119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadasivam M, Ramatchandirin B, Ayyanar A, Prahalathan C. Bacterial lipopolysaccharide differently modulates steroidogenic enzymes gene expressions in the brain and testis in rats. Neurosci Res. 2014;83:81–88. doi: 10.1016/j.neures.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, et al. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci U S A. 2006;103:18975–18980. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jegou B, Risbridger GP, de Kretser DM. Effects of experimental cryptorchidism on testicular function in adult rats. J Androl. 1983;4:88–94. doi: 10.1002/j.1939-4640.1983.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 32.Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology. 2003;144:509–517. doi: 10.1210/en.2002-220710. [DOI] [PubMed] [Google Scholar]
- 33.Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 34.Rao F, Tian H, Li W, Hung H, Sun F. Potential role of punicalagin against oxidative stress induced testicular damage. Asian J Androl. 2016;18:627–632. doi: 10.4103/1008-682X.168792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 36.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 37.Manna SK, Aggarwal BB. Lipopolysaccharide inhibits TNF-induced apoptosis: role of nuclear factor-kappaB activation and reactive oxygen intermediates. J Immunol. 1999;162:1510–1518. [PubMed] [Google Scholar]
- 38.Celino FT, Yamaguchi S, Miura C, Ohta T, Tozawa Y, Iwai T, et al. Tolerance of spermatogonia to oxidative stress is due to high levels of Zn and Cu/Zn superoxide dismutase. PLoS One. 2011;6:e16938. doi: 10.1371/journal.pone.0016938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul C, Murray AA, Spears N, Saunders PT. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction. 2008;136:73–84. doi: 10.1530/REP-08-0036. [DOI] [PubMed] [Google Scholar]
- 40.Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80:913–919. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]


