Abstract
Objective(s):
Human Wharton’s Jelly mesenchymal stem cells (hWMSCs) are undifferentiated cells commonly used in regenerative medicine. The aim of this study was to develop a reliable tool for tracking hWMSCs when utilized as therapeutics in burnt disorders and also to optimize the cell-based treatment procedure.
Materials and Methods:
The hWMSCs were first isolated from fresh umbilical cord Wharton’s jelly and cultured. The 293LTV cell line was transfected by cGFP containing lentiviral vector and the helper plasmids for production of the viral particle. The viral particles were collected to transduce the hWMSCs. The transduced cells were finally selected based on resistance to puromycin. The burned rats (n=24) were treated with cGFP expressing hWMSCs using the cell spray method, with the cells being tracked 7, 14 and 21 days later. The rats were sacrificed 7, 14 and 21 days following treatment and paraffin embedded sections prepared from the burned area for downstream pathological analyses.
Results:
The lentiviral particles carrying the cGFP gene were generated and the hWMSCs were transduced. The cGFP-expressing hWMSCs were detected in the burned tissue and the burned injuries were improved dramatically as compared to control.
Conclusion:
Because of the establishment of stably transduced cGFP expressing cells and the ability to detect cGFP for a relatively long-time interval, the method was found to be quite efficient for the purpose of cell tracking. The combination of hWMSC-based cell therapy and sterile Gauze Vaseline (GV) as covering was proven much more efficient than the traditional methods based on GV alone.
Keywords: Cell and tissue-based –therapy, Cell tracking, Lentivirus, Mesenchymal stem cells, Wound healing
Introduction
Non-healing wounds or injuries are emerging as a common and exorbitant public health issue. The problem is further complicated by the upward trend in average age in many populations. Other wound healing complications arise because of injuries due to burns, accidents and so forth. Therefore, the availability of a reliable and cost-effective approach to circumvent the aforementioned wound healing obstacles is indispensable (1, 2). Wound coverage is a key step and standard care for rescuing severe burn patients (3). On the other hand, the use of an autologous skin substitute is desirable, but can be hampered by the autogenous skin source and its preparation, which is time-consuming. Meanwhile, in case of using xenograft as a substitution, the primary problems are associated with antigen rejection reactions and the infections (4). Furthermore, bioengineered skin substitutes are still in the experimental stage. Therefore, novel and effective therapies for promoting wound healing in severe burn patients are needed.
Mesenchymal stem cells (MSCs) that can be isolated from multiple human tissues with the capacities of self-renewal and multipotent differentiation are a promising source for substitution of damaged tissues. Human Wharton’s jelly MSCs (hWMSCs), compared to other original MSCs, have many advantages including the availability of a plentiful and inexpensive source of cells, short doubling time, high capacity of proliferation, lower immunogenicity, and safety (5-7). The effect of umbilical cord MSCs on wound healing in severe burns has been studied in animal models (8-12).
On the other hand, human bone marrow MSC (BM-MSC) transplantation could effectively improve wound healing in mouse models of burn injuries. Surface wound healing was significantly accelerated when GFP-expressing BM-MSCs were applied to the wound surface in burned mice (8).
In another study, burned rats were received GFP-expressing human umbilical cord MSCs intravenously and were tracked by in vivo bioluminescence imaging (9). Wound healing was significantly improved in mice, which had received MSCs compared to a control group treated with phosphate buffered saline (PBS).
In this study, GFP-expressing hWMSCs were transplanted into burn rat models by cell spray transplantation after the creation of injury. Wound healing was monitored by taking photographs, and GFP-containing MSCs were tracked by bioluminescence imaging in the surface and internal organs over time. In the meantime, tissue biopsies for investigation of pathological changes were taken and compared with control groups.
Materials and Methods
Strains and reagents
The lentiviral vector plasmids were a gift from Tronolab (The EPFL University). The monoclonal antibodies against CD45, CD105, CD34, and CD44 were purchased from sigma (St. Louis, MO). The Mega Prep. Plasmid extraction kit was obtained from Macherey-Nagel & Co.KG (Germany). DMEM high glucose GlutaMAX™ and fetal bovine serum (FBS) were obtained from Gibco (USA). Escherichia coli (DH5α) was used for plasmid extraction. The 293LTV cell line used for the production of lentiviral particles was purchased from Iran’s Pasteur Institute (Tehran, Iran). With the informed consent and permission from the local ethics committee at Shiraz University of Medical Sciences, the umbilical cords (n=4) were obtained from full-term consenting caesarean patients at Ghadir Mother and Child Hospital (Shiraz, Iran), in sterile conditions. The adult male albino rats (n=24) were purchased from Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences (Shiraz, Iran).
Isolation of hWMSCs from Wharton’s jelly of umbilical cord
The obtained umbilical cords were washed with PBS (pH=7.2) to remove the blood, minced into 2-mm2 pieces and transferred to 10-cm2 culture plates containing DMEM F12 supplemented with 10% FBS, penicillin (100 μg/ml) and streptomycin (100 μg/ml) (explant method with some modifications; for more information see ref 13). The plates containing Wharton’s gel were incubated at 5% CO2, 37 °C and 95% of relative humidity. After reaching 70% to 80% confluence (1.5×106 cells per petri dishes, n=16), adherent cells were harvested by 0.05 % trypsin-EDTA (Gibco, Germany) and centrifuged (150 x g for 3 min). Cells were then diluted in sterile PBS for subsequent experiments.
Immunophenotyping of hWMSCs by flow cytometry
To confirm the derived MSCs, specific cell-surface antigens including CD45, CD44, CD34 and CD105 (Sigma, Germany) were probed using monoclonal antibodies and compared with cells treated with control isotype antibodies. The antibody stained cells (about 0.5 ×106 cells per petri dishes) were evaluated by FACS Calibur flow cytometer (Becton Dickinson, NJ, USA), with at least 10000 events being analyzed.
Extraction of the plasmids for the production of lentiviral particles
The plasmids used for production of lentiviral particles were transformed into E. coli DH5α for subsequent large-scale extraction. Five milliliters of the three plasmid-containing bacteria were transferred into 500 ml of fresh LB medium containing 100 µg/ml ampicillin. The cultures were grown overnight with shaking at 200 rpm and 37 °C in an orbital shaker incubator. The bacteria were harvested by centrifugation at 8000 g and 4 °C for 15 min. The resulting bacterial pellets were used for plasmid extraction using NucleoBond® PC 2000 extraction kit. The same procedure was performed for each of the three plasmids separately. The concentration of the purified plasmids was finally determined by spectrophotometric monitoring (Absorbance or A of 260 nm – A 320 nm, and purity of plasmid DNA was assessed at A260/A230 nm).
Production of lentiviral vectors
The cGFP-expressing lentiviral particles were generated by the co-transfection of 293LTV cells with lentiviral plasmids, psPAX2 (containing gag and pol genes), and pMD2.G (containing VSV-G gene) (Applied System Biosciences, USA) using calcium phosphate protocol (14). Briefly, 293LTV cells were plated in 10 ml complete media containing DMEM high glucose GlutaMAX™ and were grown until reaching 70% confluence (5×105 cells in a 10 cm cell culture plate). For each dish, 912 µl of buffered water was mixed with 33 µl of 1X TE buffer, then 21 µg of vector construct, 21 µg of pPAX2, and 10.5 µg of pPMD2.G were added to the mixture, and 105 µl of CaCl2 solution (2.5 M) with 1050 µl of 2X Hank’s buffered salt solution (HBSS) buffer were added dropwise. The solution was added into 293LTV cell culture. The medium was removed around 14-16 hr post-transfection and then the culture medium was changed. 48 hr after replacing the medium, the lentiviral particles in supernatant were collected and filtered through a 0.45 µm filter.
Transduction of hWMSCs using lentiviral vector containing cGFP gene
The hWMSCs at passage 3 with 70–80% confluence (6×104 cells per well in 4 well cell culture plates) were used for transduction. The cells were transduced with lenti-cGFP at a multiplicity of infection (MOI) of 16904. After transduction, the cells were exposed to 2 μg/ml puromycin for 2 days to obtain stable transduction.
Animal model
All procedures were approved by the committee of animal care at Shiraz University of Medical Sciences and were conducted in accordance with its established guidelines. The 24 adult male albino rats (180-200 g, 6-8 weeks old) were used. During this study, rats were kept one per coop under the retained situation (21± 2 °C, 65-70% of relative humidity and free access to water and food). The rats were subjected to intraperitoneal anesthetic induction with ketamine/xylazine (KX) (Alfasan Co, Nederland) 90 mg/kg ketamine and 9.0 mg/kg xylazine. euthanasia of animal model was performed under standard guidelines of Animal Research Advisory Committee for Rodents Using Carbon Dioxide (15).
The proximal parts of the rats’ neck were shaved. For the induction of 3rd-degree burns (20% of total body surface area or TBSA), the brass bar technique was used (The area of the square-like head of the brass bar was 1.3-cm2 capable of reaching 105 °C and chosen on the basis of the average weight of the rats used) (16). The thermal burn was used to produce the lesion by means of direct contact for 5 sec. After 12 hr, the necrosis place of wounds was excised out (with 1 mm of satellite skin) using a scalpel under the general anesthesia condition. To prevent infection, the wounds were disinfected with 70’ ethanol (for 1 sec) then washed with normal saline (for 30 sec). To prevent dehydration, wounds were sutured with sterile Gauze Vaseline (GV). Rats were euthanized at the 7, 14 and 21 days after the creation of burn wound in order to imaging and biopsy collecting processes.
hWMSCs cells spray into the wounds
The burned rats were divided randomly into 2 groups. Group one (or GV+CS or GV+ Cell Spray) received 5x105 hWMSCs+cGFP using cell spray method (17). Briefly, the wounds were then covered with sterile GV to keep them clean and prevent contamination by foreign bodies and to reduce the risk of bacterial infection. Group two (or GV) were received no hWMSCs, but the wounds were only covered with sterile GV dressing. Healing of wounds was checked every day.
Fluorescence detection of cGFP expression in tissues of the burned rats
Four rats in each group were euthanized at days 7, 14, and 21 post-hWMSCs therapy. The hWMSCs+cGFP were tracked by Kodak in vivo imaging system F-Pro IS4000MM Pro (America) (cat no 811 6634) inside the burned area. In addition to tracking the expression of cGFP in the wounds, the rats were sacrificed for pathological examination and cGFP tracking in the major organs including the heart, liver, testis, lung and kidneys.
Histological analysis of excised burn tissues
The specimens from burned tissues were isolated and fixed in 10% formalin and stored at room temperature. Then, the preserved tissues were embedded in paraffin and sectioned using microtome in a thickness of 5 µm. Following deparaffinization, the tissues were stained with haematoxylin-eosin according to standard procedures (18) and finally examined using optical microscopy.
Results
Isolation and characterization of hWMSCs
The hWMSCs were isolated successfully from umbilical cord sample, cultured and became confluent after 14 days. The hWMSCs formed a homogenous monolayer of adherent, spindle-shaped fibroblast-like cells in primary culture. The purity of isolated cells was evaluated by flow cytometry. The cells were highly positive for CD44 (99%) and CD105 (99%) and negative for CD34 (98%) and CD45 (99%) as confirmed by investigation of the surface antigens of cells in passage 3 by flow cytometry (Figure 1).
Figure 1.
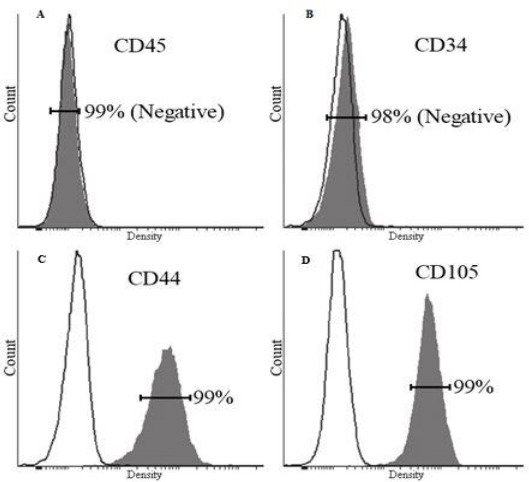
Evaluation of the cell surface antigens of hWMSCs; hWMSCs were negative for CD45 (99%) (A), CD34 (98%) (B), and positive for CD44 (99%)(C) and CD105 (99%) (D).
Production and titration of lentiviral vector-cGFP and transduction of hWMSCs
The 293LTV cell lines were co-transfected by the lentiviral expression (pPCDH-cGFP), packaging (psPAX2) and envelope (pPMD2.G) plasmids. The expression of cGFP was confirmed by fluorescence microscopy. Approximately, 70% of the transfected cells were positive for cGFP, as compared to untreated cells. cGFP-expressing cells were calculated to be 61.2% compared to the control group (Figure 2). The hWMSCs were transduced using the lentiviral particles carrying cGFP. Finally, clusters of hWMSCs were observed and used for future applications (Figure 3).
Figure 2.
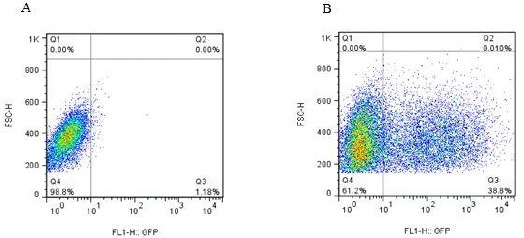
Evaluation of the lentiviral vector titration. (A) The control 293LTV cells were not treated with lentiviral vectors. About 98.8% of cells do not express cGFP as indicated in the FL1 to forward-scattered light (FSC) diagram. (B) The 293LTV cells treated with lentiviral vectors harboring the cGFP gene. As illustrated in the FL1 to FSC diagrams, 38.8% of the treated cells express the cGFP
Figure 3.
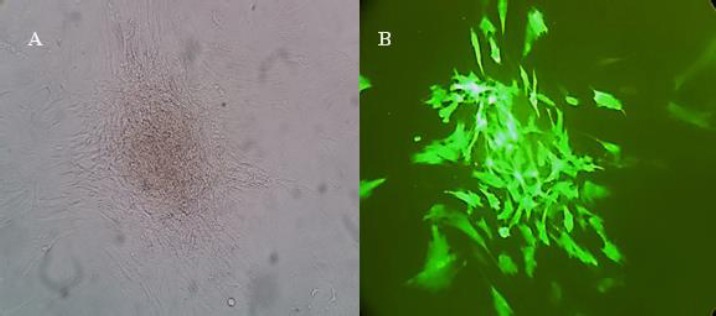
Mesenchymal stem cells (MSCs) transduced with lentiviral controls. (A) The cells were clustered after being treated with puromycin antibiotic. (B) The same cell cluster as visualized by fluorescent microscope.
In vivo tracking of hWMSCs in tissues excised out of burned area
In order to corroborate the contribution of hWMSCs to the wound healing process, their presence in the burned area was further confirmed in tissues excised out of such regions (emission = 535 nm and excitation = 470 nm) (Figure 4, 5). One, 2 and 3 weeks after treatment, tissue biopsies were obtained from the burned areas of the treated and untreated animals. In addition, other vital organs including the heart, kidneys, testes, lung, liver and skin were also investigated for the presence of cGFP. cGFP-expressing hWMSCs were detected in the tissues obtained from the burned area as patches of cells. On the other hand, no cGFP signal was observed in control rats, which had not received hWMSCs and other vital organs from both treated and untreated rats (Figure 6).
Figure 4.
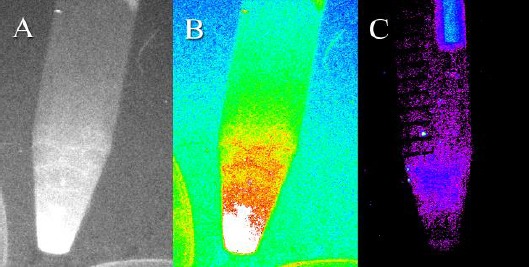
Evaluation of the fluorescent signal in cGFP-expressing human Wharton’s Jelly mesenchymal stem cells (hWMSCs) and untreated cells. Detection of the fluorescent signal in cGFP-expressing MSCs by the Kodak in vivo Imaging System F-Pro device under (A) color and (B) gray mode. (C) No fluorescent signal was detected when the control hWMSCs were evaluated using the same instrument.
Figure 5.
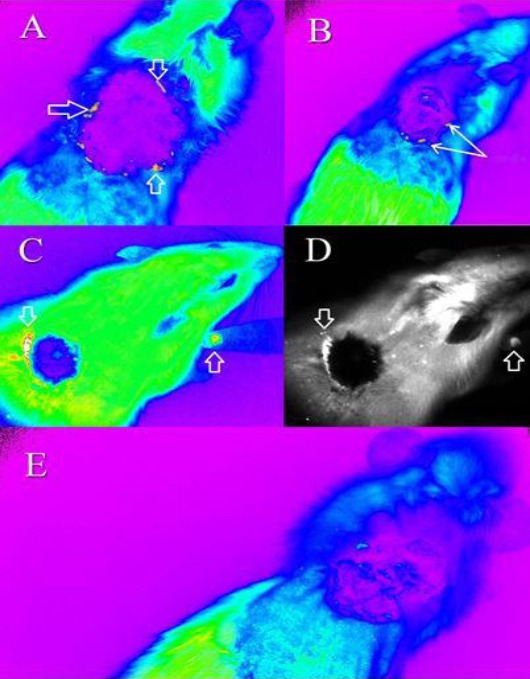
Tracking of the cGFP-expressing mesenchymal stem cells (MSCs) using the Kodak in vivo Imaging System F-Pro device. The MSCs were tracked in treated rats in color mode (A) 1 week after treatment (B) 2 weeks after treatment (C) 3 weeks after treatment. (D) Indicates the cells shown in (C) in gray mode. (E) The control rat treated with gauze vaseline after 1 week. The cGFP expressing MSCs could be detected in close proximity to the burnt area as evident by their greater intensity. The cells are indicated by the arrows.
Figure 6.
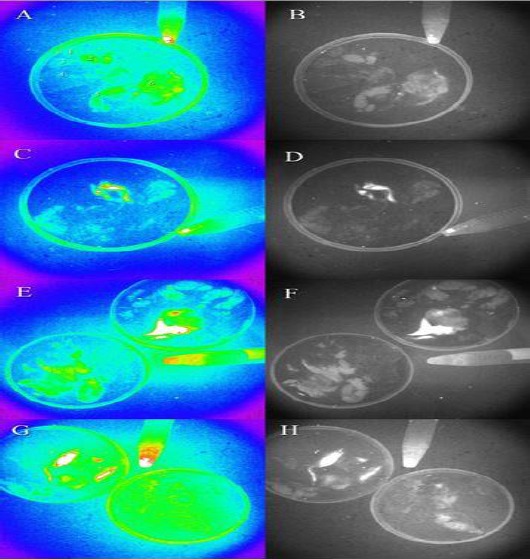
Tracking of the cGFP expressing cells in tissues excised out of the burned area. (A) No cGFP signal was detected in the tissues obtained from the control group treated only with gauze vaseline (the tissues investigated involve the kidneys, heart, lungs, liver, testes and skin designated with 1, 2, 3, 4, 5 and 6, respectively). (B) Indicates the same tissues visualized in gray mode. (C) One week after treatment, cGFP expression was detected in skin tissues excised out of the burnt area but not in other vital organs. (D) Indicates the same tissues shown in (C) under gray mode. (E) Two weeks after treatment, the cGFP expression is evident as a patch of cells and no expression is observed in the control group (the tissues in the left plate). (F) The same tissues in (E) analyzed in gray mode. (G) cGFP expression after three weeks of treatment is quite evident in several patches of cells. No expression is detectable in the control group (the plate on the right). (H) The same tissues in gray mode. The cells at the bottom of the falcon are cGFP-expressing hWMSCs
Pathological analysis
The pathological analyses performed on burned tissues treated with hWMSCs and GV indicate a high degree of re-epithelialization compared to the control group treated with GV alone (Table 1). Moreover, hemorrhage was also completely ceased by the end of the second week (or sooner) relative to the control group in which a mild hemorrhage state continued to the third week (when the last evaluation was implemented). Although the cells had been xenografted from human to the burned rats, no inflammation was observed until the third week. Furthermore, the degree of granulation tissue forming improved from early (1+) to scar (3+) states from the beginning to the completion of the cell therapy procedure in the treatment group. However, the degree of granulation tissue forming did not change in the control group and remained in the progressive state until the end of study (Figure 7 A-D).
Table 1.
Pathological studies on the wound and wound surrounding cells of the groups
| Inflammation | Hemorrhage | Granulation tissue | Re-epithelialization | Histopathology Group |
|---|---|---|---|---|
| No | 1+ (or Mild) | 1+ (or Early) | None | GV1 |
| No | 1+ | 2+ | 1+ (Incomplete or Mild) | GV2 |
| No | 1+ | 2+ | 1+ | GV3 |
| No | 1+ | 1+ | 1+ | GV+CS 1 |
| No | No | 3+ (Scar) | 2+ | GV+CS 2 |
| No | No | 3+ (Scar) | 3+ (Complete) | GV+CS 3 |
GV1, GV2, and GV3: Group GV1, 2 or 3 were control groups treated with Gauze Vaseline only, at 1st , 2nd , and 3rd week after the creation of extensive burn wound injury. GV+CS 1, GV+CS 2, and GV+CS 3: Groups were treated with human Wharton’s jelly mesenchymal stem cells (hWMSCs) and Gauze Vaseline at 1st , 2nd , and 3rd week after the creation of extensive burn wound injury
Figure 7.
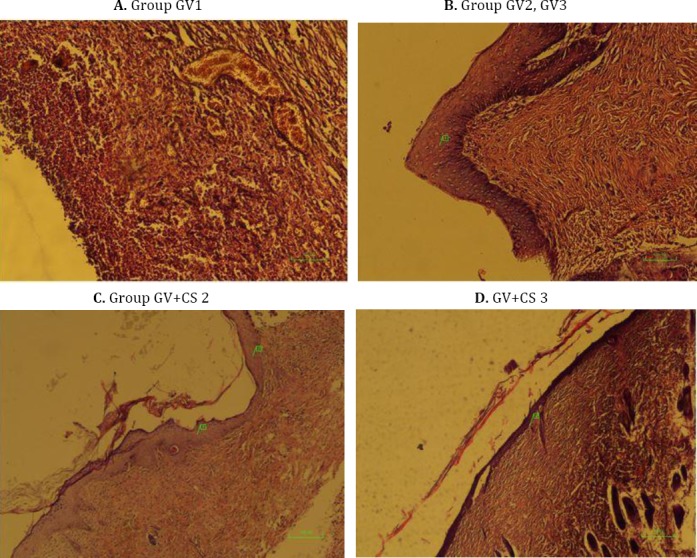
A. Group Gauze Vaseline 1 (GV1): No re-epithelialization with early granulation tissue and hemorrhage is seen (×100, H&E). B. Group GV2, GV3: Re-epithelialization begins, as acanthotic epidermis, at the margin of the ulcer (1). More granulation tissue is seen (2+ or progressive). C. Group GV+CS 2: Progressive re-epithelialization (2+) (1, 2) with mature granulation tissue (Scar) (3+) is seen (×40, H&E). D. GV+CS 3: Complete re-epithelialization (3+) (2) with mature scar tissue is seen (×40, H&E)
Discussion
The speed of burn wound healing in burned skin patients has high priority. Nowadays, MSCs have found common use in many cases of wound healing protocols (19). However, using of suitable cell source in cell therapy is still controversial (20). Many studies show that MSCs with mesodermal origin are reliable for skin injury treatments (21). There are few sources for obtaining of MSCs from the human body. So far, human bone marrow MSCs have been used in many studies to speed up the burn wound healing process (12).
A wide variety of efforts has been made hitherto to reveal the mechanisms of cell therapy in wound healing of burned patients. In this study, cGFP-expressing cells were employed to track the hWMSCs in burned rats. Finding of the present study suggests that there is no cGFP containing hWMSC in other vital organs including the heart, kidneys, testes, lung and liver. The cGFP protein emits a brighter light compared to ordinary GFP and allows detection of even a small number of cells (22). The rats were analyzed at specific time intervals and the burned areas (alongside other vital organs including the heart, liver, kidneys, testis and lung) were investigated for the presence of cGFP-expressing hWMSCs using the imaging instrument. Endpoint measurements were evaluated by detection of hairs on the burned area and by the analysis of pathological slides during this study. In many cases, emergence of hair follicles showed the complete healing of burn (or the other types of) wounds (23).
Cell therapy implemented through the administration of cells to the blood stream leads to accumulation of injected cells in other organs including the lungs (24). hWMSCs can become differentiated to cells of their destination tissue and secrete tissue-specific growth factors (25). Therefore, to direct their differentiation and secretion of growth factors in a tissue-specific manner, the cell spray technology was employed. Consequently, using the cell spray method, the hWMSCs can be localized to the burned area in the skin, differentiated to dermal cells and secrete anti-inflammatory cytokines specifically (26).
Maintaining the tissue humidity is of paramount importance in methods used for burned wound healing. Sterile GV is most commonly used to prevent the burned wound dehydration. A combination of GV and cell therapy was used and found to be much more efficient than control groups treated only with GV.
Conclusion
Cell therapy using methods of localized cell delivery heralds a new era in wound healing strategies and this study can be the basis for future burned healing protocols using hWMSCs.
Acknowledgment
We would like to thank Laboratory staff of Animal Center of Shiraz University of Medical Sciences, Shiraz, Iran for their support, and Office of Vice Chancellor for Research of Shiraz University of Medical Sciences, Shiraz, Iran for financial support.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Leclerc T, Thepenier C, Jault P, Bey E, Peltzer J, Trouillas M, et al. Cell therapy of burns. Cell Prolif. 2011;44:48–54. doi: 10.1111/j.1365-2184.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kagan RJ, Peck MD, Ahrenholz DH, Hickerson WL, Holmes J, 4th, Korentager R, et al. Surgical management of the burn wound and use of skin substitutes: an expert panel white paper. J Burn Care Res. 2013;34:e60–79. doi: 10.1097/BCR.0b013e31827039a6. [DOI] [PubMed] [Google Scholar]
- 3.Puri V, Khare NA, Chandramouli MV, Shende N, Bharadwaj S. Comparative analysis of early excision and grafting vs delayed grafting in burn patients in a developing country. J Burn Care Res. 2016;37:278–282. doi: 10.1097/BCR.0b013e31827e4ed6. [DOI] [PubMed] [Google Scholar]
- 4.Hermans MH. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference? Burns. 2014;40:408–415. doi: 10.1016/j.burns.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Troyer DL, Weiss ML. Concise review: Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong CY, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton's jelly. Reprod Biomed Online. 2007;15:708–718. doi: 10.1016/s1472-6483(10)60539-1. [DOI] [PubMed] [Google Scholar]
- 7.Baksh D, Yao R, Tuan RS. Comparison of Proliferative and Multilineage Differentiation Potential of Human Mesenchymal Stem Cells Derived from Umbilical Cord and Bone Marrow. STEM CELLS. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 8.Xue L, Xu YB, Xie JL, Tang JM, Shu B, Chen L, et al. Effects of human bone marrow mesenchymal stem cells on burn injury healing in a mouse model. Int J Clin Exp Pathol. 2013;6:1327–1336. [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Yu Y, Hou Y, Chai J, Duan H, Chu Wl, et al. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Promotes Cutaneous Wound Healing of Severe Burned Rats. PLoS ONE. 2014;9:1–7. doi: 10.1371/journal.pone.0088348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns. 2007;33:282–291. doi: 10.1016/j.burns.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Deng Z, Han S, Liu T, Wen N, Lu W, et al. Tissue-engineered skin containing mesenchymal stem cells improves burn wounds. Artif Organs. 2008;32:925–931. doi: 10.1111/j.1525-1594.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15:18–26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 13.Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, et al. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly. In Vitro Cell Dev Biol. 2012;48:75–83. doi: 10.1007/s11626-011-9480-x. [DOI] [PubMed] [Google Scholar]
- 14.Klages N, Zufferey R, Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol Ther. 2000;2:170–176. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- 15.Wong D, Makowska IJ, Weary DM. Rat aversion to isoflurane versus carbon dioxide. Biology letters. 2012;9:1–4. doi: 10.1098/rsbl.2012.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer Tufi Neder, Silva Alcino Lázaro da. A standard burn model using rats. Acta Cir. Bras. 1999:14. [Google Scholar]
- 17.Navarro FA, Stoner ML, Park CS, Huertas JC, Lee HB, Wood FM, et al. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine micro-wound model. J Burn Care Rehabil. 2000;21:513–518. doi: 10.1097/00004630-200021060-00007. [DOI] [PubMed] [Google Scholar]
- 18.Fischer A H, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 2008:3. doi: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 19.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacologica Sinica. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korbling M, Estrove Z. Adult stem cells for tissue repair-a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 21.De Girolamo L, Lucarelli E, Alessandri G, et al. Mesenchymal Stem/Stromal Cells: A New “Cells as Drugs” Paradigm. Efficacy and Critical Aspects in Cell Therapy. Current Pharmaceutical Design. 2013;19:2459–2473. doi: 10.2174/1381612811319130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marguerie E, Michael P. Scherrer, Frank D. Ferrari, Mikhail V. Matz. Very Bright Green Fluorescent Proteins from the Ponellid Copepod Pontella mimocerami. PLoS ONE. 2010;5:e11517. doi: 10.1371/journal.pone.0011517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yannas Ioannis V. Tissue and organ regeneration in adults. Springer Publishing Co; 2015. [Google Scholar]
- 24.Ankrum J, Jeffrey M K. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends in Molecular Medicine. 2010;5:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010:2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko IK, Lee SJ, Atala A, Yoo JJ. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013;45:e57. doi: 10.1038/emm.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]


