Abstract
Introduction
Laparoscopic total extraperitoneal (TEP) inguinal hernia repair is an effective and safe method for the treatment of inguinal hernia. There are very few studies on regional anesthesia methods in TEP surgery.
Aim
To compare TEP inguinal hernia repair performed when the patient was treated under spinal anesthesia (SA) with that performed under general anesthesia (GA).
Material and methods
All total of 80 patients were studied between December 2015 and March 2017. Hyperbaric bupivacaine and fentanyl were used for SA, to achieve a sensorial level of T3. Propofol, sevoflurane, rocuronium, fentanyl, and tracheal intubation were used for GA. Intraoperative events related to SA, surgical times, intra- and postoperative complications, and pain scores were recorded.
Results
The mean operative time in the SA TEP group was 70.2 ±6.7 min, which was significantly longer than the mean operative time in the GA TEP group of 67.2 ±6.2 min (p < 0.038). The mean pain scores in the SA TEP group were 0.23 ±0.42 at the first hour, 1.83 ±0.64 at 6 h and 1.28 ±0.45 at 24 h. These scores were significantly lower than the corresponding scores of 5.18 ±0.84 (p < 0.001), 2.50 ±0.55 (p < 0.001) and 1.58 ±0.55 in the GA TEP group. Generally, patients were more satisfied with SA than GA (p < 0.004).
Conclusions
Spinal anesthesia TEP is significantly less painful in the early postoperative period, leading to earlier ambulation than GA TEP. Additionally, SA TEP results in significantly less need for analgesics and better patient satisfaction results. SA TEP seems to be a better alternative than the existing GA TEP.
Keywords: general anesthesia, spinal anesthesia, laparoscopic repair
Introduction
Inguinal hernia repair is one of the most commonly performed elective surgical procedures in the world. However, there is no common consensus on which surgical technique should be used. Hernia recurrence, wound infection, scrotal edema, bleeding and chronic pain are the complications that disturb surgeons in the postoperative period. Many techniques have been described from the first inguinal hernia repair until today [1]. After laparoscopic techniques were introduced in surgery, a new era began for hernia repair. However, with the emergence of minimally invasive approaches to inguinal hernia repair, a new discussion has since been started, regarding “the best hernia repair” [2].
Laparoscopic techniques offer some advantages, such as less pain, less analgesic requirement, lower wound infection rate, better cosmetic result and early return to work, in the early postoperative period [2, 3]. In 2001, the National Institute for Clinical Excellence (NICE) [4] examined more than 40 randomized controlled trials. It was reported that the laparoscopic repair method was much better than the open inguinal hernia repair method, because it was associated with less pain and faster recovery [4]. However, the surgery cost is quite high compared to open methods. Approximately 15–20% of inguinal hernia repairs in the United States are performed laparoscopically [5]. Total extraperitoneal (TEP) inguinal hernia repair is the most preferred method among laparoscopic techniques. Contrary to other laparoscopic techniques, the abdominal cavity is not entered in the TEP technique, and therefore complications such as organ injury and postoperative ileus do not develop. However, the disadvantage of the TEP technique is that its learning curve is difficult and its consequences depend on the surgeon’s experience [6]. Total extraperitoneal is conventionally performed under general anesthesia (GA). The reason for this is that GA provides full progressive muscle relaxation [7, 8]. However, it has recently been reported that TEP has been performed under regional anesthesia [9–11]. Conversely, there are very few studies comparing regional anesthesia and GA.
Aim
In this study, we compared the advantages and disadvantages of GA and spinal anesthesia (SA) in TEP operations.
Material and methods
This retrospective study was performed at the Haseki Training and Research Hospital in Istanbul, during December 2015 to March 2017, with two surgeons experienced in laparoscopic inguinal surgery. We retrospectively analyzed the 80 patients with inguinal hernia, who underwent laparoscopic procedures. Patients were divided into 2 groups: the GA group and the SA group. Forty patients undergoing GA and 40 patients undergoing SA were included in this study. Two surgeons performed the procedures under GA or SA. Both surgeons had at least 5 years of experience in laparoscopic inguinal hernia repair methods. The possible surgical methods, such as TEP, transabdominal preperitoneal (TAPP) and open repair, were explained to the patients preoperatively. They were also informed about the risk of conversion from the laparoscopic procedure to an open technique during surgery. Informed consent forms were obtained from all patients before the surgery.
Age, gender, height, weight, body mass index (BMI), and comorbid diseases of the patients were recorded. Duration of operation, duration of hospitalization, postoperative pain levels (visual analog score – VAS), and time to enteral feeding were evaluated and recorded.
Inclusion criteria for the study were as follows: 1) age: 18–70 years, both genders, 2) surgery: elective inguinal hernia. Exclusion criteria for the study were as follows: 1) American Society of Anesthesiologists (ASA) physical health grade > II, 2) age: < 18 or > 70 years, 3) body weight > 120 kg, height < 140 cm, 4) postspinal surgery, spinal deformity, 5) history of allergy to study drugs, 6) pregnancy, 7) coagulopathy, 8) neurological disorders, 9) strangled inguinal hernia, bilateral inguinal hernia, recurrent inguinal hernia.
The Haseki Training and Research Hospital Ethical Committee approved the study (21-June-2017/513).
Preoperative evaluation
A single-dose prophylaxis, with a combination of cefazolin sodium (1,000 mg intravenous (i.v.) Flac, Cefazol, Mustafa Nevzat), was given by i.v. infusion. All surgical operations were performed by two surgeons, and anesthesia techniques were performed by two anesthesiologists. Anesthesiologists provided information about GA and SA to all patients included in the study. Moreover, they informed the patients about anxiety, shoulder pain and abdominal discomfort related to SA. Detailed information about hernia surgery and anesthesia techniques were given to all patients included in the study.
Monitoring and operative data
All patients were monitored closely, by continuous electrocardiography, noninvasive arterial blood pressure, heart rate (HR) and peripheral oxygen saturation (SpO2). All these parameters were recorded in both groups, after the patients were taken to the operating room, for baseline levels during volume replacement, prior to surgery (these parameters were recorded four times at 2-min intervals). These parameters were recorded during the anesthesia induction in group 1, and after the anesthesia procedure in group II. The patients were monitored continuously during the surgery and during the 24-h period in the hospital room. All sociodemographic characteristics, ASA physical status classification, comorbidities, length of stay, operation time (time from the incision to the last suture) and total time (time from anesthesia induction to the postanesthesia care unit (PACU) for the GA group and time from spinal puncture to the PACU for the SA group) were recorded. In addition, the maximum sensory block level was recorded for the SA group. In terms of intraoperative evaluations in both groups, hypotension was defined as a reduction of > 30% in mean arterial pressure or systolic blood pressure < 90 mm Hg, bradycardia was defined as HR < 50 beats/min, and hypoxemia was defined as SaO2 < 90%. Side effects, such as nausea, vomiting, right shoulder pain, anxiety, and abdominal discomfort, were recorded in the SA group.
The pain scores were recorded at the postoperative 1st, 6th, 12th and 24th h. These scores were also recorded at the time of discharge, and during the follow-up visits, based on VAS: 0 indicating no pain, and 10 indicating severe pain. Side effects of SA and GA, such as nausea, vomiting, anxiety, shoulder pain, headache, and urinary retention, were recorded in both groups after the surgery. The patients were contacted again at the end of the 3rd month, at which time satisfaction was rated from 1 to 5 with Likert scale (1 – very satisfied, 2 – quite satisfied, 3 – neutral, 4 – quite dissatisfied, 5 – very dissatisfied). Patient satisfaction was assessed by an independent physician. The VAS scores and postoperative adverse effects of the patients were recorded by an independent anesthesiologist, who was unaware of the patients’ group assignment.
In the SA group, midazolam (0.015–0.030 mg/kg) was given to patients with anxiety and fentanyl (1–2 µg/kg) was given to patients with shoulder pain and abdominal discomfort. Patients who developed hypotension were first treated with 250 ml of saline solution for 5 min. If patients could not be treated with fluid replacement or if the systolic blood pressure was below 90 mm Hg, 5 mg of ephedrine HCl was administered. Atropine (0.5 mg) was administered by i.v., for the treatment of bradycardia. If VAS was ≥ 4 in both groups in the postoperative period, 50 mg of tramadol was administered i.v. with 100 ml of saline solution, for 30 min.
Statistical analysis
SPSS 15.0 for Windows was used for statistical analysis. In descriptive statistics, the categorical variables were expressed as number and percentage and the numerical variables were expressed as mean, standard deviation, and median. If the numerical variables were normally distributed, Student’s t-test was used to compare two independent groups. If the numerical variables were not normally distributed, the Mann-Whitney U-test was used to compare two independent groups. The χ2 test was used to compare ratios in groups. P < 0.05 was considered statistically significant.
Surgical technique
The patients were placed in a 30° Trendelenburg position and subumbilical incision of skin and anterior fascia of the rectus, at the same side as the hernia, was performed. The rectus muscle was lateralized and a Covidien Balloon dissector (US Medtronic, Parkway, MN) and 10 mm structural balloon were inserted preperitoneally to the symphysis pubis. With a 30° optical camera, the balloon was insufflated under direct vision, and the extraperitoneal area was opened. In the midline, a 5-mm trocar was inserted at the symphysis pubis. Between this and the umbilical trocar, another midline trocar was inserted. The extraperitoneal cavity was insufflated with CO2 at 10 to 12 mm Hg pressure. The inferior epigastric vessels, symphysis pubis and inferior part of the rectus muscle were clarified, using hand tools, through 5 mm trocars. The iliopubic tract was exposed by dissecting the Cooper ligament, until it reached the femoral vein. The spermatic cord was found and the hernia sac was separated from the cord and was reduced in indirect hernias, and preperitoneal fatty tissue and hernia sac in direct hernias. To cover the myopectineal orifice, Hasselbach’s area and femoral canal orifice, a 10 × 15 cm mesh was located and fixed to the symphysis pubis with an absorbable tacker (Covidien fixation device or AbsorbaTack, US Medtronic, Parkway, MN). Number 0 vicryl was used to close the anterior rectus fascia, and the skin incision was closed with 4/0 vicryl intracutaneous stitches.
GA: Premedication was not used for any of the patients. An i.v. line was established and 10-ml/kg Ringer lactate solution was administered for 30 min, to prepare the patients for the surgery.
In the GA group, anesthesia was performed with propofol (2.0–2.5 mg/kg), fentanyl (1 µg/kg) and rocuronium (0.6 mg/kg), and then all patients were intubated with an endotracheal tube. All the patients were mechanically ventilated in a controlled mode (Vt = 6–8 ml/kg). The respiration frequency was set as 32–36 mm Hg end-tidal carbon dioxide (PETCO2). Administration of sevoflurane (1.5–2.0%) with the inspired oxygen-air fraction (FiO2 = 0.4), was used to maintain anesthesia, and rocuronium (0.015 mg/kg) was administered later, in repetitive doses. The residual neuromuscular block was antagonized with 2.0 to 2.5-mg neostigmine and 1 mg atropine, after the surgery.
SA: In all patients, SA and conscious sedation were performed. Before the surgery, 1000 ml of Ringer lactate solution infusion was administered i.v. to all patients in a routine manner. The SA was applied to the patients in a sitting position. Povidone-iodine solution was used to scrub the lumbar area. A 27-gauge, 90 mm length spinal needle (Egemen model SPPK14067 kit, Turkey) was inserted into the subarachnoid space through the L2-L3 intervertebral space. Then, 15 mg/3 ml (0.5%) of intrathecal bupivacaine HCl (20 mg Bustesin, VEM Pharmaceuticals LP, Istanbul, Turkey) was administered. The patients were then kept in a supine position for 10 min. None of the patients were administered extra local anesthetics. Intravenous midazolam (0.05 mg/kg) and fentanyl (100 µg) were used for sedation. The surgery started after the anesthesia.
Results
In 80 patients, a total of 99 hernia (50 direct; 49 indirect) were diagnosed. Many of the hernias were right-sided, with a rate of 45 (56.25%). There were 26 (52%) direct hernias in the SA group and 24 (48.97%) in the GA group. There were 24 (48%) indirect hernias in the SA group and 25 (51.03%) in the GA group (Table I).
Table I.
Demographic data of patients in both groups
| Parameter | TEP SA (n = 40) | TEP GA (n = 40) | P-values |
|---|---|---|---|
| Age [years], mean ± SD (median) | 35.0 ±11.3 (34.5) | 36.4 ±10.0 (35) | 0.553 |
| Gender, n (%): | 0.712 | ||
| Female | 3 (7.5) | 5 (12.5) | |
| Male | 37 (92.5) | 35 (87.5) | |
| Weight [kg], mean ± SD (median) | 73.4 ±10.2 (75) | 73.5 ±9.3 (74.5) | 0.991 |
| Height [cm], mean ± SD (median) | 173.9 ±6.1 (172) | 175.0 ±4.8 (175.5) | 0.252 |
| BMI [kg/m2], mean ± SD (median) | 24.0 ±2.8 (24.55) | 24.0 ±2.7 (24.35) | 0.937 |
| ASA, n (%): | 0.712 | ||
| ASA I | 35 (87.5) | 37 (92.5) | |
| ASA II | 5 (12.5) | 3 (7.5) | |
| Hernia type, n (%): | |||
| Direct hernia | 16 (40) | 15 (37.5) | |
| Indirect hernia | 14 (35) | 16 (40) | |
| Direct and indirect hernia | 10 (25) | 9 (22.5) |
SA – spinal anesthesia, GA – general anesthesia.
Patient demographics, i.e. gender, age, weight, height, BMI, and ASA scores of both groups, were not significantly different (Table I). The mean BMI of the patients in the SA group was 24.0 ±2.8 (24.55) kg/m2 and 24.0 ±2.7 (24.35) kg/m2 in the GA group (p = 0.937). The average age of the patients in the SA group was 35.0 ±11.3 (34.5) years and 36.4 ±10.0 (35) years in the GA group. There was no significant difference between the two groups, in terms of age or gender (p = 0.553, p = 0.712). In 92.5% of the patients, the dissection of the extraperitoneal space was graded easily in the SA group; however, it was difficult in 3 patients from the SA group, due to a large, indirect hernia sac and a peritoneal tear. Space was created adequately, in all patients, by using telescopic dissection.
In 92.5% of the patients, anatomical delineation of the inguinal area was satisfactory; however, it was unsatisfactory in 3 patients in the SA group (p = 0.241) because of excessive preperitoneal fat (Table II). There was no injury in the vas deferens, other cord structures, or bladder and no major vascular or bowel injury, during dissection or insertion of ports, in both groups. The triangle of doom was defined well in 92.5% of the patients (in all patients of the GA group and 92.5% of patients in the SA group (p = 0.241)) (Table II). Bleeding was graded as minimal in all patients in both groups.
Table II.
Operation details on TEP in spinal anesthesia (SA) and general anesthesia (GA)
| Variable | Total (n = 80) | SA group TEP (n = 40) | GA group TEP (n = 47) | P-value |
|---|---|---|---|---|
| Dissection of extraperitoneal space: easy | 80 (100%) | 37 (92.5%) | 40 (100%) | 0.241 |
| Anatomical delineation: satisfactory | 80 (100%) | 37 (92.5%) | 40 (100%) | 0.241 |
| Doom triangle: defined | 80 (100%) | 37 (92.5%) | 40(100%) | 0.241 |
There were no differences in the average operation time between the groups (p = 0.942). In the GA group the operation time was 55.2 ±6.9 (54) min, and in the SA group it was 55.5 ±6.7 (54) min. The total surgery time was 67.2 ±6.2 (67) min in the GA group and 70.2 ±6.7 (69) min in the SA group, which was significant (p = 0.038). None of the operations were converted into an open operation (Table III). The postoperative hospitalization period was 27.5 ±3.1 (27) h in the SA group and 27.4 ±3.8 (27) h in the GA group. There was no significant difference in the hospitalization period between the groups (p = 0.474) (Table III).
Table III.
Details (operative and postoperative)
| Parameter | SA TEP (n = 40) | GA TEP (n = 40) | P-value |
|---|---|---|---|
| Total duration [min], mean ± SD (median) | 70.2 ±6.7 (69) | 67.2 ±6.2 (67) | 0.038 |
| Surgery duration [min], mean ± SD (median) | 55.5 ±6.7 (54) | 55.2 ±6.9 (54) | 0.942 |
| Spinal time [min], mean ± SD (median) | 16.8 ±3.6 (16) | – | – |
| Hospitalization [h], mean ± SD (median) | 27.5 ±3.1 (27) | 27.4 ±3.8 (27) | 0.474 |
| Analgesics need [h], mean ± SD (median) | 5.30 ±0.79 (5.5) | 1.08 ±0.27 (1) | 0.0001 |
| Patient satisfaction, mean ± SD (median) | 4.63 ±0.49 (5) | 4.15 ±0.77 (4) | 0.004 |
| Return to work time [day], mean ± SD (median) | 7.28 ±0.72 (7) | 7.60 ±0.71 (8) | 0.032 |
When intraoperative adverse effects in the SA group were examined, it was observed that 6 (15%) patients had shoulder pain, 4 (10%) patients developed anxiety and 8 (20%) patients had abdominal discomfort. However, no patients were switched from the SA group to the GA group. In 1 (2.5%) patient, hypotension and nausea/vomiting developed after SA. This patient was treated with medication before the surgery began (Table IV).
Table IV.
Intraoperative adverse effects
| Effect | N | % |
|---|---|---|
| Shoulder pain | 6 | 15 |
| Abdominal discomfort | 8 | 20 |
| Anxiety | 4 | 10 |
| Nausea/vomiting | 1 | 2.5 |
| Hypotension | 1 | 2.5 |
Postoperative nausea vomiting was reported in 2 (5%) patients in the SA group and in 6 (15%) patients in the GA group, with no significant difference (p = 0.263). Urinary retention was determined in 4 (10%) patients and headaches in 5 (12.5%) patients in the SA group and in no patients in the GA group, with no significant difference (p = 0.116, p = 0.055). Shoulder pain was determined in 3 (7.5%) patients in the SA group and in 6 (15%) patients in the GA group, which was not significantly different (p = 0.288; Table V).
Table V.
Postoperative adverse effects
| Effect | SA TEP (n = 40) (%) | GA TEP (n = 40) (%) | P-value |
|---|---|---|---|
| Shoulder pain | 3 (7.5) | 6 (15) | 0.288 |
| Nausea/vomiting | 2 (5) | 6 (15) | 0.263 |
| Urinary retention | 4 (10) | 0 | 0.116 |
| Headache | 5 (12.5) | 0 | 0.055 |
The follow-up examinations were scheduled at 7 days, and at 3, 6, 9, and eventually, 15 months (Table VI). The follow-up examinations ranged from 9 to 15 months (mean value: 12 months). In follow-up examinations, seroma formation, infection of wound or graft, and recurrence of hernia were recorded.
Table VI.
Postoperative complications
| Complication | SA TEP (n = 40) (%) | GA TEP (n = 40) (%) | P-value |
|---|---|---|---|
| Seroma 7 days | 5 (12.5) | 3 (7.5) | 0.712 |
| Testicular/hemiscrotal swelling | 7 (17.5) | 6 (15) | 0.762 |
| Wound infection within 7 days | 3 (7.5) | 5 (12.5) | 0.712 |
| Recurrence (hernia) | 0 | 1 (2.5) | 1.000 |
Seroma was the most common problem at the 7-day follow-up examinations. There was a significant difference in seroma between the groups (p = 0.712). Seroma was observed in 5 (12.5%) patients in the SA group and in 3 (7.5%) patients in the GA group. During the follow-up examinations, 1 (16.6%) of these patients required aspiration of seroma. Superficial wound infection was detected in 8 (7.5%) patients in the umbilical port site. Superficial surgical site infection of the umbilical port was detected in 3 (7.5%) patients in the SA group and in 5 (12.5%) patients in the GA group. No significant difference was determined between the groups (p = 0.712). At the 1-month follow-up, there were no wounds, mesh infections or hernia recurrences. In the GA group, a recurrence was detected in 1 patient, in the GA group in 15-months follow-up, with no significant difference (p = 1.000; Table VI).
A significant difference was detected between the groups in seroma development (p = 0.762). Early in the postoperative period, scrotal edema developed in 32.5% of the patients (in 7 (17.5%) patients in the SA group and in 6 (15%) patients in the GA group). No correlation was detected between scrotal edema development and age, operation time, or the type of hernia, according to the Mann-Whitney U-test (p < 0.05; Table VI).
The pain scores were higher in group I than in group II, after the 1st and 6th h. The pain score at 0 and 6 h in the GA group was 5.18 ±0.84 and 2.50 ±0.55, respectively, and in the SA group the corresponding scores were 0.23 ±0.42 and 1.83 ±0.64 (Table VII). The difference between the groups was statistically significant (p = 0.001). At the 12th h, a significant difference in VAS was determined between the groups; the mean score was 1.58 ±0.55 in the GA group and 1.28 ±0.45 in the SA group (p = 0.011). The GA group patients had higher VAS scores (0.75 ±0.59) than those in the SA group (0.68 ±0.47) at 24 h postoperatively. The difference between the groups was not significant (p = 0.643; Table VII).
Table VII.
Postoperative pain scoring (VAS)
| VAS score | SA TEP (n = 40) | GA TEP (n = 40) | P-value |
|---|---|---|---|
| Post 0 VAS | 0.23 ±0.42 (0) | 5.18 ±0.84 (5) | 0.001 |
| Post 6 VAS | 1.83 ±0.64 (2) | 2.50 ±0.55 (3) | 0.001 |
| Post 12 VAS | 1.28 ±0.45 (1) | 1.58 ±0.55 (2) | 0.011 |
| Post 24 VAS | 0.68 ±0.47 (1) | 0.75 ±0.59 (1) | 0.643 |
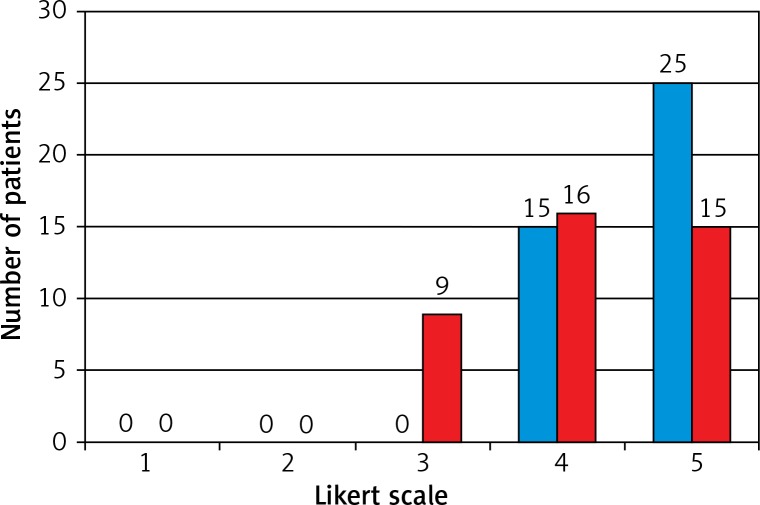
At the end of 3 months, the satisfaction of the patients was measured with a Likert scale (1–5). Most of the patients in the SA group (4.63 ±0.49) had higher satisfaction levels than the GA group (4.15 ±0.77), regarding recovery, pain, and return to normal activity. The difference between the groups was significant (p = 0.004). The distribution of the satisfaction levels of all patients is given in Figure 1.
Figure 1.
Likert scale evaluation of patient satisfaction. Blue column – SA TEP group (n = 40), red column – GA TEP group (n = 40)
Discussion
Studies continue to find the best method for inguinal hernia repair. Tension-free hernia repair (Lichtenstein) was considered to be the best technique for inguinal hernia repair before laparoscopic techniques were introduced to surgery [12]. Randomized clinical trials have shown that TEP provides better cosmetic results, less pain, less analgesic requirement and early return to work in the postoperative period compared to open hernia repair [4]. The TEP operation has some additional advantages. For example, it does not lead to intra-abdominal organ injury and adhesion because the abdominal cavity is not entered in the TEP technique [13]. Regional anesthesia, which is commonly used in open hernia repair, is not preferred by surgeons and anesthesiologists during laparoscopic operations. The reasons why surgeons do not prefer it are that patients have a fear of needles applied to their waist and request complete analgesia, the learning curve and training are easier in GA, and the surgeons believe that adequate muscle relaxation cannot be achieved [14].
The TEP and TAPP techniques are used in laparoscopic hernia repair operations. Although a wide dissection area and a comfortable work environment, due to intra-abdominal swelling, exist in the TAPP technique, unwanted complications, such as intestinal injury and adherence, can be seen [15, 16]. These complications are not observed in the TEP technique because the abdominal cavity is not entered. Considering that the surgery is performed through the extraperitoneal space in the TEP technique, there are few comparative studies on whether the dissection area and viewing distance are sufficient. In a prospective randomized trial conducted in 99 cases, by Krishna et al., the TAPP and TEP techniques were compared, the dissection area and anatomical adequacy were assessed from the surgical perspective, and it was determined that there was no significant difference between the two groups (p = 0.896). The authors documented that satisfaction was 100% in the TAPP technique but 98.1% in the TEP technique [17]. As mentioned above, there is a widespread concern, among surgeons, about whether regional anesthesia provides adequate muscle relaxation. Good results have been reported in TEP operations performed under regional anesthesia, up to now, but there is no study about the dissection area and anatomical adequacy. In a prospective clinical trial conducted in 50 cases by Donmez et al. in 2016, it was found that adequate muscle relaxation was achieved during the TEP operation performed under SA. Also, the creation of space and adequate muscle relaxation were achieved at a rate of 96% in the GA group compared to 100% in the SA group, with no significant difference between the two groups (p = 0.896) [18]. In our clinical trial, 40 cases were assessed, for ease of dissection, creation of space, dissection of space, and adequacy of anatomical planning. Satisfaction was assessed by the surgeon. It was observed that adequate muscle relaxation, which was the concern of surgeons, was provided in patients with SA and that there was no problem in terms of surgery. These parameters were higher in the GA group than the SA group. The surgical satisfaction was determined exactly. No significant difference was found in these parameters, when the two groups were compared (the GA group: 100% vs. the SA group: 92.5%; p = 0.241). However, in the SA group, the hernia sac was cut distally in 2 cases, due to an adherent scrotal hernia, and the peritoneum was opened in 1 case. The intra-abdominal gas was evacuated by Veress needle insertion in patients with shoulder pain and abdominal discomfort, due to gas escaping into the abdominal cavity, and then the operation was completed by achieving sedation with fentanyl. In the GA group, there was no problem in 3 cases in whom the peritoneum was opened, in terms of anatomical planning, creation of space and dissection.
There are very few studies comparing anesthesia techniques in TEP operations. In the study by Donmez et al., the duration of the operation was shorter in the GA group but the difference was not significant (36.16 min for the GA group vs. 40.16 min for the SA group; p = 0.063) [18]. In a prospective study performed in 30 cases by Molinelli et al., it was found that surgical duration was 55 min (38–78 min) in TEP operations performed under SA [11]. In a prospective study performed in 30 cases by Krishna et al., it was found that surgical duration was 62.13 min in TEP operations performed under GA [17]. In a very comprehensive review, the duration of the operation ranged from 55 to 95 min in TEP operations, in relation to the experience of the surgeons and the number of cases performed [19]. Similarly to other studies, in our study, both groups had a similar operation duration and there was no significant difference between the two groups (55.2 min for the GA group vs. 55.525 min for the SA group; p = 0.942). However, in our study, the total operation time was longer in the SA group than the GA group. This time was 70.2 ±6.7 (69) min in the SA group but 67.2 ±6.2 (67) min in the GA group. There was a significant difference between the two groups (p = 0.038). We believe that the total operation time was longer in the SA group because time was required to achieve appropriate motor block formation after SA was performed.
The development of seroma in the inguinal area, after hernia surgery, is an undesirable complication. In a clinical study performed by Lau and Lee, the causes of seroma formation were found to be old age, large hernia defect, extension of the hernia into the scrotum, giant scrotal hernia, and leaving the distal hernia sac in place [20]. The same authors mentioned that the incidence of seroma in the TEP operation was 7.8% in 40 cases. In a retrospective study of 44 cases by Vărcuş et al., the incidence of seroma in the TEP operation was 2.27% [21]. In a large retrospective study of 783 cases by Reiner et al., the incidence of seroma in the TEP operation was 4.7% in 37 cases [22]. Our study also supports this. Although seroma is more common in patients who have a distal hernia sac, due to the inability to lower the hernia sac extending to the scrotum, and in elderly patients, there was no significant difference between the two groups, regarding the development of seroma. Seroma developed in 5 (12.5%) cases in the SA group but in 3 (7.5%) cases in the GA group (p = 0.712).
Testicular and hemiscrotal swelling is one of the common complications of laparoscopic hernia surgery. The most important causes are giant inguinal hernia, leaving the distal hernia sac in place, scrotal hernia sac, and old age [20, 21]. In another study, scrotal swelling in TEP and TAPP operations was compared and it was 9.4% in the TEP group and 34% in the TAPP group. In a large retrospective study by Reiner et al., it was reported to develop in 23 (2.9%) patients, and it was emphasized that the rate of complications decreased with the increasing experience of the surgeon. These data for TEP operations usually belong to studies performed under GA. Conversely the data on complications related to TEP operations performed under SA are limited. In a 2016 prospective study, by Donmez et al., scrotal edema was detected in 4 (16%) patients in the SA group compared to 3 (12%) patients in the GA group, and there was no significant difference between the two groups [18]. In our study, the rate of testicular and hemiscrotal swelling in both groups was similar (SA group: 7 (17.5%); GA group: 6 (15%)). There was no significant difference between the two groups (p = 0.762).
Antibiotic prophylaxis is controversial in the surgical repair of inguinal hernia. Prospective randomized controlled trials identified that postoperative infection rates (poor wound healing, mesh infections) were 0–8.9% in the absence of antibiotic prophylaxis, and 0–8.8% in the presence of antibiotic prophylaxis [23]. In a large retrospective study, by Tamme et al., involving 5203 TEP operations and 3868 patients receiving antibiotic prophylaxis, the wound infection rate was determined as 0.08% [24]. In our study, a single preoperative antibiotic dose was given to all patients, in both groups. There was no significant difference between the two groups (p = 0.712).
It is debatable whether urinary retention is a side effect of hernia surgery or a side effect of SA. Some studies have suggested that urinary retention is associated with age and urinary retention is seen more often in elderly patients. In the study by Reiner et al., where 783 TEP operations were performed under GA, urinary retention was detected in 32 (4.2%) patients. Moreover, there was no significant difference between the genders but urinary retention was not observed in women. In this study, the age of the patients with urinary retention was found to be significantly higher than patients without urinary retention [22]. The rate of urinary retention in TEP operations performed under SA ranges between 3.33% and 18.05% [11, 25]. In our study, urinary retention was not observed in the GA group. Although all patients with urinary retention in the SA group were male, there was no significant difference between patients with and without urinary retention in terms of age and gender. Also, there was no significant difference regarding urinary retention between the groups (p = 0.116).
The main long-term issue in inguinal hernia operations is recurrence, regardless of open repair or laparoscopic repair. There are many studies about recurrence rates of both open and laparoscopic techniques [6, 7, 18, 19, 23]. In a large study performed in 2086 patients by Belyanski et al., the TEP, TAPP and modified Lichtenstein methods were compared and there was no significant difference in recurrence rates between the methods (TEP 0.42%, TAPP 1.34%, and modified Lichtenstein 1.27%) [6]. The recurrence rate of TEP operations performed under SA ranges from 0 to 3% [9, 11, 26]. However, in only one study comparing SA and GA, Donmez et al. found that there was no significant difference in recurrence rates between the two groups. In our study, while recurrence was detected in 1 patient in the GA group, no recurrence was observed in the SA group. There was no significant difference in recurrence rates between the two groups (p = 1.000).
One of the key problems faced by anesthesiologists in laparoscopic surgery performed under SA is shoulder tip pain. The exact etiology of shoulder tip pain is not known, but the most accepted theory is diaphragm irritation. Shoulder tip pain is a reflected pain and is therefore difficult to treat for anesthesiologists [27–29]. In a prospective clinical trial conducted by Bhatia et al., intrathecal dexmedetomidine and bupivacaine were compared, to reduce shoulder pain in laparoscopic cholecystectomy operations performed under SA. The study showed that excellent sedation and analgesia were provided in patients treated with dexmedetomidine and that it significantly reduced shoulder pain [30]. In the prospective randomized clinical trial of Sung et al., where patients underwent SA during the TEP operation, patients treated with intrathecal fentanyl were compared to a control group. The authors reported that the fentanyl reduced the incidence of shoulder pain by 50% and that there was a significant difference between the two groups [25]. In our study, 6 (15%) patients in the SA group had shoulder pain intraoperatively and were treated with fentanyl 50 mg (i.v.). No patients were switched from SA to GA. In the postoperative period, 3 patients in the SA group and 6 patients in the GA group had shoulder pain and were treated with tramadol 50 mg (i.v.). There was no significant difference between the two groups concerning postoperative shoulder pain.
Pain in the early postoperative period, after inguinal hernia operations, is the most common patient complaint. Many studies have proven that there was less postoperative pain in laparoscopic surgery than in open surgery [4]. Krishna et al. emphasized that postoperative pain was associated with age, gender, fixation with mesh punch, development of seroma and scrotal edema, and duration of the operation [17]. In this prospective randomized study, pain in the early postoperative period was examined in TEP and TAPP techniques. It was found that the pain score at the postoperative 1st h was lower in TEP technique than TAPP technique (p = 0.0001). However, there was no significant difference in pain scores at the postoperative 6th hour between the two groups (p = 0.108) [17]. In the study by Lau et al., age, gender, development of seroma, recurrent hernia, type of hernia, bilateral hernia and operation time were reported effective as factors affecting postoperative pain in patients undergoing a TEP operation [31]. In the prospective study by Donmez et al., pain scores at the postoperative 1st and 4th h were significantly lower in the SA group than in the GA group (p < 0.001, p = 0.02) [18]. Similarly, in our study, pain scores at the postoperative 1st, 6th and 12th h were significantly lower in the SA group than the GA group (p < 0.001, p < 0.001 and p = 0.011). In light of these results, it can be concluded that pain, being less in the SA group, depends on the continuation of the effect of SA.
There are various factors that affect patient satisfaction. Krishna et al. reported that factors affecting patient satisfaction in comparing the TEP and TAPP techniques were VAS scores, the length of hospital stay, and development of seroma and scrotal swelling. In this study, the authors used a verbal rating scale (0–3) at the end of the 3rd month and found that patient satisfaction was higher in the TEP technique (p = 0.002) [17]. It is necessary to add intraoperative adverse effects, as reasons that affect patient satisfaction, in TEP operations performed under SA. Patients feel less pain because SA would continue during the first postoperative hours in the SA group. Thus, patient satisfaction increases. In our study, patient satisfaction was higher in the SA group than the GA group. This difference was significant (4.63 ±0.49 vs. 4.15 ±0.77, p = 0.004).
As a result, contrary to surgeons’ concerns, adequate muscle relaxation can be achieved in TEP operations performed under SA. The groin area can be dissected in a relaxed way, providing appropriate anatomical space and a better viewing distance and dissection area for surgeons. However, when the peritoneum is opened, in giant scrotal hernia and recurrent hernia cases and in cases where the hernia sac cannot be separated from the cord, the patient feels pain, due to gas escaping into the abdominal cavity. Therefore, patients should be sedated. Spinal anesthesia is superior in terms of hypotension, vomiting, and pain development in the early postoperative period, when compared with GA. Another advantage of SA is that patients can follow the surgery on the monitor because they are awake.
Conclusions
Total extraperitoneal surgery performed under SA provides adequate muscle relaxation and a suitable work environment, as seen under GA. It also protects the patient from possible risks of GA. In light of the results obtained in our study, TEP operations performed under SA are safe, effective and satisfactory in terms of patient comfort.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Bassini E. Nuovo metodo sulla cura radicale dell’ernia inguinale. Arch Soc Ital Chir. 1887;4:380. [Google Scholar]
- 2.[No authors listed] Laparoscopic versus open repair of groin hernia: a randomized comparison. The MRC Laparoscopic Groin Hernia Trial Group. Lancet. 1999;354:185–90. [PubMed] [Google Scholar]
- 3.Neumayer L, Giobbie-Hurder A, Jonasson O. Open mesh versus laparoscopic mesh repair of inguinal hernia. N Engl J Med. 2004;350:1819–27. doi: 10.1056/NEJMoa040093. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Clinical Excellence . Guidance on the use of laparoscopic surgery for inguinal hernia, technological appraisal guidance. London: NICE; 2001. No. 18. [Google Scholar]
- 5.Mathavan VK, Arregui ME. Fixation versus no fixation in laparoscopic TEP and TAPP. New York: Springer; 2013. The SAGES Manual of Hernia Repair; pp. 203–12. [Google Scholar]
- 6.Belyansky I, Tsirline VB, Klima DA, et al. Prospective, comparative study of postoperative quality of life in TEP, TAPP, and modified Lichtenstein repairs. Ann Surg. 2011;254:709–14. doi: 10.1097/SLA.0b013e3182359d07. [DOI] [PubMed] [Google Scholar]
- 7.McCormick K, Scott NW, Go PM, et al. Hernia Trialists Collaboration Laparoscopic technique versus open technique for inguinal hernia repair. Cochrane Database Syst Rev. 2003;1:CD001785. doi: 10.1002/14651858.CD001785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swanstrom L. Laparoscopic surgery: laparoscopic herniorrhaphy. Surg Clin North Am. 1996;73:483–91. doi: 10.1016/s0039-6109(05)70455-2. [DOI] [PubMed] [Google Scholar]
- 9.Lau H, Wong C, Chu K, Patil NG. Endoscopic totally extraperitoneal inguinal hernioplasty under spinal anesthesia. J Laparoendosc Adv Surg Tech A. 2005;15:121–4. doi: 10.1089/lap.2005.15.121. [DOI] [PubMed] [Google Scholar]
- 10.Sinha R, Gurwara AK, Gupta SC. Laparoscopic total extraperitoneal inguinal hernia repair under spinal anesthesia: a study of 480 patients. J Laparoendosc Adv Surg Tech A. 2008;18:673–7. doi: 10.1089/lap.2007.0219. [DOI] [PubMed] [Google Scholar]
- 11.Molinelli BM, Tagliavia A, Bernstein D. Total extraperitoneal preperitoneal laparoscopic hernia repair using spinal anesthesia. JSLS. 2006;10:341–4. [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtenstein IL, Shulman AG, Amid PK, Montllor MM. The tension-free hernioplasty. Am J Surg. 1989;157:188–93. doi: 10.1016/0002-9610(89)90526-6. [DOI] [PubMed] [Google Scholar]
- 13.Takata MC, Duh QY. Laparoscopic inguinal hernia repair. Surg Clin North Am. 2008;88:157–78. doi: 10.1016/j.suc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Sinha R, Gurwara AK, Gupta SC. Laparoscopic total extraperitoneal inguinal hernia repair under spinal anesthesia: a study of 480 patients. J Laparoendosc Adv Surg Tech A. 2008;18:673–7. doi: 10.1089/lap.2007.0219. [DOI] [PubMed] [Google Scholar]
- 15.Felix EL, Harbertson N, Vartanian S. Laparoscopic hernioplasty: significant complications. Surg Endosc. 1999;13:328–31. doi: 10.1007/s004649900982. [DOI] [PubMed] [Google Scholar]
- 16.Bringman S, Blomqvist P. Intestinal obstruction after inguinal and femoral hernia repair: a study of 33, 275 operations during 1992–2000 in Sweden. Hernia. 2005;9:178–83. doi: 10.1007/s10029-004-0305-7. [DOI] [PubMed] [Google Scholar]
- 17.Krishna A, Misra MC, Bansal VK, et al. Laparoscopic inguinal hernia repair: transabdominal preperitoneal (TAPP) versus totally extraperitoneal (TEP) approach: a prospective randomized controlled trial. Surg Endosc. 2012;26:639–49. doi: 10.1007/s00464-011-1931-7. [DOI] [PubMed] [Google Scholar]
- 18.Donmez T, Erdem VM, Sunamak O, et al. Laparoscopic total extraperitoneal repair under spinal anesthesia versus general anesthesia: a randomized prospective study. Ther Clin Risk Manag. 2016;12:1599–608. doi: 10.2147/TCRM.S117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wake BL, McCormack K, Fraser C, et al. Transabdominal pre-peritoneal (TAPP) vs. totally extraperitoneal (TEP) laparoscopic techniques for inguinal hernia repair. Cochrane Database Syst Rev. 2005;1:CD004703. doi: 10.1002/14651858.CD004703.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau H, Lee F. Seroma following endoscopic extraperitoneal inguinal hernioplasty. Surg Endosc. 2003;17:1773–7. doi: 10.1007/s00464-002-8771-4. [DOI] [PubMed] [Google Scholar]
- 21.Vărcuş F, Duţă C, Dobrescu A, et al. Laparoscopic repair of inguinal hernia TEP versus TAPP. Chirurgia (Bucur) 2016;111:308–12. [PubMed] [Google Scholar]
- 22.Reiner MA, Bresnahan ER. Laparoscopic total extraperitoneal hernia repair outcomes. JSLS. 2016;20 doi: 10.4293/JSLS.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bittner R, Arregui ME, Bisgaard T, et al. Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia [International Endohernia Society (IEHS)] Surg Endosc. 2011;25:2773–843. doi: 10.1007/s00464-011-1799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamme C, Scheidbach H, Hampe C, et al. Totally extraperitoneal endoscopic inguinal hernia repair (TEP) Surg Endosc. 2003;17:190–5. doi: 10.1007/s00464-002-8905-8. [DOI] [PubMed] [Google Scholar]
- 25.Sung TY, Kim MS, Cho CK, et al. Clinical effects of intrathecal fentanyl on shoulder tip pain in laparoscopic total extraperitoneal inguinal hernia repair under spinal anaesthesia: a double-blind, prospective, randomized controlled trial. J Int Med Res. 2013;41:1160–70. doi: 10.1177/0300060513490083. [DOI] [PubMed] [Google Scholar]
- 26.Spivak H, Nudelman I, Fuco V, et al. Laparoscopic extraperitoneal inguinal hernia repair with spinal anesthesia and nitrous oxide insufflation. Surg Endosc. 1999;13:1026–9. doi: 10.1007/s004649901161. [DOI] [PubMed] [Google Scholar]
- 27.Sarli L, Costi R, Sansebastiano G, et al. Prospective randomized trial of low-pressure pneumoperitoneum for reduction of shoulder-tip pain following laparoscopy. Br J Surg. 2000;87:1161–5. doi: 10.1046/j.1365-2168.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 28.Kojima Y, Yokota S, Ina H. Shoulder pain after gynaecological laparoscopy caused by arm abduction. Eur J Anaesthesiol. 2004;21:578–9. doi: 10.1017/s0265021504267126. [DOI] [PubMed] [Google Scholar]
- 29.Donmez T, Erdem VM, Uzman S, et al. Laparoscopic cholecystectomy under spinal-epidural anesthesia vs. general anaesthesia: a prospective randomised study. Ann Surg Treat Res. 2017;92:136–42. doi: 10.4174/astr.2017.92.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatia T, Bhatia J, Attri JP, et al. Intrathecal dextmedetomidine to reduce shoulder tip pain in laparoscopic cholecystectomies under spinal anesthesia. Anesth Essays Res. 2015;9:320–5. doi: 10.4103/0259-1162.158010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Lau H, Patil NG. Acute pain after endoscopic totally extraperitoneal (TEP) inguinal hernioplasty: multivariate analysis of predictive factors. Surg Endosc. 2004;18:92–6. doi: 10.1007/s00464-003-9068-y. [DOI] [PubMed] [Google Scholar]