Abstract
Postoperative pain is one of the major complications in general and bariatric surgery, associated with ongoing problems such as ileus, pneumonia and prolonged mobilization. In this study, patients undergoing bariatric surgery were analyzed according to their postoperative pain relief regime. In one group patients were treated with a patient-controlled analgesia (PCA) device, while the other group was treated with oral and intravenous analgesic medication. The aim of this study was to analyze which postoperative pain relief therapy would be more appropriate. We chose the Cumulative Analgesic Consumption Score (CACS) and Numeric Rating Scale (NRS) for pain measurement. For better comparison, we performed a modification of CACS according to PCA treatment. We observed better pain relief in the PCA group. Furthermore, we observed an advantage of treatment with laxatives in patients treated with PCA. In conclusion, PCA devices are appropriate instruments for postoperative pain relief in bariatric patients. CACS is a practical tool for postoperative pain measurement, describing individual pain sensation more objectively, although holding further potential in modification.
Keywords: obesity, bariatric surgery, MACS, pain relief, Cumulative Analgesic Consumption Score, patient-controlled analgesia
Introduction
Postoperative ileus and pain are major complications after abdominal surgical procedures. The incidence of ileus is nearly 10.3% in laparotomy, especially in colon operations [1]. Regarding patient discharge and length of hospital stay, gastrointestinal motility is an important factor, not only in producing more costs in the healthcare system. Severe postoperative pain influences patient mobilization and causes associated complications such as pneumonia, lower vein thrombosis and also gastrointestinal motility disorders as well [2, 3].
Many individual factors may influence the sensation of pain in different patients. On the one hand, there is a strong relationship with extent of surgical trauma [4], while on the other hand, preoperative anxiety and depression lead to more postoperative pain and higher consumption of analgesic medication in both obese and non-obese patients [5].
For pain relief, patient-controlled analgesia (PCA) is used frequently in postoperative patients. With this system, patients are able to get analgesia on demand without the need for personal assistance. Furthermore, physicians are able to interpret pain intensity and need of analgesic medication by analyzing the number of overall requests and successful demands registered in an internal log [6]. Although it is a widespread technique for postoperative analgesia, studies showed that beside the advantages in open surgery, PCA devices used for pain relief may not be necessary in all surgical procedures and techniques, such as laparoscopic procedures [7]. In this context, patients treated with PCA devices have higher opioid consumption than those who received a standard oral or intravenous analgesic treatment [6]. Thus, there is a higher risk for opioid-associated adverse effects including nausea, vomiting and sedation [8, 9].
There are several tools and scores to evaluate individual pain. The most commonly used score is the Numeric Rating Scale (NRS), which ranges from 0 (no pain) to 10 (worst pain) (0 to 100 in several studies). In this score, the major problem is patient compliance as well as the patient’s mood affecting the individual sensation of pain [4, 10].
The Cumulative Analgesic Consumption Score (CACS) is a simple tool for objective measurement of the patient’s analgesics consumption. This score is not influenced by individual feelings but calculated using the number of demands and category of analgesic medication according to the WHO pain relief ladder. Previous studies showed correlations between CACS and NRS for discriminating invasiveness of surgical trauma by the magnitude of the score [11]. However, treatment with pain relief devices such as PCA is not considered in this calculation.
Aim
In this retrospective consecutive cohort study, we tried to analyze whether patients undergoing bariatric surgery have better pain relief with either PCA treatment or non-PCA analgesic medication. We investigated whether there might be an advantage in gastrointestinal motility and length of hospital stay in either of these groups. Furthermore, we were able to modify CACS to have better comparison between PCA and non-PCA treatment.
Material and methods
In this retrospective study, 61 patients undergoing laparoscopic bariatric surgery were analyzed in one cohort. Data were collected by reviewing medical records of in-patients. Patients were included by the following conditions: underwent laparoscopic bariatric surgery, age > 18 years, postoperative analgesic treatment with PCA or standard medication. Exclusion criteria were: peridural anesthesia, chronic pain syndrome or pain reliever in long-term medication, drug abuse in anamnesis. Twenty-eight patients were treated with PCA for postoperative pain relief; 33 patients were treated with standard oral or intravenous analgesia. For pain relief in the non-PCA group, standard medication according to the WHO pain relief ladder was used by the hospital standard including nonsteroidal anti-inflammatory drugs (NSAID) and non-NSAID analgesics as well as weak and strong opioids. In the PCA group, patients were also able to get additional analgesic medication on demand. Patient-controlled analgesia devices were filled with 1 mg/ml piritramide with a single demand dose of 2.5 mg. After patients received an analgesic bolus, the device was locked for 15 min and patients were only able to get a maximum of six injections within 2 h.
Data of analgesic medication, number of PCA requests, treatment with laxatives and length of hospital stay were collected. Laxatives were given when there was no bowl movement after postoperative day two. Discharge criteria were defined as full mobilization, NRS less than two and normal inflammatory markers in blood test. Additionally, on the day of the operation, as well as on postoperative days one to three, CACS was calculated by analgesic medication treatment in all patients. The score is calculated by using the number of single analgesic doses multiplied by the level of the modified WHO pain relief ladder and finally added together [11]. In the PCA group, the score was calculated using the additional analgesic medication. For better comparison, the PCA demand/get ratio was calculated and added to CACS after multiplication by the corresponding level of pain relief ladder. The result was MACS (“M” for “modified”). Mathematically, CACS and MACS can be expressed in the following way: CACS Σ = n × step of WHO pain relief ladder, e.g. 2 × WHO step III + 3 × WHO step II + 5 × WHO step I = 6 + 6 + 5 = CACS 17. MACS Σ = n × step of WHO pain relief ladder + × step of WHO pain relief ladder, e.g. 2 × WHO step II + 5 × WHO step I + 8/2 × WHO step III = 4 + 5 + 3.9 = MACS 12.9.
Statistical analysis
Statistical data analysis was performed using the Mann-Whitney U-test, χ² test and bivariate Spearman’s correlation using SPSS 24 (IBM, Armonk, NY). The retrospective study was approved by Ludwig-Maximilians-University of Munich’s ethical committee.
Results
Out of the 61 consecutively analyzed patients undergoing laparoscopic bariatric surgery, patients were divided into two groups according to the postoperative pain relief regime. Twenty-eight patients were treated with PCA for pain relief (32.1% (n = 9) men, 67.9% (n = 19) women, mean age: 47.29 ±10.78 years), and 33 patients were treated with standard oral or intravenous analgesia (9.1% (n = 3) men, 90.9% (n = 30) women, mean age: 43.58 ±11.33 years). Among patients treated with PCA, 57.1% (n = 16) underwent sleeve gastrectomy and in 42.9% (n = 12) a gastric bypass procedure was performed; the figures were respectively 72.7% (n = 24) for sleeve gastrectomy and 27.3% (n = 9) for gastric bypass in the non-PCA group. In the PCA group body mass index (BMI) was 48.78 ±8.93 kg/m² (35.3–67.7 kg/m²), and in the non-PCA group BMI was 50.82 ±8.26 kg/m² (35.0–73.6 kg/m²). There was no significant difference in age, surgical procedure or BMI. In both groups, more women underwent surgery than men (Table I).
Table I.
Demographics, surgical procedure, and body mass index in PCA and non-PCA group
| Parameter | With PCA (n = 28) | Without PCA (n = 33) | P-value | |
|---|---|---|---|---|
| Gender | Male | 32.1% (9) | 9.1% (3) | 0.024* |
| Female | 67.9% (19) | 90.9% (30) | ||
| Mean age [years] (min.–max.) | 47.29 ±10.78 (27–64) | 43.58 ±11.33 (20–63) | 0.198 | |
| Surgical procedure | Sleeve gastrectomy | 57.1% (16) | 72.7% (24) | 0.202 |
| Gastric bypass | 42.9% (12) | 27.3% (9) | ||
| Mean BMI [kg/m²] (min.–max.) | 48.78 ±8.93 (35.3–67.7) | 50.82 ±8.26 (35.0–73.6) | 0.356 |
PCA – patient-controlled analgesia, BMI – body mass index,
significant with p < 0.05.
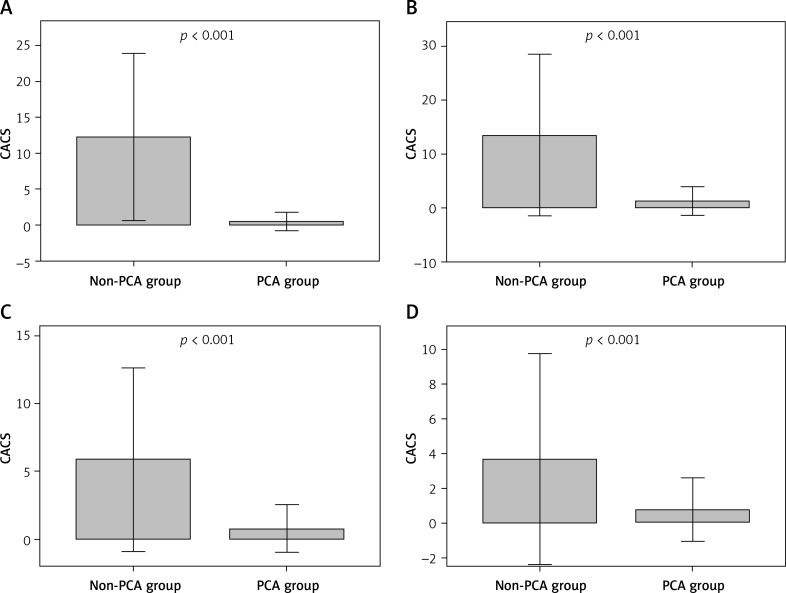
At first, CACS was analyzed on the day of surgery as well as on postoperative days one to three between the two groups. In the PCA group CACS was initially calculated by using additional demand medication. As shown in Figure 1, significantly higher CACS was detected in the non-PCA group on all days (p < 0.001). On the day of surgery, CACS in the non-PCA group was 12.24 ±5.83 (2–24) vs. 0.5 ±0.64 (0–2) in the PCA group, respectively 13.48 ±7.5 (4–38) vs. 1.29 ±1.33 (0–4) on day one, 5.88 ±3.39 (1–16) vs. 0.79 ±0.88 (0–3) on day two, and 3.68 ±3.04 (1–13) vs. 0.79 ±0.92 (0–3) on day three after the operation.
Figure 1.
CACS in non-PCA and PCA group day 0 to 3. A – Day of surgery (day 0), B – postoperative day one, C – postoperative day two, D – postoperative day three
CACS – Cumulative Analgesic Consumption Score, PCA – patient-controlled analgesia. P-value significant with < 0.05 ± 2 SD.
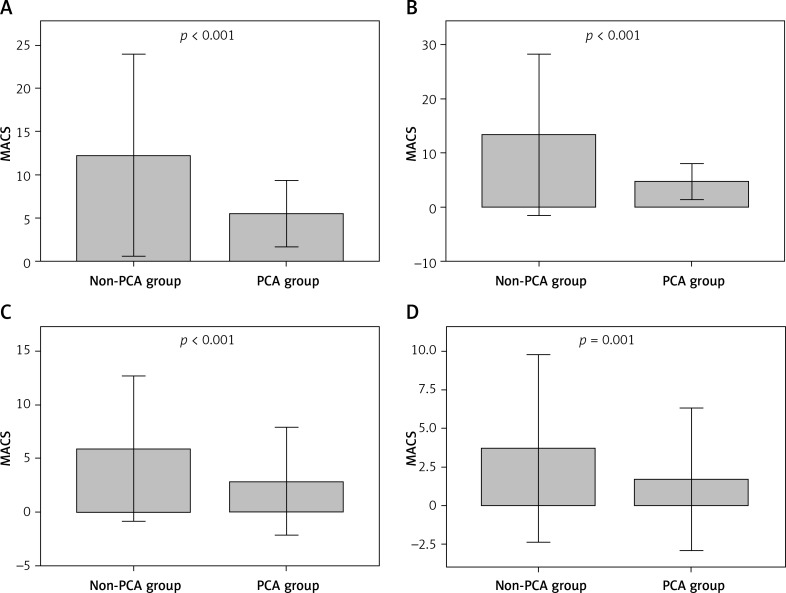
Because PCA medication was not considered within the first calculation, we performed a modified calculation of CACS using a ratio of demands and receipts of patients in the PCA group. This modified CACS was called MACS (“M” for “modified”). Again, we compared both groups, respectively CACS in the non-PCA and MACS in the PCA group, and found higher levels of CACS in the non-PCA group (days 0 to 2, p < 0.001; day 3, p = 0.001, Figure 2). Single values were 12.24 ±5.83 (2–24) in the non-PCA group vs. 5.5 ±1.92 (3–10.5) in the PCA group on the day of surgery, 13.48 ±7.5 (4–38) vs. 4.8 ±1.66 (3–10.5) on day one, 5.88 ±3.39 (1–16) vs. 2.85 ±2.52 (0–10.2) on day two and 3.68 ±3.04 (1–13) vs. 1.69 ±2.31 (0–8.2) on day three.
Figure 2.
MACS in non-PCA and PCA group day 0 to 3. A – Day of surgery (day 0), B – postoperative day one, C – postoperative day two, D – postoperative day three
MACS – modified Cumulative Analgesic Consumption Score, PCA – patient-controlled analgesia. P-value significant with < 0.05 ± 2 SD.
On days one and two after surgery, we compared values of NRS in both groups. On day one NRS in the non-PCA group was 2.19 ±1.7 (0–5) vs. 2.46 ±1.06 (1–5) in the PCA group (p = 0.653), and respectively 1.8 ±1.76 (0–6) vs. 2.35 ±1.27 (0–5) on day two (p = 0.199).
Beside pain relief, we compared length of hospital stay in both groups, and found no significant difference. In the non-PCA group the mean in-hospital-time was 6.85 ±1.23 days (5–10), while in the PCA group we observed 6.93 ±1.33 days (5–10) in hospital (p = 0.91).
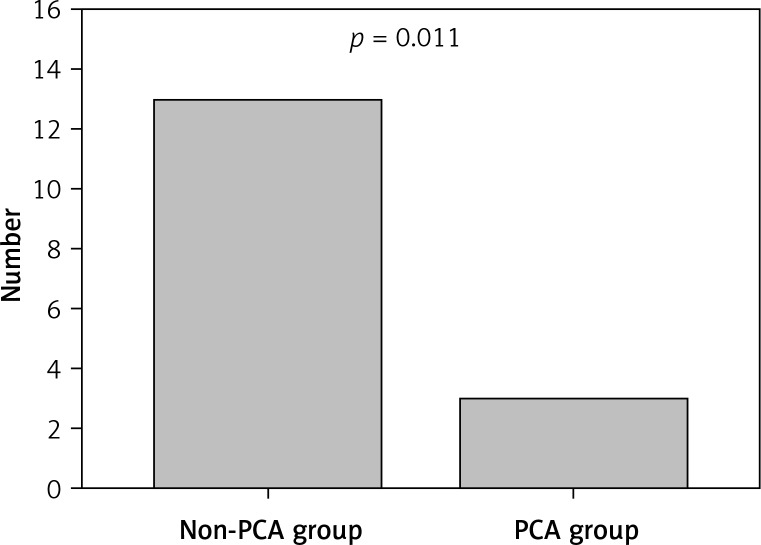
In both groups, laxative medication was given when needed. In the non-PCA group 39.4% (n = 13) of patients were treated with laxative medication, while in 60.6% (n = 20) laxatives were not necessary. In the PCA group 10.7% (n = 3) of patients needed this medication, and 89.3% (n = 25) did not. In comparison, more laxative agents were given in the non-PCA group (p = 0.011, Figure 3).
Figure 3.
Total number of patients treated with laxative medication in non-PCA and PCA group
PCA – patient-controlled analgesia. P-value significant with < 0.05.
To analyze the relation between NRS and CACS in patients undergoing bariatric surgery, we performed Spearman’s correlation, first of all without classification of postoperative pain management. We did not observe any correlation on day one (p = 0.49) or day two (p = 0.72), nor with MACS (p = 0.251 on day one, p = 0.657 on day two). The correlation between MACS and length of hospital stay showed a positive relation on postoperative day three (Spearman coefficient 0.278, p = 0.038) but no significant relation between CACS and in-hospital time.
In the non-PCA group, there was a positive correlation between CACS and NRS on day one (Spearman’s coefficient 0.43, p = 0.028) as well as between MACS and NRS (Spearman’s coefficient 0.646, p < 0.001), but not in the PCA group, either for MACS or NRS.
There was also no relation between CACS and in-hospital time in the non-PCA group. We observed a positive correlation between length of hospital stay and CACS in the PCA group (Spearman’s coefficient 0.403, p = 0.034) and between MACS and length of stay (Spearman’s coefficient 0.38, p = 0.46), on day three.
Discussion
The aim of this study was to identify sufficient pain relief and improvement of the CACS in patients undergoing bariatric surgical procedures. We compared two regimes of analgesia, on the one hand patients treated with PCA, on the other hand patients medicated with standard oral or intravenous analgesic drugs. For comparison, the CACS was used. This score is calculated by using a modified version of the WHO pain relief ladder [11]. Compared to other pain measurement scores, such as the NRS, CACS objectively describes analgesic consumption of an individual patient. We observed lower CACS and therefore less pain in patients treated with PCA. PCA medication, however, was not included in the CACS calculation, described by Schoenthaler et al. in their publication introducing the score [11]. To deal with this problem, we decided to calculate a modified MACS by using a ratio of total requests and successful demands. Schoenthaler et al. also mentioned the possibility of including PCA devices and adjunctive medication in the calculation of CACS, though never describing a specific protocol to conduct. In our opinion this small modification could also be used for other types of postoperative pain control.
Nevertheless, we also found higher MACS in the non-PCA than in the PCA group, confirming the score modification.
The NRS, being a good tool for measurement of pain relief [10], may be influenced by several striking factors. A certain degree of compliance is necessary and inner and outer factors may affect individual pain sensation. As Aceto et al. already described, postoperative pain is highly influenced by former depression and anxiety in obese and non-obese patients [5]. Although psychotropic medication has no influence on total opioid consumption or greater pain sensation, studies showed that former psychiatric hospitalization time is an independent risk factor for increased opioid requirement [12]. In this context, there might be a benefit in objective pain measurement.
In a previous study, CACS correlated well with NRS according to invasiveness of surgical trauma by analyzing pain intensity. We observed the same result on day one after the operation. According to the American Pain Society, pain measurement tools should always be used to document and monitor pain relief in analgesic treatment [2]. Patients not able to express pain intensity may be problematic in this context. Therefore, observation of analgesic consumption may be beneficial for objective analysis of individual pain, at least in the perioperative period.
One of the major complications in pain is gastrointestinal motility disorders, including those mediated by high opioid consumption. For that reason, we postulated that patients in the PCA group would have delayed beginning of bowel movement after surgery. We analyzed laxative medication treatment in both groups and found more frequent use of laxatives in the non-PCA group. Violent pain causes prolonged mobilization and associated complications such as pneumonia, deep vein thrombosis and ileus symptomatology as well [7]. And lastly, even pain itself causes less intestinal movement. Hence, we concluded that treatment with a PCA device might have an advantage according to the risk of postoperative ileus.
Postoperative pain-caused complications lead to prolonged hospitalization [1] as well as use of opioids and their adverse effects themselves [9]. In this context, we tried to examine whether higher pain sensation and therefore higher CACS (or MACS) leads to prolonged length of hospital stay (LOS) in our patient population. There was a positive correlation between CACS and LOS, but not with MACS. In subgroup analysis, we observed a relation between pain intensity and hospitalization time in the PCA group on postoperative day three. In conclusion, especially several days after surgery, pain leads to prolonged hospital stay in bariatric patients as well. It has to be remarked that there was no difference between treatment groups. Song et al. postulated longer in-hospital stay based on higher opioid consumption, but could not verify it [9]. Even though they observed a reduction in opioid treatment by combination with acetaminophen, they did not observe any influence on LOS. Choi et al. also compared a PCA treatment group with a non-PCA group. They observed slightly prolonged hospitalization in patients treated with PCA, but the difference was not significant [7]. They analyzed patients undergoing laparoscopic resection of colorectal cancer. A difference in surgical trauma according to minimal invasiveness versus open surgery in our patient population was hereby considered.
This study has several limitations, first of all its retrospective character; on the other hand, we modified CACS on our own to include PCA medication in calculation of the score. This modification has never been described in previous studies, so there is a need for prospective validation. NRS was not measured in patient mobilization, but there is a significant difference in pain intensity at rest and during movement, and guidelines recommend measurements in both conditions [2]. Finally, we have to remark that an exact dose of analgesic medication is not included in CACS calculation, although there are differences in individual drug dose [11]. For that reason, modification of CACS should be evaluated in further studies. Schug et al. reported that, according to their bodyweight, obese patients have less opioid consumption than non-obese patients. Therefore, the exact drug dose of opioids and non-opioid analgesics might be an interesting approach for further studies. According to adverse effects of opioid drugs, self-titration and dose-monitoring make PCA treatment a suitable tool in bariatric patients [13].
Conclusions
Patient-controlled analgesia devices for postoperative pain relief are suitable and sufficient tools in patients undergoing bariatric surgery. In context, CACS and MACS can help to objectify individual pain, especially in patients unable to express pain sensation. However, CACS should be interpreted in combination with clinical appearance and established pain measurement tools. Modifying this score by including other analgesic treatment options and co-analgesics should be investigated in further studies to improve this simple tool of pain measurement.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Guay J, Nishimori M, Kopp SL. Epidural local anesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery: a Cochrane review. Anesth Analg. 2016;123:1591–602. doi: 10.1213/ANE.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–57. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Andersen LP, Werner MU, Rosenberg J, Gögenur I. Analgesic treatment in laparoscopic gastric bypass surgery: a systematic review of randomized trials. Obes Surg. 2014;24:462–70. doi: 10.1007/s11695-013-1172-z. [DOI] [PubMed] [Google Scholar]
- 4.Gagliese L, Gauthier LR, Macpherson AK, et al. Correlates of postoperative pain and intravenous patient-controlled analgesia use in younger and older surgical patients. Pain Med. 2008;9:299–314. doi: 10.1111/j.1526-4637.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 5.Aceto P, Lai C, Perilli V, et al. Factors affecting acute pain perception and analgesics consumption in patients undergoing bariatric surgery. Physiol Behav. 2016;163:1–6. doi: 10.1016/j.physbeh.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 6.McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2015;6:CD003348. doi: 10.1002/14651858.CD003348.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YY, Park JS, Park SY, et al. Can intravenous patient-controlled analgesia be omitted in patients undergoing laparoscopic surgery for colorectal cancer? Ann Surg Treat Res. 2015;88:86–91. doi: 10.4174/astr.2015.88.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohl DD, Louie PK, Shah N, et al. Multimodal versus patient-controlled analgesia after an anterior cervical decompression and fusion. Spine (Phila Pa 1976) 2016;41:994–8. doi: 10.1097/BRS.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 9.Song K, Melroy MJ, Whipple OC. Optimizing multimodal analgesia with intravenous acetaminophen and opioids in postoperative bariatric patients. Pharmacotherapy. 2014;34(Suppl 1):14S–21S. doi: 10.1002/phar.1517. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 11.Schoenthaler M, Miernik A, Offner K, et al. The cumulative analgesic consumption score (CACS): evaluation of a new score to describe postsurgical analgesic consumption as a surrogate parameter for postoperative pain and invasiveness of surgical procedures. Int Braz J Urol. 2014;40:330–6. doi: 10.1590/S1677-5538.IBJU.2014.03.06. [DOI] [PubMed] [Google Scholar]
- 12.Weingarten TN, Sprung J, Flores A, et al. Opioid requirements after laparoscopic bariatric surgery. Obes Surg. 2011;21:1407–12. doi: 10.1007/s11695-010-0217-9. [DOI] [PubMed] [Google Scholar]
- 13.Schug SA, Raymann A. Postoperative pain management of the obese patient. Best Pract Res Clin Anaesthesiol. 2011;25:73–81. doi: 10.1016/j.bpa.2010.12.001. [DOI] [PubMed] [Google Scholar]