Abstract
With the development of science and technology, and development of artery bypass, methods such as cardiopulmonary cerebral resuscitation have been practiced in recent years. Despite this, some methods fail to promote or recover the function of tissues and organs, and in some cases, may aggravate dysfunction and structural damage to tissues. The latter is typical of ischemia-reperfusion (IR) injury. Lipid peroxidation mediated by free radicals is an important process of myocardial IR injury. Myocardial IR has been demonstrated to induce the formation of large numbers of free radicals in rats, which promotes the peroxidation of lipids within unsaturated fatty acids in the myocardial cell membrane. Markers of lipid peroxidation include malondialdehyde, superoxide dismutase and lactic dehydrogenase. Recent studies have demonstrated that N-acetylcysteine (NAC) is able to dilate blood vessels, prevent oxidative damage, improve immunity, inhibit apoptosis and the inflammatory response and promote glutathione synthesis in cells. NAC also improves the systolic function of myocardial cells and cardiac function, prevents myocardial apoptosis, protects ventricular remodeling and vascular remodeling, reduces opiomelanocortin levels in the serum and increases the content of nitric oxide in the serum, thus improving vascular endothelial function. Therefore, NAC has potent pharmacological activity; however, the relatively fast metabolism of NAC, along with its large clinical dose and low bioavailability, limit its applications. The present study combined NAC with medicinal activated carbons, and prepared N-acetylcysteine activated carbon sustained-release microcapsules (ACNACs) to overcome the limitations of NAC. It was demonstrated that ACNACs exerted greater effective protective effects than NAC alone on myocardial IR injury in rats.
Keywords: N-acetylcysteine, myocardial, ischemia-reperfusion, activated carbon, malondialdehyde
Introduction
Cardiovascular disease, particularly ischemic heart disease, has become a worldwide health problem affecting all economic groups of society (1). In recent years, many organs and tissues may have undergone reperfusion following ischemia with the establishment and promotion of treatment methods, including coronary artery bypass grafting, thrombolytic therapy, cardiac surgery extracorporeal circulation, cardiopulmonary cerebral resuscitation and organ transplantation (2–4). In most cases, ischemia-reperfusion (I/R) may help organs and tissues to repair themselves. However, sometimes I/R may also cause damage to them and became a serious threat to recovery. This phenomenon is called ischemia-reperfusion injury (5).
The World Health Organization predicts that by 2030, ischemic heart disease will become the second largest disease threatening human health (6). Myocardial ischemia caused by coronary artery infarction is the most important cause of ischemic heart disease (7). With social-economic development in China, changes to the living environment and lifestyle and the rise of the aging population, high incidence and mortality of chronic diseases, especially cardiovascular and cerebrovascular diseases, have been a heavy burden on society (8). Additionally, epidemiological studies showed that the age of onset tended to be younger in recent years (9).
Oxygen derived free radicals serve an important role in tissue injury during ischemia and reperfusion of the heart (10,11). There is substantial evidence that reactive oxygen including superoxide anion, hydrogen peroxide and hydroxyl radicals are responsible for myocardial injury during ischemia-reperfusion (12–14). MDA is a product of lipid peroxidation that indirectly reflects the generation of free radicals and injury degree of myocardial tissues (15); SOD is scavenging agent of superoxide radicals, which serves an important protective role in anti myocardial cell injury (16). At the same time, myocardial cell membrane lipid peroxidation increases cell membrane permeability and a large amount of LDH in cells is leaked into the intercellular space and body fluid (17).
N-acetylcysteine (NAC) is an acetyl compound of L-cysteine with an active mercapto group (18). In the past it has been used clinically as a mucolytic in respiratory diseases (19,20). More recently, studies have demonstrated that NAC dilates blood vessels, prevents oxidative damage, improves immunity, inhibits apoptosis and the inflammatory response and promotes the synthesis of glutathione in cells (21–25). With regard to the liver, NAC has been demonstrated to exert strong anti-fibrotic effects and preventative effects against fatty liver disease (26–28). With regard to the heart, NAC may improve the systolic function of myocardial cells and cardiac function, resist myocardial apoptosis, protect ventricular remodeling and vascular remodeling, reduce opiomelanocortin levels in the serum and improve the content of nitric oxide (NO) in the serum, and thus improve vascular endothelial function (29–33). Therefore, NAC has strong pharmacological effects (34,35), though its unfavorable effects include its relatively fast metabolism in the body, large clinical dose and low bioavailability, and side effects such as flush, nausea and vomiting (35–37). By contrast, medicinal activated carbons are sufficiently absorbed and biocompatible (35), and slowly release drugs during the metabolism of absorbed drugs, which overcomes the limitations of acetylcysteine and improves drug action time and bioavailability (38). The present study prepared N-acetylcysteine activated carbon sustained-release microcapsules (ACNACs) (39) by effectively combining NAC with medicinal activated carbons through an orthogonal experiment. The curative effect of ACNAC in rat liver has previously been documented (26,27,40–41). To determine the effect of ACNAC in the heart, the current study investigated the protective effect of ACNAC on myocardial ischemia-reperfusion (IR) injury in rats.
Materials and methods
Drugs and equipment
NAC was from Wuhan Grand Hoyo Co., Ltd. (Wuhan, China; batch number: 20110607); medicinal activated carbon was from Zhejiang Hangzhou Hangmu Timber Industry Co., Ltd. (Hangzhou, China; batch number: 120907) metoprolol tartrate injection was from Shandong East San Lu Pharmaceutical Co., Ltd. (Jining, China); malondialdehyde (MDA) assay kit (cat no. A003-1), superoxide dismutase (SOD) assay kit (cat no. A001-3), lactic dehydrogenase (LDH) assay kit (cat no. A020-2), NO assay kit (cat no. A012-1), NO synthase (NOS) assay kit (cat no. A014-1-1 and Coomassie brilliant blue protein assay kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); a Sartorius BS124S precision balance and PowerLab biological signal acquisition and analysis system were from AD Instruments (ML880, Sydney, Australia); a HX-300 small animal respiratory ventilator was from Chengdu Taimeng Technology Co., Ltd. (Chengdu, China); an Electrothermal Constant-temperature Dry Box was from Tianjin City Taisite Instrument Co., Ltd. (Tianjin, China); and a rotary paraffin microtome was from Jinhua Yidi Medical Appliance Co. Ltd. (YD-1508, Jinhua, China).
Preparation of animal model
A total of 64 male Sprague-Dawley rats (weight, 226.835±21.646 g; 45 days old) were used. Animals were obtained from the Experimental Animal Center of the Zhejiang Academy of Medical Sciences (Hangzhou, China), and had access to a standard commercial diet and water ad libitum with the exception of preoperative fasting for 12 h. Rats were kept in rooms maintained at 22±1°C under a 12-h light/dark cycle throughout the experiments. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (42), and approved by the Animal Care Committee of Xixi Hospital of Hangzhou Affiliated to Zhejiang University of Traditional Chinese Medicine (Hangzhou, China). The rats were randomly divided into the following eight groups (n=8 per group): Normal, sham, IR, metoprolol (Meto) control, NAC control, ACNAC1 (low-dose), ACNAC2 (moderate-dose) and ACNAC3 (high-dose).
The animals in groups ACNAC1, ACNAC2 and ACNAC3 were administered with 20, 40, 80 mg/kg ACNACs, respectively, via gavage. The animals in the NAC and Meto control groups were administered with an equivalent concentration of 80 mg/kg NAC and 20 mg/kg metoprolol solvent, respectively, via gavage. Normal saline of equivalent volume was administered to animals in the IR and sham groups. IR models were prepared 30 min after drug treatments, as described previously (43,44). All groups received ligature sutures, and the ligature in all groups excluding the normal and sham groups were tensioned after 5 min. The ligatures were loosened after 45 min, and limb lead electrocardiogram (ECG) and carotid blood pressure were observed for 2 h, after which a plastic tubing ball end a mosquito clamp was used to oppress and ligature the anterior descending left coronary artery. During the experiment, cyanosis in the left ventricular posterior wall and an increased ST section in a synchronous lead ECG were defined as signs of successful ligation, and a gradual change of cyanosis to red in the left ventricular posterior wall and 50% decrease in the ECG ST section were defined as signs of successful reperfusion. Rats were euthanized via exposure to gradually increasing concentrations of isoflurane and carbon dioxide gas (30% gradual-fill chamber vol/min) (45,46). Blood and heart tissue samples were then immediately stored at −80°C for later use. The number of animals used and their suffering was minimized. Following the procedure, animals were treated and specimens were prepared in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Determination of SOD, MDA and LDH indices
At 2 h after reperfusion, the heart was isolated and immediately washed with normal saline, and ~100 mg myocardial tissue from the ischemic area was cut and ground to obtain a 10% tissue homogenate. The mixture was homogenized 15 times at 4°C prior to centrifugation at 1,0625 × g for 15 min at 4°C. The supernatant was retained. SOD activity and MDA content were determined with SOD and MDA assay kits, respectively, and measured with a UV visible light photometer, according to the manufacturer's instructions. At 2 h after reperfusion, abdominal aorta blood was also collected and centrifuged at 1,0625 × g for 15 min at 4°C to obtain serum. LDH level was measured with a UV visible light photometer, according to the instructions of the LDH assay kit.
Determination of NO content and NOS activity
The reserved serum samples (stored at −80°C) were thawed at room temperature and NO content was) was measured according to the kit instructions, based on measurements of optical density (OD) obtained using a 721 Spectrophotometer (Shanghai Optical Instrument Factory, Co., Ltd., Shanghai, China) at a wavelength of 550 nm. The following formula was used: NO content in serum (µmol/l)=(OD value of sample tube-OD value of blank tube)/(OD value of standard tube-OD value of blank tube)x standard tube concentration (20 µmol/l)x sample dilution.
The reserved heart samples were thawed at room temperature and myocardial tissue homogenate was prepared. The mixture was homogenized 15 times at 4°C prior to centrifugation at 10,625 × g for 15 min at 4°C. The supernatant was retained and the OD values of total NOS (TNOS) in the sample tube, inducible NOS (iNOS) in the sample tube and constitutive NOS (cNOS) in the sample tube were determined with a 721 Spectrophotometer at a wavelength of 530 nm. Protein content (in mg prot/l) in the sample tubes was also measured with the Coomassie brilliant blue kit. Measurements were obtained with a 721 Spectrophotometer at wavelength 595 nm. The following formula was used:
Recording of reperfusion arrhythmia duration
The judgment of arrhythmia on Lambeth, Conventions standards, and arrhythmia reperfusion scores were assigned according to the scoring system by Walker et al (47). The system is as follows: 0, No arrhythmia; 1, Accidental ventricular premature contraction, VPC; 2, Frequent VPC; 3, Accidental ventricular tachycardia, VT; 4, Frequent VT or Accidental Ventricular fibrillation (VF); and 5, Frequent VF or death.
Hematoxylin and eosin staining of myocardial tissues to detect pathological changes
Left ventricular anterior wall tissue from each group was treated in 4% paraformaldehyde for 24 h at room temperature, dehydrated with alcohol prepared according to a set gradient (75, 85, 90, 95 and 100%) and then embedded in paraffin blocks. Sections 4 µm thick were deparaffinated in dimethylbenzene, embedded in xylene I for 20 min, xylene II for 20 min, absolute ethyl alcohol I for 10 min, absolute ethyl alcohol II for 10 min, 95% alcohol for 5 min, 90% alcohol for 5 min, 80% alcohol for 5 min and 70% alcohol for 5 min successively prior to washing with water. Cell nuclei were stained with hematoxylin, and the cell cytoplasm was stained with eosin. Sections were then dehydrated and sealed with neutral resin. A light microscope (magnification, ×200; Nikon Corporation; Tokyo, Japan) was used to observe the structures of myocardial tissues from each group.
Statistical analysis
Measurement data were presented as the mean ± standard deviation, and statistical analysis was performed with SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was used for comparison of multiple groups, and the LSD method was used as a post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Determination of SOD, MDA and LDH indices
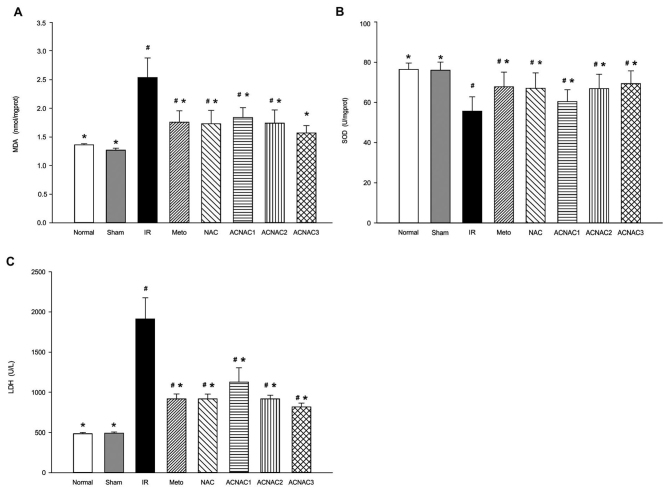
The SOD activation and MDA and LDH levels are presented in Fig. 1. Compared with the normal group, the IR group exhibited significantly reduced SOD (76.41±3.19 vs. 55.66±7.11 U/mg prot; P<0.05) activation and significantly increased MDA (1.36±0.02 vs. 2.54±0.34 nmol/mg prot; P<0.05) content and serum LDH (484±16 vs. 1,915±262 U/l; P<0.05) levels; the difference in SOD (76.41±3.19 vs. 75.97±4.06 U/mg prot; P>0.05) activation, MDA (1.36±0.02 vs. 1.27±0.03 nmol/mg prot; P>0.05) and serum LDH levels (484±16 vs. 491±16 U/l; P>0.05) in the Sham group was not significant; the NAC group exhibited significantly reduced SOD (76.41±3.19 vs. 67.07±7.60 U/mg prot; P<0.05) activation and significantly increased MDA (1.36±0.02 vs. 1.73±0.23 nmol/mg prot; P<0.05) content and serum LDH (484±16 vs. 917±61 U/l; P<0.05) levels; the Meto group exhibited significantly reduced SOD (76.41±3.19 vs. 67.82±7.21 U/mg prot; P<0.05) activation and significantly increased MDA (1.36±0.02 vs. 1.76±0.20 nmol/mg prot; P<0.05) content and serum LDH (484±16 vs. 917±62 U/l; P<0.05) levels; the ACNAC1 group exhibited significantly reduced SOD (76.41±3.19 vs. 60.43±5.89 U/mg prot; P<0.05) activation and significantly increased MDA (1.36±0.02 vs. 1.84±0.17 nmol/mg prot; P<0.05) content and serum LDH (484±16 vs. 1,128±177 U/l; P<0.05) levels; the ACNAC2 group exhibited significantly reduced SOD (76.41±3.19 vs. 66.82±7.14 U/mg prot; P<0.05) activation and significantly increased MDA (1.36±0.02 vs. 1.741±0.232 nmol/mg prot; P<0.05) content and serum LDH (484±16 vs. 918±46 U/l; P<0.05) levels; the difference in SOD (76.41±3.19 vs. 69.33±6.09 U/mg prot; P>0.05) and MDA (1.36±0.02 vs. 1.57±0.13 nmol/mg prot; P>0.05) content in the ACNAC3 group was not significant, while the serum LDH (484±16 vs. 818±49 U/l; P<0.05) was significantly increased.
Figure 1.
Effect of ACNAC on the activation of SOD, MDA and LDH in the myocardium of IR rats. (A) MDA content, (B) SOD activity and (C) LDH levels were determined following exposure of the rat groups to IR. Data are presented as the mean ± standard deviation. *P<0.05 vs. IR group, #P<0.05 vs. normal group. SOD, superoxide dismutase; MDA, malondialdehyde; LDH, lactate dehydrogenase; IR, ischemia-reperfusion; Meto, metoprolol; NAC, N-acetylcysteine; ACNAC, N-acetylcysteine activated carbon release microcapsule; ACNAC1/2/3, ACNAC low/moderate/high-dose groups.
In the Sham group, SOD (75.97±4.06 vs. 55.67±4.29 U/mg prot; P<0.05) activation was significantly increased and MDA (1.27±0.03 vs. 2.54±0.41 nmol/mg prot; P<0.05) content and serum LDH (491±16 vs. 1915±262 U/l; P<0.05) levels were significantly reduced when compared with the IR group. In the NAC group, SOD (67.07±7.60 vs. 55.67±4.29 U/mg prot; P<0.05) activation was significantly increased and MDA (1.73±0.23 vs. 2.54±0.41 nmol/mg prot; P<0.05) content and serum LDH (917±61 vs. 1,915±262 U/l; P<0.05) levels were significantly reduced when compared with the IR group. In the Meto group, SOD (67.82±7.21 vs. 55.67±4.29 U/mg prot; P<0.05) activation was significantly increased and MDA (1.76±0.20 vs. 2.54±0.41 nmol/mg prot; P<0.05) content and serum LDH (917±62 vs. 1915±262 U/l; P<0.05) levels were significantly reduced when compared with the IR group. In the ACNAC1 group, SOD (60.43±5.89 vs. 55.67±4.29 U/mg prot; P<0.05) activation was significantly increased and MDA (1.84±0.17 vs. 2.54±0.41 nmol/mg prot; P<0.05) content and serum LDH (1,128±177 vs. 1,915±262 U/l; P<0.05) levels were significantly reduced when compared with the IR group. In the ACNAC2 group, SOD (66.82±7.14 vs. 55.67±4.29 U/mg prot; P<0.05) activation was significantly increased and MDA (1.74±0.23 vs. 2.54±0.41 nmol/mg prot; P<0.05) content and serum LDH (918±46 vs. 1,915±262 U/l; P<0.05) levels were significantly reduced when compared with the IR group. In the ACNAC3 group, SOD (70.49±6.22 vs. 55.67±4.29 U/mg prot; P<0.05) activation was significantly increased and MDA (1.57±0.15 vs. 2.54±0.41 nmol/mg prot; P<0.05) content and serum LDH (818±49 vs. 1,915±262 U/l; P<0.05) levels were significantly reduced when compared with the IR group. The difference between the SOD (67.82±7.21 vs. 67.07±7.60 U/mg prot; P>0.05) activation, MDA (1.76±0.20 vs. 1.73±0.23 nmol/mg prot; P>0.05) content and serum LDH (917±62 vs. 917±61 U/l; P>0.05) in the Meto and NAC groups, was not significant. The difference between the SOD (67.82±7.21 vs. 66.82±7.14 U/mg prot; P>0.05) activation, MDA (1.76±0.20 vs. 1.74±0.23 nmol/mg prot; P>0.05) content and serum LDH (917±62 vs. 918±46 U/l; P>0.05) in the Meto and ACNAC2 groups was not significant.
Determination of NO content and NOS activity
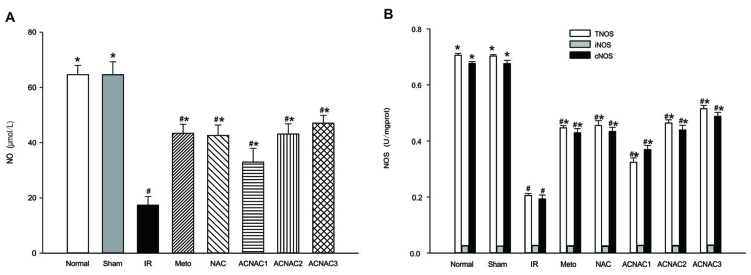
The NO content and NOS activity are presented in Fig. 2. Compared with the normal group, the NO content of the IR group was significantly reduced (64.667±3.31 vs. 17.41±3.06 µmol/l; P<0.05); the NO content of the Sham group was not significantly reduced (64.67±3.31 vs. 64.60±4.70 µmol/l; P>0.05); the NO content of the NAC group was significantly reduced (64.67±3.31 vs. 42.63±3.78 µmol/l; P<0.05); the NO content of the Meto group was significantly reduced (64.67±3.31 vs. 43.44±3.22 µmol/l; P<0.05); the NO content of the ACNAC1 group was significantly reduced (64.67±3.31 vs. 32.94±5.01 µmol/l; P<0.05); the NO content of the ACNAC2 group was significantly reduced (64.67±3.31 vs. 43.13±3.66 µmol/l; P<0.05); the NO content of the ACNAC3 group was significantly reduced (64.67±3.31 vs. 47.08±2.83 µmol/l; P<0.05).
Figure 2.
Effect of ACNAC on NO content and NOS activity in the myocardium of IR rats. (A) NO content; (B) TNOS, iNOS and cNOS activity. Data are presented as the mean ± standard deviation.*P<0.05 vs. IR group, #P<0.05 vs. normal group. NO, nitric oxide; NOS, nitric oxide synthase; T/i/cNOS, total/inducible/constitutive NOS; IR, ischemia-reperfusion; Meto, metoprolol; NAC, N-acetylcysteine; ACNAC, N-acetylcysteine activated carbon release microcapsule; ACNAC1/2/3, ACNAC low/moderate/high-dose groups.
Compared with the IR group, the NO content of the Sham group was significantly increased (17.41±3.06 vs. 64.60±4.70 µmol/l; P<0.05); the NO content of the NAC group was significantly increased (17.41±3.06 vs. 42.63±3.78 µmol/l; P<0.05); the NO content of the Meto group was significantly increased (17.41±3.06 vs. 43.44±3.22 µmol/l; P<0.05); the NO content of the ACNAC1 group was significantly increased (17.41±3.06 vs. 32.94±5.01 µmol/l; P<0.05); the NO content of the ACNAC2 group was significantly increased (17.41±3.06 vs. 43.13±3.66 µmol/l; P<0.05); and the NO content of the ACNAC3 group was significantly increased (17.41±3.06 vs. 47.08±2.83 µmol/l; P<0.05).
Regarding NOS activity, the activity of TNOS (0.205±0.008 vs. 0.706±0.006 U/mg prot; P<0.05) and cNOS (0.193±0.014 vs. 0.677±0.006 U/mg prot; P<0.05) was significantly reduced in the IR group when compared with the normal group, while iNOS activity did not differ significantly between the two groups (0.026±0.002 vs. 0.025±0.001 U/mg prot; P>0.05). Regarding NOS activity, the activity of TNOS (0.703±0.005 vs. 0.706±0.006 U/mg prot; P>0.05), cNOS (0.676±0.012 vs. 0.677±0.006 U/mg prot; P>0.05) and iNOS (0.024±0.001 vs. 0.025±0.001 U/mg prot; P>0.05) activity did not different significantly between the Sham group and the normal group. Regarding NOS activity, the activity of TNOS (0.455±0.017 vs. 0.706±0.006 U/mg prot; P<0.05) and cNOS (0.434±0.015 vs. 0.677±0.006 U/mg prot; P<0.05) was significantly reduced in the NAC group when compared with the normal group, while iNOS (0.023±0.003 vs. 0.025±0.001 U/mg prot; P>0.05) activity did not different significantly between the two groups. Regarding NOS activity, the activity of TNOS (0.447±0.008 vs. 0.706±0.006 U/mg prot; P<0.05) and cNOS (0.429±0.015 vs. 0.677±0.006 U/mg prot; P<0.05) was significantly reduced in the Meto group when compared with the normal group, while iNOS (0.024±0.002 vs. 0.025±0.001 U/mg prot; P>0.05) activity did not different significantly between the two groups. Regarding NOS activity, the activity of TNOS (0.324±0.015 vs. 0.706±0.006 U/mg prot; P<0.05) and cNOS (0.369±0.015 vs. 0.677±0.006 U/mg prot; P<0.05) was significantly reduced in the ACNAC1 group when compared with the normal group, while iNOS (0.026±0.002 vs. 0.025±0.001 U/mg prot; P>0.05) activity did not differ significantly between the two groups. Regarding NOS activity, the activity of TNOS (0.464±0.011 vs. 0.706±0.006 U/mg prot; P<0.05) and cNOS (0.439±0.017 vs. 0.677±0.006 U/mg prot; P<0.05) was significantly reduced in the ACNAC2 group when compared with the normal group, while iNOS (0.025±0.002 vs. 0.025±0.001 U/mg prot; P>0.05) activity did not differ significantly between the two groups. Regarding NOS activity, the activity of TNOS (0.515±0.011 vs. 0.706±0.006 U/mg prot; P<0.05) and cNOS (0.488±0.014 vs. 0.677±0.006 U/mg prot, P<0.05) was significantly reduced in the ACNAC3 group when compared with the normal group, while iNOS (0.027±0.002 vs. 0.025±0.001 U/mg prot; P>0.05) activity did not different significantly between the two groups.
Compared with the IR group, the activity of TNOS (0.205±0.008 vs. 0.703±0.005 U/mg prot, P<0.05) and cNOS (0.193±0.014 vs. 0.676±0.012 U/mg prot; P<0.05) in the Sham group was significantly increased, while iNOS (0.026±0.002 vs. 0.024±0.001 U/mg prot; P>0.05) activity did not differ significantly between the two groups. Compared with the IR group, the activity of TNOS (0.205±0.008 vs. 0.455±0.017 U/mg prot; P<0.05) and cNOS (0.193±0.014 vs. 0.434±0.015 U/mg prot; P<0.05) in the NAC group was significantly increased, while iNOS (0.026±0.002 vs. 0.023±0.003 U/mg prot; P<0.05) was significantly decreased. Compared with the IR group, the activity of TNOS (0.205±0.008 vs. 0.447±0.008 U/mg prot; P<0.05) and cNOS (0.193±0.014 vs. 0.429±0.015 U/mg prot; P<0.05) in the Meto group was significantly increased, while iNOS (0.026±0.002 vs. 0.024±0.002 U/mg prot; P>0.05) activity did not differ significantly between the two groups. Compared with the IR group, the activity of TNOS (0.205±0.008 vs. 0.324±0.015 U/mg prot; P<0.05) and cNOS (0.193±0.014 vs. 0.369±0.015 U/mg prot; P<0.05) in the ACNAC1 group was significantly increased, while iNOS (0.026±0.002 vs. 0.026±0.002 U/mg prot; P>0.05) activity did not differ significantly between the two groups. Compared with the IR group, the activity of TNOS (0.205±0.008 vs. 0.464±0.011 U/mg prot; P<0.05) and cNOS (0.193±0.014 vs. 0.439±0.017 U/mg prot; P<0.05) in the ACNAC2 group was significantly increased, while iNOS (0.026±0.002 vs. 0.025±0.002 U/mg prot; P>0.05) activity did not differ significantly between the two groups.
Compared with the IR group, the activity of TNOS (0.205±0.008 vs. 0.515±0.011 U/mg prot; P<0.05) and cNOS (0.193±0.014 vs. 0.488±0.014 U/mg prot; P<0.05) in the ACNAC3 group was significantly increased, activity did not differ significantly between the two groups.
Effect of ACNACs on the duration and score of reperfusion arrhythmia
The reperfusion arrhythmia scores are presented in Fig. 3. The reperfusion arrhythmia score of the IR group was significantly increased when compared with the normal group (4.16±0.57 vs. 0 min; P<0.05). The difference in reperfusion arrhythmia score of the Sham group when compared with the normal group was not significant (0.34±0.05 vs. 0 min; P>0.05). The reperfusion arrhythmia score of the NAC group was significantly increased when compared with the normal group (2.25±0.60 vs. 0 min; P<0.05). The reperfusion arrhythmia score of the Meto group was significantly increased when compared with the normal group (2.22±0.40 vs. 0 min; P<0.05). The reperfusion arrhythmia score of the ACNAC1 group was significantly increased when compared with the normal group (3.57±0.63 vs. 0 min; P<0.05). The reperfusion arrhythmia score of the ACNAC2 group was significantly increased when compared with the normal group (2.50±0.43 vs. 0 min; P<0.05). The reperfusion arrhythmia score of the ACNAC3 group was significantly increased when compared with the normal group (1.78±0.36 vs. 0 min; P<0.05).
Figure 3.
Effect of ACNAC on the duration and score of reperfusion arrhythmia. (A) Duration of reperfusion arrhythmia; (B) score of reperfusion arrhythmia. Data are presented as the mean ± standard deviation. *P<0.05 vs. IR group, #P<0.05 vs. normal group. IR, ischemia-reperfusion; Meto, metoprolol; NAC, N-acetylcysteine; ACNAC, N-acetylcysteine activated carbon release microcapsule; ACNAC1/2/3, ACNAC low/moderate/high-dose groups; S, score.
The reperfusion arrhythmia score of the Sham group was significantly decreased when compared with the IR group (0.34±0.05 vs. 4.16±0.57 min; P<0.05). The reperfusion arrhythmia score of the NAC group was significantly decreased when compared with the IR group (2.25±0.60 vs. 4.16±0.57 min; P<0.05). The reperfusion arrhythmia score of the Meto group was significantly decreased when compared with the IR group (2.25±0.60 vs. 4.16±0.57 min; P<0.05). The reperfusion arrhythmia score of the ACNAC1 group was significantly decreased when compared with the IR group (3.57±0.63 vs. 4.16±0.57 min; P<0.05). The reperfusion arrhythmia score of the ACNAC2 group was significantly decreased when compared with the IR group (2.50±0.43 vs. 4.16±0.57 min; P<0.05). The reperfusion arrhythmia score of the ACNAC3 group was significantly decreased when compared with the IR group (1.78±0.36 vs. 4.16±0.57 min; P<0.05).
In addition, compared with the normal group, the duration of reperfusion arrhythmia in the IR group was significantly increased (0 vs. 446.56±21.81 sec; P<0.05). Compared with the normal group, the difference in duration of reperfusion arrhythmia in the Sham group was not significant (0 vs. 4.94±0.49 sec; P<0.05). Compared with the normal group, the duration of reperfusion arrhythmia in the NAC group was significantly increased (0 vs. 88.64±9.85 sec; P<0.05). Compared with the normal group, the duration of reperfusion arrhythmia in the Meto group was significantly increased (0 vs. 89.71±5.87 sec; P<0.05). Compared with the normal group, the duration of reperfusion arrhythmia in the ACNAC1 group was significantly increased (0 vs. 198.85±17.92 sec; P<0.05). Compared with the normal group, the duration of reperfusion arrhythmia in the ACNA2 group was significantly increased (0 vs. 89.15±14.89 sec; P<0.05). Compared with the normal group, the duration of reperfusion arrhythmia in the ACNAC3 group was significantly increased (0 vs. 84.71±12.92 sec; P<0.05).
In the Sham group, the duration of reperfusion arrhythmia was significantly reduced when compared with the IR group (4.94±0.49 vs. 446.56±21.81; P<0.05). In the NAC group, the duration of reperfusion arrhythmia was significantly reduced when compared with the IR group (88.64±9.85 vs. 446.56±21.81; P<0.05). In the Meto group, the duration of reperfusion arrhythmia was significantly reduced when compared with the IR group (89.71±5.87 vs. 446.56±21.81; P<0.05). In the ACNAC1 group, the duration of reperfusion arrhythmia was significantly reduced when compared with the IR group (198.85±17.92 vs. 446.56±21.81; P<0.05). In the ACNAC2 group, the duration of reperfusion arrhythmia was significantly reduced when compared with the IR group (89.15±14.89 vs. 446.56±21.81; P<0.05). In the ACNAC3 group, the duration of reperfusion arrhythmia was significantly reduced when compared with the IR group (84.71±12.92 vs. 446.56±21.81; P<0.05).
Pathological changes in the myocardial tissues
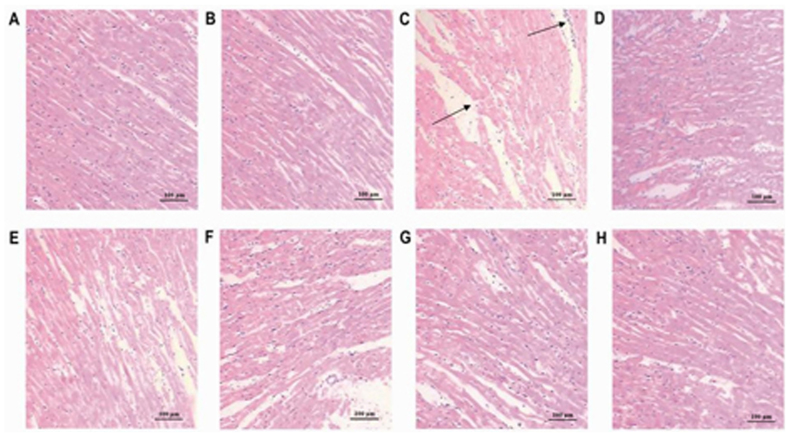
HE staining was used to identify pathological changes in the myocardium following ACNAC treatment and IR injury (Fig. 4). Cardiac muscle fibers in the normal and sham-operated groups (Fig. 4A and B) were complete and exhibited a regular arrangement, clear structure and uniform coloring. The morphology of the nucleus appeared normal, the cell membrane was complete, and no degeneration or necrosis of cells was observed. In the IR group (Fig. 4C), the cardiac muscle fibers exhibited uneven coloring and a disordered arrangement, and some cells were broken and necrotic. Rupture, dispersal and disappearance of the nucleus were also observed, and there was edema of the intercellular space and a high level of inflammatory cell infiltration. Compared with the IR group, the cardiac muscle fibers in the Meto and ACNAC3 groups (Fig. 4D and H) were relatively complete. Fiber arrangement was relatively ordered with uniform coloring, however, a few inflammatory cells had infiltrated and the intercellular space exhibited mild edema. Compared with the IR group, there was an improvement in non-uniform staining and disorganization of myocardial fibers and some of the damaged cells were reduced in the NAC, ACNAC1 and ACNAC2 group (Fig. 4E-G).
Figure 4.
Hematoxylin and eosin staining of the myocardium from different rat groups (magnification, ×200). (A) Normal group; (B) Sham group; (C) IR group; (D) Meto group; (E) NAC group; (F) ACNAC1 group; (G) ACNAC2 group; (H) ACNAC3 group. IR, ischemia-reperfusion; Meto, metoprolol; NAC, N-acetylcysteine; ACNAC, N-acetylcysteine activated carbon release microcapsule; ACNAC1/2/3, ACNAC low/moderate/high-dose groups.
Discussion
The majority of coronary heart diseases involving myocardial damage are caused by a reduction in the volume of coronary blood flow and imbalances in myocardial oxygen demand due to coronary artery lesion (48,49). Coronary heart disease is among the main diseases that threaten human health worldwide (50). At present, thrombolytic therapy, coronary artery intervention therapy and coronary artery bypass grafting are the more effective therapeutic strategies for acute myocardial infarction, and generally these technologies are capable of saving dying ischemic myocardial cells, decreasing infarct size and improving heart function (51). Treatment of myocardial reperfusion injury using the above methods is a current focus of research in the medical community (52,53). Lipid peroxidation mediated by free radicals is an important step during myocardial IR injury. It has been observed in rats that myocardial IR leads to the formation of a large number of free radicals (54–58). This promotes lipid peroxidation in unsaturated fatty acids of the myocardial cell membrane, and produces lipid peroxides that react with intracellular structures, including nucleic acid and proteins, which ultimately changes cell structure and function and leads to myocardial cell damage (59,60). The content of MDA is also increased, which interferes with the cell membrane and leads to greater damage to cell membrane structure and function (61). This typically manifests as a change in cell membrane fluidity and permeability, leakage of myocardial enzymes, reduction of cell membrane ATP activity, disorders of ion transportation and abnormal ion distribution (62). In turn, aberrant ion distribution due to intracellular calcium overload may stimulate after depolarizations and trigger activity, inducing arrhythmia and cardiac dysfunction (63). In addition, impairment of energy metabolism caused by ischemic myocardial necrosis and mitochondrial dysfunction may lead to a decrease in cardiac function (64). Therefore, the content of MDA may indirectly reflect the generation of oxygen free radicals and degree of tissue damage (65,66). LDH, as a specific enzyme of the myocardial cytoplasm, is an additional marker of myocardial damage, as the leakage of LDH typically occurs only when the cell membrane is damaged. Thus the degree of LDH leakage may indirectly reflect the degree of myocardial damage (67,68). Furthermore, SOD is an intracellular antioxidant, which removes superoxide anions and protects the body against oxidative damage by free radicals, and thus the level of SOD may indirectly reflect the body's ability to scavenge oxygen free radicals and prevent lipid peroxidation (69,70).
Results of the present study indicated that compared with the IR group, SOD activation was significantly increased in rats of the ACNAC3 group, while MDA content and serum LDH were significantly decreased. SOD activation, MDA content and serum LDH levels did not differ significantly among the Meto, NAC and three ACNAC groups. These results suggest that ACNAC may inhibit peroxidation by free radicals and stimulate oxidase activation in myocardial tissue. This is a preliminary indication that lipid peroxidation mediated by free radicals is an important underlying mechanism of ACNAC regarding its alleviative role in myocardial ischemia injury.
NO, which is synthesized by a family of NOS enzymes, including neuronal, inducible, and endothelial NOS (n/i/eNOS), serves a key role in cardiovascular physiology and pathology (71). eNOS has been reported to inhibit the progression of myocardial infarction (72), ameliorate myocardial I/R injury (73) and left ventricular hypertrophy (74,75), and prevent the onset of heart failure (76). However, it remains controversial whether NO exerts a protective or cytotoxic effect in myocardial IR injury (77,78). Though as an important signal molecule in the body, a specific concentration range of NO is required to protect and maintain cardiac muscle cells and cardiac function (79–81).
L-arginine is the substrate of NOS, and the reaction generates citruline and NO. In signaling pathways involving NO, NOS is a key rate-limiting enzyme. Due to its differential expression pattern in different tissues, NOS is as cNOS and iNOS, of which cNOS is also divided into nNOS and eNOS. eNOS primarily serves roles in the regulation of arterial blood pressure and blood flow (82). eNOS is mainly expressed in myocardial cells, vascular endothelial cells, the endocardium and platelets, and it maintains physiological function through continuous synthesis of basic NO (82–84). iNOS is mainly expressed in endothelial and vascular smooth muscle cells (85,86). Previous studies have indicated that ischemia and reperfusion may induce endothelial cell damage and dysfunction and activate endogenous NOS inhibitors, thus reducing the total activity of NOS (82) and eNOS (87–90). When the activity of iNOS is increased, it may produce high levels of NO; however, the activation process of iNOS is relatively slow and requires 4–6 h to be expressed abundantly, reaching a peak some 48 (91–93).
It has been previously observed that in addition to the direct effect of NO on myocardial systolic function (94–96), NO influences myocardial cell oxygen metabolism (97), regeneration (98), hypertrophy (99) and apoptosis (100). It may also enhance the mechanical efficiency of the myocardium (101) and reduce myocardial oxygen consumption (102). In the process of IR, NO maybe a protective agent in the heart, and may prevent IR-related tissue damage (103).
NAC is a molecule containing a sulfhydryl group, and it may interact with the electrophilic group of reactive oxygen species, which generates a sulfhydryl intermediate. In this way, NAC serves a direct role as an antioxidant, alleviates oxidative stress-related injury to tissues and enhances the biological function of NO by preventing reactions between NO and free radicals (104,105).
Spectrophotometry data in the present study demonstrated that compared with the IR group, the NO content, TNOS and cNOS activity of the ACNAC3 group were significantly increased, while iNOS activity did not differ significantly. Due to the relatively long activation process of iNOS, the unaltered expression of iNOS may have been due to the short reperfusion period of 2 h used in the current study. These data suggest that ACNAC may alleviate myocardial ischemia injury by increasing the NO content of myocardial tissue, which may be initially increased by the promotion of TNOS activity in the myocardial tissue. ACNAC also reduced arrhythmia score and shortened reperfusion arrhythmia duration, which may be beneficial to the recovery of heart function and indicates the protective effects of ACNAC against arrhythmia.
The present study preliminarily concluded that the underlying mechanism of ACNAC regarding its alleviative role in myocardial reperfusion injury may be related to its effects on lipid peroxidation mediated free radicals, and the NO pathway. However, whether ACNAC is related to other mechanisms requires further comprehensive studies.
Acknowledgements
The present study was supported by the Hangzhou Science and Technology Development Project (grant nos. 20130633B09, 20140633B29 and 20142013A60).
References
- 1.Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu P, Zhang J, Yu S, Luo Z, Hua F, Yuan L, Zhou Z, Liu Q, Du X, Chen S, et al. Protective Effect of sevoflurane postconditioning against cardiac ischemia/reperfusion injury via ameliorating mitochondrial impairment, oxidative stress and rescuing autophagic clearance. PLoS One. 2015;10:e0134666. doi: 10.1371/journal.pone.0134666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Wu W, Li Q, Chen C, Zhou R, Qiu Y, Luo M, Tan Z, Li S, Chen G, et al. High-dose polymerized hemoglobin fails to alleviate cardiac ischemia/reperfusion injury due to induction of oxidative damage in coronary artery. Oxid Med Cell Longev. 2015;2015:125106. doi: 10.1155/2015/125106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu T, Li D, Jiang D. Targeting cell signaling and apoptotic pathways by luteolin: Cardioprotective role in rat cardiomyocytes following ischemia/reperfusion. Nutrients. 2012;4:2008–2019. doi: 10.3390/nu4122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun D, Huang J, Zhang Z, Gao H, Li J, Shen M, Cao F, Wang H. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS One. 2012;7:e33491. doi: 10.1371/journal.pone.0033491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta R, Oki E, Endo K, Biedermann V, Ren J, Kufe D. XIAP regulates DNA damage-induced apoptosis downstream of caspase-9 cleavage. J Biol Chem. 2000;275:31733–31738. doi: 10.1074/jbc.M910231199. [DOI] [PubMed] [Google Scholar]
- 7.Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, Chiribiri A. Coronary artery anomalies overview: The normal and the abnormal. World J Radiol. 2016;8:537–555. doi: 10.4329/wjr.v8.i6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun YQ, Jiang AL, Chen SM, Li H, Xing HY, Wang F. Quality of life and self-care in elderly patients with cardiovascular diseases: The effect of a traditional Chinese medicine health educational intervention. App Nur Res. 2017;38:134–140. doi: 10.1016/j.apnr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Cao XQ. Preventive strategies of ischemic cardiovascular disease oriented by risk predictive model (unpublished PhD thesis) Zhengzhou University; Zhengzhou: 2016. [Google Scholar]
- 10.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 11.Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989;80:1115–1127. doi: 10.1161/01.CIR.80.5.1115. [DOI] [PubMed] [Google Scholar]
- 12.Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J Biol Chem. 1990;265:6656–6663. [PubMed] [Google Scholar]
- 13.Ruuge EK, Ledenev AN, Lakomkin VL, Konstantinov AA, Ksenzenko MYu. Free radical metabolites in myocardium during ischemia and reperfusion. Am J Physiol. 1991;261(4 Suppl):S81–S86. doi: 10.1152/ajplung.1991.261.4.L81. [DOI] [PubMed] [Google Scholar]
- 14.Takemura G, Onodera T, Ashraf M. Quantification of hydroxyl radical and its lack of relevance to myocardial injury during early reperfusion after graded ischemia in rat hearts. Circ Res. 1992;71:96–105. doi: 10.1161/01.RES.71.1.96. [DOI] [PubMed] [Google Scholar]
- 15.He H, Li N, Zhao Z, Han F, Wang X, Zeng Y. Ischemic postconditioning improves the expression of cellular membrane connexin 43 and attenuates the reperfusion injury in rat acute myocardial infarction. Biomed Rep. 2015;3:668–674. doi: 10.3892/br.2015.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YX, Liu JN, Shen MZ, Li R, Zhang RH, Zhai YL, Zhao M, Wang YM, Wang XM. Effects of EDRV and DIDS on reactive oxygen species levels in acute ischemia-reperfusion injured myocardium. Chin J Pathophysiol. 2011;27:648–652. (In Chinese) [Google Scholar]
- 17.Li H, Liu Z, Wang J, Wong GT, Cheung CW, Zhang L, Chen C, Xia Z, Irwin MG. Susceptibility to myocardial ischemia reperfusion injury at early stage of type 1 diabetes in rats. Cardiovasc Diabetol. 2013;12:133. doi: 10.1186/1475-2840-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhouib Elbini I, Jallouli M, Annabi A, Gharbi N, Elfazaa S, Lasram MM. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci. 2016;151:359–363. doi: 10.1016/j.lfs.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Chen XQ, Jin YY, Tang G, editors. The New Pharmacology. People's Medical Publishing House; Beijing: 2011. p. 428. [Google Scholar]
- 20.Blasi F, Page C, Rossolini GM, Pallecchi L, Matera MG, Rogliani P, Cazzola M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Res Med. 2016;117:190–197. doi: 10.1016/j.rmed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Yang MJ. Progress of N-acetylcysteine clinical application. Guide Chin Med. 2015;13:49. (In Chinese) [Google Scholar]
- 22.Astiz M, de Alaniz MJ, Marra CA. The oxidative damage and inflammation caused by pesticides are reverted by lipoic acid in rat brain. Neurochem Int. 2012;61:1231–1241. doi: 10.1016/j.neuint.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Mecdad AA, Ahmed MH, ElHalwagy MEA, Afify MMM. A study on oxidative stress biomarkers and immunomodulatory effects of pesticides in pesticide-Sprayers. Egy J Foren Sci. 2011;1:93–98. [Google Scholar]
- 24.Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: Attenuation by N-acetylcysteine. Exp Neurol. 2008;210:560–576. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Jia L, Wang J, Shi JP, Wang F, Fang H, Zhu M, Lou G. The effect of N-acetylcysteine nano-carbon on anti-oxidative capacity in non-alcoholic steatohepatitis rats. Chin J Exp Clin Virol. 2014;28:4–6. (In Chinese) [Google Scholar]
- 27.Fang HY, Zhuang RX, Xi JJ, Sun J, Shao Y, Shi Z, Pan X, Wang F. Role of treatment on liver fibrosis of activated carbon N-acetylcysteine microcapsule. Chin J Clin Pharm Ther. 2015;20:976–980. (In Chinese) [Google Scholar]
- 28.Demiroren K, Dogan Y, Kocamaz H, Ozercan IH, Ilhan S, Ustundag B, Bahcecioglu IH. Protective effects of L-carnitine, N-acetylcysteine and genistein in an experimental model of liver fibrosis. Clin Res Hepatol Gastroenterol. 2014;38:63–72. doi: 10.1016/j.clinre.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Zhang G, Ren J. Effect of N-acetylcysteine on cardiac function and myocardial energy metabolism of heart failure rabbits. Med J Wuhan Uni. 2014;5:678–688. [Google Scholar]
- 30.Yi X, Cui X, Wu P, Wang S, Wang G, Yang X, Yang F, Zheng S, Li Z. Effects of N-acetylcysteine on apoptosis induced by myocardial ischemia reperfusion injury in rats' heart transplantation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:1234–1239. (In Chinese) [PubMed] [Google Scholar]
- 31.Yi X, Li ZQ, Yang SL, Wu PY, Wang S, Cui X, Guan X, Yang X, Zheng S. Effects of N-acetylcysteine on modulating matrix metalloproteinase in myocardial ischemia reperfusion injury during heart transplantation in rats. J New Med. 2014;9:593–596. [Google Scholar]
- 32.Yang Y. Effects of N-acetylcysteine on reactive oxygen species-induced apoptosis in H9c2 cells. Modern Med J Chin. 2015;1:50–52. [Google Scholar]
- 33.Li XB. Effects of N-acetylcysteine on cardiac remodeling in hypertensive. Nanjing Medical University; Nanjing: 2007. [Google Scholar]
- 34.Machado MV, Kruger L, Jewell ML, Michelotti GA, Tde A Pereira, Xie G, Moylan CA, Diehl AM. Vitamin B5 and N-Acetylcysteine in nonalcoholic steatohepatitis: A preclinical study in a dietary mouse model. Dig Dis Sci. 2016;61:137–148. doi: 10.1007/s10620-015-3871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu QL, Zhang YG, Yang LZ, Sun L. Intraperitoneal chemotherapy with mitomycin C bound to activated carbon nanoparticles for nude mice bearing human gastric carcinoma. Zhonghua Zhong Liu Za Zhi. 2006;28:257–260. (In Chinese) [PubMed] [Google Scholar]
- 36.Zhang J, Liu M, Lin W, Li XQ. Study on clinical safety of different doses of N-acetylcysteine in treatment of patients with severe liver diseases. Yao Wu Bu Liang Fan Ying Za Zhi. 2009;11:87–90. (In Chinese) [Google Scholar]
- 37.Wang SM, Geng JB, Wang M, Li Z, Zhang Z, He CL. Therapeutic effects of Acetylcysteine on patients with drug induced hepatitis. Gan Zang. 2017;22:32–34. (In Chinese) [Google Scholar]
- 38.Zhong Y, Shuzheng M, Yingge Z. Using activated carbon nanoparticles to decrease the genoloxicity and teratogenicity of anticancer therapeutic agents. J Nanosci Nanotechnol. 2010;10:8603–8609. doi: 10.1166/jnn.2010.2492. [DOI] [PubMed] [Google Scholar]
- 39.Fang H, Zhuang R, Pan X, Sun J, Xi J, Wang F, Shi T, Liu S. Preparation technology screening of activated carbon N-acetylcysteine microcapsule. Chin Pharm. 2016;27:955–958. [Google Scholar]
- 40.Wang F, Zhuang RX, Xi JJ, Fang HY, Gao J. Preparation and distribution in mice of acetylcysteine nanoparticles. Chin J Pharma. 2012;43:572–576. (In Chinese) [Google Scholar]
- 41.Wang FG, Shen YQ, Zhuang RX, Xi JJ, Fang HY. Study on preparation of acetylcysteine nanoparticles. Strait Pharmaceut J. 2013;25:15–17. [Google Scholar]
- 42.Shen Y, Qin H, Chen J, Mou L, He Y, Yan Y, Zhou H, Lv Y, Chen Z, Wang J, Zhou YD. Postnatal activation of TLR4 in astrocytes promotes excitatory synaptogenesis in hippocampal neurons. J Cell Biol. 2016;215:719–734. doi: 10.1083/jcb.201605046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang YG, Song HY, Li Y, Yang SJ. Protective effect of GSTT preconditioning on myocardial ischemia-reperfusion injury in rats. Chin Pharma Bull. 2010;26:714–718. [Google Scholar]
- 44.Shi TT, Bai JP, Liang YQ, Zhang HZ, Yu KM, Li CP. Effect of apigenin on the cardiomyocyte apoptosis in rats with ischemia and reperfusion and the expression of Bcl-2, Bax, Caspase-3. Chin Pharma Bull. 2011;27:666–671. [Google Scholar]
- 45.Stutler SA, Johnson EW, Still KR, Schaeffer DJ, Hess RA, Arfsten DP. Effect of method of euthanasia on sperm motility of mature Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2007;46:13–20. [PubMed] [Google Scholar]
- 46.Thomas AA, Flecknell PA, Golledge HD. Combining nitrous oxide with carbon dioxide decreases the time to loss of consciousness during euthanasia in mice-refinement of animal welfare? PLoS One. 2012;7:e32290. doi: 10.1371/journal.pone.0032290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DW, et al. The Lambeth Conventions: Guidelines for the study of arrhythmias in ischemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 48.Infante T, Forte E, Schiano C, Cavaliere C, Tedeschi C, Soricelli A, Salvatore M, Napoli C. An integrated approach to coronary heart disease diagnosis and clinical management. Am J Transl Res. 2017;9:3148–3166. [PMC free article] [PubMed] [Google Scholar]
- 49.Gong CF. High quality low-dose myocardial CT perfusion imaging method (unpublished PhD thesis) Southern Medical University; 2013. [Google Scholar]
- 50.Müller-Nordhorn J, Willich SN. Coronary heart disease. In: Quah SR, editor. Reference Module in Biomedical Sciences: International Encyclopedia of Public Health. 2nd edition. Academic Press; Oxford: 2017. pp. 159–167. [Google Scholar]
- 51.Xing K. Cardioprotective effect of anisodamine against myocardial ischemia/reperfusion injury and its mechanism on cardiomyocytesapoptosis (unpublished PhD thesis) Hebei Medical University; 2015. [Google Scholar]
- 52.Levitsky S. Protecting the myocardial cell during coronary revascularization. Circulation. 2006;114(Suppl I):I339–I343. doi: 10.1161/CIRCULATIONAHA.105.001685. [DOI] [PubMed] [Google Scholar]
- 53.Ota S, Nishikawa H, Takeuchi M, Nakajima K, Nakamura T, Okamoto S, Setsuda M, Makino K, Yamakado T, Nakano T. Impact of nicorandil to prevent reperfusion injury in patients with acute myocardial infarction: Sigmart multicenter angioplasty revascularization trial (SMART) Circ J. 2006;70:1099–1104. doi: 10.1253/circj.70.1099. [DOI] [PubMed] [Google Scholar]
- 54.Álvarez P, Tapia L, Mardones LA, Pedemonte JC, Farías JG, Castillo RL. Cellular mechanisms against ischemia reperfusion injury induced by the use of anesthetic pharmacological agents. Chem Biol Interact. 2014;218:89–98. doi: 10.1016/j.cbi.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Xiong N, Wei M. Study on protection of ginkgolide B against myocardial ischemia reperfusion injury and its mechanism. Chin J Mod Appl Pharm. 32:289–294. [Google Scholar]
- 56.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Gao XQ, Xue L. Study on the protective mechanisms ofrosiglitazone on myocardial ischemia reperfusion injury inrabbits. Chin J Mod Appl Pharm. 2014;31:265–270. (In Chinese) [Google Scholar]
- 58.Zhao JF, Zhu J, Chen W, Lu DZ. Effect of tanshinone IIA on Notch signal pathway in the neonatal rat cardiac myocytes of ischemic/reperfusion injury. Chin J Mod Appl Pharm. 2014;31:1435–1439. (In Chinese) [Google Scholar]
- 59.Gross GJ, Kersten JR, Warliter DC. Mechanisma of postischemic contractile dysfunction. Ann Thor Surg. 1999;68:1898–1904. doi: 10.1016/S0003-4975(99)01035-8. [DOI] [PubMed] [Google Scholar]
- 60.Kaplán P, Lehotský J, Racay P. Role of sarcoplasmic reticulum in the contractile dysfunction during myocardial ischemia and reperfusion. Physiol Res. 1997;46:333–339. [PubMed] [Google Scholar]
- 61.Yang P. Study on mechanism and the protective effect of tanshinone IIA and Prohibitin against myocardial ischemia/reperfusion injury (unpublished PhD thesis) Southern Medical University; 2010. [Google Scholar]
- 62.Zhang X, Liang X, Lin X, Zhang S, Huang Z, Chen C, Guo Y, Xuan F, Xu X, Huang R. Mechanism of the protective effect of Yulangsan flavonoid on myocardial ischemia/reperfusion injury in rats. Cell Phy Bio. 2014;34:1050–1062. doi: 10.1159/000366320. [DOI] [PubMed] [Google Scholar]
- 63.Gómez-Hurtado N, Domínguez-Rodríguez A, Mateo P, Fernández-Velasco M, Val-Blasco A, Aizpún R, Sabourin J, Gómez AM, Benitah JP, Delgado C. Beneficial effects of leptin treatment in a setting of cardiac dysfunction induced by transverse aortic constriction in mouse. J Physiol. 2017;595:4227–4243. doi: 10.1113/JP274030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y. Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARγ/FUNDC1/mitophagy pathways. J Pineal Res. 2017;63:e12438. doi: 10.1111/jpi.12438. [DOI] [PubMed] [Google Scholar]
- 65.Guo Q, Chen SY, Zhang ZM, Yao XJ. Study on association between ischemia-reperfusion and oxygen free radicals. Shaanxi Yi Xue Za Zhi. 2006;35:932–941. (In Chinese) [Google Scholar]
- 66.Kanemoto Y, Nakase H, Akita N, Sakaki T. Effects of anti-intercellular adhesion molecule-1 antibody on reperfusion injury induced by late reperfusion in the rat middle cerebral artery occlusion model. Neurosurgery. 2002;51:1034–1041. doi: 10.1097/00006123-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 67.Mohanty IR, Arya DS, Gupta SK. Withania somnifera provides cardioprotection and attenuates ischemia-reperfusion induced apoptosis. Clin Nutr. 2008;27:635–642. doi: 10.1016/j.clnu.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J, Jang HS, Park KM. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am J Physiol Renal Physiol. 2010;298:F158–F166. doi: 10.1152/ajprenal.00474.2009. [DOI] [PubMed] [Google Scholar]
- 70.Guo C, Tong L, Xi M, Yang H, Dong H, Wen A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol. 2012;144:768–774. doi: 10.1016/j.jep.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 71.Lee J, Bae EH, Ma SK, Kim SW. Altered nitric oxide system in cardiovascular and renal diseases. Chonnam Med J. 2016;52:81–90. doi: 10.4068/cmj.2016.52.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Waard MC, van der Velden J, Boontje NM, Dekkers DH, van Haperen R, Kuster DW, Lamers JM, de Crom R, Duncker DJ. Detrimental effect of combined exercise training and eNOS overexpression on cardiac function after myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1513–H1523. doi: 10.1152/ajpheart.00485.2008. [DOI] [PubMed] [Google Scholar]
- 73.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, van Haperen R, de Crom R, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–H282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 74.Ozaki M, Kawashima S, Yamashita T, Hirase T, Ohashi Y, Inoue N, Hirata K, Yokoyama M. Overexpression of endothelial nitric oxide synthase attenuates cardiac hypertrophy induced by chronic isoproterenol infusion. Circ J. 2002;66:851–856. doi: 10.1253/circj.66.851. [DOI] [PubMed] [Google Scholar]
- 75.Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, Jans P, Scherrer-Crosbie M, Picard MH, Szelid Z, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94:1256–1262. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- 76.Couto GK, Britto LR, Mill JG, Rossoni LV. Enhanced nitric oxide bioavailability in coronary arteries prevents the onset of heart failure in rats with myocardial infarction. J Mol Cell Cardiol. 2015;86:110–120. doi: 10.1016/j.yjmcc.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 77.Stefanelli C, Pignatti C, Tantini B, Stanic I, Bonavita F, Muscari C, Guarnieri C, Clo C, Caldarera CM. Nitric oxide can function as either a killer molecule or an antiapoptotic effector in cardiomyocytes. Biochim Biophys Acta. 1999;1450:406–413. doi: 10.1016/S0167-4889(99)00045-2. [DOI] [PubMed] [Google Scholar]
- 78.Herbertson MJ, Werner HA, Walley KR. Nitric oxide synthase inhibition partially prevents decreased LV contractility during endotoxemia. Am J Physiol. 1996;270:H1979–H1984. doi: 10.1152/ajpheart.1996.270.6.H1979. [DOI] [PubMed] [Google Scholar]
- 79.Wang D, Yang XP, Liu YH, Carretero OA, LaPointe MC. Reduction of myocardial infarct size by inhibition of inducible nitric oxide synthase. Am J Hypertens. 1999;12:174–182. doi: 10.1016/S0895-7061(98)00235-0. [DOI] [PubMed] [Google Scholar]
- 80.Wang X. Effect of cAM and NO signals on the mechanism of post conditioning cardio protection (unpublished PhD thesis) Southern Medical University; 2011. [Google Scholar]
- 81.Su W, Zhang Y, Zhang Q, Xu J, Zhan L, Zhu Q, Lian Q, Liu H, Xia ZY, Xia Z, Lei S. N-acetylcysteine attenuates myocardial dysfunction and postischemic injury by restoring caveolin-3/eNOS signaling in diabetic rats. Cardiovasc Diabetol. 2016;15:146. doi: 10.1186/s12933-016-0460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amrani M, Chester AH, Jayakumar J, Schyns CJ, Yacoub MH. L-arginine reverses low coronary reflow and enhances postischaemic recovery of cardiac mechanical function. Cardiovasc Res. 1995;30:200–204. doi: 10.1016/S0008-6363(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 83.Han Y, Zhang W, Tang Y, Bai W, Yang F, Xie L, Li X, Zhou S, Pan S, Chen Q, et al. l-Tetrahydropalmatine, an active component of Corydalis yanhusuo W.T. Wang, protects against myocardial ischaemia-reperfusion injury in rats. PLoS One. 2012;7:e38627. doi: 10.1371/journal.pone.0038627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang W, Han Y, Meng G, Bai W, Xie L, Lu H, Shao Y, Wei L, Pan S, Zhou S, et al. Direct renin inhibition with aliskiren protects against myocardial ischemia/reperfusion injury by activating nitric oxide synthase signaling in spontaneously hypertensive rats. J Am Heart Assoc. 2014;3:e000606. doi: 10.1161/JAHA.113.000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan JY, Chan SH, Chang AY. Differential contributions of NOS isoforms in the rostral ventrolateral medulla to cardiovascular responses associated with mevinphos intoxication in the rat. Neuropharmacology. 2004;46:1184–1194. doi: 10.1016/j.neuropharm.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 86.Jiang MY. The effect and mechanism of medullary NO/NOS system on the treatment of myocardial ischemia by electroacupuncture in rats. Fudan University; 2011. [Google Scholar]
- 87.Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 88.Anderson TJ, Meredith IT, Ganz P, Selwyn AP, Yeung AC. Nitric oxide and nitrovasodilators: Similarities, differences and potential interactions. J Am Coll Cardiol. 1994;24:555–566. doi: 10.1016/0735-1097(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 89.Wang QD, Morcos E, Wiklund P, Pernow J. L-arginine enhances functional recovery and Ca(2+)-dependent nitric oxide synthesis activity after ischemia and reperfusion in the rat heart. J Cardiovasc Pharmacol. 1997;29:291–296. doi: 10.1097/00005344-199702000-00020. [DOI] [PubMed] [Google Scholar]
- 90.Giraldez RR, Panda A, Xia Y, Sanders SP, Zweier JL. Decreased nitric-oxide synthesis activity causes impaired endothelium-dependent relaxation in the postischemic heart. J Biol Chem. 1997;272:21420–21426. doi: 10.1074/jbc.272.34.21420. [DOI] [PubMed] [Google Scholar]
- 91.Wildhirt SM, Weismueller S, Schulze C, Conrad N, Kornberg A, Reichart B. Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: Evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc Res. 1999;43:698–711. doi: 10.1016/S0008-6363(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 92.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation-the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/S0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 93.Balligand J, Ungureanu-Longrois D, Simmons WW, Pimental D, Malinski TA, Kapturczak M, Taha Z, Lowenstein CJ, Davidoff AJ, Kelly RA, et al. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J Biol Chem. 1994;269:27580–27588. [PubMed] [Google Scholar]
- 94.Gan YQ. Attenuation of LPS-induced cardiac injury in mice by Taurine. Uni South Chin. 2014;5 [Google Scholar]
- 95.Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. doi: 10.1016/S0163-7258(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 96.Sears CE, Ashley EA, Casadei B. Nitric oxide control of cardiac function: Is neuronal nitric oxide synthase a key component? Philos Trans R Soc Lond B Biol Sci. 2004;359:1021–1044. doi: 10.1098/rstb.2004.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: Ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 98.Tada H, Thompson CI, Recchia FA, Loke KE, Ochoa M, Smith CJ, Shesely EG, Kaley G, Hintze TH. Myocardial glucose uptake is regulated by nitric oxide via endothelial nitric oxide synthase in Langendorff mouse heart. Circ Res. 2000;86:270–274. doi: 10.1161/01.RES.86.3.270. [DOI] [PubMed] [Google Scholar]
- 99.Kanno S, Kim PK, Sallam K, Lei J, Billiar TR, Shears LL., II Nitric oxide facilitates cardiomyogenesisin mouse embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12277–12281. doi: 10.1073/pnas.0401557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andreka P, Nadhazi Z, Muzes G, Szantho G, Vandor L, Konya L, Turner MS, Tulassay Z, Bishopric NH. Possible therapeutic targets in cardiac myocyte apoptosis. Curr Pharm Des. 2004;10:2445–2461. doi: 10.2174/1381612043383908. [DOI] [PubMed] [Google Scholar]
- 102.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marbán E, et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 103.Shi TT. The protective effect of apigenin against myocardial ischemia reperfusion apoptosis in rat (unpublished PhD thesis) Shanxi Medical University; 2011. [Google Scholar]
- 104.Tian YQ. The effects and mechanisms of free fatty acids on blood pressure and cardiac function in rats (unpublished PhD thesis) Shandong University; 2011. [Google Scholar]
- 105.Lahera V, Khraibi AA, Romero JC. Sulfhydryl group donors potentiate the hypotensive effect of acetylcholine in rats. Hypertension. 1993;22:156–160. doi: 10.1161/01.HYP.22.2.156. [DOI] [PubMed] [Google Scholar]