Abstract
The aim of the present study was to investigate the protective effect of chicoric acid on oxidative stress and inflammation in rats with cerebral ischemia-reperfusion injury. A cerebral ischemia-reperfusion injury rat model was created via transient middle cerebral artery occlusion (MCAO) and rats were treated with various doses of chicoric acid (0, 1, 10 and 100 mg/kg). Neurological deficits and infarct volume were used to estimate the protective effects of chicoric acid treatment. Levels of reactive oxygen species (ROS), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, nitric oxide (NO) and prostaglandin E2 (PGE2) were assessed. Western blot analysis was also used to measure the expression of cyclooxygenase (COX)-2, p38-mitogen activated protein kinase (MAPK), c-Jun, phosphorylated protein kinase B (p-AKT) and AKT. Chicoric acid exposure was observed to reduce neurological deficits and infarct volume in rats with cerebral ischemia-reperfusion injury. In addition, ROS production and inflammation were significantly suppressed following treatment with chicoric acid. Chicoric acid was demonstrated to significantly inhibit the upregulation of NO and PGE2 levels in rats following MCAO. Furthermore, chicoric acid significantly suppressed the MCAO-induced promotion of COX-2, p38-MAPK and c-Jun protein expression and enhanced the inhibition of p-AKT/AKT. These results suggest that chicoric acid has a protective effect, preventing oxidative stress and inflammation in rats with cerebral ischemia-reperfusion injury via the p38-MAPK, c-Jun and AKT signaling pathways.
Keywords: chicoric acid, cerebral ischemia-reperfusion injury, cyclooxygenase-2, p38-mitogen activated protein kinase, c-Jun
Introduction
Cerebrovascular disease is one of the most common diseases globally (1). With high morbidity, disability rates and death rates, cerebrovascular disease has a negative impact on quality of life and public health (1). Ischemic cerebrovascular disease occurs when stenosis or blockade occurs in the major arteries that supply blood to the brain (2). This results in oxygen deprivation to the brain leading to ischemia or necrosis (2). Following cerebral ischemic stroke, hemoperfusion of the ischemic region may be achieved by dissolution of the thrombus or recanalization (3). However, organizational functions are not recovered in certain patients and the damage is aggravated. Cerebral ischemia-reperfusion (I/R) injury is a complex pathophysiological procedure (4). A previous study have reported that brain cell injuries caused by cerebral blood flow interruption and reperfusion is a rapid cascade reaction that involves a number of steps, including energy barrier, acidosis, increased excitatory amino acid release, the formation of free radicals and the expression of apoptotic genes (4). These steps interact to form a feedback loop (5) and ultimately lead to apoptosis or necrosis.
The mechanisms of cerebral I/R injury are complicated and interrelated (6). An important step in the pathogenesis of cerebral I/R injuries is a series of events known as the oxidative-inflammatory cascade (OIC), which is triggered by the interaction between oxidative stress and the inflammatory response (6). During OIC, pressure sensitivity kinase as protein kinase C and protein kinase are activated by reactive oxygen species (ROS), which subsequently stimulate the expression of inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6 and monocyte chemotactic protein-1 (7,8). These inflammatory factors further stimulate the production of ROS and a positive feedback loop is formed (7).
In cerebral I/R injuries, the inflammatory cascade reaction caused by local ischemic injury is an important pathophysiological process following cerebral ischemia (9). With the occurrence of inflammation post-ischemia and the destruction of blood brain barrier, white blood cells are able to enter the brain and may induce inflammatory responses (9). A number of inflammatory factors, including TNF-α, IL-1, IL-6 and inducible nitric oxide synthase (iNOS), induce cerebral ischemic injuries as well as stimulating the expression of a number of adhesion molecules, facilitating infiltration and triggering the inflammatory response (10).
Chicoric acid is a caffeoyl derivative with potential anti-diabetic properties (11). Chicoric acid induces the apoptosis of 3T3-L1 preadipocytes and mediates ROS, phosphatidylinositide 3-kinases (PI3K)/protein kinase B (AKT) and p38-mitogen-activated protein kinase (MAPK) signaling pathways (12,13).
The aim of the present study was to investigate the protective effect of chicoric acid on oxidative stress and inflammation in rats with cerebral I/R injury.
Materials and methods
Animals
A total of 50 adult male Sprague Dawley rats (280±20 g, 12–14 weeks old) were purchased from the Animal Center of Hebei Cangzhou Central Hospital (Cangzhou, China). Animal experiments were approved by the Ethics Committee of Cangzhou Central Hospital (Cangzhou, China) and were performed in accordance with the National Institute of Health Guidelines. Rats were housed at 22–25°C with 55–60% humidity, were subjected to a 12-h light/dark cycle and had free access to food and water.
Grouping and cerebral I/R rat model
Rats were randomly divided into the following groups (n=10): Negative control group, cerebral I/R injury group and cerebral I/R injury with chicoric acid treatment group (1, 10 or 100 mg/kg). Rats were injected with 35 mg/kg pentobarbital sodium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A cerebral I/R rat model was induced by transient middle cerebral artery occlusion (MCAO) for 1 h as described previously (14). In the negative control group, surgery was performed in the same way without inserting threads. In the cerebral I/R damage group, the rats were injected with 200 µl normal saline. In the cerebral I/R damage with chicoric acid treatment group, the rats were injected with 1, 10 or 100 mg/kg/day of chicoric acid (Sigma-Aldrich; Merck KGaA) for 1 week.
Neurological deficits
Neurological deficits were evaluated following 1 day of treatment post-surgery and were scored on a 5-point scale. Scoring was performed as follows: 0, No deficit; 1, mild deficit-failure of left forepaw extension; 2, moderate deficit-circling to the left; 3, severe deficit-falling to the left; 4, critical deficit-depressed consciousness, no spontaneous walking (15).
Infarct volume
Rats were sacrificed and brain tissue samples were harvested. Brain tissues was fixed with 4% paraformaldehyde for 24 h at room temperature, and tissue samples were sliced into 2 mm sections and stained with 2% 2,3,5-triphenyltetrazolium chloride in the dark for 30 min at 37°C. The infarct area was demarcated as follows: Infarct volume (%) = (volumeipsilateral - volumecontralateral)/volumecontralateral × 100.
Histopathological assessment
Brains tissue samples were harvested, fixed with 4% paraformaldehyde for 24 h at room temperature and embedded in paraffin. Hippocampal tissues were separated and the hippocampal embedded tissue samples were cut into 6 µm sections and stained with hematoxylin and eosin (H&E) at 37°C for 15 min. H&E tissue samples were examined under a light microscope (BX51; magnification, ×10; Olympus Corp., Tokyo, Japan) and images were captured using a DP72 camera (Olympus Corp.).
Measurement of ROS
Brain tissue samples were harvested, homogenated and fractionated by centrifugation at 2,000 × g for 5 min at 4°C. The separated upper layer was incubated with 2′,7′-dichlorodihydrofluorescein diacetate (Nanjing Jiancheng Biology Engineering Institute Co., Ltd., Nanjing, China) in buffer solution (Nanjing Jiancheng Biology Engineering Institute Co., Ltd.) at 37°C for 30 min. ROS production was measured using an Olympus fluorescence microscope (magnification, ×10; Olympus Corp.).
Measurement of TNF-α and IL-1 β in the circulation
A total of 100 µl of venous blood was collected and fractionated by centrifugation at 2,000 × g for 5 min at 4°C. The separated upper plasma layer was collected and the levels of TNF-α (cat. no. PT516) and IL-1β (cat. no. PI303) were analyzed using an ELISA microplate reader at an absorbance of 450 nm.
Measurement of NO and prostaglandin E2 (PGE2)
Brains tissue samples were quickly separated, homogenated with Griess reagent (Sigma-Aldrich; Merck KGaA) and 100 µl of bronchoalveolar lavage fluid obtained from rats, and subsequently incubated for 10 min at room temperature. The concentration of NO was determined at 540 nm using an ELISA microplate reader and NO kit (cat. no. A012-1; Nanjing Jiancheng Biology Engineering Institute). The PGE2 concentrations were determined using a prostaglandin E2 assay kit (cat. no. H099; Nanjing Jiancheng Biology Engineering Institute).
Western blot analysis
Brain tissue samples were separated and homogenated with liquid nitrogen. Protein content was determined using a BCA assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins (50 µg) were separated by SDS-PAGE (10–12%) and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with PBS containing 1% Tween-20 and 5% non-fat dried milk for 1 h at room temperature, and subsequently incubated with the following primary antibodies: Anti-cyclooxygenase (COX)-2 (sc-7951, 1:1,000), anti-phosphorylated (p)-c-Jun (sc-7980-R, 1:1,000), anti-AKT (sc-8312, 1:2,000), anti-p-AKT (sc-135651, 1:1,500; all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-β-actin (1:1,000; Sangon Biotech Co., Ltd., Shanghai, China) overnight at 4°C. Membranes were subsequently incubated with goat anti-rabbit IgG-horserasish peroxidase secondary antibody (sc-2004, 1:15,000; Santa Cruz Biotechnology, Inc.) for 2 h at 37°C. β-actin was used as an internal control.
Statistical analysis
Data are presented as the mean ± standard error of the mean and were analyzed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Statistically significant differences were determined using one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Protective effect of chicoric acid on neurological deficits in rats with cerebral I/R injury
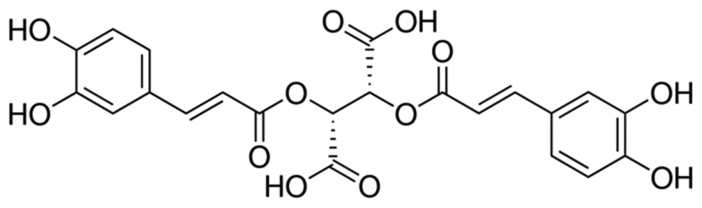
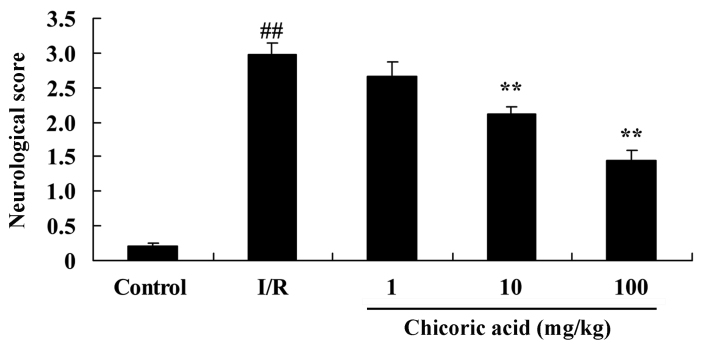
The chemical structure of chicoric acid is presented in Fig. 1. The neurological deficit score was significantly higher in the cerebral I/R injury group compared with the control group (P<0.01; Fig. 2). However, treatment with 10 or 100 mg/kg of chicoric acid significantly inhibited the cerebral I/R injury-induced neurological deficits in rats (P<0.01; Fig. 2). No significant difference was observed between the cerebral I/R injury and 1 mg/kg chicoric acid groups (Fig. 2).
Figure 1.
Chemical structure of chicoric acid.
Figure 2.
Effect of chicoric acid on neurological deficits in rats with I/R. ##P<0.01 vs. control group and **P<0.01 vs. I/R group. I/R, cerebral ischemia-reperfusion injury.
Protective effect of chicoric acid on infarct volume in rats with cerebral I/R injury
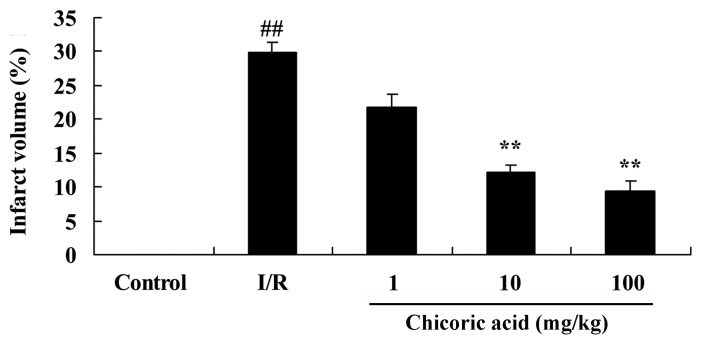
The infarct volume of rats in the cerebral I/R injury group was significantly higher compared with the control group (P<0.01; Fig. 3). However, treatment with 10 or 100 mg/kg of chicoric acid significantly reduced infarct volume in rats with cerebral I/R injury (P<0.01; Fig. 3). No significant difference was observed between the cerebral I/R injury and 1 mg/kg chicoric acid groups (Fig. 3).
Figure 3.
Effect of chicoric acid on infarct volume in rats with I/R. ##P<0.01 vs. control group and **P<0.01 vs. I/R group. I/R, cerebral ischemia-reperfusion injury.
Protective effect of chicoric acid on hippocampus injury in rats with cerebral I/R injury
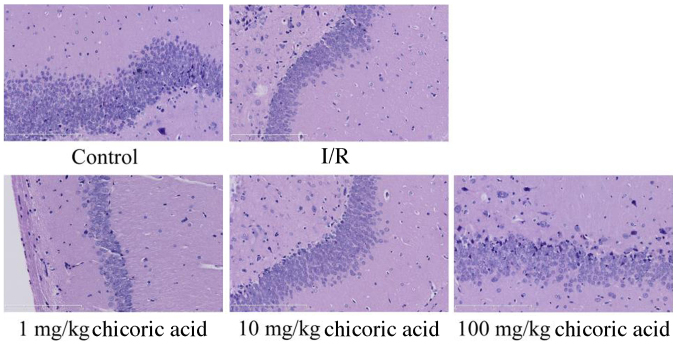
Cerebral I/R injury and the inhibition of the number of neuronal cells in the hippocampus were observed in the cerebral I/R injury group compared with the control group (Fig. 4). Treatment with 1 mg/kg chicoric acid had no marked effect on hippocampus injury and the number of neuronal cells compared with the cerebral I/R injury group (Fig. 4). However, treatment with 10 or 100 mg/kg chicoric acid markedly inhibited the extent of I/R injury and the number of neuronal cells in rats following MCAO (Fig. 4).
Figure 4.
Effect of chicoric acid on hippocampus injury in rats with I/R (magnification, ×10). I/R, cerebral ischemia-reperfusion injury.
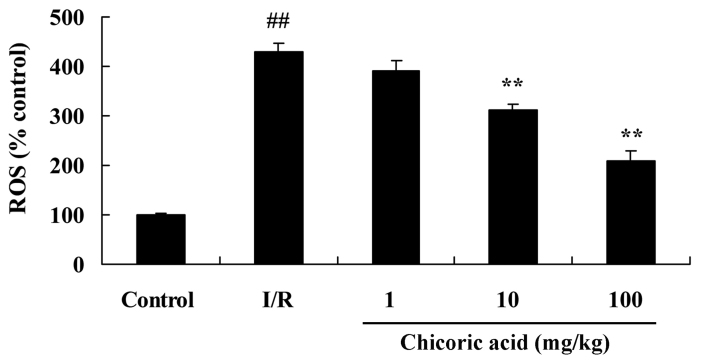
Effect of chicoric acid on ROS expression in rats with cerebral I/R injury
ROS production was significantly higher in the cerebral I/R injury group compared with the control group (P<0.01; Fig. 5). However, pretreatment with 10 or 100 mg/kg of chicoric acid significantly reduced ROS production in rats with cerebral I/R injury (P<0.01; Fig. 5). No significant difference was observed between the 1 mg/kg chicoric acid and cerebral I/R injury groups (Fig. 5).
Figure 5.
Effect of chicoric acid on ROS expression in rats with I/R. ##P<0.01 vs. control group; **P<0.01 vs. I/R group. ROS, reactive oxygen species; I/R, cerebral ischemia-reperfusion injury.
Protective effect of chicoric acid on inflammation in rats with cerebral I/R injury
Compared with the control group, TNF-α and IL-1β levels were significantly increased in the cerebral I/R injury group (P<0.01; Fig. 6). Treatment with 10 or 100 mg/kg of chicoric acid significantly suppressed the levels of TNF-α and IL-1β in cerebral I/R injury rats (P<0.01; Fig. 6). However, no significant differences in TNF-α or IL-1β levels were observed between the 1 mg/kg chicoric acid and cerebral I/R injury groups (Fig. 6).
Figure 6.
Effect of chicoric acid on inflammation in rats with I/R. Chicoric acid attenuates the levels of (A) TNF-α and (B) IL-1β in rats with cerebral ischemia-reperfusion injury. ##P<0.01 vs. control group; **P<0.01 vs. I/R group. I/R, cerebral ischemia-reperfusion injury; TNF, tumor necrosis factor; IL, interleukin.
Effect of chicoric acid on NO levels in rats with cerebral I/R injury
NO levels in rats in the cerebral I/R injury group were significantly increased compared with control rats (P<0.01; Fig. 7). Treatment with 10 or 100 mg/kg of chicoric acid significantly reduced NO levels in rats with cerebral I/R injury (P<0.01; Fig. 7). No significant differences in NO levels were observed between the 1 mg/kg chicoric acid and cerebral I/R injury groups (Fig. 7).
Figure 7.
Effect of chicoric acid on NO levels in rats with I/R. ##P<0.01 vs. control group; **P<0.01 vs. I/R group. NO, nitric oxide; I/R, cerebral ischemia-reperfusion injury.
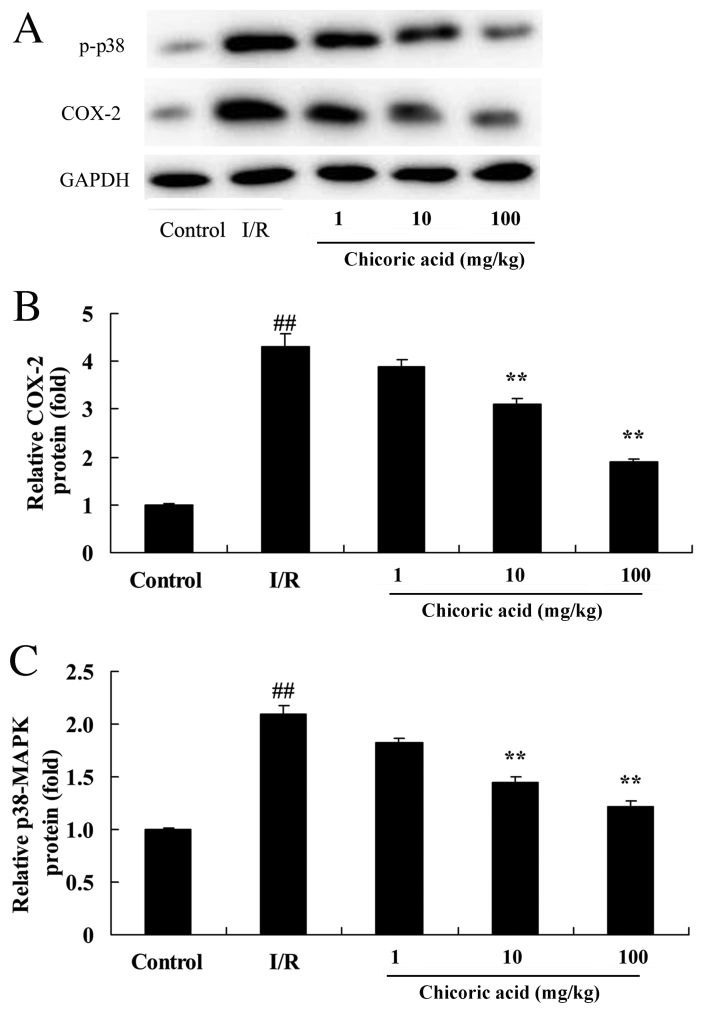
Effect of chicoric acid on COX-2 and p-p38-MAPK levels in rats with cerebral I/R injury
COX-2 and p-p38-MAPK protein levels were significantly increased in the cerebral I/R injury group compared with control rats (P<0.01; Fig. 8). Treatment with 10 or 100 mg/kg chicoric acid significantly decreased COX-2 and p-p38-MAPK levels in rats with cerebral I/R injury (P<0.01; Fig. 8). No significant differences in COX-2 and p-p38-MAPK levels were observed between the 1 mg/kg chicoric acid and cerebral I/R injury groups (Fig. 8).
Figure 8.
Effect of chicoric acid on COX-2 and p-p38-MAPK level in rats with I/R. (A) Western blot analysis of COX-2 and p-p38-MAPK levels in rats with cerebral ischemia-reperfusion injury. Statistical analysis of (B) COX-2 and (C) p-p38-MAPK levels. ##P<0.01 vs. control group; **P<0.01 vs. I/R group. COX, cyclooxygenase; p, phosphorylated; MAPK, mitogen activated protein kinase; I/R, cerebral ischemia-reperfusion injury.
Effect of chicoric acid on PGE2 levels in rats with cerebral I/R injury
The results of the present study reveal that PGE2 levels were significantly increased in the cerebral I/R injury group compared with the control group (P<0.01; Fig. 9). Treatment with 10 or 100 mg/kg chicoric acid significantly attenuated the MCAO-induced upregulation of PGE2 in rats (P<0.01; Fig. 9). No significant differences were observed in PGE2 levels between the 1 mg/kg chicoric acid and cerebral I/R injury groups (Fig. 9).
Figure 9.
Effect of chicoric acid on PGE2 levels in rats with I/R. ##P<0.01 vs. control group; **P<0.01 vs. I/R group. PGE2, prostaglandin E2; I/R, cerebral ischemia-reperfusion injury.
Effect of chicoric acid on p-c-Jun and p-AKT levels in rats with cerebral I/R injury
The expression of p-c-Jun was upregulated in rats with cerebral I/R injury compared with control rats (P<0.01; Fig. 10A and B). Treatment with 10 or 100 mg/kg chicoric acid significantly attenuated the MCAO-induced increase in p-c-Jun (P<0.01; Fig. 10A and B), whereas no significant difference was observed in the 1 mg/kg chicoric acid group compared with the cerebral I/R injury group. Levels of p-AKT protein were significantly lower in the cerebral I/R group compared with the control group (P<0.01; Fig. 10A and C). Treatment with 10 or 100 mg/kg chicoric acid significantly increased p-AKT protein expression in cerebral I/R injury rats (P<0.01; Fig. 10A and C); however, no significant difference was observed between the 1 mg/kg chicoric acid and cerebral I/R injury groups.
Figure 10.
Effect of chicoric acid on p-c-Jun and p-AKT levels in rats with I/R. (A) Western blot analysis of p-c-Jun and p-AKT levels in rats with cerebral ischemia-reperfusion injury. Statistical analysis of (B) p-c-Jun and (C) p-AKT levels. ##P<0.01 vs. control group; **P<0.01 vs. I/R group. P, phosphorylated; AKT, protein kinase B; I/R, cerebral ischemia-reperfusion injury.
Discussion
Cerebrovascular disease has high morbidity, death rates and disability rates worldwide, posing a serious threat to public health (14). Approximately 2 million individuals suffer from cerebrovascular disease worldwide and >1.5 million succumb each year (14). Furthermore, the morbidity is on the increase with improvements in living standards and lifestyle changes (1). Cerebrovascular disease may be classified as hemorrhagic or cerebrovascular. Cerebral atherosclerosis and inflammation of the cerebral artery are major causes of cerebral I/R injury and cause the highest morbidity. The majority of patients who suffer cerebral ischemia have several sequelae (15). In the present study, the protective effect of chicoric acid on neurological deficits and infarct volume in rats with cerebral I/R injury was investigated.
As a rate-limiting enzyme, COX is able to control the speed of thromboxane and prostaglandin synthesis by arachidonic acid (16). It has previously been reported that the expression of COX-1 is maintained at the same level following local cerebral ischemic injury (16). This suggests that certain factors, including ischemia, oxygen deficit and inflammation, do not induce COX-1 upregulation. Compared with COX-1, the expression of COX-2 in brain tissues is relatively low under normal physiological conditions (17). Following cerebral ischemic injury, the expression of COX-2 is upregulated to several times the normal level (17). The results of the present study suggest that chicoric acid has a protective effect following cerebral I/R injury, ameliorating increases in ROS generation, inflammation and NO and COX-2 levels in rats. It has also been reported that chicoric acid induces the apoptosis of 3T3-L1 preadipocytes and mediates COX-2, ROS, PI3K/AKT and p38-MAPK signaling pathways (13). Kour and Bani (11) revealed that chicoric acid suppressed inflammation in chronically stressed mice. Landmann et al (18) suggested that chicoric acid is able to reduce acute alcohol-induced steatosis via the induction of iNOS in the liver.
As a crucial catalyst of COX-2, PGE2 is a marker of COX-2 activity (19). A previous study reported that changes in PGE2 levels in the frontoparietal cortex were correlated with changes in the expression pattern of COX-2 in the ischemic hemisphere of a rat model of focal I/R injury (20); PGE2 may therefore be used as a marker of COX-2 activity. COX-2 inhibitor is able to suppress the metabolism of arachidonic acid via inhibiting COX-2 activity (21), thereby reducing the production of PGE2 and alleviating inflammatory response injuries. It may also relieve the degree of brain tissue injury and encephaledema to protect brain tissues (21). The results of the present study demonstrate that chicoric acid has a protective effect on PGE2 levels in rats with cerebral I/R injury. Park et al (21) reported that luteolin and chicoric acid inhibited inflammatory responses via suppressing PGE2 in lipopolysaccharide-stimulated RAW 264.7 cells. Furthermore, Jiang et al suggested that chicoric acid has a protective effect on a rat model of collagen-induced arthritis, functioning via PGE-2, TNF-α and IL-1β (22).
It has been reported that the transduction pathways of cascade p38-MAPK are located in the central nervous system and may be associated with the recovery of cerebral I/R injuries (23). Furthermore, a previous study demonstrated that the expression peak of the p38-MAPK signaling pathway occurred 1 h following the appearance of stimulating factors (23). The p38-MAPK signaling pathway has also been demonstrated to regulate COX-2 expression in other diseases, including pneumonia and hepatitis (24). In the present study, chicoric acid suppressed p38-MAPK levels in rats with cerebral I/R injury. Similarly, Park et al (21) reported that chicoric acid inhibits inflammatory responses via p38-MAPK and c-Jun.
The results of the present suggest that chicoric acid inhibits c-Jun expression in rats with cerebral I/R injury. JUN is a member of the immediate early gene family (23). Its expression product is a vasodilator-stimulated phosphoprotein of the cell nucleus, which functions as an intermediary in the external stimuli response and a marker of activation in nerve cells (25). c-Jun mRNA expression levels may be used as an effective index to judge neuronal function following injury to nerve cells (21).
The PI3K/AKT signaling pathway is associated with cell differentiation, proliferation, apoptosis and migration (26); excessive activation of this pathway may therefore result in cell malfunction. The PI3K/AKT signaling pathway induces the phosphorylation of AKT via a series of activations and induces various biological functions (27). AKT serves a crucial role in preventing cell apoptosis, as well as regulating glycometabolism and protein synthesis (28). Furthermore, Schlernitzauer et al (12) demonstrated that chicoric acid may function as an antioxidant via the MAPK and AKT pathways in L6 myotubes. The results of the present study reveal that chicoric acid activates the PI3K/AKT pathway and may be used as an effective treatment for cerebral I/R injury.
References
- 1.Zhang L, Xing T, Geng F, Du L, Wang J. Preliminary application of hybrid operation in the treatment of carotid artery stenosis in patients with complex ischemic cerebrovascular diseases. Int J Clin Exp Pathol. 2014;7:5355–5362. [PMC free article] [PubMed] [Google Scholar]
- 2.Li XQ, Wang J, Fang B, Tan WF, Ma H. Intrathecal antagonism of microglial TLR4 reduces inflammatory damage to blood-spinal cord barrier following ischemia/reperfusion injury in rats. Mol Brain. 2014;7:28. doi: 10.1186/1756-6606-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb M, Bonova P, Danielisova V, Nemethova M, Burda J, Cizkova D. Brain-derived neurotrophic factor blood levels in two models of transient brain ischemia in rats. Gen Physiol Biophys. 2013;32:139–142. doi: 10.4149/gpb_2013008. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Qu Z, Prakash R, Chung C, Ma H, Hoda MN, Fagan SC, Ergul A. Comparative analysis of the neurovascular injury and functional outcomes in experimental stroke models in diabetic Goto-Kakizaki rats. Brain Res. 2013;1541:106–114. doi: 10.1016/j.brainres.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad A, Khan MM, Raza SS, Javed H, Ashafaq M, Islam F, Safhi MM, Islam F. Ocimum sanctum attenuates oxidative damage and neurological deficits following focal cerebral ischemia/reperfusion injury in rats. Neurol Sci. 2012;33:1239–1247. doi: 10.1007/s10072-012-0940-1. [DOI] [PubMed] [Google Scholar]
- 6.Collino M, Aragno M, Mastrocola R, Gallicchio M, Rosa AC, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Modulation of the oxidative stress and inflammatory response by PPAR-gamma agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol. 2006;530:70–80. doi: 10.1016/j.ejphar.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Won MH, Kang TC, Jeon GS, Lee JC, Kim DY, Choi EM, Lee KH, Choi CD, Chung MH, Cho SS. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999;836:70–78. doi: 10.1016/S0006-8993(99)01611-X. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai-Yamashita Y, Shigematsu K, Yamashita K, Niwa M. Expression of MCP-1 in the hippocampus of SHRSP with ischemia-related delayed neuronal death. Cell Mol Neurobiol. 2006;26:823–831. doi: 10.1007/s10571-006-9077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y, Dong L, Wu C, Qin J, Li S, Wang C, Shao X, Huang D. Buyang Huanwu Decoction fraction protects against cerebral ischemia/reperfusion injury by attenuating the inflammatory response and cellular apoptosis. Neural Regen Res. 2013;8:197–207. doi: 10.3969/j.issn.1673-5374.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdallah DM, Nassar NN, Abd-El-Salam RM. Glibenclamide ameliorates ischemia-reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus. Brain Res. 2011;1385:257–262. doi: 10.1016/j.brainres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Kour K, Bani S. Augmentation of immune response by chicoric acid through the modulation of CD28/CTLA-4 and Th1 pathway in chronically stressed mice. Neuropharmacology. 2011;60:852–860. doi: 10.1016/j.neuropharm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Schlernitzauer A, Oiry C, Hamad R, Galas S, Cortade F, Chabi B, Casas F, Pessemesse L, Fouret G, Feillet-Coudray C, et al. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS One. 2013;8:e78788. doi: 10.1371/journal.pone.0078788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao H, Wang J, Yuan L, Xiao C, Wang Y, Liu X. Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric Food Chem. 2013;61:1509–1520. doi: 10.1021/jf3050268. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery B, Eisenberger MA, Rettig MB, Chu F, Pili R, Stephenson JJ, Vogelzang NJ, Koletsky AJ, Nordquist LT, Edenfield WJ, et al. Androgen receptor modulation optimized for response (ARMOR) phase I and II studies: Galeterone for the treatment of castration-resistant prostate cancer. Clin Cancer Res. 2016;22:1356–1363. doi: 10.1158/1078-0432.CCR-15-1432. [DOI] [PubMed] [Google Scholar]
- 15.Vasarainen H, Lahdensuo K, Savolainen R, Ruutu M, Taari K, Rannikko A. Diffusion-weighted magnetic resonance imaging in prostate cancer patients on active surveillance one year after diagnosis and before repeat biopsy. Scand J Urol. 2013;47:456–461. doi: 10.3109/21681805.2013.765910. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]
- 17.Iadecola C, Sugimoto K, Niwa K, Kazama K, Ross ME. Increased susceptibility to ischemic brain injury in cyclooxygenase-1-deficient mice. J Cereb Blood Flow Metab. 2001;21:1436–1441. doi: 10.1097/00004647-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Landmann M, Kanuri G, Spruss A, Stahl C, Bergheim I. Oral intake of chicoric acid reduces acute alcohol-induced hepatic steatosis in mice. Nutrition. 2014;30:882–889. doi: 10.1016/j.nut.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 19.van Dijk B, Potier E, van DIjk M, Langelaan M, Papen-Botterhuis N, Ito K. Reduced tonicity stimulates an inflammatory response in nucleus pulposus tissue that can be limited by a COX-2-specific inhibitor. J Orthop Res. 2015;33:1724–1731. doi: 10.1002/jor.22946. [DOI] [PubMed] [Google Scholar]
- 20.Rumzhum NN, Rahman MM, Oliver BG, Ammit AJ. Sphingosine 1-phosphate increases COX-2 expression and PGE secretion: Effects on beta-adrenergic receptor desensitization. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2014-0443OC. [DOI] [PubMed] [Google Scholar]
- 21.Park CM, Jin KS, Lee YW, Song YS. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J Pharmacol. 2011;660:454–459. doi: 10.1016/j.ejphar.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Jiang L, Li W, Wang Y, Zhang X, Yu D, Yin Y, Xie Z, Yuan Y. Effects of cichoric acid extract from Echinacea purpurea on collagen-induced arthritis in rats. Am J Chin Med. 2014;42:679–692. doi: 10.1142/S0192415X1450044X. [DOI] [PubMed] [Google Scholar]
- 23.Jie P, Hong Z, Tian Y, Li Y, Lin L, Zhou L, Du Y, Chen L, Chen L. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015;6:e1775. doi: 10.1038/cddis.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang YF, Yang HY, Ko TL, Hsu YF, Sheu JR, Ou G, Hsu M. Valproic acid suppresses lipopolysaccharide-induced cyclooxygenase-2 expression via MKP-1 in murine brain microvascular endothelial cells. Biochem Pharmacol. 2014;88:372–383. doi: 10.1016/j.bcp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Haeusgen W, Boehm R, Zhao Y, Herdegen T, Waetzig V. Specific activities of individual c-Jun N-terminal kinases in the brain. Neuroscience. 2009;161:951–959. doi: 10.1016/j.neuroscience.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 26.You L, Gu W, Chen L, Pan L, Chen J, Peng Y. MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2014;7:7249–7261. [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira AC, Robaina MC, Rezende LM, Severino P, Klumb CE. Histone deacetylase inhibitor prevents cell growth in Burkitt's lymphoma by regulating PI3K/Akt pathways and leads to upregulation of miR-143, miR-145, and miR-101. Ann Hematol. 2014;93:983–993. doi: 10.1007/s00277-014-2021-4. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Chu ES, Chen HY, Man K, Go MY, Huang XR, Lan HY, Sung JJ, Yu J. microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget. 2015;6:7325–7338. doi: 10.18632/oncotarget.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]