Abstract
Congenital heart disease (CHD) is a problem in the structure of the heart that is present at birth. Due to advances in interventional cardiology, CHD may currently be without surgery. The present study aimed to explore the molecular mechanism underlying CHD. A total of 200 cases of CHD treated by transcatheter closure as well as 200 controls were retrospectively assessed. Serum cytokines prior to and after treatment were assessed by reverse-transcription quantitative polymerase chain reaction analysis. Furthermore, the levels of proteins associated with c-Jun N-terminal kinase (JNK) and nuclear factor (NF)-κB were assessed by western blot analysis and immunohistochemistry. Furthermore, an animal model of CHD in young pigs was successfully constructed and treated with inhibitors of JNK and/or NF-κB to investigate the roles of these pathways in CHD. The results revealed that tumor necrosis factor-α, interleukin (IL)-6 and IL-8 were significantly elevated in the experimental group following transcatheter closure treatment, compared with those in the healthy control group, and the serum levels of the anti-inflammatory cytokine IL-10 were significantly reduced. Phosphorylated c-Jun and p65 levels in the experimental group were notably higher in the experimental group compared with the control group, but were restored to normal levels following transcatheter closure treatment. Similar results were also obtained in the pig model. The results of the present study suggested that the CHD-associated changes in cytokines, as well as their recovery following transcatheter closure treatment were associated with the JNK and NF-κB signaling pathways. The present study may provide further understanding of the underlying molecular mechanisms in CHD and propose a potential novel target for the treatment of CHD.
Keywords: congenital heart disease, transcatheter closure, cytokines, animal model, nuclear factor-κB signaling pathway, c-Jun N-terminal kinase signaling pathway
Introduction
Congenital heart disease (CHD), also known as a congenital heart anomaly, is a structural aberration of the heart that exists at birth (1). Its symptoms depend on the specific type of anomaly and therefore symptoms may vary widely, ranging from none to those of life-threatening severity (2). CHD is also a major cause of infant mortality worldwide (3). Previous studies have demonstrated that 28% of all major congenital anomalies consist of heart defects (4) and the most practical measurement of CHD occurrence is birth prevalence per 1,000 live births (5). The surveillance of birth defects in China revealed that in 1996 CHD was the 5th most common birth defect and it became the leading birth defect in 2009 (6–8).
At present, CHD is thought to be induced by genetic, lifestyle and environmental factors, including maternal obesity, diabetes, toxicant exposure and alterations in anti-oxidant capacity (9). These well-known risk factors are also associated with immune dysregulation (10). Due to advances in interventional cardiology, certain congenital heart anomalies no longer require surgical treatment and are treated via percutaneous interventions (11,12). There have been numerous studies on CHD, however studies reporting on changes of inflammatory cytokines in patients with CHD following transcatheter closure treatment are lacking. It has been reported that CHD patients with a broad spectrum of pathologies demonstrated certain clinical features and a typical pattern of neurohormonal activation, which is characteristically found in chronic heart failure (CHF) (13). It still remains elusive whether the similarities between adult CHD and CHF extend to the inflammatory cytokine system (14). Certain studies reported that tumor necrosis factor (TNF)-α levels were elevated in a small pediatric cohort with CHD (15). However, only few studies have investigated the level of inflammatory cytokine system activity in CHD patients (16–23).
Materials and methods
Ethics statement
The present study was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (Zhengzhou, China). Written informed consent was provided by all subjects.
Subjects
A total of 200 consecutive patients with CHD [107 males, 93 females; age, 13–41 years (29.6±15.2)] admitted to the Third Affiliated Hospital of Zhengzhou University (Zhengzhou, China) between September 2013 and February 2014 were included in this retrospective study. None of the patients had any unassociated abnormalities (not CDH-specific) or pulmonary hypertension. The cardiac diagnoses of all patients were made on the basis of clinical and laboratory examinations, including telecardiography, electrocardiography and echocardiography. The cases treated with transcatheter closure were retrospectively analyzed. Results prior to treatment as well as those at 6 and 12 months were included. Control data were taken from 200 normal individuals [110 males and 90 females; age, 12–39 years (25.42±13.28)] who received a physical examination at the Third Affiliated Hospital of Zhengzhou University between September 2013 and February 2014.
Fasting blood samples were obtained and collected from the antecubital vein using a lightly fitting tourniquet. Blood (3 ml) was drawn from each subject after fasting for 12 h. Samples were collected in a sterile tube and sera were obtained immediately after clotting of samples, filled in sterile tubes and stored at −80°C until use.
Serum biochemical analyses
TNF-α (cat. no. DTA00C), interleukin (IL)-6 (cat. no. D6050), IL-8 (cat. no. DY208) and IL-10 (cat. no. D1000B) levels were measured using commercially available ELISA assay kits (R&D Systems, Minneapolis, MN, USA). The results are presented in pg/ml or U/l.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the serum of subjects from the experimental group and the control group using an RNA extraction kit (cat. no. DP412; Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's protocol. Detection of the copy number of the mRNA of cytokines was performed as previously described (24).
Immunohistochemical (IHC) staining
Randomly selected samples of peripheral blood mononuclear cells were subjected to IHC staining and detection. IHC was performed as previously described (25).
Western blot analysis
Total protein was extracted and quantitated using a BCA Protein Assay reagent (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Western blot analysis was performed as previously described (25). The membranes were incubated at 4°C overnight with the following primary antibodies: Anti-c-Jun antibody (1:1,000; ab32137), anti-p-c-Jun antibody (1:1,000; ab32385), anti-IKBa antibody (1:1,000; ab32518), anti-p-IΚBα antibody (1:10,000; (ab133462), anti-P65 antibody (2.5 µg/ml; ab19870), anti-p-P65 antibody (1:10,000; ab76302) (all from Abcam, Cambridge, MA, USA). This was followed by incubation with the corresponding horseradish peroxidase-conjugated secondary antibodies: anti-rabbit (ab6721) or anti-mouse (ab6785) immunoglobulin G antibodies (both 1:5,000; Beyotime Institute of Biotechnology, Haimen, China) at 37°C for 45 min. The target bands were visualized by using an enhanced chemiluminescence kit (Qihai Biotech, Shanghai, China) and the associated band intensities were analyzed using Gel-Pro-Analyzer software version 4.0 (Media Cybernetics, Inc., Rockville, MD, USA). β-actin was used as the internal control.
Animal model of CHD
A hybrid method was employed to generate the animal model of CHD in young pigs, as previously described (26). A total of 50 animals (4–8 weeks old; 10–12 kg) were provided by the Third Affiliated Hospital of Zhengzhou University (Zhengzhou, China), CHD was successfully induced in 43 animals and 40 animals were divided into four groups (n=10/group) with 3 animals in reserve. All animals were housed in isolated cages under a 12 h light/dark cycle at 23–25°C, humidity 50–70% with free access to food and water according to the Guide for the Care and Use of Laboratory Animals (27). A group of 10 normal young pigs was used for determination of serum cytokine levels as a blank control group. Over 3 months following surgery, a group of CHD model animals with catheter closure treatment received continuous injection of normal saline as a control group. A second group of the CHD models received continuous injection of pyrrolidine dithiocarbamate (PDTC; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), a nuclear factor (NF)-κB inhibitor, over 3 months following catheter closure treatment. Another group of CHD models received continuous injection of SP600125 (Sigma-Aldrich; Merck KGaA), a c-Jun N-terminal kinase (JNK) inhibitor, and a further group of CHD models received continuous combined injection of PDTC and SP600125 over 3 months following catheter closure treatment. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Third Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
Statistical analysis
Quantitative data are expressed as the mean ± standard deviation. SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Comparison between two groups was performed using Student's t-test. Comparisons among three or more groups were performed using one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics of patients
All 200 cases of CHD were subjected to transcatheter closure treatment at the Third Affiliated Hospital of Zhengzhou University (Zhengzhou, China) between September 2013 and February 2014. The baseline patient characteristics are presented in Table I. The mean patient age in the experimental group was 29.6±15.2 years and that in the control group was 65.42±3.28 years.
Table I.
Characteristics of patients in the experimental group and control group at baseline.
| Characteristic | Experimental group | Control group |
|---|---|---|
| Sex | ||
| Male | 107 | 110 |
| Female | 93 | 90 |
| Age (years) | 29.6±15.2 | 25.42±13.28 |
| Height (cm) | 172±10.13 | 175±9.26 |
| Body weight (kg) | 65.2±10.15 | 68.6±14.5 |
Values are expressed as n or as the mean ± standard deviation.
Transcatheter closure treatment restores cytokine levels in CHD patients
Peripheral blood mononuclear cell samples and serum were obtained from patients in the experimental group and the control group. ELISA demonstrated that serum inflammatory cytokine levels of TNF-α, IL-6 and IL-8 in the experimental group after transcatheter closure treatment were significantly elevated compared with those in the healthy control group without treatment. Conversely, the serum levels of the anti-inflammatory cytokine IL-10 were significantly reduced. Of note, at 6 months and 12 months after transcatheter closure treatment, TNF-α, IL-6 and IL-8 levels in patients' serum were significantly reduced, and IL-10 rose compared with the control. The levels of all of the cytokines were nearly restored (Table II).
Table II.
Serum cytokine levels in patients with congenital heart disease prior to and after transcatheter closure treatment as well as in the control group.
| Experimental group | ||||
|---|---|---|---|---|
| Cytokine | Control | Baseline | 6 months | 12 months |
| TNF-α | 877±129 | 1,936±214a | 1,311±211a | 866±242 |
| IL-6 | 599±289 | 1,699±299a | 1,196±313a | 561±189 |
| IL-8 | 623±92 | 1,268±104a | 972±272a | 413±113a |
| IL-10 | 1,191±296 | 499±211a | 791±363a | 1,278±202 |
P<0.05 vs. Control. TNF, tumor necrosis factor; IL, interleukin.
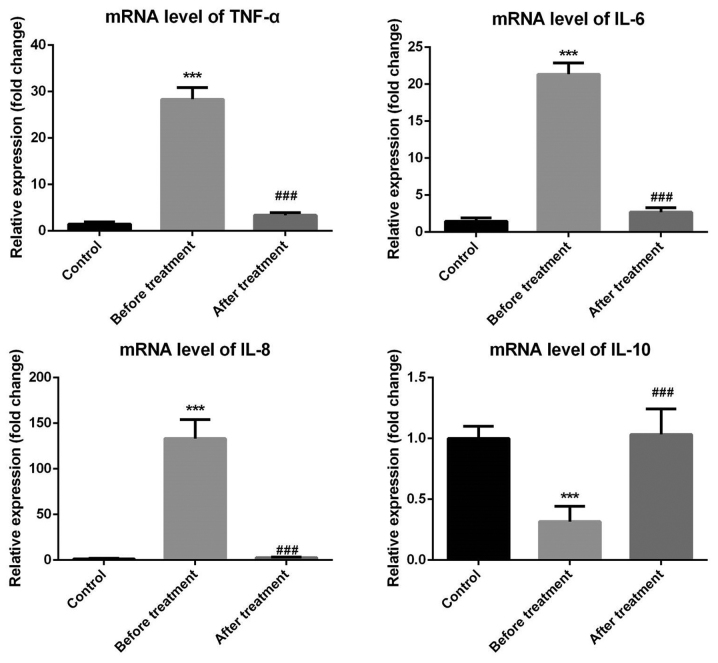
To further confirm the changes of cytokines in the patients, RT-qPCR was performed and the results demonstrated that the mRNA levels of TNF-α, IL-6 and IL-8 in the experimental group were significantly upregulated, while those of IL-10 were significantly reduced compared with those in the control group. At 6 and 12 months after transcatheter closure treatment, the mRNA levels of TNF-α, IL-6, IL-8 and IL-10 returned to normal levels (Fig. 1).
Figure 1.
Cytokine mRNA levels prior to and following transcatheter closure treatment as well as in the control group. TNF, tumor necrosis factor; IL, interleukin. ***P<0.001 vs. the control group; ###P<0.001 vs. the before treatment group.
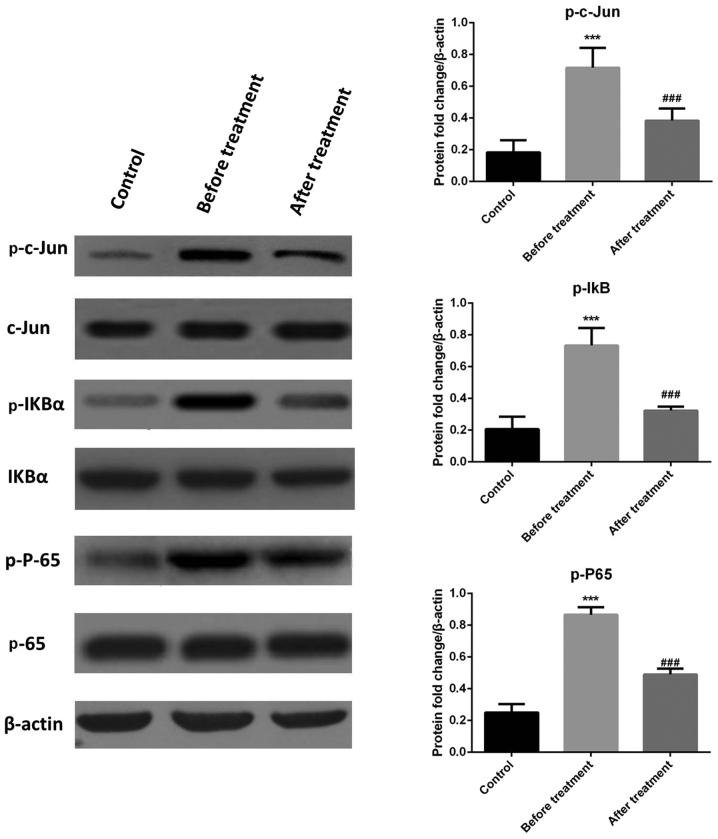
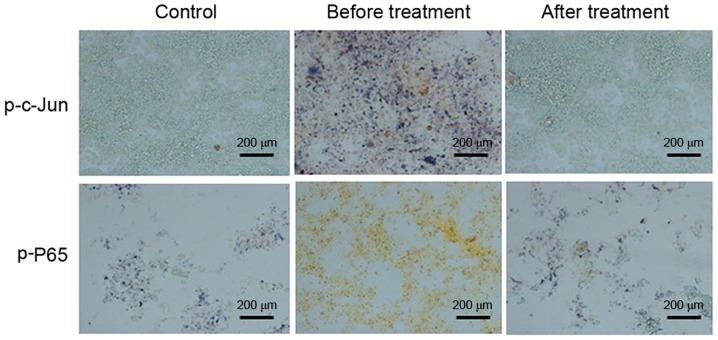
To explore the molecular mechanisms associated with the changes in cytokine levels of CHD patients after transcatheter closure treatment, peripheral blood mononuclear cells were subjected to western blot analysis and IHC staining. The western blot results demonstrated that phosphorylated (p)-c-Jun, p65 and p-IκBa levels in the experimental group were higher than in the control group, and were restored to normal levels after transcatheter closure treatment (Fig. 2). IHC analysis was conducted 6 months following treatment and the results indicated that the p-c-Jun- and p-65-positive cell rate in peripheral blood mononuclear cells was higher prior to treatment compared with that in the control group and decreased following treatment (Fig. 3). These results indicate that the JNK and NF-κB signaling pathways were activated in patients with CHD, and that transcatheter closure treatment may notably decrease the activation of these pathways.
Figure 2.
Levels of c-Jun N-terminal kinase and nuclear factor-κB pathway proteins prior to and after transcatheter closure treatment as well as in the control group. p-IκB, phosphorylated inhibitor of nuclear factor κB. ***P<0.001 vs. the control group; ###P<0.001 vs. the before treatment group.
Figure 3.
Immunostaining for p-P65 and p-c-Jun in the experimental and control groups 6 months following transcatheter closure treatment (scale bar, 200 µm). p, phosphorylated.
Animal model of CHD
Next, an animal model of CHD was successfully constructed in 40 young pigs via a hybrid method according to standard protocols and divided into four groups. The serum cytokine levels of TNF-α, IL-6 and IL-8 were elevated following transcatheter closure treatment, while the levels of the anti-inflammatory cytokine IL-10 dropped. Injection of PDTC (NF-κB inhibitor) or SP600125 (JNK inhibitor) alone only partly restored TNF-α, IL-6, IL-8 or IL-10 compared with normal levels. Of note, combination treatment with PDTC and SP600125 restored TNF-α, IL-6, IL-8 and IL-10 to normal levels (Table III). These results suggested that the CHD-associated changes in cytokines as well as their recovery after transcatheter closure treatment are associated with the JNK and NF-κB signaling pathways.
Table III.
Levels of cytokines in the model of congenital heart disease in young pigs.
| Experimental group | |||||
|---|---|---|---|---|---|
| Cytokine | Control | Vehicle treated | PDTC | SP600125 | PDTC + SP600125 |
| TNF-α | |||||
| Baseline | 689±172 | 1,312±191a | 1,311±211a | 1,196±313a | 1,301±328a |
| 6 months | 691±296 | 472±133 | 984±191a | 967±211a | 456±98 |
| IL-6 | |||||
| Baseline | 531±298 | 1,699±299a | 1,531±298a | 1,588±139a | 1,761±189a |
| 6 months | 581±112 | 482±133 | 884±11a | 867±111a | 456±98 |
| IL-8 | |||||
| Baseline | 431±298 | 1,788±299a | 1,453±298a | 1,688±139a | 1,861±189a |
| 6 months | 481±112 | 482±133 | 974±11a | 967±111a | 356±98 |
| IL-10 | |||||
| Baseline | 1,431±298 | 288±29a | 453±298a | 488±139a | 461±189a |
| 6 months | 1,481±112 | 1,482±133 | 974±113a | 967±111a | 1,356±98 |
P<0.05 vs. Control. TNF, tumor necrosis factor; IL, interleukin; PDTC, pyrrolidine dithiocarbamate.
Discussion
TNF-α, IL-6, IL-8 and IL-10 are important inflammatory or anti-inflammatory cytokines, which contribute to numerous signaling pathways (JNK, NF-κB) to regulate certain biological processes (28–33). The present study provided a better understanding of the roles of cytokines in patients with CHD; the results indicated the presence of a certain degree of immune activation, causing an increase in the levels of inflammatory cytokines and a decrease in anti-inflammatory cytokine levels to result in inflammation. Following catheter closure treatment, the patients' immune and inflammatory status was gradually restored to normal levels.
An increasing number of studies have demonstrated that the occurrence of inflammation is closely associated with the expression of genes linked to inflammation (34,35). The JNK (36–41) and NF-κB (42–47) signaling pathways are two important pathways that have roles in the occurrence and development of inflammation and are associated with heart diseases such as heart failure (48). Cytokine changes caused by CHD may synergistically occur via these two pathways (48,49). Changes of cytokines in patients with CHD may be due to the coordination of members of the NF-κB and the JNK signaling pathway. Wang et al (50) demonstrated that fasudil hydrochloride hydrate, a rho-kinase inhibitor, suppressed isoproterenol-induced heart failure in rats via the JNK and Extracellular signal-regulated kinase 1/2 signaling pathways. Gu et al (51) demonstrated that hypoxia-induced SOCS3 inhibited NF-κB activation in congenital heart disease.
Of note, the NF-κB and JNK signaling pathways were not activated in patients with CHD following transcatheter closure treatment. To a certain degree, the abnormal changes of cytokines in patients with CHD and their recovery following transcatheter closure treatment are dependent on the coordination of JNK and NF-κB signaling pathways.
The present study had certain limitations. Sampling of a larger amount of serum from the same patient with CHD would have been more beneficial to assess more cytokines to better elucidate the potential underlying mechanism.
In conclusion, the results of the present study suggested a certain degree of immune activation in patients with CHD, resulting in an increase in inflammatory cytokine levels and a decrease in anti-inflammatory cytokine levels. Transcatheter closure treatment gradually restored the patients' inflammatory status. Changes in the levels of cytokines in patients with CHD may be regulated by the NF-κB and JNK signaling pathways. Taken together, the results of the present study indicated that CHD is associated with an upregulated inflammatory response, providing a theoretical basis for further research.
References
- 1.Fahed AC, Gelb BD, Seidman JG, Seidman CE. Genetics of congenital heart disease: The glass half empty. Circ Res. 2013;112:707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam AM, Ahmed I, Ahmed M, et al. Congenital heart surgery: Analysis of cases. 2016;130:30. [Google Scholar]
- 3.Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. doi: 10.1016/j.jacc.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 4.Dolk H, Loane M, Garne E, European surveillance of congenital anomalies (EUROCAT) working group Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–849. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 5.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Rudan I, Chan KY, Zhang JS, Theodoratou E, Feng XL, Salomon JA, Lawn JE, Cousens S, Black RE, Guo Y, et al. Causes of deaths in children younger than 5 years in China in 2008. Lancet. 2010;375:1083–1089. doi: 10.1016/S0140-6736(10)60060-8. [DOI] [PubMed] [Google Scholar]
- 7.Qu Y, Liu X, Zhuang J, Chen G, Mai J, Guo X, Ou Y, Chen J, Gong W, Gao X, et al. Incidence of congenital heart disease: The 9-year experience of the guangdong registry of congenital heart disease, China. PLoS One. 2016;11:e0159257. doi: 10.1371/journal.pone.0159257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai L, Zhu J, Liang J, Wang YP, Wang H, Mao M. Birth defects surveillance in China. World J Pediatr. 2011;7:302–310. doi: 10.1007/s12519-011-0326-0. [DOI] [PubMed] [Google Scholar]
- 9.Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, Romano-Adesman A, Bjornson RD, Breitbart RE, Brown KK, et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature. 2013;498:220–223. doi: 10.1038/nature12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole CR, Yutzey KE, Brar AK, Goessling LS, Van Vickle-Chavez SJ, Cunningham MW, Eghtesady P. Congenital heart disease linked to maternal autoimmunity against cardiac myosin. J Immunol. 2014;192:4074–4082. doi: 10.4049/jimmunol.1301264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braunwald E, Bristow MR. Congestive heart failure: Fifty years of progress. Circulation. 2000;102(20 Suppl 4):IV14–IV23. doi: 10.1161/01.CIR.102.suppl_4.IV-14. [DOI] [PubMed] [Google Scholar]
- 12.Sharma R, Bolger AP, Li W, Davlouros PA, Volk HD, Poole-Wilson PA, Coats AJ, Gatzoulis MA, Anker SD. Elevated circulating levels of inflammatory cytokines and bacterial endotoxin in adults with congenital heart disease. Am J Cardiol. 2003;92:188–193. doi: 10.1016/S0002-9149(03)00536-8. [DOI] [PubMed] [Google Scholar]
- 13.Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, Kemp M, Coats AJ, Anker SD, Gatzoulis MA. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation. 2002;106:92–99. doi: 10.1161/01.CIR.0000020009.30736.3F. [DOI] [PubMed] [Google Scholar]
- 14.Tatebe S, Fukumoto Y, Sugimura K, Miura Y, Nochioka K, Aoki T, Yamamoto S, Shimokawa H. Clinical profiles of chronic heart failure with adult congenital heart disease. J Cardiac Failure. 2012;18:S156. doi: 10.1016/j.cardfail.2012.08.163. [DOI] [Google Scholar]
- 15.Afify MF, Mohamed GB, El-Maboud MA, Abdel-Latif EA. Serum levels of ghrelin, tumor necrosis factor-α and interleukin-6 in infants and children with congenital heart disease. J Trop Pediatr. 2009;55:388–392. doi: 10.1093/tropej/fmp036. [DOI] [PubMed] [Google Scholar]
- 16.McMurray J, Abdullah I, Dargie HJ, Shapiro D. Increased concentrations of tumour necrosis factor in ‘cachectic’ patients with severe chronic heart failure. Br Heart J. 1991;66:356–358. doi: 10.1136/hrt.66.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, Cassani G, Visioli O. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–1486. doi: 10.1161/01.CIR.92.6.1479. [DOI] [PubMed] [Google Scholar]
- 19.Kubota T, Mctiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res. 1997;81:627–635. doi: 10.1161/01.RES.81.4.627. [DOI] [PubMed] [Google Scholar]
- 20.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/S0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 21.Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220–225. doi: 10.1161/01.CIR.103.2.220. [DOI] [PubMed] [Google Scholar]
- 22.Aukrust P, Ueland T, Lien E, Bendtzen K, Müller F, Andreassen AK, Nordøy I, Aass H, Espevik T, Simonsen S, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–382. doi: 10.1016/S0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- 23.Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45:183–193. doi: 10.1536/jhj.45.183. [DOI] [PubMed] [Google Scholar]
- 24.Latorre E, Mendoza C, Matheus N, Castro M, Grasa L, Mesonero JE, Alcalde AI. IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine. 2013;61:778–784. doi: 10.1016/j.cyto.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Choi Y, Park J, Choi Y, Ko YS, Yu DA, Kim Y, Pyo JS, Jang BG, Kim MA, Kim WH, Lee BL. c-Jun N-terminal kinase activation has a prognostic implication and is negatively associated with FOXO1 activation in gastric cancer. Bmc Gastroenterol. 2016;16:59. doi: 10.1186/s12876-016-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Liu Y, Lv X, Yu C, Li X. A novel hybrid method for creating a porcine model of cyanotic congenital heart defect with decreased pulmonary blood flow. J Surg Res. 2009;154:262–266. doi: 10.1016/j.jss.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council, corp-author. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington, DC: 2012. pp. 267–268. [Google Scholar]
- 28.Ji RR, Gereau RW, IV, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Lin A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 31.Bogoyevitch MA, Kobe B. Uses for JNK: The many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akella R, Moon TM, Goldsmith EJ. Unique MAP Kinase binding sites. Biochim Biophys Acta. 2008;1784:48–55. doi: 10.1016/j.bbapap.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monroy CM, Cortes AC, Lopez MS, D'Amelio AM, Jr, Etzel CJ, Younes A, Strom SS, El-Zein RA. Hodgkin disease risk: Role of genetic polymorphisms and gene-gene interactions in inflammation pathway genes. Mol Carcinog. 2011;50:36–46. doi: 10.1002/mc.20747. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, Duggal P, Allen C, Chuang R, Ehsanian R, et al. ΔNp63 versatilely regulates a Broad NF-κB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res. 2011;71:3688–3700. doi: 10.1158/0008-5472.CAN-10-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Slevin M, Elasbali AB, Turu M Miguel, Krupinski J, Badimon L, Gaffney J. Identification of differential protein expression associated with development of unstable human carotid plaques. Am J Pathol. 2006;168:1004–1021. doi: 10.2353/ajpath.2006.050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm M, Xu Z, Kukekov NV, Gire S, Greene LA. Proapoptotic Nix activates the JNK pathway by interacting with POSH and mediates death in a Parkinson disease model. J Biol Chem. 2007;282:1288–1295. doi: 10.1074/jbc.M607038200. [DOI] [PubMed] [Google Scholar]
- 39.Smith WW, Gorospe M, Kusiak JW. Signaling mechanisms underlying Abeta toxicity: Potential therapeutic targets for Alzheimer's disease. Cns Neurol Disord Drug Targets. 2006;5:355–361. doi: 10.2174/187152706784111515. [DOI] [PubMed] [Google Scholar]
- 40.Størling J, Binzer J, Andersson AK, Züllig RA, Tonnesen M, Lehmann R, Spinas GA, Sandler S, Billestrup N, Mandrup-Poulsen T. Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia. 2005;48:2039–2050. doi: 10.1007/s00125-005-1912-2. [DOI] [PubMed] [Google Scholar]
- 41.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance; Proc Natl Acad Sci USA; 2006; pp. 10741–10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 2006;177:7485–7496. [PubMed] [Google Scholar]
- 43.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.CIR.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 44.Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307–314. doi: 10.1016/S0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- 45.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 46.Haudek SB, Bryant DD, Giroir BP. Differential regulation of myocardial NF kappa B following acute or chronic TNF-alpha exposure. J Mol Cell Cardiol. 2001;33:1263–1271. doi: 10.1006/jmcc.2001.1388. [DOI] [PubMed] [Google Scholar]
- 47.Bradham WS, Moe G, Wendt KA, Scott AA, Konig A, Romanova M, Naik G, Spinale FG. TNF-α and myocardial matrix metalloproteinases in heart failure: Relationship to LV remodeling. Am J Physiol Heart Circ Physiol. 2002;282:H1288–H1295. doi: 10.1152/ajpheart.00526.2001. [DOI] [PubMed] [Google Scholar]
- 48.Lagyal CM, Guia TD, Ayuyao F, Bautista M. Prolonged mechanical ventilation among children with congenital heart disease undergoing cardiac surgery in philippine heart center: A risk factors analysis. Am J Respir Crit Care Med. 2011;183:A1700. [Google Scholar]
- 49.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: Role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang N, Guan P, Zhang JP, Li YQ, Chang YZ, Shi ZH, Wang FY, Chu L. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses isoproterenol-induced heart failure in rats via JNK and ERK1/2 pathways. J Cell Biochem. 2011;112:1920–1929. doi: 10.1002/jcb.23112. [DOI] [PubMed] [Google Scholar]
- 51.Gu Q, Kong Y, Yu ZB, Bai L, Xiao YB. Hypoxia-induced SOCS3 is limiting STAT3 phosphorylation and NF-κB activation in congenital heart disease. Biochimie. 2011;93:909–920. doi: 10.1016/j.biochi.2011.02.009. [DOI] [PubMed] [Google Scholar]