Abstract
The current study aimed to investigate ethanolic extracts from the following medicinal plant species cultivated in western Romania: Melissa officinalis L., Rosmarinus officinalis L. (RO) and Salvia officinalis L. (SO). Antioxidant activity, total phenolics content and a profile of the main hydroxycinnamic acids (HCAs), including caffeic, ferulic, coumaric and rosmarinic acids, was determined for each plant extract. The in vitro antimicrobial activity against four bacterial strains (Escherichia coli, Listeria-, Pseudomonas aeruginosa and Staphylococcus aureus), and the effect on cell viability in two melanoma cell lines (B164A5 murine melanoma and A375 human melanoma) was also assessed. The results indicated that total phenolics content was 73.76–274.73 mg GAE·g−1 and the antioxidant activity was 2.32–2.87 mM Fe2+·100 g−1. There was found a strong positive correlation (R=0.9691) between total phenolics content and the antioxidant activity in the investigated samples. Regarding the HCA profile obtained by high performance liquid chromatography, the results demonstrated that rosmarinic acid represents the main identified compound. The ethanolic extracts of RO and SO exhibited antibacterial activity against Gram positive and Gram negative bacteria. RO was the most effective in terms of decreasing the cell viability of murine and human melanoma cell lines, while the HCAs did not exhibit any effect on cell viability. These findings suggest that plant extracts from the Lamiaceae family may used in the clinic as natural antibacterial agents.
Keywords: Melissa officinalis L., Rosmarinus officinalis L., Salvia officinalis L., total polyphenols, hydroxycinnamic acids, antioxidant, antibacterial, B16 4A5 murine melanoma, A375 human melanoma
Introduction
Pure natural compounds or different types of plant extracts have been studied over a long period of time for a large range of therapeutic effects (1,2). Furthermore, lifestyle and dietary habits serve a vital role in the prevention and treatment of a number of pathologies, including cancer (3).
The Lamiaceae family represents the most important source of plants that have a calming and relaxing effect. Its members also strengthen and stimulate, and have specific effects associated with a particular organ or system. It has been demonstrated that the active compounds present in plants from the Lamiaceae family have natural antibacterial (4,5), antioxidant (6,7), antifungal (8) and antitumor effects (9,10) which suggests that they may be viable alternatives to synthetic products in the therapy of various diseases. Thus, the proven benefits on human health represent the main reason why various medicinal plant species belonging to the Lamiaceae family are increasingly exploited nowadays, in various formulations. These approaches have revealed not only therapeutic effects similar to those exhibited by synthetic chemical molecules, but have not produced any side effects and may thus be used over longer periods of time (7,9). Species of the Lamiaceae family contain hydroxycinnamic acids (HCAs), including rosmarinic (RS), ferulic (FE), caffeic (CA) and coumaric (CU) acids and phytochemical compounds with biological potential, including antibacterial, antiviral, antioxidant, anti-inflammatory, antidepressive, anticancer, antiangiogenic and antihepatotoxic effects (10).
Cutaneous melanoma is an aggressive skin malignant tumor induced by changes in skin melanocytes, and its incidence rate is steadily increasing globally (11). Notably, the cutaneous melanoma is one of the most common cancers in young adults, particularly in young women (12). Although, the average age at diagnosis is 63, melanoma is not uncommon among those younger than 30 years old (13). The American Cancer Society's estimates in the United States for 2017 ~87,110 new cases of melanoma will be diagnosed (~52,170 in men and 34,940 in women) and ~9,730 people are expected to succumb to fatality (~6,380 men and 3,350 women) (11). Furthermore, metastatic melanoma has indicated high resistance to cytotoxic agents, which typically results in a poor prognosis (14). Therefore, the finding of natural and sustainable anti-cancer compounds for improving the melanoma prognosis represents an important research issue. Plant extracts rich in bioactive compounds may significantly contribute to improve conventional chemical treatments (9,10). In this line, a growing number of investigations on different species from Lamiaceae family, confirmed their pharmacological potential to be used as phytochemical extracts in the treatment and management of cutaneous melanoma (10,14,15).
The aim of the present study was to analyze the antioxidant activity (AA), total phenols content (TP) and profile of selected HCAs in ethanol extracts of Melissa officinalis L. (MO), Rosmarinus officinalis L. (RO), Salvia officinalis L. (SO) and the antimicrobial activity against a panel of four relevant pathogenic microorganisms, which constitute typical residents of skin and intestinal flora. The effect on cell viability of extracts and the main HCAs was also determined for the B16 4A5 murine melanoma and A375 human melanoma cell lines.
Materials and methods
Plant samples
The whole aerial parts of the investigated medicinal plant species (MO, RO and SO) were collected in 2014 from the experimental field at Banat's University of Agricultural Sciences and Veterinary Medicine, King Michael I of Romania (Timisoara, Romania; 21013′E longitude, 45045′N latitude). From each species, ~500 g fresh material was used. Additionally, voucher specimens, defined as a representative sample of an expertly identified plant that is deposited and stored at a facility from which researchers may later obtain the specimen for examination and further study (16), were identified and 20 plants are deposited in a temperature-controlled herbarium (22–25°C and 30–40% relative humidity) of the Department of Agricultural Technologies, Banat's University of Agricultural Sciences and Veterinary Medicine ‘King Michael I of Romania’ from Timişoara.
Preparation of extracts
Plant material was air dried at 25°C and ground to a fine powder using a grinder (GM 2000; Grindomix; Retsch Technology GMbH, Haan, Germany). The powdered material (2 g) was extracted with 20 ml 70% ethanol for 30 min at 60°C using an ultrasonic water bath (FALC Instruments, Treviglio, Italy). Extracts were filtered using Whatman membrane filters nylon 0.45 µm, 30 mm (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and stored at 2–4°C for subsequent antioxidant, antimicrobial and anticancer experiments.
AA evaluation
The evaluation of AA was performed using the ferric reducing antioxidant power (FRAP) assay according to a previously published protocol (17). FRAP reagent (1.5 ml; Sigma-Aldrich; Merck KGaA) and 50 µl plant extract were vortexed 6 min at 3,200 rotation/min using Vortex Genie 2 (Scientific Industries, Inc., Bohemia, NY, USA) and reaction mixtures were measured at a wavelength of 595 nm using a UV-VIS spectrophotometer (Specord 205; Analytik Jena AG, Jena, Germany). A calibration curve was used in the range of 1–50 mM Fe2+·100 g−1 dry matter (d.m). The regression equation was: y=3.41+x-0.02 and the coefficient of correlation was R2=0.9991. Ethanol was used as a control sample. All experiments were performed in triplicate and the results were expressed as a mean value.
TP evaluation
A total of 0.5 ml extract was treated with 1.25 ml Folin-Ciocalteu reagent (Sigma-Aldrich; Merck KGaA) diluted 1:10 with water. The sample was incubated for 5 min at room temperature and 1 ml Na2CO3 60 g·l−1 was added. Following 30 min of incubation at 50°C, the absorbance of samples was measured at 750 nm using an UV-VIS spectrophotometer (Specord 205; Analytik Jena) (18). The calibration curve was obtained using gallic acid (GA) (Sigma-Aldrich; Merck KGaA) as the standard with a concentration range of 0.03–1 mg·ml−1 and a blank ethanol control (Sigma-Aldrich; Merck KGaA). The regression equation was: y=1.92·x-0.10 and the coefficient of correlation R2=0.9980. The results were expressed in mg GAE·g−1 d.m. All experiments were performed in triplicate.
HCA determination
The levels of primary HCAs FE, CA, RS and CU were determined using an LC-Shimadzu chromatograph (Shimadzu, Kyoto, Japan) equipped with a degasser (DGU-20AS), binary pump (LC-20AD), column thermostat (CTO-20AC), autosampler (SIL 20-A) and SPD-10A UV detector (Shimadzu). A sample of 10 µl was injected at a flow rate of 1 ml/min. The chromatographic conditions were: PREVAIL column 150×4.6 mm (Alltech Associates, Inc., Deerfield, IL, USA). Two mobile phases were used to elute the compounds. Mobile phase A contained CH3OH:CH3COOH:H2O at a ratio 90:2:8 (v/v) and mobile phase B contained CH3OH:CH3COOH:H2O at a ratio 10:2:88 (v/v). Elution of the compounds through chromatographic column was performed with a concentration gradient, which implied the use of the mobile phase B in the first 10 min, followed by a mixture of mobile phases B and A in the proportion (85:15, v/v) for 10–15 min, followed by a mixture of mobile phases B and A in the proportion (50:50, v/v) for 15–20 min, followed by a mixture of mobile phases B and A in the proportion (30:70, v/v) for 20–25 min and then an isocratic program with 80% mobile phase B until the 40th min. The wavelengths of maximum absorbance were 280 nm and 320 nm. The calibration curves were produced in the range of 1–10 µg·ml−1. The regression equations were as follows: y=2.36·x-2.34 (CA), y=2.00·x-2.65 (CU), y=2.96·x-5.28 (FE), y=4.22·x-2.02 (RS). R2 was >0.98 for all regression curves. The limit of detection representing the amount of compounds that may be detected with a signal to noise ratio (S/N) ≥3 was 0.5 µg·ml−1 for CA, CU and FE and 0.4 µg·ml−1 for RS. The limit of quantification representing the lowest concentration for which S/N ≥5 was 0.7 µg·ml−1 for CA, CU and FE and 0.6 µg·ml−1 for RS. The results were expressed in mg·g−1 d.m. Experiments were performed in duplicate. All standards were prepared in methanol (Merck KGaA) and all reagents and solvents used were analytical grade chemicals. Standards of RS, CA, FE and CU were purchased from Sigma-Aldrich, Merck KGaA.
Antibacterial activity
A total of four bacterial strains that grow primarily on skin and constitute part of the intestinal flora and food contaminants (4) were used to determine the antibacterial effect of medicinal plant extracts. The following bacterial strains were used: Escherichia coli (cat. no. 25922), Listeria monocytogenes (cat. no. 19114), Pseudomonas aeruginosa (cat. no. 27853) and Staphylococcus aureus (cat. no. 25923; all American Type Culture Collection [ATCC], Manassas, VA, USA). The agar wells procedure was employed (4). Strains were kept at −80°C as stock cultures. Prior to each experiment, bacteria were cultured on Brain Heart Infusion (BHI) agar (Oxoid; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and incubated at 37°C for 24 h. Strains were then revived using BHI broth (Oxoid; Thermo Fisher Scientific, Inc.) at 37°C for 24 h. Following this, 2 ml bacterial suspension adjusted according to the turbidity of 0.5 McFarland standard was transferred to 200 ml Muller Hinton (MH) agar (Oxoid; Thermo Fisher Scientific, Inc.) at 45°C. MH agar (25 ml) was then poured into Petri dishes and left to solidify. Four wells were made using a sterile hole puncher (5 mm diameter) in each agar plate. Ethanolic MO, RO and SO plant extracts (80 µl) was then placed in each well and 70% ethanol was used as the control. Two different concentrations of RS were also tested: 100 µg·ml−1 and 1,000 µg·ml−1. Plates were incubated at 37°C for 24 h. The diameter of zone inhibition was then measured and recorded. Experiments were performed in duplicate.
MTT cell viability assay
B164A5 murine melanoma (Sigma-Aldrich; Merck KGaA) and A375 human melanoma cell lines (cat no. CRL-1619™; ATCC) were seeded on a 96-well culture plate at a cellular density of 6,000 cells/well and attached to the bottom of the well overnight. After 24 h, 100 µl of fresh Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) containing 50 and 100 µg·ml−1 plant extracts or HCAs [dissolved in dimethyl sulfoxide (DMSO); Sigma-Aldrich; Merck KGaA] was added to cells and incubated for 72 h at 37°C. Ethanol was used as a control sample. The medium was supplemented with 10% fetal calf serum (PromoCell GmbH, Heidelberg, Germany) and 1% penicillin/streptomycin mixture (10,000 IU/ml; PromoCell GmbH). Melanoma cells were then assayed using 10 µl 5 mg·ml−1 MTT solution from the MTT-based in vitro toxicology assay kit (Tox-1; Sigma-Aldrich; Merck KGaA). Intact mitochondrial reductase converted and precipitated MTT as blue crystals during a 4 h contact period. The precipitated crystals were dissolved in 100 µl lysis solution provided by the manufacturer (Sigma-Aldrich; Merck KGaA). The reduced MTT was spectrophotometrically analyzed at a wavelength of 570 nm, using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Wells with untreated cells were considered as a reference for viability, while DMSO, which was used to prepare stock solutions of the tested substances was also added to cells for the evaluation of cell viability. All in vitro experiments were carried out on two microplates in quadruplicate for each tested substance, as well as controls.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis was performed using the Statistical Analysis System 8.1 (SAS Institute, Inc., Cary, NC, USA). One way analysis of variance was applied to evaluate statistical significance regarding the reduction in cell viability following treatment with selected extracts *, ** and *** indicate P<0.05, P<0.01 and P<0.001, respectively, compared to control group. Pearson's correlation coefficient was also calculated between antioxidant parameters. P<0.05 was considered to indicate a statistically significant difference.
Results and Discussion
Antioxidant properties
The TP and FRAP values of natural extracts are presented in Table I. SO exhibited the highest TP value (274.73±2.53 mg GAE·g−1 d.m.) followed by RO (86.05±0.40 mg GAE·g−1 d.m.) and MO (73.76±2.80 mg GAE·g−1 d.m.). The antioxidant properties of medicinal plants belonging to the Lamiaceae family have been previously highlighted (11,19). Total phenolic content in MO extract ranged between 20.16–38.77 mg GAE/g depending on flowering stages (11). The results reported by Mohamadi et al (19) indicated higher phenolic compound in MO extract compared with SO extract (31 mg GAE/g d.m. in MO and 26 mg GAE/g d.m.in SO) and rosmarinic acid contents (22 mg/g d.m. in MO and 18 mg RA/g d.m. in SO). A previous study indicated the antioxidant properties of metanolic extracts from MO and SO on the stability of soybean oil, and results suggested the higher antioxidant activity of the MO extracts were indicated compared with SO (0.043 mg/ml extract of MO and 0.061 mg/ml extract of SO was necessary to obtain 50% of DPPH degradation) (19). TP content in medicinal plants belonging to the Lamiaceae family from Romania ranged between 40–60 mg GAE·g−1 d.m. according to a previous study (20). Rasmy et al (21) indicated 94.35 mg GAE·g−1 TP in SO alcoholic extracts while other studies indicated that the TP content was 61–137 mg GAE·g−1 (22) and 21.74–93.65 mg GAE·g−1 (23). Lower values (14.53–33.22 mg GAE·g−1) were detected in Lamiaceae species from Iran (24). The differences registered among the values reported by previous studies may be due to the pedological and agro-technical conditions.
Table I.
TP and AA of medicinal plants.
| Plant species | AA (mMFe2+·100 g−1 d.m.) | P-value | TP (mg GAE·g−1d.m.) | P-value |
|---|---|---|---|---|
| RO | 2.32±0.57 | (RO/MO) 0.75 | 86.05±0.40 | (RO/MO) 0.001 |
| MO | 2.47±0.54 | (MO/SO) 0.63 | 73.76±2.80 | (MO/SO) 2.27×10−8 |
| SO | 2.87±1.19 | (RO/SO) 0.52 | 274.73±2.53 | (RO/SO) 8.28×10−8 |
Data are expressed as the mean ± standard deviation, n=3. TP, total phenolic content; AA, antioxidant assay; d.m., dry matter; RO, Rosmarinus officinalis L.; MO, Melissa officinalis L.; SO, Salvia officinalis L.
SO extract exhibited the highest FRAP content (2.87 mM Fe2+·100 g−1 d.m.) followed by MO (2.47 mM Fe2+·100 g−1 d.m.) and RO (2.32 mM Fe2+·100 g−1 d.m.). Similar results were reported by Derakhshani et al (24) in extracts from different species of SO (2.49–3.7 mM Fe2+·100 g−1 d.m.).
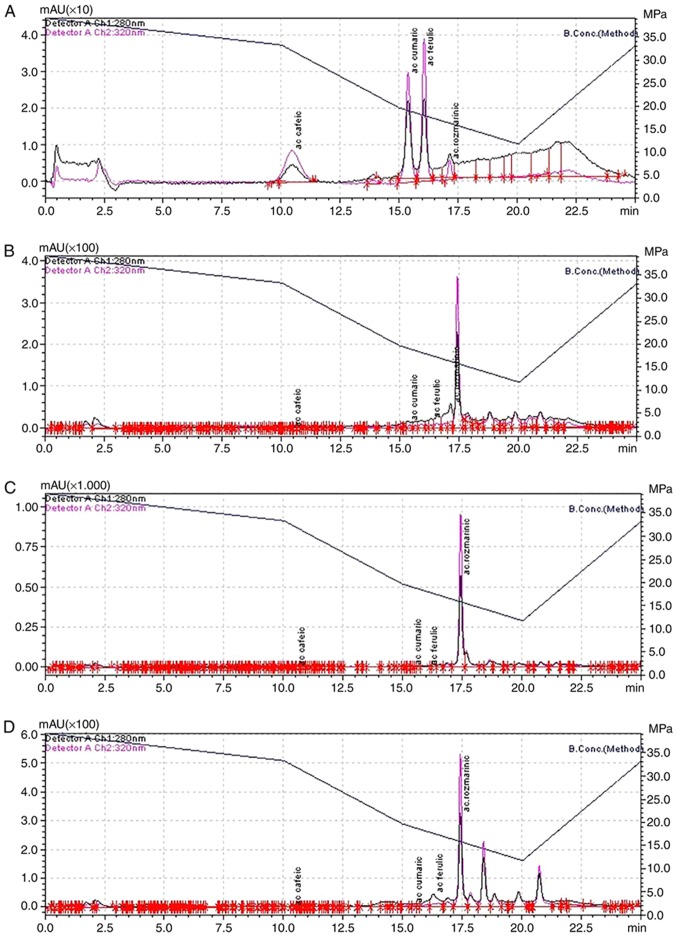
The HCA profile demonstrated that RS is the main phenolic acid in Lamiaceae plant extracts (Fig. 1). The content of RS in analyzed extracts was 9.63 mg·g−1 d.m. in MO, 14.91 mg·g−1d.m. in SO and 3.31 mg·g−1 d.m. in RO. The majority of our results are in agreement with the results reported in previous studies, which demonstrated variation in the RS content detected in Lamiaceae extracts in the range 3.48–90.52 mg·g−1 d.m. (19,25–28). The only exception were the results concerning RO extract. Agro-techniques and fertilization treatments are responsible for the increase in RS content in medicinal plants (29).
Figure 1.
Chromatograms of standards and samples. Chromatographs for (A) Caffeic (tr=10.4), (B) coumaric (tr=15.4), (C) ferulic (tr=16.4) and (D) rosmarinic (tr=17.2) acids. Wavelengths of 280 and 320 nm are indicated by the pink and black lines, respectively. tr, retention time.
The levels of FE and CU acids were decreased compared with the level of RS acid in the MO, RO and SO groups (Table II). The FE content ranged between 0.08 and 0.54 mg·g−1d.m. with the highest value recorded in the MO extract, which is consistent with studies performed by Oniga et al (30) where the range obtained was 0.36–0.56 mg·g−1 d.m., Proestos and Komaitis (31) where the range was 0.34–6.9 mg·g−1 d.m. and Baciu et al (32) where the content was determined as 0.15 mg·g−1d.m. The level of CU was also detected in the same range of values as FE, with the minimum value recorded in SO extract (0.091±0.90 mg·g−1d.m.) and the maximum value recorded in the RO extract (0.585±0.57 mg·g−1 d.m.). Similar results have been reported in extracts from medicinal plants cultivated in Europe (33–35).
Table II.
HCA content (mg·g−1 d.m.) of medicinal plants extracts.
| HCA | CA | CU | P-value | FE | P-value | RS | P-value |
|---|---|---|---|---|---|---|---|
| MO | Nd | 0.212±0.02 | (MO/RO) 0.0005 | 0.540±0.04 | (MO/RO) 0.0001 | 9.632±1.25 | (MO/RO) 0.0010 |
| RO | Nd | 0.585±0.05 | (RO/SO) 0.0001 | 0.138±0.01 | (RO/SO) 0.018 | 3.310±0.24 | (RO/SO) 0,0001 |
| SO | Nd | 0.091±0.01 | (MO/SO) 0.002 | 0.080±0.1 | (MO/SO) 0.0004 | 14.910±0.90 | (MO/SO) 2.7×10−5 |
Data are expressed as the mean ± standard deviation, n=2. CA values were Nd as their concentrations were lower than the quantification limit (0.04 mg·g−1 d.m.). RO, Rosmarinus officinalis L.; MO, Melissa officinalis L.; SO, Salvia officinalis L.; HCA, hydroxycinnamic acid; RS, rosmarinic acid; FE, ferulic acid; CA, caffeic acid; CU, coumaric acid; Nd, not detectable; d.m., dry matter.
As indicated in Table III, there was a significant negative correlation for the concentration of HCAs and antioxidant activity for FE/CU (−0.1695), FE/RS (−0.0640), FE/AA (−0.4307) (P<0.05); a significant moderate negative correlation for TP/CU (−0.6489), TP/FE (−0.6397) (P<0.05); a significant strong negative correlation for AA/CU (−0.8163), RS/CU (−0.9726) (P<0.05) and a significant strong positive correlation for TP/AA (0.9691), RS/AA (0.9282) and RS/TP (0.8079) (P<0.05).
Table III.
Correlation between hydroxycinnamic acid concentration and antioxidant activity.
| TP | CU | FE | RS | |
|---|---|---|---|---|
| Correlation | R | |||
| CU | −0.6489 | |||
| FE | −0.6397 | −0.1695 | ||
| RS | 0.8079 | −0.9726 | −0.0640 | |
| AA | 0.9691 | −0.8163 | −0.4307 | 0.9282 |
Data are expressed as the mean ± standard deviation. Correlation between CA and AA was not investigated because the CA values were not detectable (the values were lower than the quantification limit (0.04 mg·g−1 d.m.) S, rosmarinic acid; FE, ferulic acid; CU, coumaric acid; TP, total phenolic content; AA, antioxidant assay; R, correlation coefficient. P<0.05.
These results demonstrated that the activities of RS among its constituents provided a strong contribution to antioxidant efficiency and value, indicating that RS had the greatest AA. By contrast, FE and CU had a small contribution to the total antioxidant capacity of the extracts.
Antibacterial evaluation
Many previous studies have focused on the antimicrobial potential of medicinal plants or pure active phytocompounds (36–38), however few studies have associated this effect with a particular chemical composition or specifically with the HCA content. Therefore, the antimicrobial activity against a panel of four relevant pathogenic microorganisms associated with RS content was investigated in the current study. The results demonstrated that RO and SO extracts exhibit an antibacterial effect against Gram positive and Gram negative bacteria (Table IV). RO extract exhibited the greatest antibacterial effect against L. monocytogenes with an inhibition zone diameter of 15±1.2 mm, while the weakest antibacterial effect was detected against E. coli with an inhibition zone diameter of 8±0.3 mm. The greatest antibacterial effect for SO was recorded against S. aureus with an inhibition zone diameter of 15±0.3 mm, while the weakest antibacterial effect was detected again against E. coli with an inhibition zone diameter of 7±0.6 mm. The antibacterial effect of the MO extract was similar to the control.
Table IV.
Antimicrobial activity of extracts.
| Inhibition zone diameter (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plant extract | Escherichia coli | P-value | Listeria monocytogenes | P-value | Pseudomonas aeruginosa | P-value | Staphylococcus aureus | P-value |
| C (70% ethanol) | 0 | 0 | 0 | 0 | ||||
| MO | 0 | 0 | 0 | 0 | ||||
| RO | 8±0.3 | (RO/SO) 0.06 | 15±1.2 | (RO/SO) 0.006 | 12±1.0 | (RO/SO) 0.31 | 12±0.9 | (RO/SO)0.005 |
| SO | 7±0.6 | (C/SO) 3.59 ×10−5 | 11±0.6 | (C/SO) 5.90×10−6 | 11±0.2 | (C/SO) 5.31×10−6 | 15±0.3 | (C/SO) 1.04×10−7 |
| RS 100 µg/ml | 0 | 0 | 0 | 0 | ||||
| RS 1,000 µg/ml | 0a | 0 | 0 | 0 | ||||
Data are expressed as the mean ± standard deviation. RO, Rosmarinus officinalis L.; MO, Melissa officinalis L.; SO, Salvia officinalis L.; RS, rosmarinic acid, C, control.
By contrast, the results obtained concerning bacterial growth in the presence of two concentrations of RS indicated that it is not responsible for the antimicrobial activity of analyzed plant extracts. However, previous studies have demonstrated an active role of RS against S. aureus (39). In the present study, it is hypothesized that the antibacterial effect of RO and SO extracts was due to the synergism exercised by the presence of different bioactive compounds within the extract. A similar observation was made for the antimicrobial effect of different essential plant oils in the study by Hossain et al (40). This may be an advantage because different microorganisms cannot acquire resistance to multiple biological compounds (8).
Reduction in cell viability
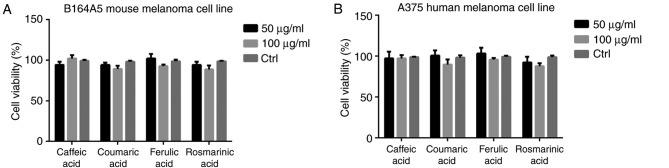
Following an incubation period of 72 h, the A375 human melanoma cell line became more sensitive to RO extract compared with the B164A5 mouse melanoma cell line. The viability of the human melanoma cell line was 56% at a concentration of 50 µg·ml−1 RO and 45.5% at the concentration of 100 µg·ml−1 RO compared with a viability of 70% at a concentration of 50 µg·ml−1 RO and 61% at a concentration of 100 µg·ml−1 RO for the murine cell line. At the concentration of 100 µg·ml−1 SO, a viability of 71% for A375 melanoma cell line and 65.5% for the B164A5 mouse melanoma cell line was indicated. Less effective was the MO extract, namely at the concentration of 100 µg·ml−1 MO, the cell viability of the A375 melanoma cell line was 79.5% and for the B164A5 mouse melanoma cell line this was 77.5% (Fig. 2). Treatment with 100 µg·ml−1 RO significantly decreased the cell viability compared with the control in case of the A375 human melanoma cell line group (P<0.01). Additionally, 100 µg·ml−1 SO treatment significantly decreased the cell viability compared with the control group in case of the same tested cell line (P<0.05). Furthermore, 100 µg·ml−1 RO and 100 µg·ml−1 SO treatment significantly decreased the cell viability compared with the control group in case of B164A5 mouse melanoma cell line (P<0.05). The reduction in viability in each melanoma cell line between cells treated with different concentrations of MO and the control group was not significant. The reduction in viability in each melanoma cell line between cells treated with 100 µg·ml−1 SO or RO and the control group was significant, although this reduction was more marked in RO compared with SO. The difference in viability between the control group and cells treated with different concentrations of HCAs was not significant in the two melanoma cell lines (Fig. 3). RO extract has been previously described to reduce the viability of M14 and A375 melanoma cell lines at concentrations ranging from 20–80 µg·ml−1 (14). Additionally, in the same study it was demonstrated that RO extract containing 31.7% carnosic acid, which is the major polyphenol responsible for AA, was able to confer ultraviolet radiation protection (14). Furthermore, it was demonstrated that RO extract reduced the viability of A375 human melanoma cell line, which directly correlated with the time check point (24, 48 or 72 h) and concentration (1:240, 1:480 or 1:960 dilution) (15). Huang et al (41) indicated that carnosol, the major compound of RO extract responsible for antioxidant and chemopreventive activity, downregulates nuclear factor-κB and c-Jun, thus suppressing the MMP9. These mechanisms are correlated with the direct effect of inhibiting the invasion of B16/F10 mouse melanoma cells.
Figure 2.
Cell viability following treatment with plant extracts. Cell viability of (A) B164A5 mouse melanoma and (B) A375 human melanoma cell lines following treatment with 50 and 100 µg·ml−1 Rosmarinus officinalis L., Melissa officinalis L. and Salvia officinalis L. plant extracts. Values are presented as the mean ± standard deviation. All in vitro experiments were performed on two microplates in quadruplicate for each tested substance, as well as control samples. *P<0.05, **P<0.01 as indicated. Ctrl, control.
Figure 3.
Cell viability following treatment with HCAs. Cell viability of (A) B164A5 mouse melanoma and (B) A375 human melanoma cell lines following treatment with 50 and 100 µg·ml−1 caffeic acid, coumaric acid, ferulic acid and rosmarinic acid. Values are presented as the mean ± standard deviation. All in vitro experiments were carried out on two microplates in quadruplicate for each tested substance, as well as control samples. No statistically significant differences were observed between groups.
In conclusion, the results of the current study indicated that the ethanolic extracts from selected medicinal species belonging to the Lamiaceae family exhibited a high AA and RS content. The antibacterial potential of RO and SO extracts against Gram positive and Gram negative bacteria suggest that they may be viable alternatives to synthetic products in the treatment of various diseases. The RO extract exhibited a stronger effect in terms of reducing the viability of the murine and human melanoma cell lines compared with SO and MO extracts and the B164A5 murine melanoma cell line had an improved response to the extracts compared with the A375 human melanoma cell line. The results also demonstrated that HCAs are not responsible for the reduction in viability caused by the plant extracts investigated in the current study. These findings suggest that plant extracts from the Lamiaceae family may be used in the clinic as natural antibacterial agents.
Acknowledegments
This study was supported by project no. 2763/30.04.2015, in the internal competition of research projects from Banat's University of Agricultural Sciences and Veterinary Medicine ‘King Michael I of Romania’ from Timişoara, session 2015 and a grant of the Romanian National Authority for Scientific Research and Innovation (CNCS/CCCDI/UEFISCDI) (National University Research Council/Consultative College for Research, Development and Innovation/Executive Unit for Financing Higher Education, Research, Development and Innovation) (grant no. PN III-P2-2.1.BG-2016-0126), within PNCDI III, BG-15.
References
- 1.Danciu C, Berkó S, Varju G, Balázs B, Kemény L, Németh IB, Cioca A, Petruș A, Dehelean C, Cosmin CI, et al. The effect of electroporation of a lyotroic liquid crystal genistein-based formulation in the recovery of murine melanoma lesions. Int J Mol Sci. 2015;16:15425–15441. doi: 10.3390/ijms160715425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nacsa-Farkas E, Kerekes E, Kerekes EB, Krisch J, Popescu R, Vlad DC, Ivan P, Vágvölgyi C. Antifungal effect of selected European herbs against Candida albicans and emerging pathogenic non-albicans Candida species. Acta Biol Szeged. 2014;58:61–64. [Google Scholar]
- 3.Tiulea C, Peev C, Brezovan D, Dehelean D, Motoc A. A comparison regarding antiproliferative action between soy total extract and genistein. Rom J Morphol Embryol. 2011;52(3 Suppl):S1065–S1069. [PubMed] [Google Scholar]
- 4.Stanojević D, Čomić L, Stefanović O, Solujić S, Sukdolak S. In vitro synergistic antibacterial activity of Melissa officinalis L. and some preservatives. Span J Agric Res. 2010;8:109–115. doi: 10.5424/sjar/2010081-1149. [DOI] [Google Scholar]
- 5.Pop A, Muste S, Muresan C, Pop C, Salanta L. Comparative study regarding the importance of sage (Salvia officinalis L.) in terms of antioxidant capacity and antimicrobial activities. Hop Med Plants. 2013;21:1–2. [Google Scholar]
- 6.Kamdem JP, Adeniran A, Boligon AA, Klimaczewski CV, Elekofehinti OO, Hassan W, Ibrahim M, Waczuk EM, Meinerz DF, Athayde ML. Antioxidant activity, genotoxicity and cytotoxicity evaluation of lemon balm (Melissa officinalis L.) ethanolic extract: Its potential role in neuroprotection. Ind Crops Prod. 2013;51:26–34. doi: 10.1016/j.indcrop.2013.08.056. [DOI] [Google Scholar]
- 7.Lin JT, Chen YC, Lee YC, Hou Rolis CW, Chen FL, Yang DJ. Antioxidant, anti-proliferative and cyclooxygenase-2 inhibitory activities of ethanolic extracts from lemon balm (Melissa officinalis L.) leaves. LWT-Food Sci Technol. 2012;49:1–7. doi: 10.1016/j.lwt.2012.04.009. [DOI] [Google Scholar]
- 8.Stević T, Berić T, Šavikin M, Soković M, Gođevac I, Dimkić I, Stanković S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind Crops Prod. 2014;55:116–122. doi: 10.1016/j.indcrop.2014.02.011. [DOI] [Google Scholar]
- 9.de Sousa AC, Alviano DS, Blank AF, Alves PB, Alviano CS, Gattass CR. Melissa officinalis L. essential oil: Antitumoral and antioxidant activities. J Pharm Pharmacol. 2004;56:677–681. doi: 10.1211/0022357023321. [DOI] [PubMed] [Google Scholar]
- 10.De P, Baltas M, Bedos-Belval F. Cinnamic acid derivatives as anticancer agents-a review. Curr Med Chem. 2012;18:1672–1703. doi: 10.2174/092986711795471347. [DOI] [PubMed] [Google Scholar]
- 11.www.cancer.org/cancer/melanoma-skin-cancer/about/key-statitics.html AmericanCancer Society: Key statistics for melanoma skin cancer. [Google Scholar]
- 12.Weir HK, Marrett LD, Cokkinides V, Barnholtz-Sloan J, Patel P, Tai E, Jemal A, Li J, Kim J, Ekwueme DU. Melanoma in adolescents and young adults (ages 15–39 years): United States, 1999–2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S38–S49. doi: 10.1016/j.jaad.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop JN, Harland M, Randerson-Moor J, Bishop DT. Management of familial melanoma. Lancet Oncol. 2007;8:46–54. doi: 10.1016/S1470-2045(06)71010-5. [DOI] [PubMed] [Google Scholar]
- 14.Russo A, Lombardo L, Troncoso N, Garbarino J, Cardile V. Rosmarinus officinalis extract inhibits human melanoma cell growth. Nat Prod Comm. 2009;4:1707–1710. [PubMed] [Google Scholar]
- 15.Cattaneo L, Cicconi R, Mignogna G, Giorgi A, Mattei M, Graziani G, Ferracane R, Grosso A, Aducci P, Schininà ME, Marra M. Anti-proliferative effect of Rosmarinus officinalis L. extract on human melanoma A375 cells. PLoS One. 2015;10:e0132439. doi: 10.1371/journal.pone.0132439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culley TM. Why vouchers matter in botanical research. Appl Plant Sci. 2013;1 doi: 10.3732/apps.1300076. pii: apps.1300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 19.Mohamadi S, Kiarostami K, Bokaii Nazem Z. The study of antioxidant property of metanolic extracts of Melissa officinalis L. and Salvia officinalis L. on stability of soybean oil. J Agroaliment Proc Technol. 2014;20:293–297. [Google Scholar]
- 20.Spiridon I, Bodirlau R, Teaca CA. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent Eur J Biol. 2011;6:388–396. [Google Scholar]
- 21.Rasmy NM, Hassan AA, Foda MI, El-Moghazy MM. Assessment of the antioxidant activity of sage (Salvia officinalis L.) extracts on the shelf life of mayonnaise. World J Dairy Food Sci. 2012;7:28–40. [Google Scholar]
- 22.Veličković DT, Karabegović IT, Stojicevic SS, Lazić ML, Marinkovic VD, Veljkovic VB. Comparison of antioxidant and antimicrobial activities of extracts obtained from Salvia glutinosa L. and Salvia officinalis. Hem Ind. 2011;65:599–605. doi: 10.2298/HEMIND110412034V. [DOI] [Google Scholar]
- 23.Bejeli M, Rowshan V, Zakerin A. Comparison of total phenolic content and antioxidant activity of five salvia species by FRAP and DPPH assay. Int J Pharm Pharm Sci. 2012;4 [Google Scholar]
- 24.Derakhshani Z, Hassani A, Pirzad A, Abdollahi R, Dalkani M. Evaluation of phenolic content and antioxidant capacity in some medicinal herbs cultivated in Iran. Bot Serb. 2012;36:117–122. [Google Scholar]
- 25.Kontogianni VG, Tomic G, Nikolic I, Nerantzaki AA, Sayyad N, Stosic-Grujicic S, Stojanovic I, Gerothanassis IP, Tzakos AG. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013;136:120–129. doi: 10.1016/j.foodchem.2012.07.091. [DOI] [PubMed] [Google Scholar]
- 26.Shang YJ, Liu BY, Zhao MM. Details of the antioxidant mechanism of hydroxycinnamic Acids. Czech J Food Sci. 2015;33:210–216. doi: 10.17221/611/2014-CJFS. [DOI] [Google Scholar]
- 27.Vladimir-Knežević S, Blažeković B, Kindl M, Vladić J, Lower-Nedza AD, Brantner AH. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules. 2014;19:767–782. doi: 10.3390/molecules19010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Štefan MB, Rodríguez JV, Blažeković B, Kindl M. Total hydroxycinnamic acids assay: Prevalidation and application on Lamiaceae species. Food Anal Method. 2013;6:1–117. [Google Scholar]
- 29.Kiani MH, Mokhtari A, Zeinali H, Abbasnejad A. Rosmarinic acid and anthocyanin content improvement by foliar application of Fe and Zn fertilizer in Lemon balm (Melissa officinalis L.) IJABBR. 2014;2:1525–1530. [Google Scholar]
- 30.Oniga I, Vlase L, Toiu A, Benedec D, Duda M. Evaluation of phenolic acid derivatives and essential oil content in some Melissa officinalis L. varieties. Farmacia. 2010;58:764–769. [Google Scholar]
- 31.Proestos C, Komaitis M. Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC coupled to diode array detector (DAD) and GC-MS after Silylation. Foods. 2013;2:90–99. doi: 10.3390/foods2010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baciu A, Ranga F, Fetea F, Zagrean F, Socaciu C. Fingerprint and quantification of phenolic dervatives in Melissa off. and Calendula off. extracts in relation to their antioxidant potential. Hop Med Plants. 2012;20:80–86. [Google Scholar]
- 33.Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- 34.Hanganu D, Vlase L, Filip L, Sand C. The study of some phenolic compounds from Melissa officinalis. Rev Med Chir Soc Med Nat. 2008;2:523–527. [PubMed] [Google Scholar]
- 35.Miron LT, Herrero M, Ibáñez E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J Chromatogr A. 2013;1288:1–9. doi: 10.1016/j.chroma.2013.02.075. [DOI] [PubMed] [Google Scholar]
- 36.Pavel IZ, Danciu C, Oprean C, Dehelean CA, Muntean D, Csuk R, Danina MM. In vitro evaluation of the antimicrobial ability and cytotoxicity on two melanoma cell lines of a benzylamide derivative of maslinic acid. Anal Cell Pathol (Amst) 2016;2016:2787623. doi: 10.1155/2016/2787623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danciu C, Borcan F, Soica C, Zupko I, Csányi E, Ambrus R, Muntean D, Sass C, Antal D, Toma C, Dehelean C. Polyurethane microstructures-a good or bad in vitro partner for the isoflavone genistein? Nat Prod Commun. 2015;10:951–954. [PubMed] [Google Scholar]
- 38.Oprean C, Zambori C, Borcan F, Soica C, Zupko I, Minorics R, Bojin F, Ambrus R, Muntean D, Danciu C, et al. Anti-proliferative and antibacterial in vitro evaluation of the polyurethane nanostructures incorporating pentacyclic triterpenes. Pharm Biol. 2016;54:2714–2722. doi: 10.1080/13880209.2016.1180538. [DOI] [PubMed] [Google Scholar]
- 39.Slobodníková L, Fialová S, Hupková H, Grancai D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat Prod Comm. 2013;8:1747–1750. [PubMed] [Google Scholar]
- 40.Hossain F, Follett P, Vu Dang K, Harich M, Salmieri S, Lacroix M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2015;53:24–30. doi: 10.1016/j.fm.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappaB and c-Jun. Biochem Pharmacol. 2005;69:22132. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]