Abstract
Insulin resistance is associated with impaired endothelial regeneration in response to mechanical injury. We recently demonstrated that insulinlike growth factor–binding protein-1 (IGFBP1) ameliorated insulin resistance and increased nitric oxide generation in the endothelium. In this study, we hypothesized that IGFBP1 would improve endothelial regeneration and restore endothelial reparative functions in the setting of insulin resistance. In male mice heterozygous for deletion of insulin receptors, endothelial regeneration after femoral artery wire injury was enhanced by transgenic expression of human IGFBP1 (hIGFBP1). This was not explained by altered abundance of circulating myeloid angiogenic cells. Incubation of human endothelial cells with hIGFBP1 increased integrin expression and enhanced their ability to adhere to and repopulate denuded human saphenous vein ex vivo. In vitro, induction of insulin resistance by tumor necrosis factor α (TNFα) significantly inhibited endothelial cell migration and proliferation. Coincubation with hIGFBP1 restored endothelial migratory and proliferative capacity. At the molecular level, hIGFBP1 induced phosphorylation of focal adhesion kinase, activated RhoA and modulated TNFα-induced actin fiber anisotropy. Collectively, the effects of hIGFBP1 on endothelial cell responses and acceleration of endothelial regeneration in mice indicate that manipulating IGFBP1 could be exploited as a putative strategy to improve endothelial repair in the setting of insulin resistance.
IGFBP1 ameliorated insulin resistance–induced defects in re-endothelialization in vivo and impairment of endothelial migration and proliferation in vitro via FAK and RhoA activation.
Functional and structural integrity of the endothelial monolayer plays a critical role in vascular homeostasis. Damage to the endothelium by exposure to vascular risk factors or mechanical trauma predisposes to a range of abnormalities, including atherosclerosis (1), bypass graft failure (2), restenosis (3), and stent thrombosis (4). Regeneration of damaged endothelium following injury is essential to prevent adverse remodeling and is mediated by two broad mechanisms: proliferation and migration of local endothelial cells (5, 6) and recruitment of circulating cells to the injured vessel (7). The latter include endothelial colony-forming cells, which are fully committed to the endothelial lineage and can form mature vascular networks, and myeloid angiogenic cells (MACs), which exhibit a macrophage/monocyte-like phenotype and contribute to endothelial repair through the secretion of proangiogenic cytokines (8).
Type 2 diabetes mellitus is associated with both dysfunctional vascular endothelial regeneration and a high risk for cardiovascular events. Insulin resistance has emerged as a major player in diabetes-related vasculopathy, not least through its strong association with endothelial dysfunction (9). Although diabetes is strongly associated with defective vascular repair, we identified that insulin resistance per se is sufficient to jeopardize endothelial regeneration after arterial injury (10). Endothelial regeneration following mechanical wire–induced arterial injury was impaired in mice heterozygous for deletion of the insulin receptor (IR+/−)—explained at least in part through reduced mobilization of MACs (10). Recognition of the adverse effect of insulin resistance on endothelial repair processes led us to question whether insulin sensitization might enhance endothelial regeneration in the setting of insulin resistance.
Insulinlike growth factor–binding protein 1 (IGFBP1) is one of a family of circulating proteins that confer spatial and temporal regulation of insulinlike growth factor (IGF) bioavailability but that can also orchestrate cellular responses independent of their modulation of IGF actions (11). At the structural level, IGF-independent actions of IGFBP1 have been ascribed to an Arg-Gly-Asp (RGD) motif within its C-terminal domain, which can interact with cell surface integrins and promote migratory responses in certain cell types (12, 13). However, potential effects of IGFBP1 on migratory responses have not previously been studied in endothelial cells.
From the functional perspective, an inhibitory effect of insulin on hepatic IGFBP1 synthesis has led to IGFBP1 being implicated in glucose regulation (14). The circulating concentration of IGFBP1 has been proposed as a biomarker of insulin sensitivity (15, 16). In epidemiological studies, low plasma IGFBP1 concentrations have been strongly predictive of the prospective development of type 2 diabetes (17–19). We recently identified direct actions of the RGD domain of IGFBP1 in augmenting insulin signaling and insulin-stimulated glucose uptake (20). Human studies also indicate a link between low circulating IGFBP1 concentration and risk for cardiovascular disease (16, 21). Conversely, in the setting of acute myocardial infarction, IGFBP1 levels predict mortality; however, the effect may be confounded by association with elevated levels of copeptin (22, 23).
In preclinical studies, we have demonstrated that IGFBP1 plays a favorable role in both insulin sensitivity and vascular function (24). Transgenic expression of human IGFBP1 in mice was associated with whole-body and vascular insulin sensitization, increased basal nitric oxide (NO) bioavailability, lower blood pressure, and reduced susceptibility to atherosclerosis (24).
Here we hypothesized that increasing the concentration of IGFBP1 would ameliorate the detrimental effects of insulin resistance on endothelial repair. To investigate this, we assessed endothelial regeneration in IR+/− mice expressing human IGFBP1 (hIGFBP1) subjected to arterial injury and evaluated the effects of hIGFBP1 on the functional properties of endothelial cells in vitro.
Materials and Methods
Chemicals and antibodies
The antibodies used for immunoblotting are listed in Table 1. Chemicals were purchased from Sigma Chemical/Sigma-Aldrich (St. Louis, MO), unless otherwise specified. Human IGFBP1 and IGF-1 were purchased from GroPep (Adelaide, Australia). Recombinant hIGFBP1 was expressed in Expi293F cells (Life Technologies, Carlsbad, CA) by using the SUMOStar expression system (LifeSensors, Malvern, PA) and purified as described previously (20). Site-directed mutagenesis of the hIGFBP-1expressing plasmid was performed by using the QuikChange Lightning kit (Agilent Technologies) using primers 5′-TCCAGAGATCTGGGGAGACCC-3′ and 5′-GGGTCTCCCCAGATCTCTGGA-3′ to generate the Trp-Gly-Asp (WGD) hIGFBP1 expression construct.
Table 1.
List of Antibodies Used, Manufacturer, Catalog Number, and Research Resource Identifier (RRID)
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No. | RRID | Species Raised, Monoclonal or Polyclonal | Dilution |
|---|---|---|---|---|---|
| Sca-1 | FITC rat anti-mouse Ly-6A/E | BD Pharmingen, 557405 | AB_396688 | Rat, monoclonal | 1:5000 |
| Rat IgG2a isotype control | FITC rat IgG2a, κ isotype control | BD Pharmingen, 553929 | AB_395144 | Rat, monoclonal | 1:5000 |
| Flk-1 | PE rat anti-mouse Flk-1 | BD Pharmingen, 561052 | AB_2034023 | Rat, monoclonal | 1:5000 |
| Rat IgG2a isotype control | PE rat IgG2a, κ isotype control | BD Pharmingen, 553930 | AB_479719 | Rat, monoclonal | 1:5000 |
| CD16/CD32 | Mouse BD Fc block | BD Pharmingen, 553152 | AB_398533 | Rat, monoclonal | 1:10 |
| Akt | Akt | Cell Signaling, 9272 | AB_329827 | Rabbit, polyclonal | 1:1000 |
| pAkt | Phospho-Akt (Ser473) (D9E) XP | Cell Signaling, 4060 | AB_2315049 | Rabbit, monoclonal | 1:2000 |
| FAK | FAK | Cell Signaling, 3285 | AB_2269034 | Rabbit, polyclonal | 1:20 000 |
| pFAK | Phospho-FAK (Tyr397) (D20B1) | Cell Signaling, 8556 | AB_10891442 | Rabbit, monoclonal | 1:2000 |
| β-Actin | β-Actin antibody (C4) | Santa Cruz, Sc-47778 | AB_626632 | Mouse, monoclonal | 1:1000 |
Abbreviations: pAKT, phospho Akt; pFAK, phospho focal adhesion kinase.
Animals
IR+/− mice (25) were bred in-house from founder animals originating from the Medical Research Council Mammalian Genetics Unit (Harwell, Oxfordshire, United Kingdom). hIGFBP1 transgenic (tg) mice were originally generated by Crossey et al. (26) at King’s College London and subsequently backcrossed to a C57BL/6J background for multiple generations. IR+/− and hIGFBP1 mice were intercrossed to generate IR+/−hIGFBP1tg mice. Animals were maintained as heterozygotes on a C57BL/6 background in a conventional animal facility with a 12-hour light/dark cycle and received a standard laboratory diet. Male wild-type, IR+/−, hIGFBP1tg, and IR+/−hIGFBP1tg littermate mice (aged 12 to 16 weeks) were compared. Genotyping was performed by using polymerase chain reaction on ear notch genomic DNA, with the primers described previously (24, 27). All procedures were approved by the Animal Welfare and Ethical Review Committee at the University of Leeds and were carried out in accordance with the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012.
Plasma IGFBP1 concentration
Circulating concentration of IGFBP1 was measured in plasma of nonfasted animals using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (IGFBP1 ELISA kit ab100539, Abcam, Cambridge, United Kingdom).
Vascular injury
Mice were anesthetized with isoflurane (2.5% to 5%) before a small incision was made in the midthigh to permit isolation of the femoral artery (28). After arteriotomy made by using iris scissors (World-Precision Instruments, Sarasota, FL), a 0.014-inch-diameter angioplasty guide wire with tapered tip (Hi-torque Cross-it XT, Abbott-Vascular, Abbott, IL), was introduced. The angioplasty guide wire was advanced 3 cm, and three passages were performed per mouse, resulting in complete arterial denudation. The guide wire was removed and the suture was tightened rapidly. The vessel was then ligated, and the skin was closed with a continuous suture. The contralateral artery underwent an identical sham operation, without passage of the wire. Animals received postoperative analgesia with buprenorphine (0.25 mg/kg).
Assessment of endothelial regeneration by en face microscopy
Mice were anesthetized 5 days after wire injury, and 50 μL of 0.5% Evans blue dye was injected into the inferior vena cava. The mice were perfused/fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) before the femoral arteries (injured and uninjured) were harvested. The vessels were opened longitudinally. The areas stained and unstained in blue were measured in a 5-mm injured segment beginning 5 mm distal to the aortic bifurcation, and the percentage areas were calculated by using ImageProPlus 7.0 software (Media Cybernetics, Bethesda, MD).
Mononuclear cell isolation and culture
Isolation of mononuclear cells (MNCs) from 1 mL of blood, obtained from the vena cava under terminal anesthesia, was done by using Histopaque-1083 (Sigma-Aldrich Gillingham, Dorset, UK) density gradient centrifugation. MNCs were seeded on fibronectin 24-well plates (BD Biosciences, Wokingham, Berkshire, UK) at a density of 1 × 106 cells/well. Cells were cultured in endothelial cell growth medium 2 (EGM-2) medium supplemented with EGM-2 Bullet kit (Lonza, Basel, Switzerland) in addition to 20% fetal calf serum (FCS).
Spleens obtained from mice under terminal anesthesia were minced mechanically. MNCs were isolated by density gradient centrifugation, as described above. After washing steps, cells were seeded on fibronectin 24-well plates at a seeding density of 8 × 106 cells/well and cultured as described above.
Tibias and femurs were flushed three times in Dulbecco’s modified Eagle medium with a 26-gauge needle to collect bone marrow. MNCs were isolated by density gradient centrifugation as described above. After washing steps, cells were seeded on fibronectin 24-well plates at a seeding density of 1 × 106 cells/well and cultured as described above.
MAC characterization
After 4 days' incubation at 37°C in 5% CO2, gentle washing with PBS discarded nonadherent cells and adherent cells were resuspended in medium. At day 7, attached cells from peripheral blood, spleen, and bone marrow were stained for the uptake of 1,1′-dioctadecy-3,3,3′,3′-tetramethyllindocarbocyanine–labeled acetylated low-density lipoprotein (DiI-Ac-LDL) (Molecular Probes, Invitrogen, Carlsbad, CA) and lectin from Ulex europaeus fluorescein isothiocyanate (FITC) conjugate (Sigma-Aldrich). Cells were first incubated with DiI-Ac-LDL at 37°C for 3 hours and later fixed with 4% paraformaldehyde for 10 minutes. Cells were washed and reacted with lectin for 1 hour. After staining, cells were quantified by examining 10 random high-power fields (HPFs) and double-positive cells were identified as MACs.
MAC function: adhesion assay
To assess adhesion, 50,000 MACs were resuspended in EGM-2 medium, plated onto 24-well plates coated with indicated substrates, and incubated for 1 hour at 37°C. After washing three times with PBS, attached cells were counted. Adhesion was evaluated as the mean number of attached cells per HPF (×100).
Fluorescence-activated cell sorter enumeration of Sca-1/Flk-1 cells
Murine saphenous vein blood samples (100 µL) were incubated with PharmLyse (BD Biosciences, San Jose CA) at room temperature. After centrifugation, mononuclear cells (MNCs) were resuspended in fluorescence-activated cell sorter buffer and incubated with FcR blocker (BD Biosciences) at 4°C. As per protocol, appropriate volumes of the antibodies, or their respective isotype controls, were added for 10 minutes at 4°C: FITC anti-mouse Sca-1 and phycoerythrin (PE) anti-mouse Flk-1 (BD Biosciences). MACs were enumerated by using flow cytometry (FACS Calibur, BD Biosciences) to quantify dual-stained Sca-1/Flk-1 cells. Isotype control specimens were used to define the threshold for antigen presence and to subtract nonspecific fluorescence. The cytometer was set to acquire 100,000 events within the lymphocyte gate, defined by typical light scatter properties.
Ex vivo saphenous vein adhesion assay
Saphenous vein segments were obtained with informed consent from patients undergoing coronary artery bypass graft surgery at Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, after ethical approval. Human coronary artery endothelial cells (HCAECs) (Promocell, Heidelberg, Germany) stained with CellTracker CM-Dil (Invitrogen, Eugene, OR) were incubated for 1 hour at a fixed density (250,000 cells/mL) in full (20%) endothelial cell growth medium (ECGM) suspension with control vehicle or hIGFBP1 (500 ng/mL) (GroPep, Adelaide, Australia). After 1 hour, 50,000 cells from each treatment group were seeded onto a denuded segment of human saphenous vein and incubated for 5 minutes. After 5 minutes, the cell suspension was gently washed with PBS; Hoechst stain was then added to the saphenous vein segment, and the segment was finally resuspended in full (20%) ECGM. Images were obtained by confocal microscopy of the vein segments, with assessment of the number of cells adhering to the saphenous vein matrix.
Endothelial cell adhesion assays
The potential for hIGFBP1 to modulate adhesion was investigated by using adhesion assays performed with HCAECs. HCAEC suspensions (100,000 cells/mL) were seeded onto sterile glass coverslips. Vehicle-treated wells contained 1% FCS and treatment wells contained hIGFBP1 (500 ng/mL in 1% FCS). Adherent cells in one vehicle-treated well and one corresponding IGFBP-1 well were fixed after 2 hours, 4 hours, and 6 hours of incubation at 37°C in 4% paraformaldehyde and stained with hematoxylin and eosin for 1 minute. Finally, the coverslips were mounted onto microscope slides using glycerol gelatin, and 10 random fields containing the adherent cells were counted at ×400 magnification.
To investigate the potential for hIGFBP1 to modulate adhesion to individual extracellular matrix components, human umbilical vein endothelial cells (HUVECs) were seeded at 50,000 cells per well of 24-well plates coated with fibronectin (Corning, Corning, NY 354411), collagen I (Thermo Fisher Scientific, Waltham, MA, A1142802), collagen IV (Corning, 734-0127), or vitronectin (coated in house by using C395 (Novoprotein, Summit, NJ) at 1 µg/well) and incubated for 30 minutes at 37°C. Cells were washed once with PBS and attached cells were counted. Adhesion was evaluated as the mean number of attached cells per HPF (×40). The involvement of focal adhesion kinase (FAK) was investigated by using the FAK inhibitor PZ0117 (Sigma-Aldrich; 100 nmol/L).
Integrin-mediated cell adhesion
Cell surface subunit or heterodimer integrins were quantified by using an integrin-mediated cell adhesion array kit (ECM532, Millipore). HCAECs were grown to confluence in T-75 flasks. Once confluent, cells were harvested by using Gibco® Cell Dissociation Buffer. HCAECs were coincubated with or without hIGFBP1 (500 ng/mL) for 1 hour before 100,000 cells were added to the integrin antibody-coated and control wells and incubated for 2 hours at 37°C. Unbound cells were then washed off and the adherent cells stained. The optical density of nuclear stain extracts was measured at 540 nm (optical density, 540 nm) on an MRX TC 2 microplate reader (Dynex Technologies, Worthing, United Kingdom).
Migration assays
Migration of HCAECs and HUVECs was investigated in 12-well plates using a modification of a "scratch wound" method. Briefly, duplicate scratches were made with a sterile 1-mL pipette tip in confluent endothelial monolayers [having been quiesced in medium containing 1% fetal bovine serum (FBS) for 16 hours], reference points were etched in the dishes, and images were captured (0 hours). Cells were then exposed to hIGFBP1 (500 ng/mL), tumor necrosis factor α (TNFα) (10 ng/mL), or both in combination with 10% FBS endothelial cell growth medium in a tissue-culture incubator for an additional 48 hours. Further images were then captured by aligning the dishes with the reference point made at time 0 hours, and images were acquired at 24 hours and 48 hours. Quantification was achieved by counting the number of cells that had migrated beyond a fixed distance from the initial wound edge.
Endothelial cell migration was also studied by using a modified Boyden chamber technique, as we have described previously (29). HCAECs or HUVECs (100,000) were loaded in the upper chamber in medium supplemented with 20% FBS. The lower chamber contained 20% FBS with hIGFBP1 (500 ng/mL) or vascular endothelial growth factor (VEGF) (50 ng/mL). After incubation for 6 hours at 37°C in a tissue-culture incubator, duplicate membranes were processed and evaluated by counting migrated cells on the underside of the membrane in 10 random fields under high-power (×400) light microscopy.
Cell proliferation assays
HCAEC and HUVEC proliferation assays were performed by seeding cells in 24-well culture plates at a density of 20,000 cells per well in full endothelial growth medium (20% FBS). After 30 to 32 hours, incubated cells were quiesced in medium containing 1% FBS for 16 hours. Cells were then exposed to control growth medium (20% FBS) and hIGFBP1 (500 ng/mL), TNFα (0.1 ng/mL), or both in combination. Medium and chemicals were replaced on days 2 and 4, and viable cell number was determined in triplicate wells on day 5 by using trypan blue and a hemocytometer. In additional experiments, proliferation was assessed in HUVECs by an 5-ethynyl-2′-deoxyuridine (EdU) kit in accordance with the manufacturer’s instructions (Click-iT EdU Alexa Fluor 488 Imaging Kit; C10337; Invitrogen). The proliferative response to hIGFBP1 (500 ng/mL) with or without IGF-1 (18 nmol/L) (GroPep) were studied. Involvement of FAK was investigated by using the FAK inhibitor PZ0117 (Sigma-Aldrich; 100 nmol/L).
Western blotting for phospho-FAK and phospho-Akt
HUVECs after 4 hours of serum starvation were incubated with or without hIGFBP1 or WGD-hIGFBP1 (500 ng/mL) for 15 minutes. Protein was extracted in lysis buffer and quantified by using the protein bicinchoninic acid assay (Sigma-Aldrich). Then, 30 µg of protein were separated by electrophoresis through 4%–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (Invitrogen Life Technologies, Carlsbad, CA) and blotted onto polyvinylidene fluoride membranes. Immunoblots were performed as previously described (30) by using antibodies listed in Table 1. Inhibition by TNFα of insulin-stimulated Akt phosphorylation was determined in HUVECs. Cells were incubated with TNFα (10 ng/mL) for the indicated durations (30 to 120 minutes) and then stimulated with insulin (100 nmol/L for 15 minutes) to assess the effects of TNFα on insulin-induced Akt phosphorylation.
RhoA activity assay
HCAECs were seeded at 100,000 cells/well into six-well plates. On reaching 80% confluence, cells were serum starved overnight and then treated with hIGFBP1 for the following times: 0 minutes, 10 minutes, 20 minutes, and 40 minutes. After treatment, the medium was aspirated and washed thrice with ice-cold PBS, with care taken to remove all residual PBS. Cells were then lysed with 120 µL of ice-cold lysis buffer (1:100 of protease inhibitor:lysis buffer), harvested and transferred into microcentrifuge tubes on ice. The samples were centrifuged at 10,000g, 4°C for 2 minutes. Twenty microliters of lysate was taken off and stored at 4°C for protein quantification; the remainder was used to assess RhoA activity assay by the RhoA G-LISA kit (Cytoskeleton, Inc., Denver, CO) according to the manufacturer’s instructions.
Endothelial cell actin fiber anisotropy assessment
HCAECs were seeded at 12,000 cells/well in gelatin-coated Lab-Tek (Brendale, Australia) eight-well chamber slides, then grown for 48 hours in ECGM/20% FCS. Subsequently, cells were incubated for 24 hours in ECGM/10% FCS alone or with hIGFBP1 (500 ng/mL), TNFα (10 ng/mL; PeproTech, Rocky Hill, NJ) or both proteins. Before fixation, cells were washed once with PBS at 37°C and then treated with 3% paraformaldehyde in PBS (warmed to 37°C) for 20 minutes. To remove unreacted paraformaldehyde, cells were washed three times with PBS, incubated for 10 minutes with 50 mM NH4Cl in PBS, then washed three additional times with PBS. Before staining, cells were permeabilized for 4 minutes with 0.2% Triton X 100 in PBS followed by three PBS washes, then blocked for 30 minutes with 0.2% fish skin gelatin (FSG) in PBS (FSG/PBS). Actin filaments were stained with FITC-phalloidin (Enzo Life Science, Farmingdale, NY) at 1.5 μg/mL final concentration in FSG/PBS for 1 hour, followed by three washes with FSG/PBS, one wash in PBS, and one wash in deionized water. Cells were mounted with Duolink In Situ Mounting Medium with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) and actin filaments, and cell nuclei were imaged on Delta Vision widefield deconvolution system (Applied Precision, Issaquah, WA), and Zeiss LSM 700 laser-scanning confocal microscopes. On the Delta Vision microscope, images were acquired with a ×40 1.35–numerical aperture (NA) oil objective at 0.2-µm z-intervals, and 1024 × 1024–pixel resolution images were processed with 10 cycles of deconvolution (conservative model) before generation of an 8-bit maximum-intensity projection for analysis. On the LSM 700 microscope, images were acquired with a ×40 1.3-numerical aperture oil objective and were scanned at 1024 × 1024–pixel resolution at 8 bits per pixel. Optical section thickness was set at 1 Airy unit and z-step at 0.48 µm.
The Fibril Tool plug-in for ImageJ software (National Institutes of Health, Bethesda, MD) (31) was used to analyze the anisotropy of actin fibers in FITC-phalloidin images. Whole cells with intact nuclei were included in the analysis. Cells were subdivided into two to eight subregions (corresponding to major fiber cluster alignments and avoiding the nucleus and saturated areas) by using the polygon tool, and anisotropy for each cell was calculated as an area-weighted average of subregions.
Data analysis
Results are expressed as mean ± standard error of the mean. Data were demonstrated to be normally distributed by using the Shapiro-Wilk test. Comparisons within groups were made by using paired Student t tests and between groups by using unpaired Student t tests or repeated-measures analysis of variance with post hoc Newman-Keuls tests, as appropriate. A P value < 0.05 was considered to indicate a statistically significant difference.
Results
Transgenic expression of hIGFBP1 ameliorates the detrimental effects of insulin resistance on endothelial regeneration
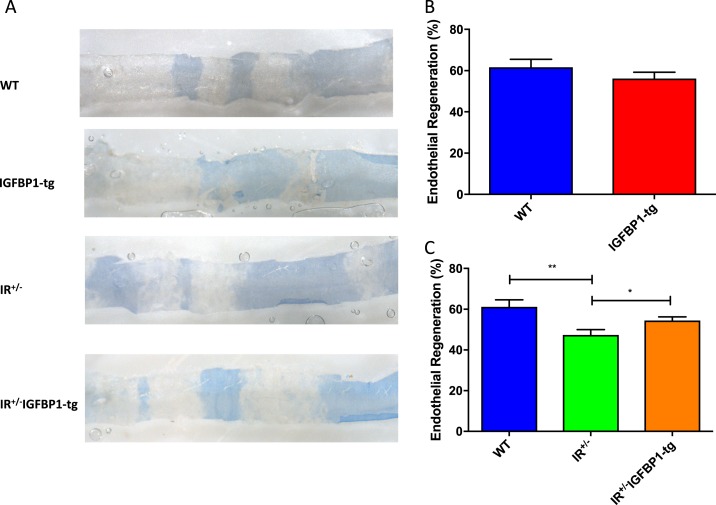
Endothelial regeneration was quantified in murine femoral arteries 5 days after wire-induced arterial injury, which we determined as the time point at which re-endothelialization was maximally impaired in insulin-resistant mice (10). Endothelial regeneration did not differ between hIGFBP1tg and wild-type (WT) animals (Fig. 1A and 1B). Because whole-body metabolic phenotype is not altered in hIGFBP1tg mice (24), this indicates that increasing hIGFBP1 does not alter endothelial regeneration in metabolically normal animals. In contrast, impaired endothelial regeneration observed in IR+/− mice was significantly improved by overexpression of hIGFBP1 (Fig. 1A and 1C). Plasma concentration of IGFBP1 was significantly increased in hIGFBP1tg mice and was similarly increased in insulin-resistant IR+/− mice overexpressing hIGFBP1 (Supplemental Fig. 1 (458.2KB, docx) ).
Figure 1.
Endothelial regeneration after wire injury of the femoral artery. IGFBP-1 rescues endothelial regeneration in insulin-resistant mice. (A) Representative in situ Evans blue staining 5 days after vascular injury (blue staining indicates denuded endothelium) in WT, IGFBP1-tg, IR+/− and IR+/− IGFBP1-tg mice (magnification ×20). (B) Endothelial regeneration 5 days after vascular injury in WT and IGFBP-1tg mice (n = 7 mice per group). No significant difference was seen between WT and IGFBP-1tg mice. (C) Endothelial regeneration 5 days after vascular wire injury in WT, IR+/− and IR+/−IGFBP1-tg mice (n = 5 to 10 per group). Data shown are mean +/− SEM. *P < 0.05; **P < 0.01.
Enhanced endothelial regeneration in hIGFBP1-expressing insulin resistant mice is not attributable to changes in abundance or function of circulating angiogenic progenitor cells
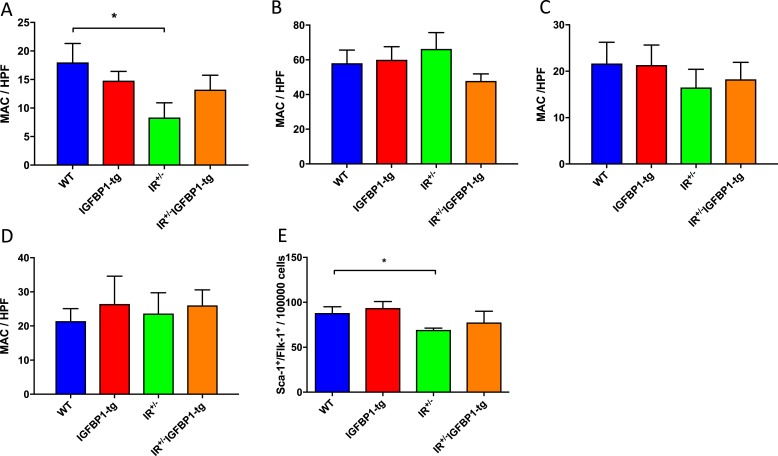
Endothelial regeneration following mechanical injury is accomplished by an orchestrated cellular response comprising the proliferation and migration of vessel-wall resident endothelial cells and the recruitment of circulating cells with angiogenic potential (7). We have previously reported that impaired endothelial regeneration in IR+/− mice is associated with reduced abundance of circulating MACs and decreased mobilization of Sca-1+/Flk-1+cells from bone marrow (10). We therefore investigated whether changes in abundance of MACs were responsible for enhanced endothelial regeneration in mice expressing hIGFBP1 in the current study. We found no difference between WT and hIGFBP1tg mice in abundance of blood-derived MACs (Fig. 2A). The yield of circulating MACs was reduced in IR+/− mice but was not significantly modified by the expression of hIGFBP1 (Fig. 2A). The yield of MACs from bone marrow and spleen was similar in all groups of mice (Fig. 2B and 2C). Adhesion of MACs to fibronectin-coated plates was uninfluenced by genotype (Fig. 2D). The abundance of Sca-1+/Flk-1+ cells in the MNC fraction of blood was measured by flow cytometry and was similar in all groups of mice (Fig. 2E).
Figure 2.
Progenitor cell abundance and function. (A–C) Enumeration of MACs derived from blood, spleen, and bone marrow by cell culture after 7 days. Numbers of (A) peripheral blood–derived (n = 5 to 6), (B) spleen-derived (n = 6), and (C) bone marrow–derived (n = 6 to 9) cultured MACs from uninjured mice are shown. *P < 0.05. (D) Adhesion capacity of spleen-derived MACs expressed as number of cells adhering to fibronectin-coated plates (n = 5 to 6). No significant difference between groups was observed. (E) Enumeration of circulating Sca-1+/Flk-1+ cells. Number of Sca-1+/Flk-1+ cells was quantified in peripheral blood by flow cytometry. *P < 0.05. (n = 6). Data shown are mean +/− SEM.
Acute exposure to hIGFBP1 increases adherence of human endothelial cells to human vessels and upregulates availability of integrins
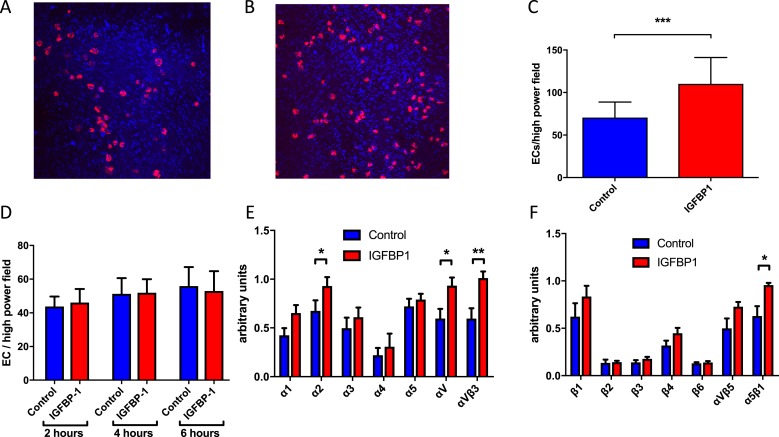
To examine whether hIGFBP1 directly modulates the reparative function of native endothelial cells, we first examined the effects of short-term incubation with hIGFBP1 on the adhesive properties of endothelial cells. We investigated the ability of HCAECs to adhere to endothelium-denuded segments of human saphenous vein. We found that HCAECs were adherent to denuded saphenous vein after 5 minutes (Fig. 3A). Preincubation with hIGFBP1 (500 ng/mL; 1 hour) resulted in a significant increase in the number of adherent cells (Fig. 3B and 3C). In contrast to the modulatory effect of hIGFBP1 on adhesion to a denuded vessel, incubation with hIGFBP1 had no effect on adhesion of HCAECs to uncoated glass coverslips (Fig. 3D). Because adhesion of endothelial cells to extracellular matrix is critically dependent on the interaction of matrix components (e.g., fibronectin) with cell surface integrins (32), we quantified functional integrin abundance by using an integrin-mediated cell adhesion array kit. We found that incubation of HCAECs with hIGFBP1 (500 ng/mL; 1 hour) led to a significant increase in the cell surface abundance of α2 and αV integrin subunits and of αVβ3 and α5β1 integrins (Fig. 3E and 3F). To further characterize the upregulation of endothelial cell adhesion by hIGFBP1, we investigated the effects of hIGFBP1 on adherence of HUVECs to individual extracellular matrix (ECM) components in vitro. There was a trend toward increased adhesion on several matrices, but we were not able to identify a dominant ECM constituent to which hIGFBP1 preferentially increased adhesion (Supplemental Fig. 2 (458.2KB, docx) ).
Figure 3.
hIGFBP1 improves adhesion of human endothelial cells to denuded human saphenous vein and upregulates cell-surface integrins. (A and B) Representative images of cell-tracker–labeled HCAECs adherent to denuded saphenous vein after preincubation with (A) control medium or (B) hIGFBP1 (500 ng/mL) for 60 minutes (magnification ×10). (C) Significantly more cells were adherent to the saphenous vein after preincubation with hIGFBP1 (500 ng/mL; 60 minutes) (n = 5). ***P < 0.001. (D) Adhesion of HCAECs to glass coverslips. HCAECs were incubated in 1% FCS with or without hIGFBP1 (500 ng/mL) for indicated times before cells were fixed with paraformaldehyde and stained with hematoxylin and eosin. Adherent cells were quantified in 10 random fields at ×400 magnification. There were no significant differences between control and hIGFBP1-treated cells at each time point. (E and F) Cell-surface integrin expression. HCAECs were incubated with or without hIGFBP1 (500 ng/mL) for 1 hour before quantification of cell-surface integrins by using an integrin-mediated cell adhesion array kit (Millipore). Expression of α-integrins (E) and β-integrins (F) are indicated (n = 6). Data shown are mean +/− SEM. *P < 0.05; **P < 0.01.
hIGFBP1 ameliorates insulin-resistance induced endothelial migratory and proliferative defects in human endothelial cells
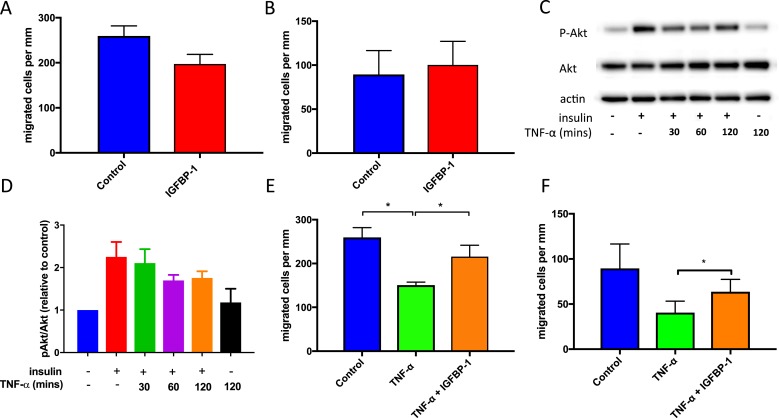
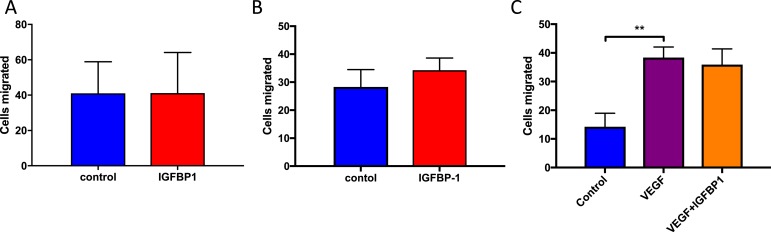
Regeneration of injured endothelium by local resident endothelial cells depends on their ability to migrate and proliferate to form a neo-endothelium. In linear wound assays, we found that incubation with hIGFBP1 (500 ng/mL; 1 hour) did not influence migration of HUVECs or HCAECs (Fig. 4A and 4B). To mimic the biochemical milieu to which endothelial cells are exposed in insulin-resistant states in vivo, we preincubated HUVECs with TNFα to inhibit the insulin signaling pathway (Fig. 4C and 4D). Incubation with TNFα (10 ng/mL) inhibited migration of HUVECs (Fig. 4E) and HCAECs (Fig. 4F), which was ameliorated in both types of endothelial cell by coincubation with hIGFBP1 (500 ng/mL) (Fig. 4E and 4F). We also investigated whether hIGFBP1increased migration of endothelial cells in a modified Boyden chamber assay. Migration of HUVECs and HCAEC did not differ, indicating that hIGFBP1 does not act as a chemotactic stimulus for endothelial cells (Fig. 5A and 5B). Similarly, hIGFBP1 did not modulate the migratory response of HCAEC to the potent chemotactic stimulus VEGF (Fig. 5C).
Figure 4.
hIGFBP1 abrogates TNFα-induced inhibition of endothelial cell (EC) migration. (A and B) No significant difference in migration in response to hIGFBP1 (500 ng/mL; 48 hours) was observed in (A) HUVECs or (B) HCAECs in a scratch wound healing assay (n = 3). (C and D) Preincubation with TNFα (10 ng/mL) for the indicated times inhibited insulin-stimulated (100 nmol/L; 15 minutes) Akt phosphorylation in HUVECs. (C) Representative immunoblot and (D) mean data of phospho Akt (pAkt)/Akt ratio are shown. (E and F) hIGFBP1 (500 ng/mL) partially restored endothelial migratory responses after exposure to TNFα (10 mg/mL) in scratch wound assays. (E) HUVECs (n = 9). *P < 0.01. (F) HCAECs (n = 6). *P < 0.05. Data shown are mean +/− SEM.
Figure 5.
IGFBP-1 does not act as chemotactic agent for endothelial cell migration in Boyden chamber assays. (A) Effect of hIGFBP1 (500 ng/mL; 6 hours) on migration in HUVECs (n = 3). No significant difference was seen. (B) Effect of hIGFBP1 (500 ng/mL; 6 hours) on migration in HCAECs (n = 5). P = 0.07. (C) Effects of IGFBP-1 (500 ng/mL; 6 hours) and VEGF (50 ng/mL; 6 hours) on cell migration (HCAECs) (n = 5). Control vs. VEGF: ** P < 0.01; VEGF vs. VEGF + IGFBP-1: no significant difference. Data shown are mean +/− SEM.
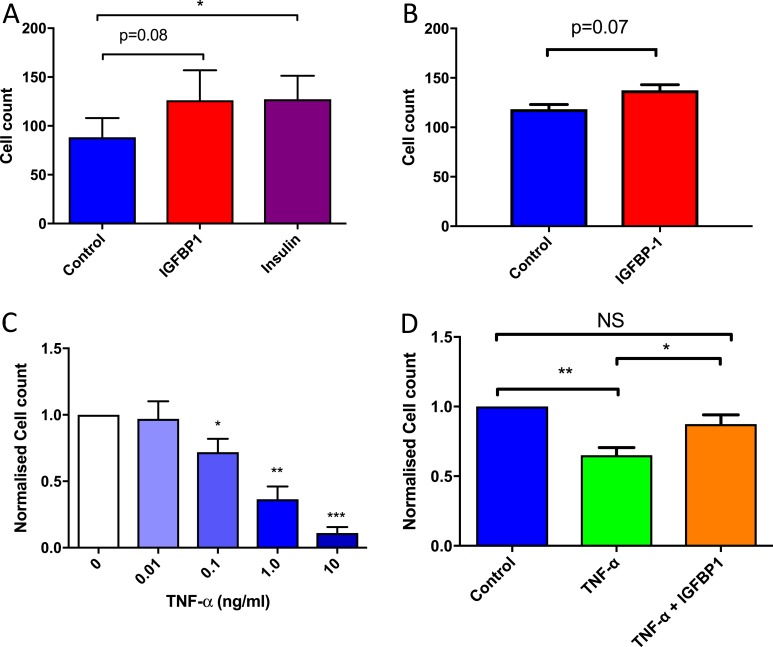
In both HUVECs and HCAECs, incubation with hIGFBP1 led to a trend toward increased cell proliferation assessed by cell counting, which did not reach statistical significance (Fig. 6A and 6B). Incubation with TNFα inhibited proliferation of HCAECs in a concentration-dependent manner (Fig. 6C). The antiproliferative effects of TNFα were ameliorated by incubation of HCAECs with hIGFBP1 (Fig. 6D).
Figure 6.
hIGFBP1 improves endothelial cell proliferation in a proinflammatory setting. (A) HUVEC proliferation. Quiesced cells treated with 2.5% FCS supplemented with insulin (100 nmol/L) or hIGFBP1 (500 ng/mL). Cells counted after 5 days with insulin or hIGFBP1 treatment (n=4). *P < 0.05. (B) HCAEC proliferation. Quiesced cells treated with 20% FCS supplemented with vehicle or 500 ng/mL hIGFBP1. Cells counted after 5 days with control or hIGFBP1 treatment (n = 4). (C) Concentration-dependent effect of TNFα on inhibition of proliferation in HCAECs. Cells counted after 5 days following TNFα treatment (0.01 to 10 ng/mL) (n = 3). (D) HCAEC proliferation. Quiesced cells treated with 20% FCS supplemented with TNFα (1 ng/mL), hIGFBP1 (500 ng/mL), or a combination of TNFα (1 ng/mL) and IGFBP1 (500 ng/mL). Cells counted after 5 days. Analysis of variance: P < 0.01. Post hoc: **P < 0.01; *P < 0.05 (n = 6). Data shown are mean +/− SEM. Abbreviation: NS, not significant.
Proreparative effects of hIGFBP1 on the endothelium depend on its RGD domain and FAK
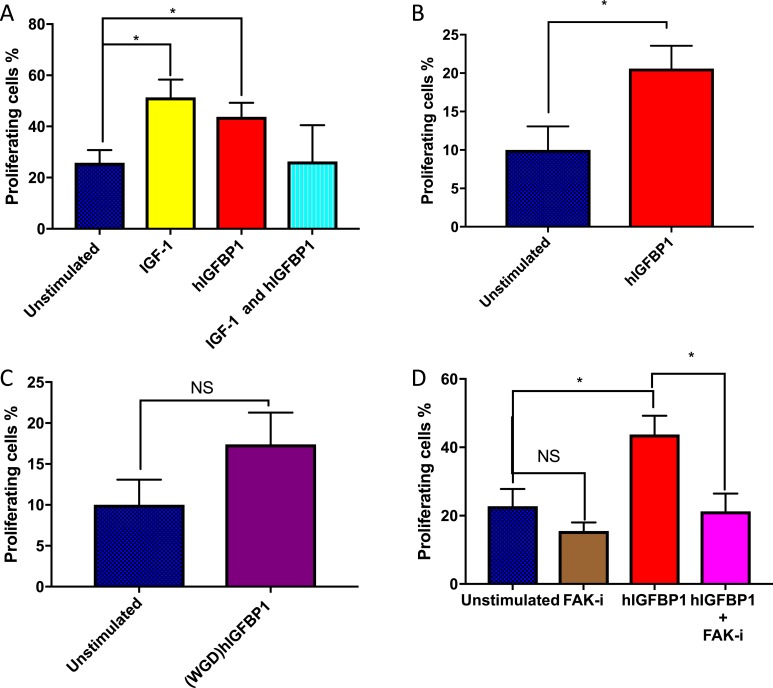
To further explore the molecular basis of the proreparative effects of hIGFBP1 in the endothelium, we used an EdU assay to quantify the effects of hIGFBP1 on proliferative responses in HUVECs. We found a significant dose-dependent increase in cell proliferation at hIGFBP1 concentrations of 100 to 500 ng/mL (Supplemental Fig. 3 (458.2KB, docx) ). Because IGFBPs are known to act variably as IGF modulators or to act independently of IGF contingent on context, we next compared the effects of IGF-1 and hIGFBP1 on endothelial cell proliferation. Equimolar concentrations of IGF-1 and hIGFBP1 both individually stimulated proliferation to a similar extent, but there was no evidence of an additive effect (Fig. 7A). We then mutated the RGD domain of IGFBP1, responsible for binding to cell surface integrins, to a nonfunctional WGD domain incapable of integrin binding (20). Stimulation of endothelial cell proliferation by hIGFBP1 was ameliorated by RGD→WGD mutation (Fig. 7B and 7C). FAK is an important signaling node downstream of integrins and is known to mediate proangiogenic signaling in endothelial cells (33). Inhibition of FAK abrogated the stimulatory effect of hIGFBP1 on cell proliferation (Fig. 7D) and reduced adhesion to collagen IV in the presence of hIGFBP1 (Supplemental Fig. 2F (458.2KB, docx) ).
Figure 7.
IGF-independent effects of hIGFBP1 and involvement in its RGD domain and FAK on endothelial cell proliferation. (A) hIGFBP1 (500 ng/L) and IGF-1 (18 nM) both independently stimulated proliferation of HUVECs on an EdU assay. There was no additive effect of IGF-1 and hIGFBP1 on cell proliferation. (B and C) WT hIGFBP1 stimulated proliferation of HUVECs. Proliferation was not significantly increased by hIGFBP1 when the RGD domain was mutated to WGD. (D) The positive effect of hIGFBP1 on proliferation of HUVECs was abrogated by the focal FAK inhibitor (FAK-i) PZ0117 (100 nmol/L) (n = 4). *P < 0.05. Abbreviation: NS, not significant. Data shown are mean +/− SEM.
Acute exposure to hIGFBP1 leads to phosphorylation of FAK, activation of RhoA, and modulation of F-actin organization in endothelial cells
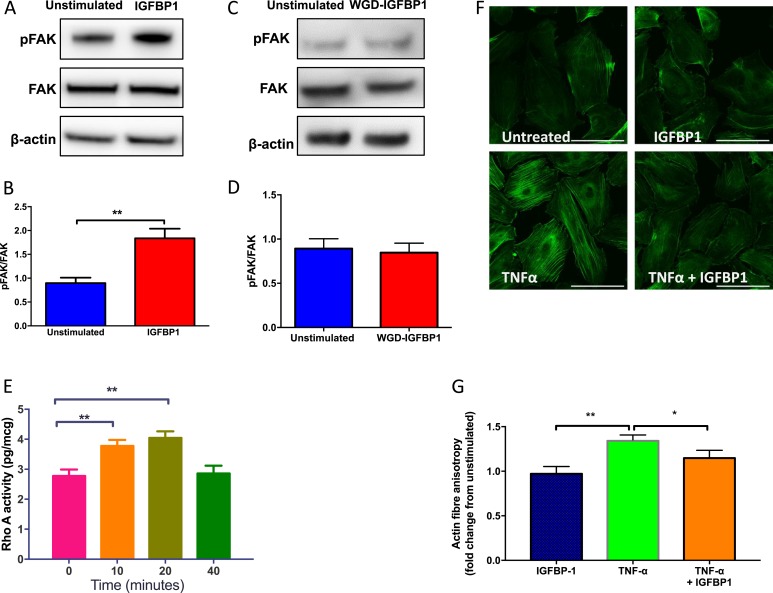
Because the RGD motif of hIGFBP1 is capable of interaction with cell-surface integrins, we investigated whether hIGFBP1 activates outside-in integrin-mediated signaling in endothelial cells. We found that incubation of HUVECs with hIGFBP1 led to acute phosphorylation of the critical integrin signaling intermediary FAK (Fig. 8A and 8B). The non–integrin-binding WGD mutant hIGFBP1 had no effect on FAK phosphorylation (Fig. 8C and 8D). Endothelial cell migration depends on cytoskeletal rearrangements in which the small guanosine triphosphatase (GTPase) RhoA plays a critical role (34). We observed rapid time-dependent activation of RhoA in HUVECs in response to hIGFBP1 (Fig. 8E). The cytoskeletal rearrangements associated with endothelial cell motility are complex, involving formation and dissolution of focal adhesions and the remodeling of actin filaments. We assessed cytoskeletal remodeling by quantifying actin filament anisotropy. Incubation with TNFα led to a significant increase in actin filament anisotropy, which was inhibited by coincubation with hIGFBP1 (Fig. 8 F and 8G).
Figure 8.
hIGFBP1 stimulates phosphorylation of FAK, activates the small GTPase RhoA, and ameliorates TNFα induced cytoskeletal rearrangement in endothelial cells. (A and B) hIGFBP1 (500 ng/mL; 15 minutes) induced rapid 397Tyr phosphorylation of FAK in HUVECs. (A) Representative immunoblot. (B) Mean data for phospho-FAK (pFAK)/FAK ratio (n = 6). *P < 0.05. (C and D) Mutation of the RGD domain of IGFBP1 to WGD (incapable of integrin binding) abrogates phosphorylation of FAK. (C) Representative immunoblot. (D) Mean data for pFAK/FAK ratio (n = 6). (E) hIGFBP1 (500 ng/mL) induced time-dependent activation of RhoA in HCAECs (n = 5). **P < 0.01. (F and G) Effects of TNFα (10 ng/mL) and hIGFBP1 (500 ng/mL) on actin fiber anisotropy in HUVECs. (F) Representative images (scale bar represents 100 µm). (G) Mean data from four repeat experiments with 188 to 287 cells per experiment. *P < 0.05; **P < 0.01.
Discussion
This study demonstrated that transgenic expression of hIGFBP1 partially reversed the endothelial regenerative dysfunction in insulin-resistant IR+/− mice. This was not explained by modulation of the abundance or function of MACs, which are known to be impaired in IR+/− mice (10). In vitro, we observed favorable effects of hIGFBP1 on multiple endothelial cell functional properties integral to endothelial regeneration, including increased adhesion to ECM and amelioration of the detrimental effects of insulin resistance on proliferative and migratory responses. At the molecular level, hIGFBP1 induced integrin signaling through rapid phosphorylation of FAK, increased activity of the small GTPase RhoA and modulated the effects of the proinflammatory cytokine TNFα on cytoskeletal remodeling in endothelial cells. Collectively, these findings further support the emerging concept of a vasculo-protective role for IGFBP1 and raise the possibility that increasing IGFBP1 concentration may be a strategy to improve endothelial repair in insulin resistant states.
Damage to the vascular endothelium can result from diverse insults, including exposure to the adverse biochemical milieu associated with the presence of vascular risk factors or mechanical trauma associated with surgical or percutaneous revascularization procedures. Endogenous repair mechanisms, which mitigate against the development of atherosclerosis, thrombosis, and restenosis, are deficient in the presence of diabetes (35). We previously reported that endothelial regeneration following arterial injury was impaired in insulin-resistant mice (IR+/−), in which reduced NO bioavailability and defective mobilization of MACs from bone marrow contributed to the reduced abundance of circulating MACs (10). In a separate study, we reported that hIGFBP1 improved vascular insulin sensitivity and increased vascular NO bioavailability in IR+/− mice (24). Intriguingly, the enhanced repair observed in IR+/− mice expressing hIGFBP1 in the current study was not explained by changes in the abundance or adhesive properties of circulating MACs. Although we cannot exclude the possibility of changes in other classes of circulating progenitor cells, our data suggest that effects of hIGFBP1 on local endothelial cells per se may predominate in the modulation of endothelial repair. In keeping with this suggestion, the contribution of circulating progenitor cells to endogenous endothelial regeneration has been questioned (36, 37), reigniting interest in the long-recognized contribution of local, mature endothelial cells to endothelial repair (5, 6, 38).
Circulating concentrations of IGFBP1 have been associated with both metabolic regulation and cardiovascular disease. In nondiabetic humans, low levels of IGFBP1 predict the subsequent development of diabetes (17–19). However, data linking IGFBP1 levels with cardiovascular disease development are conflicting (15, 22, 23) It is therefore important to address whether IGFBP1 directly affects the function of vascular cells. We observed a significant increase in plasma IGFBP1 levels in hIGFBP1-transgenic mice, which were similarly increased in IR+/− mice overexpressing hIGFBP1. The levels achieved in hIGFBP1tg mice in the current dataset are around twofold higher than circulating levels in healthy nonobese humans (39) and are substantially higher than those in obese C57BL6 mice (24).
IGFBP1 modulates migratory and/or proliferative responses in a range of cell types, predominantly through interaction of the RGD sequence within its C-terminal domain with α5β1 integrin (12, 13, 40–42). In keeping with a critical effect of the RGD sequence of IGFBP1 on diverse cellular responses, we recently demonstrated that hIGFBP1 directly modulates insulin signaling and insulin-stimulated glucose uptake in skeletal muscle cells through an RGD-dependent mechanism (20). However, this study reports a modulatory effect of IGFBP1 on functional responses in endothelial cells. Several fundamental actions pertinent to endothelial repair, including adhesion, migration, and proliferation, were favorably modulated by hIGFBP1 in this study.
In an ex vivo model of endothelial regeneration, short-term incubation with hIGFBP1 significantly increased the proportion of endothelial cells adherent to endothelium-denuded human saphenous vein. The concentration of hIGFBP1 used in the cellular experiments was only slightly higher that the levels achieved in the transgenic mice, indicating that important vascular effects of IGFBP1 can be achieved at physiological or modestly supraphysiological concentrations. In keeping with the key role of integrins in the adhesion of endothelial cells to the ECM, cell surface expression of integrins αVβ3, αVβ5, and α5β1 was increased after incubation with hIGFBP1. Interaction of the RGD motif of IGFBP1 with integrins is well described (12, 13); however, this study reports that IGFBP1 increases cell surface integrin expression. Although other members of the IGF-binding protein family regulate integrin expression at the transcriptional level (43), the change in cell surface integrin expression observed here is likely to be too rapid to be explained by transcriptional regulation and may reflect recycling of intracellular integrins to the cell membrane (44). In vitro, we were unable to identify a dominant ECM component to which hIGFBP1 preferentially increased endothelial cell adhesion. We speculate that the proadhesive action we observed in saphenous veins ex vivo is due to additive minor effects on multiple ECM components or to a selective effect on an ECM constituent we have not selectively studied.
To determine whether a functional RGD domain of IGFBP1 is essential for its stimulatory effects on endothelial repair, we mutated RGD to WGD, which is incapable of binding integrins (20). The ability of IGFBP1 to stimulate endothelial cell proliferation and activate downstream signaling pathways was abrogated by loss of a functional RGD domain. The findings of the current study therefore add to the growing body of evidence that the IGFBP1-mediated effects in endothelial cells and skeletal muscle are RGD-integrin mediated.
To mimic insulin resistance in vitro, we exposed cells to the cytokine TNFα. In keeping with previous reports (45, 46), TNFα inhibited migratory and proliferative responses of endothelial cells. Incubation with hIGFBP1 partially restored endothelial migration in a scratch wound assay. However, no effect of hIGFBP1 was evident on the Boyden chamber assay, suggesting that hIGFBP1 augments the motility of endothelial cells rather than acting as a chemotactic agent in vascular repair. hIGFBP1 improved endothelial cell proliferation in the presence of TNFα.
It is notable that the favorable effects of hIGFBP1 on endothelial regeneration in this study were restricted to insulin-resistant IR+/− mice with no detectable effect apparent when hIGFBP1 was expressed in insulin-sensitive mice. Similarly, modulatory effects of hIGFBP1 on endothelial proliferative and migratory responses were apparent in vitro only after we induced insulin resistance by incubation with TNFα. Although the proreparative effects of hIGFBP1 may be attributable to hIGFBP1-mediated insulin sensitization in endothelium, as occurs in skeletal muscle (20), this would not readily explain the modulatory effects of hIGFBP1 observed in vitro, which were carried out in the absence of exogenous insulin. The failure of hIGFBP1 to improve endothelial regeneration in the insulin-sensitive state may therefore reflect the fact the reparative processes are already maximal in this setting. How IGFBP1 interacts with endothelial cells at the molecular level remains poorly understood. We demonstrated that IGFBP1 induces NO generation in endothelial cells independently of IGF by activation of the Akt pathway (24). Other groups have shown that IGFBP1 can activate integrin-mediated intracellular signaling through its C-terminal RGD sequence and induce migratory or proliferative responses in a range of cell types, including Chinese hamster ovary cells (12), oligodendrocytes (13), trophoblasts (40), breast cancer cells (41), and schwannoma cells (42). In keeping with activation of downstream integrin signaling by IGFBP1 in the endothelium, we observed rapid RGD-dependent phosphorylation of FAK, a nonreceptor tyrosine kinase, which becomes tyrosine-phosphorylated during integrin activation and is believed to play a vital role in integrin signal transduction (33, 47, 48). Inhibition of FAK abrogated the proproliferative effects of hIGFBP1. FAK promotes cell migration and angiogenic responses through interaction with a pool of intracellular signaling proteins, including c-Src, phosphatidylinositol 3-kinase, and Rho GTPase family members, which are associated with assembly and disassembly of actin cytoskeleton (49). Coordinated remodeling of actin filaments through dynamic regulation of filament assembly and disassembly is required for endothelial cells to mobilize after vascular injury. In response to hIGFBP1, we observed rapid and time-dependent activation of the small GTPase RhoA, which acts as a key player in cytoskeletal rearrangement and endothelial cell migration (34).
Consistent with the known inhibitory effect of TNFα on actin remodeling and endothelial cell migration (50), we observed a significant increase in actin filament anisotropy after TNFα stimulation. This was abrogated by costimulation with hIGFBP1, providing further evidence that IGFBP1 modulates cytoskeletal remodeling and thereby promotes endothelial repair.
In summary, we have demonstrated that IGFBP1 abrogates the inhibitory effects of insulin resistance on endothelial repair in vivo and exerts multiple favorable effects on the reparative phenotype of endothelial cells in vitro. These findings add to our previous description of insulin-sensitizing and antiatherosclerotic effects of IGFBP1, are consistent with the argument that low levels of IGFBP1 are permissive for the development of vascular disease, and suggest that raising IGFBP1 levels may be an appropriate strategy to promote endothelial repair.
Acknowledgments
Financial Support: This work was funded by a British Heart Foundation Clinical Research Training Fellowship for A.A. R.M.C. holds a British Heart Foundation Intermediate Clinical Research Fellowship. M.T.K. holds a British Heart Foundation Chair in Cardiology. S.B.W. holds a European Research Council Starting Grant.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DiI-Ac-LDL
- 1,1′-dioctadecy-3,3,3′,3′-tetramethyllindocarbocyanine–labeled acetylated low-density lipoprotein
- ECGM
- endothelial cell growth medium
- ECM
- extracellular matrix
- EdU
- 5-ethynyl-2′-deoxyuridine
- FAK
- focal adhesion kinase
- FBS
- fetal bovine serum
- FCS
- fetal calf serum
- FITC
- fluorescein isothiocyanate
- FSG
- fish skin gelatin
- GTPase
- guanosine triphosphatase
- HCAEC
- human coronary artery endothelial cell
- hIGFBP1
- insulinlike growth factor–binding protein-1
- HPF
- high-power field
- HUVEC
- human umbilical vein endothelial cell
- IGF
- insulinlike growth factor
- IGFBP1
- insulinlike growth factor–binding protein-1
- IR+/−
- heterozygous for deletion of the insulin receptor
- MAC
- myeloid angiogenic cell
- MNC
- mononuclear cell
- NO
- nitric oxide
- PBS
- phosphate-buffered saline
- RGD
- Arg-Gly-Asp
- tg
- transgenic
- TNFα
- tumor necrosis factor α
- VEGF
- vascular endothelial growth factor
- WGD
- Trp-Gly-Asp
- WT
- wild-type.
References
- 1.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl). 2004;82(10):671–677. [DOI] [PubMed] [Google Scholar]
- 2.Manchio JV, Gu J, Romar L, Brown J, Gammie J, Pierson RN III, Griffith B, Poston RS. Disruption of graft endothelium correlates with early failure after off-pump coronary artery bypass surgery. Ann Thorac Surg. 2005;79(6):1991–1998. [DOI] [PubMed] [Google Scholar]
- 3.Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, Serruys P. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44(4):733–739. [DOI] [PubMed] [Google Scholar]
- 4.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435–2441. [DOI] [PubMed] [Google Scholar]
- 5.Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979;41(5):407–418. [PubMed] [Google Scholar]
- 6.Itoh Y, Toriumi H, Yamada S, Hoshino H, Suzuki N. Resident endothelial cells surrounding damaged arterial endothelium reendothelialize the lesion. Arterioscler Thromb Vasc Biol. 2010;30(9):1725–1732. [DOI] [PubMed] [Google Scholar]
- 7.Krankel N, Luscher TF, Landmesser U. Novel insights into vascular repair mechanisms. Curr Pharm Des. 2014;20(14):2430–2438. [DOI] [PubMed] [Google Scholar]
- 8.Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med. 2017;6(5):1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–1904. [DOI] [PubMed] [Google Scholar]
- 10.Kahn MB, Yuldasheva NY, Cubbon RM, Smith J, Rashid ST, Viswambharan H, Imrie H, Abbas A, Rajwani A, Aziz A, Baliga V, Sukumar P, Gage M, Kearney MT, Wheatcroft SB. Insulin resistance impairs circulating angiogenic progenitor cell function and delays endothelial regeneration. Diabetes. 2011;60(4):1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol Metab. 2009;20(4):153–162. [DOI] [PubMed] [Google Scholar]
- 12.Jones JI, Gockerman A, Busby WH Jr, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci USA. 1993;90(22):10553–10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesik D, De Keyser J, Bron R, Fuhler GM. Insulin-like growth factor binding protein-1 activates integrin-mediated intracellular signaling and migration in oligodendrocytes. J Neurochem. 2010;113(5):1319–1330. [DOI] [PubMed] [Google Scholar]
- 14.Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216(3):319–357. [DOI] [PubMed] [Google Scholar]
- 15.Maddux BA, Chan A, De Filippis EA, Mandarino LJ, Goldfine ID. IGF-binding protein-1 levels are related to insulin-mediated glucose disposal and are a potential serum marker of insulin resistance [published correction appears in Diabetes Care 2006;29(11):257190]. Diabetes Care. 2006;29(7):1535–1537. [DOI] [PubMed] [Google Scholar]
- 16.Heald AH, Cruickshank JK, Riste LK, Cade JE, Anderson S, Greenhalgh A, Sampayo J, Taylor W, Fraser W, White A, Gibson JM. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia. 2001;44(3):333–339. [DOI] [PubMed] [Google Scholar]
- 17.Petersson U, Ostgren CJ, Brudin L, Brismar K, Nilsson PM. Low levels of insulin-like growth-factor-binding protein-1 (IGFBP-1) are prospectively associated with the incidence of type 2 diabetes and impaired glucose tolerance (IGT): the Söderåkra Cardiovascular Risk Factor Study. Diabetes Metab. 2009;35(3):198–205. [DOI] [PubMed] [Google Scholar]
- 18.Lewitt MS, Hilding A, Ostenson C-G, Efendic S, Brismar K, Hall K. Insulin-like growth factor-binding protein-1 in the prediction and development of type 2 diabetes in middle-aged Swedish men. Diabetologia. 2008;51(7):1135–1145. [DOI] [PubMed] [Google Scholar]
- 19.Lewitt MS, Hilding A, Brismar K, Efendic S, Ostenson C-G, Hall K. IGF-binding protein 1 and abdominal obesity in the development of type 2 diabetes in women. Eur J Endocrinol. 2010;163(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haywood NJ, Cordell PA, Tang KY, Makova N, Yuldasheva NY, Imrie H, Viswambharan H, Bruns AF, Cubbon RM, Kearney MT, Wheatcroft SB. Insulin-like growth factor binding protein 1 could improve glucose regulation and insulin sensitivity through its RGD domain. Diabetes. 2017;66(2):287–299. [DOI] [PubMed] [Google Scholar]
- 21.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114–120. [DOI] [PubMed] [Google Scholar]
- 22.Mellbin LG, Rydén L, Brismar K, Morgenthaler NG, Ohrvik J, Catrina SB. Copeptin, IGFBP-1, and cardiovascular prognosis in patients with type 2 diabetes and acute myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care. 2010;33(7):1604–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallander M, Norhammar A, Malmberg K, Ohrvik J, Rydén L, Brismar K. IGF binding protein 1 predicts cardiovascular morbidity and mortality in patients with acute myocardial infarction and type 2 diabetes. Diabetes Care. 2007;30(9):2343–2348. [DOI] [PubMed] [Google Scholar]
- 24.Rajwani A, Ezzat V, Smith J, Yuldasheva NY, Duncan ER, Gage M, Cubbon RM, Kahn MB, Imrie H, Abbas A, Viswambharan H, Aziz A, Sukumar P, Vidal-Puig A, Sethi JK, Xuan S, Shah AM, Grant PJ, Porter KE, Kearney MT, Wheatcroft SB. Increasing circulating IGFBP1 levels improves insulin sensitivity, promotes nitric oxide production, lowers blood pressure, and protects against atherosclerosis. Diabetes. 2012;61(4):915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, José PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12(1):106–109. [DOI] [PubMed] [Google Scholar]
- 26.Crossey PA, Jones JS, Miell JP. Dysregulation of the insulin/IGF binding protein-1 axis in transgenic mice is associated with hyperinsulinemia and glucose intolerance. Diabetes. 2000;49(3):457–465. [DOI] [PubMed] [Google Scholar]
- 27.Wheatcroft SB, Shah AM, Li J-M, Duncan E, Noronha BT, Crossey PA, Kearney MT. Preserved glucoregulation but attenuation of the vascular actions of insulin in mice heterozygous for knockout of the insulin receptor. Diabetes. 2004;53(10):2645–2652. [DOI] [PubMed] [Google Scholar]
- 28.Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, Bailey MA, Yuldasheva NY, Ludlow MJ, Cubbon RM, Li J, Futers TS, Morley L, Gaunt HJ, Marszalek K, Viswambharan H, Cuthbertson K, Baxter PD, Foster R, Sukumar P, Weightman A, Calaghan SC, Wheatcroft SB, Kearney MT, Beech DJ. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. 2017;8(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter KE, Naik J, Turner NA, Dickinson T, Thompson MM, London NJM. Simvastatin inhibits human saphenous vein neointima formation via inhibition of smooth muscle cell proliferation and migration. J Vasc Surg. 2002;36(1):150–157. [DOI] [PubMed] [Google Scholar]
- 30.Imrie H, Abbas A, Viswambharan H, Rajwani A, Cubbon RM, Gage M, Kahn M, Ezzat VA, Duncan ER, Grant PJ, Ajjan R, Wheatcroft SB, Kearney MT. Vascular insulin-like growth factor-I resistance and diet-induced obesity. Endocrinology. 2009;150(10):4575–4582. [DOI] [PubMed] [Google Scholar]
- 31.Boudaoud A, Burian A, Borowska-Wykręt D, Uyttewaal M, Wrzalik R, Kwiatkowska D, Hamant O. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat Protoc. 2014;9(2):457–463. [DOI] [PubMed] [Google Scholar]
- 32.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. [DOI] [PubMed] [Google Scholar]
- 33.Shen T-L, Park AY-J, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan J-L. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169(6):941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VWM. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol. 2003;23(2):211–217. [DOI] [PubMed] [Google Scholar]
- 35.Cubbon RM, Kahn MB, Wheatcroft SB. Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clin Sci (Lond). 2009;117(5):173–190. [DOI] [PubMed] [Google Scholar]
- 36.Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93(2):223–231. [DOI] [PubMed] [Google Scholar]
- 37.Tsuzuki M. Bone marrow-derived cells are not involved in reendothelialized endothelium as endothelial cells after simple endothelial denudation in mice. Basic Res Cardiol. 2009;104(5):601–611. [DOI] [PubMed] [Google Scholar]
- 38.Lindner V, Reidy MA, Fingerle J. Regrowth of arterial endothelium. Denudation with minimal trauma leads to complete endothelial cell regrowth. Lab Invest. 1989;61(5):556–563. [PubMed] [Google Scholar]
- 39.Borai A, Livingstone C, Ferns G. Reference change values for insulin and insulin-like growth factor binding protein-1 (IGFBP-1) in individuals with varying degrees of glucose tolerance. Scand J Clin Lab Invest. 2013;73(4):274–278. [DOI] [PubMed] [Google Scholar]
- 40.Gleeson LM, Chakraborty C, McKinnon T, Lala PK. Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein Kinase pathway. J Clin Endocrinol Metab. 2001;86(6):2484–2493. [DOI] [PubMed] [Google Scholar]
- 41.Perks CM, Newcomb PV, Norman MR, Holly JM. Effect of insulin-like growth factor binding protein-1 on integrin signalling and the induction of apoptosis in human breast cancer cells. J Mol Endocrinol. 1999;22(2):141–150. [DOI] [PubMed] [Google Scholar]
- 42.Ammoun S, Schmid MC, Zhou L, Ristic N, Ercolano E, Hilton DA, Perks CM, Hanemann CO. Insulin-like growth factor-binding protein-1 (IGFBP-1) regulates human schwannoma proliferation, adhesion and survival. Oncogene. 2012;31(13):1710–1722. [DOI] [PubMed] [Google Scholar]
- 43.Lee H-J, Lee J-S, Hwang SJ, Lee H-Y. Insulin-like growth factor binding protein-3 inhibits cell adhesion via suppression of integrin β4 expression. Oncotarget. 2015;6(17):15150–15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23(5):607–614. [DOI] [PubMed] [Google Scholar]
- 45.López-Marure R, Bernal AE, Zentella A. Interference with c-myc expression and RB phosphorylation during TNF-mediated growth arrest in human endothelial cells. Biochem Biophys Res Commun. 1997;236(3):819–824. [DOI] [PubMed] [Google Scholar]
- 46.Krasinski K, Spyridopoulos I, Kearney M, Losordo DW. In vivo blockade of tumor necrosis factor-alpha accelerates functional endothelial recovery after balloon angioplasty. Circulation. 2001;104(15):1754–1756. [DOI] [PubMed] [Google Scholar]
- 47.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89(18):8487–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42(1):283–323. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Guan J-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63(8):610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang E, Heo K-S, Woo C-H, Lee H, Le N-T, Thomas TN, Fujiwara K, Abe J. MK2 SUMOylation regulates actin filament remodeling and subsequent migration in endothelial cells by inhibiting MK2 kinase and HSP27 phosphorylation. Blood. 2011;117(8):2527–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]