Abstract
Diacylglycerol (DAG) has been reported to reduce the serum lipid and glucose levels more effectively compared with triacylglycerol (TAG). The present study examined the long-term effects of dietary DAG on rats with type 2 diabetic nephropathy (DN). The type 2 DN model was established by administering a high-fat and high-calorie diet along with an intraperitoneal injection of 35 mg/kg body weight streptozocin, and the disease developed over the following 8 weeks. Sprague-Dawley rats were then randomly divided into the control, TAG, low-dose DAG (Low-DAG) and high-dose DAG (High-DAG) groups. Blood glucose (BG), lipid levels and renal function parameters were then measured. The results revealed that the body weight in the High-DAG group was significantly reduced compared with the TAG group, while there was no significant difference in the food intake between TAG and DAG groups. BG, advanced glycation end products (AGEs), TAG, low-density lipoprotein cholesterol, urine protein and urine albumin levels were significantly reduced, while high-density lipoprotein cholesterol levels were significantly increased in the two DAG groups. In addition, hematoxylin-eosin stained glomeruli presented decreased glomerular enlargement and mesangial expansion in the DAG groups. Immunohistochemical detection revealed that the levels of transforming growth factor-β1 and connective tissue growth factor in renal tissues of the DAG groups were also significantly reduced compared with the TAG group. These findings indicate that DAG oils can significantly reduce BG levels and the deposition of AGEs in renal tissue, as well as regulate the levels of transforming growth factor-β1 and connective tissue growth factor, thus delaying the progression of nephropathy.
Keywords: diacylglycerol, diabetic nephropathy, advanced glycation end products, transforming growth factor-β1
Introduction
Diabetes mellitus (DM) is considered to be a metabolic disorder that is characterized by hyperglycemia (1). In 2014, the incidence of DM was estimated to be 366 million cases worldwide, and it is expected to become the seventh leading cause of mortality by 2025 (2). Diabetic nephropathy (DN) is a severe vascular complication of DM, which frequently leads to end-stage renal disease and considerably increases cardiovascular disease risk and mortality (1). As the mechanisms that lead to DN remain unclear, a significant amount of research is ongoing to establish strategies for its prevention and treatment. It has been hypothesized that high glucose levels in diabetes often lead to non-enzymatic glycosylation reactions between glucose and protein molecules, and generate advanced glycosylation end products (AGEs) (3). AGE deposition can directly lead to structural changes in the kidneys, including incrassation of the glomerular basement membrane, glomerular hypertrophy and glomerulosclerosis (3). In addition, AGEs are able to combine with the receptor for AGEs (RAGE), activating the expression of transforming growth factor-β1 (TGF-β1) and damaging cell metabolic activities (4). In diabetes, TGF-β1 serves a key role in mediating the hypertrophic and fibrotic/sclerotic manifestations of DN (5,6). Furthermore, connective tissue growth factor (CTGF) is a major autocrine growth factor that can be induced by TGF-β1 (7), also leading to kidney tissue fibrosis.
Diacylglycerol (DAG), which consists mainly of 1,3-DAG, naturally exists in various cooking oils (8,9). Compared with triacylglycerol (TAG), DAG has been reported to possess several nutritional properties associated with serum lipids and body fat metabolism (10–13). In addition, DAG reduces the serum TAG concentration at 2, 4 and 6 h after meals in a manner that is positively correlated with the daily intake (14). A previous study reported that a 3-month intake of DAG oil significantly reduced fasting serum TAG in patients with type 2 diabetes and hypertriglyceridemia (15). Furthermore, previous evidence suggested that high TAG levels contribute to inflammatory cell infiltration, which may lead to glomerular sclerosis and fibrosis (16). Serum glucose was also significantly improved in patients with higher glucose levels at baseline (>7.00 mmol/l) in the DAG oil group (17). Yamamoto et al reported that diet therapy with DAG oil delayed the progression of renal failure in type 2 diabetic patients with nephropathy (18). However, to the best of our knowledge, no previous studies have examined the effects of DAG on DN in type 2 diabetes, and little is known regarding the underlying mechanism.
In the present study, the influence of long-term intake of DAG on DN in type 2 diabetic rats was examined, and the effects of DAG and TAG on serum glucose, lipids and renal function parameters were compared. In addition, the study researched the effects of DAG on kidney tissue pathology, as well as the effects of DAG on the levels of AGEs, TGF-β1 and CTGF. The study aimed to characterize the possible underlying mechanism and to provide important data for the potential use of DAG for the prevention and treatment of DN.
Materials and methods
DAG and TAG oils
The DAG and TAG oils used in the current study were purchased from Jinan Dowin Chemical Technology Co., Ltd. (Jinan, China). The fatty acid composition of DAG oil was similar to that of TAG oil (soybean oil), as shown in Table I. The DAG oil consisted of ~90% DAG and 10% TAG, and the DAG component was comprised of 1,3-DAG and 1,2-DAG at a ratio of 7:3 (w/w).
Table I.
Fatty acid composition of test oils (%).
| Lipid numbersa | TAG oil | DAG oil |
|---|---|---|
| C16:0 | 4.25 | 3.02 |
| C16:1 | 0.02 | 0.30 |
| C18:0 | 1.38 | 1.05 |
| C18:1 | 30.6 | 32.9 |
| C18:2 | 54.6 | 53.6 |
| C18:3 | 8.79 | 8.79 |
| C20:0 | 0.23 | 0.22 |
| C22:1 | 0.07 | 0.06 |
| Other | 0.06 | 0.06 |
No. of carbon atoms against the no. of double bonds in the fatty acids. TAG, triacylglycerol; DAG, diacylglycerol.
Animals
Sprague-Dawley male rats (180–220 g) at 7-weeks of age were provided by the Laboratory Animal Center of The School of Public Health, Shandong University (Jinan, China; production permit no. SCXK (lu) 20090001). All rats were maintained under natural light/dark at 18–28°C and a humidity of 40–60%. Food and water were provided ad libitum. Animal experiments were performed with the approval of the Ethics Committee of Shandong University.
Reagents
Streptozocin was provided by Sigma-Aldrich (Merck KGaA, Shanghai, China). Rabbit anti-rat TGF-β1 (1:100; ab92486) and goat anti-rat CTGF polyclonal antibodies (1:100; ab6992) were provided by Jinan Yanda Biological Technology Co., Ltd. (Jinan, China).
Animal model and experimental protocol
The animal model was established using the method described by Haseena et al (19) with certain modifications. A total of 40 rats were randomly divided into the control, TAG, low-dose DAG (Low-DAG) and high-dose DAG (High-DAG) groups (n=10 in each group; 5 rat/cage). The control group received a TAG diet without DN induction. Rats in the TAG, Low-DAG and High-DAG groups were administered a high-fat diet for 5 weeks (20), and then DN was induced with streptozocin (35 mg/kg body weight in 0.1 M citrate buffer, pH=4.5) (21). DN was defined as a tail blood glucose (BG) concentration of ≥16.7 mmol/l after 72 h and a results of quantitative test for 24 h urine protein (Upro) of >30 mg/24 h (normal values: BG, 5.96±1.81 mmol/l; Upro, 7–12 mg/24 h). After establishing the DN model, rats in the TAG group received a TAG diet, while rats in the Low-DAG and High-DAG groups received a diet with low and high doses of DAG, respectively, for a further 8 weeks. All rats were provided food and water ad libitum. Diet compositions are demonstrated in Table II. The intervention period for experimental animals was 8 weeks (22,23).
Table II.
Compositions of diets (%).
| Ingredients | High fat | TAG | Low-DAG | High-DAG |
|---|---|---|---|---|
| TAG | 10 | 10 | 5 | – |
| DAG | – | – | 5 | 10 |
| Lard | 10 | – | – | – |
| Sucrose | 20 | – | – | – |
| Cholesterol | 4.8 | – | – | – |
| Cholates | 0.2 | – | – | – |
| Casein | 13 | 20 | 20 | 20 |
| Cellulose | 2.6 | 4 | 4 | 4 |
| Minerals | 2.25 | 3.5 | 3.5 | 3.5 |
| Vitamins | 0.65 | 1 | 1 | 1 |
| Potato starch | 36.5 | 61.5 | 61.5 | 61.5 |
TAG, triacylglycerol; DAG, diacylglycerol.
Specimen collection
The rats were housed individually in metabolic cages to collect 24-h urine samples prior to sacrifice. Samples were stored at −70°C for subsequent assays. Food intake was measured on a per-cage basis per week. Blood was collected every 4 weeks, and serum was separated (4,000 × g, 4°C, 10 min) and used for biochemical assays. Serum was stored at −70°C following centrifugation. In addition, the left kidney was extracted and weighed, and the kidney index (KI) was calculated as follows: KI=left kidney weight (g)/body weight (kg). Neutral formalin (10%) was injected into the upper-right kidney for pathological sectioning and analysis.
Biochemical analysis
BG level was assayed using a BG meter (Roche Diagnostics, Shanghai, China) after the rats were fasted, while levels of triglyceride (TG), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC) and total cholesterol (TC) were determined using an automatic biochemistry analyzer (Olympus 5400; Olympus Optical, Tokyo, Japan) at 0, 4 and 8 weeks. Serum creatinine (SCr), blood urea nitrogen (BUN), glycohemoglobin A1c (HbA1c) and Upro were determined using an automatic biochemistry analyzer (Olympus 5400) at 8 weeks. Urine albumin (Ualb) was determined with a fully automatic protein analyzer (Perlong Medical Equipment Co., Ltd., Nanjing, China) at 8 weeks. Insulin and AGEs were assayed with enzyme linked immunosorbent assay (ELISA) kits (C507166 and C506595; Sangon Biotech Co., Ltd., Shanghai, China) at 8 weeks.
Histopathological examination
Kidney specimens from all experimental groups were fixed in 10% buffered formalin and processed for paraffin sectioning. Sections with a ~5-µm thickness were stained with hematoxylin and eosin (H&E) and subjected to photomicroscopic assessment.
TGF-β1 and CTGF expression levels in renal tissues
TGF-β1 and CTGF protein levels in renal tissues were measured using the method described by Wang et al (24) with a few modifications. The streptavidin-peroxidase immunohistochemical method was used for semi-quantitative analyses. Briefly, paraffin-embedded kidney sections were dewaxed and incubated in water followed by 3% H2O2 for 20 min in the dark. The sections were then washed with distilled water and phosphate-buffered saline (PBS) for 5 min each. For the antigen retrieval, sections were incubated for 3 min in a domestic microwave oven in citrate buffer and then cooled immediately, followed by PBS washing (twice, 5 min each). Sections were incubated at 37°C for 30 min. Rabbit anti-rat TGF-β1 and goat anti-rat CTGF polyclonal antibodies (dilution, 1:100 in PBS) were added to the samples, and slides were incubated overnight at 4°C. Subsequent to washing five times (3 min each) with PBS, biotin-labeled secondary antibody (1:2,000; D111057; Sangon Biotech Co., Ltd.) was added to the sections and incubated at 37°C for 30 min. The sections were then washed with PBS for five times (3 min each) and incubated at 37°C for 30 min after addition of SP. Diaminobenzidine was used for colorimetric analysis. Hematoxylin counterstaining (1–5 min) was performed following routine dehydration, transparency and mounting. Sections of glomeruli were randomly selected under a microscope with 400-fold magnification.
The immunohistochemistry results were analyzed using a secondary scoring method (25), according to two categories, as follows: i) Scoring according to the percentage of positive cells: 0, <5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; and 4, >75%; ii) scoring according to the degree of positive staining: 0, negative (colorless); 1, weakly positive (pale yellow); 2, moderate positive (yellow); and 3, strong positive (brown). The final scores were calculated by adding the values for the two categories.
Statistical analysis
All data are presented as the means ± standard deviation, and were analyzed by multiple sample comparison methods, namely analysis of variance. Multiple comparison between the groups was performed using Fisher's least significant difference method. Statistically significant differences were determined based on a threshold of P<0.05. Analyses were conducted using the software package IBM SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Body weight and food intake subsequent to establishing the DN model
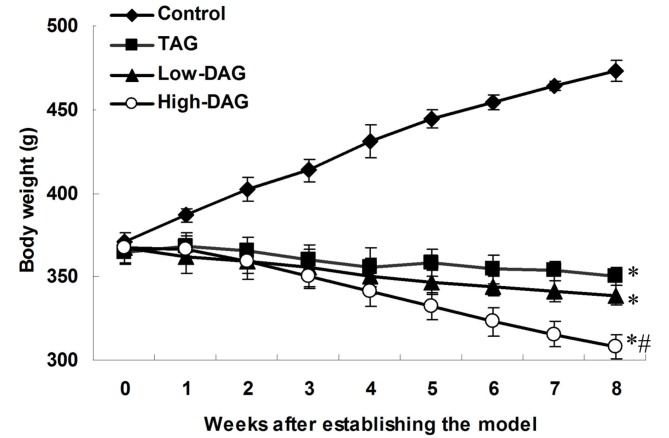
Alterations in body weight after establishing the DN model are shown in Fig. 1. Rats in the control group continued to gain weight, and were heavier in comparison with the TAG, Low-DAG and High-DAG rats throughout the study period. By contrast, the body weight of rats in the TAG, Low-DAG and High-DAG groups was gradually reduced during the follow-up period. Compared with the control group, body weight in the TAG, Low-DAG and High-DAG groups was significantly reduced (P<0.05). In addition, when compared with the TAG group, body weight in the High-DAG group was significantly reduced (P<0.05).
Figure 1.
Body weight of rats subsequent to establishing the diabetic nephropathy model. Data are presented as the means ± standard deviation (n=10). *P<0.05 vs. control group; #P<0.05 vs. TAG group. DAG, diacylglycerol; TAG, triacylglycerol.
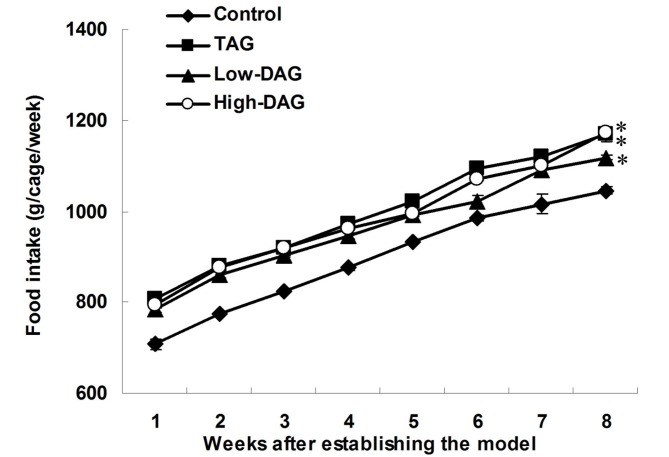
Food intake after establishing the DN model is shown in Fig. 2. The food intake in all groups increased during the follow-up period, but it was significantly lower in the control group compared with the TAG, Low-DAG and High-DAG groups (P<0.05). However, there was no significant difference in the food intake among the TAG, Low-DAG and High-DAG groups (P>0.05).
Figure 2.
Food intake subsequent to establishing the diabetic nephropathy model. Data are presented as the means ± standard deviation (n=10). *P<0.05 vs. control group. DAG, diacylglycerol; TAG, triacylglycerol.
Parameters associated with glucose and lipid metabolism
Table III demonstrates the levels of various parameters associated with glucose and lipid metabolism. In the High-DAG group, the BG, TC and TAG levels were significantly reduced during 8 weeks of treatment (P<0.05), whereas the changes in HDLC and LDLC levels were not significantly different compared with the TAG group. In the Low-DAG group, TAG levels were significantly decreased during the 8-week treatment (P<0.05), while changes in the levels of BG, TC, HDLC, and LDLC were non-significant. In addition, compared with the TAG group, BG and TAG levels in the two DAG groups were significantly reduced at 8 weeks (P<0.05), while HDLC levels were significantly increased (P<0.05). Repeated measurements revealed that there was a significant correlation between the time and treatment oil with the levels of BG, TG, HDLC and LDLC in each group (P<0.05).
Table III.
Parameters associated with glucose and lipid metabolism (n=10).
| Group | BG (mmol/l) | TAG (mmol/l) | TC (mmol/l) | HDLC (mmol/l) | LDLC (mmol/l) |
|---|---|---|---|---|---|
| Control | |||||
| 0 weeks | 4.78±0.27 | 1.05±0.39 | 2.71±0.12 | 1.00±0.14 | 0.64±0.06 |
| 4 weeks | 4.75±0.34 | 1.03±0.44 | 2.73±0.13 | 1.03±0.13 | 0.65±0.11 |
| 8 weeks | 4.81±0.33 | 0.97±0.52 | 2.75±0.09 | 1.06±0.11 | 0.66±0.09 |
| TAG | |||||
| 0 weeks | 20.18±1.14a | 2.93±0.19a | 3.54±0.28a | 0.71±0.09a | 1.11±0.15a |
| 4 weeks | 20.95±1.78a | 2.99±0.18a | 3.64±0.26a | 0.66±0.07a | 1.14±0.16a |
| 8 weeks | 20.97±2.61a | 3.10±0.22a | 3.68±0.24a | 0.66±0.09a | 1.18±0.16a |
| Low-DAG | |||||
| 0 weeks | 20.23±1.39a | 3.08±0.14a | 3.53±0.28a | 0.77±0.08a | 1.11±0.15a |
| 4 weeks | 19.93±1.25a | 3.03±0.13a | 3.45±0.19a,b | 0.82±0.09a,b | 1.10±0.12a |
| 8 weeks | 19.77±0.92a | 2.89±0.20a | 3.34±0.20a,b | 0.83±0.08a,b | 1.06±0.20a |
| High-DAG | |||||
| 0 weeks | 20.73±2.04a | 3.04±0.21a | 3.55±0.31a | 0.73±0.08a | 1.11±0.15a |
| 4 weeks | 19.29±1.71a–c | 2.85±0.20a | 3.35±0.19a,b | 0.76±0.08a,b | 1.05±0.16a |
| 8 weeks | 18.36±0.81a–c | 2.45±0.29a–c | 3.21±0.15a–c | 0.79±0.11a,b | 1.08±0.19a |
| P-value | |||||
| Time | <0.0001 | <0.0001 | 0.32 | 0.05 | 0.81 |
| Oil | <0.0001 | <0.0001 | 0.48 | <0.0001 | <0.0001 |
| Time × oil | <0.0001 | <0.0001 | 0.41 | 0.026 | 0.023 |
P<0.05 vs. control group
P<0.05 vs. TAG group.
P<0.05 vs. 0 weeks in each group. Quantitative data are expressed as the mean ± standard deviation (n=10). Analysis of variance was used to analyze the difference between weeks 0 and 8, while multiple comparison between the groups was performed using the least significant difference t-test method. TAG, triacylglycerol; DAG, diacylglycerol; BG, blood glucose; TC, total cholesterol; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol.
Chemical and histological findings at 8 weeks post-DN induction
The effects of DAG on the levels of SCr, BUN, HbA1c, insulin, KI and AGEs are shown in Table IV. Compared with the control group, the levels of SCr, BUN, HbA1c, insulin, KI and AGEs in the TAG group were significantly increased (P<0.05). In addition, compared with the TAG group, the levels of SCr, BUN, HbA1c, insulin, KI and AGEs in the Low-DAG group were decreased, but this difference was non-significant (P>0.05); by contrast, the levels of insulin, KI and AGEs in the High-DAG group were significantly decreased (P<0.05). Thus, DAG oil demonstrated suppressive effects on the levels of SCr, BUN, HbA1c, insulin, KI and AGEs, which are associated with the renal function in DN rats.
Table IV.
Chemical and histological findings at 8 weeks after diabetic nephropathy induction.
| Parameter | Control | TAG | Low-DAG | High-DAG |
|---|---|---|---|---|
| SCr (umol/l) | 73.6±4.14 | 194.5±13.53a | 191.9±9.23a | 186.6±6.87a |
| BUN (mmol/l) | 8.79±1.79 | 15.14±1.86a | 14.83±1.11a | 14.48±0.71a |
| HbA1c (%) | 5.13±0.79 | 13.57±1.50a | 12.77±1.36a | 11.93±1.47a |
| Insulin (mmol/l) | 1.11±0.07 | 1.68±0.11a | 1.60±0.12a | 1.53±0.15a,b |
| KI (g/kg) | 5.18±0.60 | 10.89±1.14a | 9.93±2.43a | 7.44±1.63a,b |
| AGEs (ng/mg) | 22.31±1.85 | 46.96±3.02a | 44.32±3.60a | 33.21±3.58a,b |
P<0.05 vs. control group
P<0.05 vs. TAG group. Quantitative data are expressed as the mean ± standard deviation (n=10), and were analyzed by one-way analysis of variance. Multiple comparisons between the groups were performed using the least significant difference t-test method. TAG, triacylglycerol; DAG, diacylglycerol; SCr, serum creatinine; BUN, blood urea nitrogen; HbA1c, glycohemoglobin A1c; KI, kidney index; AGEs, advanced glycation end products.
Upro and Ualb excretion at 8 weeks post-DN induction
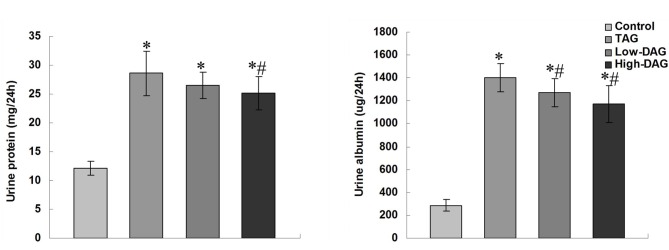
The effects of DAG on the levels of Upro and Ualb are shown in Fig. 3. Compared with the control group, the levels of Upro and Ualb in the TAG group were significantly increased (P<0.05). When compared with the TAG group, Upro levels in the High-DAG group were significantly reduced (P<0.05), but were not significant altered in the Low-DAG group (P>0.05). By contrast, Ualb levels were significantly reduced both in the Low-DAG and High-DAG groups (P<0.05).
Figure 3.
Urinary protein and urine albumin excretion at 8 weeks after diabetic nephropathy induction. Data are presented as the means ± standard deviation (n=10). *P<0.05 vs. control group; #P<0.05 vs. TAG group. DAG, diacylglycerol; TAG, triacylglycerol.
Pathological changes in renal tissues of the experimental rats assessed by H&E staining
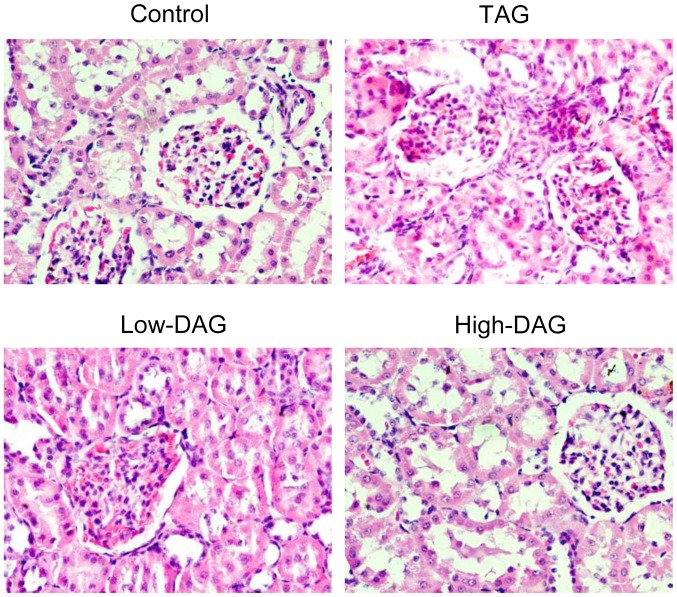
Rat kidney tissues were examined by electron microscopy, and representative images are demonstrated in Fig. 4. H&E-stained glomeruli presented expanded mesangium in all DN groups compared with the control group. The TAG group animals were the most affected, as the glomerular volume increased, the glomerular mesangial broadened, the matrix was evidently increased, and the proximal convoluted tubule epithelial cells disappeared. Lower degrees of glomerular enlargement and mesangial expansion were detected in the Low-DAG and High-DAG groups in comparison with the TAG group.
Figure 4.
Pathological changes in renal tissues of experimental rats stained with hematoxylin-eosin (magnification, ×400). Ten different sections were analyzed in each group. DAG, diacylglycerol; TAG, triacylglycerol.
Immunohistochemical staining of TGF-β1 in renal tissues from experimental rats
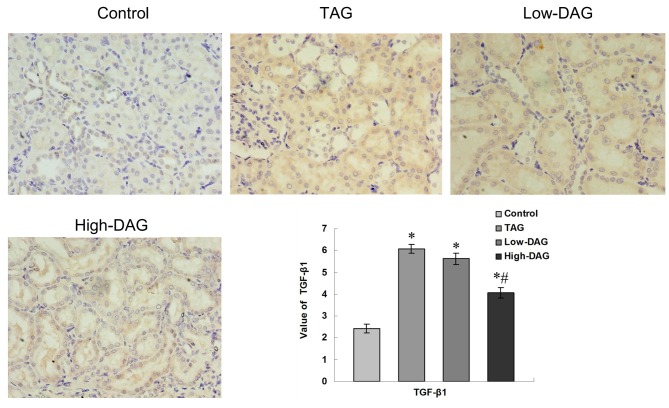
Representative immunohistochemistry slides for TGF-β1 expression and the results of the quantitative analysis are illustrated in Fig. 5. Light staining was observed in the control group, whereas significantly increased staining was detected in the TAG group and moderate staining in the High-DAG group. Reduced expression levels of TGF-β1 in the glomerular, extracellular matrix (ECM) and renal tubular areas were observed in the DAG groups compared with the TAG group. Quantitative analysis of the TGF-β1-stained area revealed that there was a significant increase in all groups compared with the control group (P<0.05). However, the High-DAG group demonstrated significantly reduced staining in comparison with the TAG group (P<0.05). Furthermore, a reduction in TGF-β1 staining was also observed in the Low-DAG group as compared with the TAG group, but this difference was not statistically significant (P>0.05).
Figure 5.
Immunohistochemical staining of TGF-β1 in renal tissues from experimental rats (magnification, ×400). Quantification of the value of the TGF-β1-stained area was analyzed using a secondary scoring method. Data are represented as the mean ± standard deviation (n=10). *P<0.05 vs. control group; #P<0.05 vs. TAG group. DAG, diacylglycerol; TAG, triacylglycerol; TGF-β1, transforming growth factor-β1.
Immunohistochemical staining of CTGF in renal tissues from experimental rats
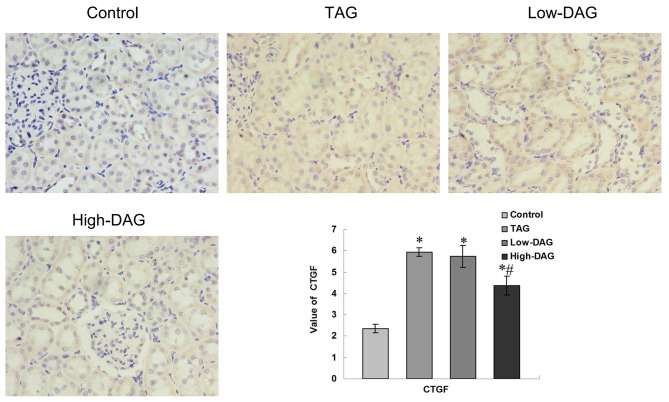
Representative immunohistochemistry slides for CTGF and the quantitative analysis results are demonstrated in Fig. 6. Light staining for CTGF was observed in the control group, with significantly increased staining detected in the TAG group and moderate staining in the High-DAG group. Decreased expression of CTGF in the glomerular, ECM and renal tubular regions were observed in the DAG groups as compared with the TAG group. Quantitative analysis of the CTGF-stained area revealed that, compared with the control group, there was a significant increase in all DN groups (P<0.05). The High-DAG group, however, demonstrated a significant reduction in CTGF expression as compared with the TAG group (P<0.05).
Figure 6.
Immunohistochemical staining of CTGF in renal tissues from experimental rats (magnification, ×400). Quantification of the value of the CTGF-stained area was analyzed using a secondary scoring method. Data are represented as the mean ± standard deviation (n=10). *P<0.05 vs. control group; #P<0.05 vs. TAG group. DAG, diacylglycerol; TAG, triacylglycerol; CTGF connective tissue growth factor.
Discussion
DAG naturally exists in various cooking oils (8,9); however, its ingestion has metabolic characteristics that are distinct from TAG, and these effects are most evident in nutrition metabolism. Nagao et al reported that dietary DAG, in contrast to TAG, decreased the body weight in healthy men (12). In addition, Murase et al reported that dietary DAG suppresses the accumulation of body weight induced by high-fat and high-sucrose diet in C57BL/6J mice (26). In the present study, the effects of DAG on type 2 DN rats were investigated, and it was observed that rats in the control group without DN continued to gain weight, while model rats continued to lose weight during the follow-up period. These findings are similar to the observations of Danda et al (6). In addition, the present study indicated that DAG oil intake reduced the body weight levels compared with TAG intake. However, there was no significant difference in the food intake between the TAG and DAG diet groups. Furthermore, it was confirmed that fatty acid compositions did not differ significantly between TAG oil and DAG oil, suggesting that reduced accumulation of body weight in the DAG group was not solely associated with reduced energy intake, and that this difference may be due to the disparate metabolic characteristics.
Previous studies have indicated that DAG oil intake can reduce the postprandial elevation in serum lipid levels compared with TAG intake (10). In addition, serum glucose is also significantly improved in patients with higher glucose levels at the baseline (>7.00 mmol/l) in the DAG oil group (17). Numerous experimental studies have reported that DAG oils have an effective activity that can delay diabetes and hyperlipidemia (10,12,14). However, to the best of our knowledge, there are no previous studies available on the effects of DAG on DN in type 2 diabetes. In the present study, consistent with previous findings (12,17), DAG oil consumption was found to significantly reduce the fasting BG levels in animals with type 2 diabetes with nephropathy. The delayed progression of renal failure by DAG oil may have resulted from the improvement in abnormal glucose metabolism, a predictor of progressive renal dysfunction (17). In addition, HbA1c levels, a relatively stable indicator of DM (26), were decreased in the High-DAG group in the current study, suggesting that DAG oils may be effective activities in delaying DN progression. It was also observed that TAG and TC levels were significantly reduced, while HDLC levels were significantly increased compared with the TAG group, suggesting that DAG oils can improve the abnormal lipid metabolism, which is associated with the pathogenesis of DN, and these findings were similar to previously published results (10). The present research also found that insulin levels were significantly reduced in the two DAG groups. Previous trials revealed that DAG significantly reduced the serum insulin (P=0.036) and insulin resistance (homeostasis model assessment) in patients with a normal weight (17), suggesting that hyperglycemia functions synergistically with other factors, such as insulin, to alter the course of kidney disease (27).
Indices associated with renal metabolism were also investigated in the current study. Animals with type 2 diabetes present hyperglycemia and abnormal Upro excretion, while the hyperglycemia appears to be a major factor in determining the Upro levels (28). High level of Ualb is an evidence of existing nephropathy, which is also associated with the generalized vascular pathology. There is a progressive increase of renal risk with increasing Ualb excretion rates (27). Thickening of the glomerular basement membrane causing textural abnormalities and abnormal chemical composition has been considered to be the major cause of Ualb (28). The present study demonstrated that feeding DAG oils to experimental rats significantly reduced Upro, Ualb and KI levels compared with the TAG group (P<0.05), while reduced glomerular enlargement and mesangial expansion were detected in the DAG treatment groups compared with the TAG groups. These results suggested that DAG oils can improve the hemodynamics and then improve the injury of the renal structure, which is associated with pathogenesis of DN.
The possible underlying metabolic mechanism was also analyzed in the current study. AGEs are substances created by proteins and glucose, and serve a pivotal role in the development of DN (29). The AGE deposition can directly lead to structural changes in the kidneys, such as the incrassation of glomerular basement membrane, glomerular hypertrophy and glomerulosclerosis (3). In addition, AGEs can combine with RAGE, which activates the expression of TGF-β1, and then damage cell metabolic activities (30). Dysregulation of growth factors appears to be critical in mediating glomerular extracellular mesangial matrix accumulation, also serving an important role in DN pathogenesis (4). In vitro studies have demonstrated that high glucose concentrations induce the expression of TGF-β1, CTGF and vascular endothelial growth factor (30). A study on transgenic rats revealed that TGF-β1 may cause glomerular sclerosis based on in situ hybridization findings (31). TGF-β1, in particular, serves an important role during the course of DN (32,33), and has been reported to promote kidney mesangial cell hypertrophy in renal tissues, drive ECM production and affect the ECM metabolism (34). Furthermore, TGF-β1 induces the mRNA and protein expression of CTGF (35), which is a major autocrine growth factor (7) that can also lead to kidney tissue fibrosis. In the present study, AGE levels were significantly increased in the TAG group compared with the control group. Immunohistochemical staining of growth factors in renal tissues revealed significantly increased TGF-β1 and CTGF expression levels in the TAG group. One possible mechanism of diabetic kidney disease may involve the high BG level, which is a characteristic of DN, causing abnormal glucose metabolism and then inducing AGEs. The AGE deposition may then lead to structural changes in the kidneys directly. In addition, AGEs can combine with RAGE, which then activates the expression of TGF-β1 and CTGF, with high TGF-β1 levels leading to glomerular enlargement and glomerulosclerosis. Kidney structure lesions can also lead to a higher glomerular filtration rate, increasing SCr, BUN, Ualb and Upro levels. Furthermore, abnormal lipid metabolism leads to lipid deposits in the renal mesangial area, drives ECM production and then results in glomerulosclerosis.
In conclusion, the present study observed that DAG oils functions via a slower metabolic pathway, significantly improved abnormal BG and lipid metabolism, reduced the generation of AGEs, and decreased the levels of TGF-β1 and CTGF. These changes prevented glomerular enlargement and glomerulosclerosis, and ultimately delayed the process of kidney failure. This provided important information for the use of DAG oils to improve DN condition in clinical practice. Further studies to investigate the specific underlying mechanism are warranted for the application of DAG oils in order to improve the quality of life in diabetic patients.
Acknowledgements
The current study was financially supported by a grant from the National Natural Sciences Foundation of China (no. 31201343). The authors would like to thank Dr Qiu Bin, Dr Tao Haiteng, Dr Zong Aizhen and Dr Liu Wei of Shandong Academy of Agricultural Science for their help with experiment guidance and article review.
Glossary
Abbreviations
- AGEs
advanced glycation end products
- BG
blood glucose
- BUN
blood urea nitrogen
- CTGF
connective tissue growth factor
- DAG
diacylglycerol
- DM
diabetes mellitus
- DN
diabetic nephropathy
- HDLC
high-density lipoprotein cholesterol
- H&E
hematoxylin-eosin
- HbA1c
glycohemoglobin A1c
- KI
kidney index
- LDLC
low-density lipoprotein cholesterol
- SCr
serum creatinine
- TAG
triacylglycerol
- TC
total cholesterol
- TGF-β1
transforming growth factor-β1
- Ualb
urine albumin
- Upro
urine protein
References
- 1.Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H, Sun X, Mao L, Xiang H. Attenuating effect of Fufang Xueshuantong Capsule on kidney function in diabetic nephropathy model. J Nat Med. 2013;67:86–97. doi: 10.1007/s11418-012-0654-y. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Li Y. Advances in research on risk and prognosis of diabetic nephropathy. J Integrated Tradit Western Med Nephrol. 2015;16:169–171. (In Chinese) [Google Scholar]
- 3.D'Agati V, Schmidt AM. RAGE and the pathogenesis of chronic Kidney disease. Nat Rev Nephrol. 2010;6:352–360. doi: 10.1038/nrneph.2010.54. [DOI] [PubMed] [Google Scholar]
- 4.Meng J, Sakata N, Imanaga Y, Takebayashi S, Nagai R, Horiuchi S. Carboxymethyllysine in dermal tissues of diabetic and nondiabetic patients with chronic renal failure: Relevance to glycoxidation damage. Nephron. 2001;88:30–35. doi: 10.1159/000045955. [DOI] [PubMed] [Google Scholar]
- 5.Hocevar BA, Howe PH. Analysis of TGF-beta-mediated synthesis of extracellular matrix components. Methods Mol Biol. 2000;142:55–65. doi: 10.1385/1-59259-053-5:55. [DOI] [PubMed] [Google Scholar]
- 6.Danda RS, Habiba NM, Rincon-Choles H, Bhandari BK, Barnes JL, Abboud HE, Pergola PE. Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int. 2005;68:2562–2571. doi: 10.1111/j.1523-1755.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease: The case for transforming growth factor-b as a key mediator. Diabetes. 1995;44:1139–1146. doi: 10.2337/diabetes.44.10.1139. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Nabey AA, Shehata AAY, Ragab MH, Rossell JB. Glycerides of cottonseed oils from Egyptian and other varieties. Riv Ital Delle Sostanze Grasse. 1992;69:443–447. [Google Scholar]
- 9.D'alonzo RP, Kozarek WJ, Wade RL. Glyceride composition of processed fats and oils as determined by glass capillary gas chromatography. J Am Oil Chem Soc. 1982;59:292–295. doi: 10.1007/BF02662229. [DOI] [Google Scholar]
- 10.Taguchi H, Watanabe H, Onizawa K, Nagao T, Gotoh N, Yasukawa T, Tsushima R, Shimasaki H, Itakura H. Double-blind controlled study on the effects of dietary diacylglycerol on postprandial serum and chylomicron triacylglycerol responses in healthy humans. J Am Coll Nutr. 2000;19:789–796. doi: 10.1080/07315724.2000.10718079. [DOI] [PubMed] [Google Scholar]
- 11.Tada N, Watanabe H, Matsuo N, Tokimitsu I, Okazaki M. Dynamics of postprandial remnant-like lipoprotein particles in serum after loading of diacylglycerols. Clin Chim Acta. 2001;311:109–117. doi: 10.1016/S0009-8981(01)00583-6. [DOI] [PubMed] [Google Scholar]
- 12.Nagao T, Watanabe H, Goto N, Onizawa K, Taguchi H, Matsuo N, Yasukawa T, Tsushima R, Shimasaki H, Itakura H. Dietary diacylglycerol suppresses accumulation of body fat compared with triacylglycerol in men in a double-blind controlled trial. J Nutr. 2000;130:792–797. doi: 10.1093/jn/130.4.792. [DOI] [PubMed] [Google Scholar]
- 13.Maki KC, Davidson MH, Tsushima R, Matsuo N, Tokimitsu I, Umporowicz DM, Dicklin MR, Foster GS, Ingram KA, Anderson BD, et al. Consumption of diacylglycerol oil as part of a reduced-energy diet enhances loss of body weight and fat in comparison with consumption of a triacylglycerol control oil. Am J Clin Nutr. 2002;76:1230–1236. doi: 10.1093/ajcn/76.6.1230. [DOI] [PubMed] [Google Scholar]
- 14.Xu T, Li X, Ma X, Zhang Z, Zhang T, Li D. Effect of diacylglycerol on postprandial serum triacylglycerol concentration: A meta-analysis. Lipids. 2009;41:161–168. doi: 10.1007/s11745-008-3258-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto K, Asakawa H, Tokunaga K, Watanabe H, Matsuo N, Tokimitsu I, Yagi N. Long-term ingestion of dietary diacylglycerol lowers serum triacylglycerol in type II diabetic patients with hypertriglyceridemia. J Nutr. 2001;131:3204–3207. doi: 10.1093/jn/131.12.3204. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama M, Tanigawa K, Murata T, Kobayashi Y, Tada E, Suzuki I, Nakabou Y, Kuwahata M, Kido Y. Dietary polyunsaturated fatty acids slow the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Nutr Res. 2010;30:217–225. doi: 10.1016/j.nutres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Xu T, Takase H, Tokimitsu I, Zhang P, Wang Q, Yu X, Zhang A. Diacylglycerol-induced improvement of whole-body insulin sensitivity in type 2 diabetes mellitus: A long-term randomized, double-blind controlled study. Clin Nutr. 2008;27:203–211. doi: 10.1016/j.clnu.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Tomonobu K, Asakawa H, Tokunaga K, Hase T, Tokimitsu I, Yagi N. Diet therapy with Diacylglycerol oil Delays the progression of renal failure in type 2 diabetic patients with nephropathy. Diabetes Care. 2006;29:417–419. doi: 10.2337/diacare.29.02.06.dc05-2223. [DOI] [PubMed] [Google Scholar]
- 19.Haseena BHK, Kanchana KG, Shanthi Deepa P, Sachdanandam P. Nephroprotective effect of Semecarpus anacardium on diabetic nephropathy in type 2 diabetic rats. Comp Clin Pathol. 2014;23:443–449. doi: 10.1007/s00580-012-1639-7. [DOI] [Google Scholar]
- 20.Xie W, Du L. High-cholesterol diets impair short-term retention of memory in alloxan-induced diabetic mice, but not acquisition of memory in prediabetic mice. Life Sci. 2005;77:481–495. doi: 10.1016/j.lfs.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Tada N, Shoji K, Takeshita M, Watanabe H, Yoshida H, Hase T, Matsuo N, Tokimitsu I. Effects of diacylglycerol ingestion on postprandial hyperlipidemia in diabetes. Clin Chim Acta. 2005;353:87–94. doi: 10.1016/j.cccn.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Liu YY, Mao L, Mo QZ. Experimental NIDDM rat model. Zhonghua Neifenmi Daixie Zazhi. 1990;6:115. (In Chinese) [Google Scholar]
- 23.Guo XH, Liu ZH, Li H, Li LS. Type 2 diabetes mellitus induced by diets and its features of renal involvement in rat. Zhongguo Tangniaobing Zazhi. 2002;10:291. (In Chinese) [Google Scholar]
- 24.Wang ZS, Gao F, Lu FE. Effect of ethanol extract of rhodiola rosea on the early nephropathy in type 2 diabetic rats. J Huazhong Univ Sci Technolog Med Sci. 2013;33:375–378. doi: 10.1007/s11596-013-1127-6. [DOI] [PubMed] [Google Scholar]
- 25.Fang WK, Chen B, Xu XE, Liao LD, Wu ZY, Shen J, Xu LY, Li EM. Altered expression and localization of desmoglein 3 in esophageal squamous cell carcinoma. Acta Histochem. 2014;116:803–809. doi: 10.1016/j.acthis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Murase T, Mizuno T, Omachi T, Onizawa K, Komine Y, Kondo H, Hase T, Tokimitsu I. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice. J Lipid Res. 2001;42:372–378. [PubMed] [Google Scholar]
- 27.Monnler L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 28.Tobe SW, McFarlane PA, Naimark DM. Microalbuminuria in diabetes mellitus. Can Med Ass J. 2002;167:499–503. [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf G, Ritz E. Diabetic nephropathy in type 2 diabetes prevention and patient management. J Am Soc Nephrol. 2003;14:1396–1405. doi: 10.1097/01.ASN.0000065639.19190.CF. [DOI] [PubMed] [Google Scholar]
- 30.Mogensen CE. Microalbuminuria, blood pressure and diabetic renal disease: Origin and development of ideas. Diabetologia. 1999;42:263–285. doi: 10.1007/s001250051151. [DOI] [PubMed] [Google Scholar]
- 31.Abboud HE. Growth factors and diabetic nephrology: An overview. Kidney Int Suppl. 1997;60(Suppl):S3–S6. [PubMed] [Google Scholar]
- 32.Wahab NA, Schaefer L, Weston BS, Yiannikouris O, Wright A, Babelova A, Schaefer R, Mason RM. Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia. 2005;48:2650–2660. doi: 10.1007/s00125-005-0006-5. [DOI] [PubMed] [Google Scholar]
- 33.Park I, Kiyomoto H, Abboud SL, Abboud HE. Expression of transforming growth factor-beta and type IV collagen in early streptozotocin-induced diabetes. Diabetes. 1997;46:473–480. doi: 10.2337/diabetes.46.3.473. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert RE, Wilkinson-Berka JL, Johnson DW, Cox A, Soulis T, Wu LL, Kelly DJ, Jerums G, Pollock CA, Cooper ME. Renal expression of transforming growth factor-beta inducible gene-h3 in normal and diabetic rats. Kidney Int. 1998;54:1052–1062. doi: 10.1046/j.1523-1755.1998.00081.x. [DOI] [PubMed] [Google Scholar]
- 35.Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002;61:51–60. doi: 10.1046/j.1523-1755.2002.00103.x. [DOI] [PubMed] [Google Scholar]