Abstract
Breast cancer represents one of the most common forms of cancer in women worldwide, with an increase in the number of newly diagnosed patients in the last decade. The role of fatty acids, particularly of a diet rich in ω-3 and ω-6 polyunsaturated fatty acids (PUFAs), in breast cancer development is not fully understood and remains controversial due to their complex mechanism of action. However, a large number of animal models and cell culture studies have demonstrated that high levels of ω-3 PUFAs have an inhibitory role in the development and progression of breast cancer, compared to ω-6 PUFAs. The present review focused on recent studies regarding the correlation between dietary PUFAs and breast cancer development, and aimed to emphasize the main molecular mechanisms involved in the modification of cell membrane structure and function, modulation of signal transduction pathways, gene expression regulation, and antiangiogenic and antimetastatic effects. Furthermore, the anticancer role of ω-3 PUFAs through the modulation of microRNA expression levels was also reviewed.
Keywords: breast cancer, ω-3 and ω-6 polyunsaturated fatty acids, signal transduction pathways, microRNA expression, diet and health effects
1. Introduction
Breast cancer is a highly prevalent cancer in women worldwide, with ~1.6 million new cases diagnosed in 2015 (1). Globally, the incidence of breast cancer appears higher in industrialized countries, with the majority of cases being observed in Western Europe, Australia and New Zeeland, and North America (2). According to the National Institute of Statistics, 90% of women have their disease diagnosed in an advanced form, which dramatically decreases their chances of survival and their quality of life (3–5).
Breast cancer incidence is further increased as a response to multiple toxic environmental exposures or the presence of certain environmental factors, including radiation, mutagens or carcinogens (6,7). Meanwhile, epigenetic and genetic alterations may occur due to an unbalanced diet (1). Mammary cancer development and progression is directly affected by dietary habits and environmental exposure (1,6,8,9). Advances in new generation technologies, particularly in the fields of transcriptomics and metabolomics (10,11), have markedly facilitated the pursuit to elucidate the influence of diet at the molecular level (12). This may eventually contribute to the health evaluation for particular nutritional components, with the final purpose of developing novel functional food products (12,13).
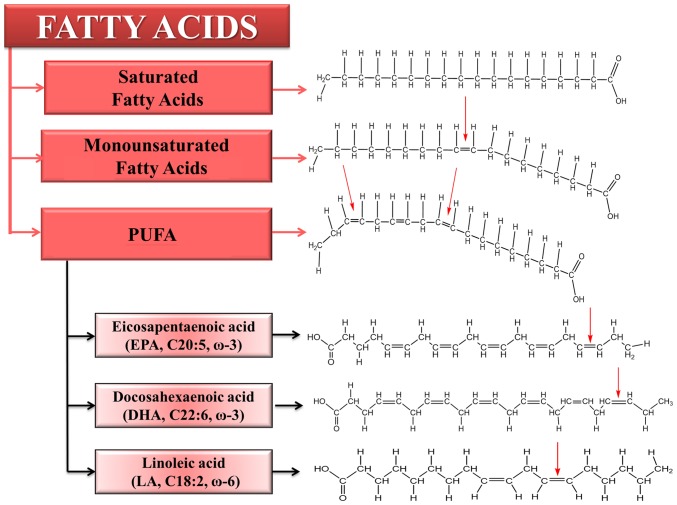
Presently there are a broad range of ongoing nutrigenomics studies focusing on detecting the mechanisms on which nutrient and gene interactions are based. Such studies may lead to the identification of genetic variants used for the discovery and development of novel biomarkers for specific and personalized diet prescriptions for each patient (14,15). A classic example is related to the Mediterranean diet, which is associated with reduced mortality rates for a wide range of pathologies, such as cancer (8). According to this, the increased olive oil consumption in a Mediterranean diet is linked to a reduced risk of breast cancer (16–18), due to the beneficial actions of polyunsaturated fatty acids (PUFAs). The main PUFAs are presented in Fig. 1. Therefore, the favorable effects of PUFAs have been demonstrated by epidemiological and experimental studies worldwide. The purpose of the present review was to summarize these findings.
Figure 1.
Types of fatty acids with emphasis on the main class of PUFAs. The difference between fatty acids is determined by the presence of double bonds. Eicosapentaenoic acid and docosahexaenoic acid are characterized by the double bond in three positions, also known as ω-3 PUFAs, while linoleic acid has the first double bond in position 6, also known as ω-6 PUFAs. PUFA, polyunsaturated fatty acid.
2. ω-3 and ω-6 fatty acid balance in a healthy diet
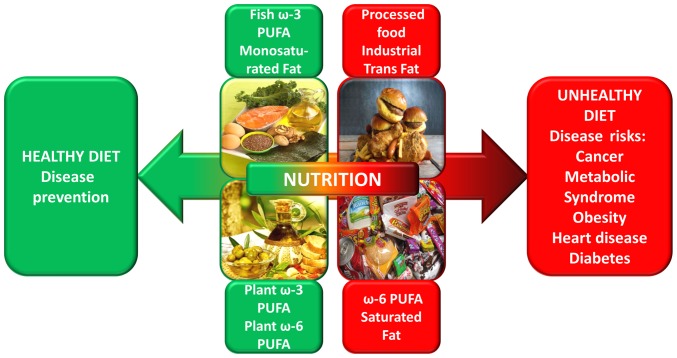
It is well known that modern society diets are dominated by processed foods and vegetable oils with high levels of ω-6 and low levels of ω-3 PUFAs (19). A proportion of 2:1 for the case of ω-6:ω-3 PUFAs is believed to have been present in our ancestors' diet, a ratio that today has been markedly altered to 10:1 because of unhealthy dietary habits (14). Overconsumption of ω-6 PUFAs, and an increased proportion of ω-6:ω-3 PUFA ratio observed in general in Western diets, leads to the activation of pathogenesis mechanisms for a wide range of pathologies (Fig. 2), including cardiovascular diseases, metabolic or immune pathologies, and cancer (14,20). Thus, the risk of cancer may be abridged by limiting the consumption of foods containing ω-3 fatty acids (21,22).
Figure 2.
Impact of dietary PUFAs in disease prevention or risk. The diagram emphasizes the importance of a balanced diet to maintain a healthy condition. The human body is unable to synthesize ω-6 and ω-3 PUFAs and they may only be obtained from a balanced diet. The amounts and balance of PUFAs in the diet are important for maintenance of and improving health due to their role in the body's functions, including immune and inflammatory responses, blood lipid levels, blood pressure and blood clotting. PUFA, polyunsaturated fatty acid.
For elongation and desaturation reactions, there is a competition for the same enzymes between the two types of fatty acids. High levels of ω-6 PUFAs result in an inhibition of the elongation and desaturation of ω-3 PUFAs (20–22). This competition between ω-3 and ω-6 PUFAs reveals the importance of low ratios of ω-3:ω-6, compared to the individual fatty acid concentrations in human organisms (21–29).
3. Implication of ω-3 and ω-6 fatty acids in breast cancer
The role of a fatty acid-rich diet in the development and progression of breast cancer is not well understood and remains challenging, particularly since the information from human studies is limited. In vitro cell culture investigations or in vivo animal models have demonstrated the tumor suppressive role of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA); however, the inhibitory role of ω-3 PUFAs in cancer is yet to be fully elucidated and requires further investigation (3,23,24).
PUFAs are essential nutrients, and include ω-6 fatty acids, such as linoleic and arachidonic acids (AA), as well as ω-3 fatty acids, including EPA and DHA acids. They have been demonstrated to have notable roles in modulating key cellular and molecular processes (Tables I and II) due to the fact that they are essential precursors of the cell membrane and interfere with other mediators of the inflammatory response (25,26). By adapting the fatty acid composition of the cells, a wide range of aspects related to cell metabolism may be controlled (24,25).
Table I.
Principle mechanisms of ω-3 polyunsaturated fatty acids in breast cancer.
| Mechanism | Key target/gene | (Refs.) |
|---|---|---|
| Changes of cell membrane properties | Bcl-2; procaspase-8 | (18,37) |
| Modulation of intracellular signaling pathways | FAK, NF-κB, MAPK, COX-2 | (33,82) |
| Regulation of gene expression | EGFR, Her-2, Erk 1/2, AKT PTEN, Bcl-2, PDCD4, NF-κB | (70,110–112) |
| Antimetastatic and antiangiogenic activity | EZH2, VEGF, E-cadherin | (36,103) |
| Regulation of miR expression | miR-21, miR-26a/b, miR19b, miR146b, miR183 | (34,42,110) |
Bcl-2, B-cell lymphoma 2; FAK, focal adhesion kinase; NF-κB, nuclear factor κB; MAPK, mitogen-activated protein kinase; COX-2, cyclooxygenase 2; EGFR, epidermal growth factor receptor; Erk, extracellular signal-regulated kinase; PTEN, phosphatase and tensin homolog; PDCD4, programmed cell death 4; EZH2, enhancer of zeste 2; VEGF, vascular epithelial growth factor; miR, microRNA.
Table II.
Principle mechanisms related to pro-carcinogenic effects of ω-6 polyunsaturated fatty acids in breast cancer.
| Mechanism | Key/target gene | (Refs.) |
|---|---|---|
| Lipid peroxidation, DNA adducts | Redox-cycling of 4-hydroxyestradiol | (21,26,37) |
| Regulation of gene expression | p21WAF1/CIP1, MAPK, TGF-β, TLR | (21,42) |
| Antimetastatic and antiangiogenic activity | VEGF, FGF, HIF-α, E-cadherin | (21,41,122) |
| Regulation of miR expression | MiR19b, miR146b, miR1835p, let-7a, | (42,109) |
| miR-23b, miR-27a/b, miR-21, let-7 |
MAPK, mitogen-activated protein kinase; TGF-β, transforming growth factor-β; TLR, toll-like receptor; VEGF, vascular epithelial growth factor; FGF, fibroblast growth factor; HIF-α, hypoxia-inducible factor-α; miR, microRNA.
Despite their distinct physiologic and metabolic characteristics, ω-6 and ω-3 PUFAs cannot be endogenously produced by the human body, and thus must be obtained from the diet; however, these should preferably be obtained in the correct ratio (21). This has been supported by multiple epidemiological studies, where a reduced ratio of ω-6:ω-3 PUFAs has been indicated to have beneficial effects (25–27). Studies have also demonstrated that a modern diet is related to estrogen receptor (ER) negative breast cancer risk among taller women (≥160 cm tall) (28).
EPA and DHA are present in marine organisms, particularly in ocean fish. According to a 2011 United Nations report, global fish consumption increased with a yearly average of 17 kg/person (1,30–32). In the nutritional etiology of breast cancer, ω-3 fatty acids of fish origin have been demonstrated to have a significant role as protective factors, being associated with a 14% reduction in the risk of developing this malignancy (29–31). When comparing tumor and normal breast tissue in terms of their fatty acid content, higher levels of ω-6 PUFAs were observed in malignant tissues (33). The study conclusion was that an increased expression level of the enzyme Δ-6 desaturase is desired, as well as an abundance of ω-6 PUFA precursors (32).
Preclinical studies have offered a higher understanding of the effects of PUFAs, particularly in the etiology of breast cancer (Fig. 3). These studies have attempted to explain the cancer-related preventive activity of ω-3 PUFAs (Table I), and the association between ω-6 PUFAs and procarcinogenic effects (Table II) in breast malignancies (18), leading to the alteration of gene expression patterns, as well as dysregulations of microRNA (miRNA) sequences. PUFAs were demonstrated to have effects on the composition of the plasma membrane (18,33,34), increased cellular oxidative stress (14), gene expression modifications (35,36), alterations to intracellular signaling pathways (37,38), antiangiogenic and antimetastatic activity (39–43).
Figure 3.
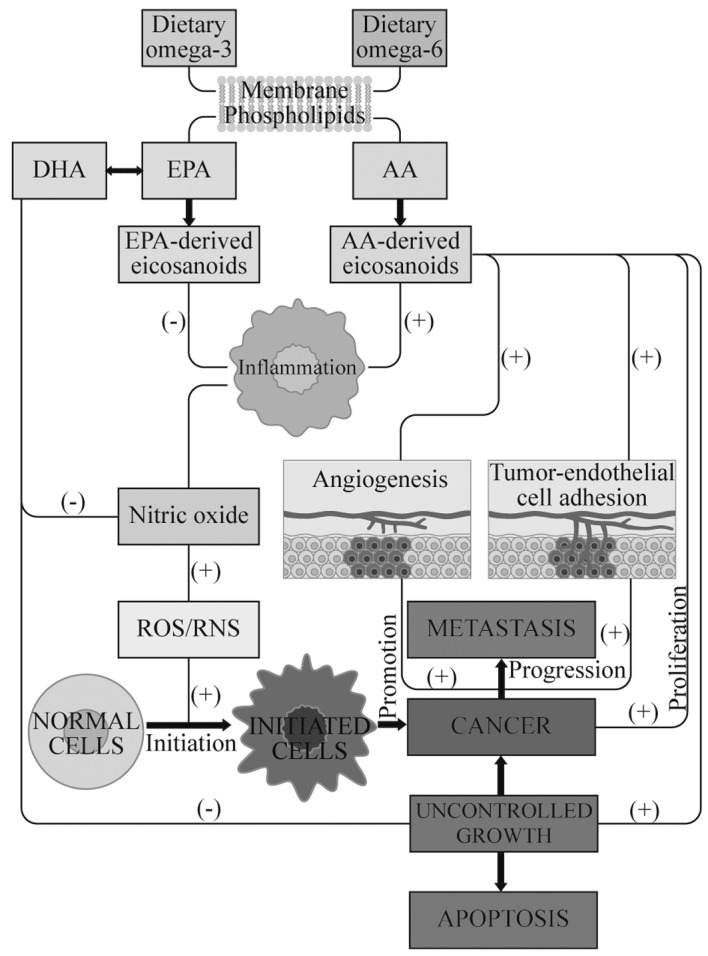
Potential mechanisms of action of ω-3 and ω-6 PUFAs in tumorigenesis, related to the activation of inflammation and production of ROS, which finally leads to the activation of cell proliferation, a predisposition for carcinogenesis and distant metastasis in breast cancer. PUFAs may stimulate (+) or suppress (−) pathways. Dietary ω-3 PUFAs suppress the inflammatory process, stimulate apoptosis, inhibit metastasis and tumor proliferation, and also upregulate the gene expression of antioxidant enzymes. In tumor cells, phospolipase A2, cyclooxygenase 2 and lipoxygenases are overexpressed and induce the overproduction of AA (20:4n-6)-derived eicosanoids, which lead to inflammatory processes. The production of nitric oxide is elevated in inflammation and is involved in the initiation and the progression of carcinogenesis. Nitric oxide may be responsible for tumor growth and metastasis due to its ability to stimulate tumor cell angiogenesis. ω-3 PUFAs reduce the desaturation and elongation of linoleic acid (18:2n-6) to AA. ROS, reactive oxygen species; PUFA, polyunsaturated fatty acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; RNS, reactive nitrogen species.
4. Modifications of cell membrane structure and function
Cell membrane integrity and alterations in signal transduction are important cellular processes in which ω-3 PUFAs are involved, and these cellular changes lead to reduced cell proliferation, the induction of apoptosis and an increased degree of unsaturation (38). Cell membrane structures, their fluidity and permeability, are affected in a notable manner by higher densities of ω-6 fatty acids (44).
High concentrations of ω-6 PUFAs have a potent effect on cell functions by damaging different ion transporters and channels, such as the ones for Ca2+ (38). Increased amounts of ω-6 PUFAs reduce the number of Ca2+ channels (45), which damages the fluidity of membranes and affects the function of specific integral and membrane-bound proteins (45–47).
The incorporation of ω-3 PUFAs, particularly EPA and DHA, is able to modify the degree of lipid peroxidation in cell membranes, altering the formation of lipid rafts and suppressing raft-associated cell signal transduction (37). The susceptibility to peroxidation is determined by the degree of unsaturation of the membrane phospholipid fatty acids. High unsaturation causes increased cell oxidative stress and disrupts physiological signaling pathways by leading to malignant transformation (48,49).
5. Modulation of signal transduction pathways
A clinical study revealed that ω-3 and ω-6 PUFAs have similar biochemical activity and require the same elongase, desaturase, cyclooxygenase and lipoxygenase enzymes (50). Enzymatic conversion into eicosanoids, compounds with notable roles in cell differentiation and growth, is among the most important cellular functions of PUFAs (50–60). Studies have demonstrated that a diet rich in ω-6 PUFAs has strong promoting effects on breast cancer development (50–59). The carcinogenic effects of high levels of ω-6 PUFAs were correlated with increased ratios of eicosanoids (48). Studies have also indicated that eicosanoid compounds, including prostaglandins (PG), thromboxane (TX), leukotrienes (LT), hydroxyl fatty acids and lipoxins, are produced in higher quantities than those of ω-3 PUFAs, due to the high amounts of ω-6 PUFAs present in Western diets (20,51,52).
Eicosanoids derived from ω-6 PUFAs have been demonstrated to have pro-inflammatory and pro-carcinogenic effects as compared to ω-3 PUFA-derived lipid mediators (48,53). In obese individuals, ω-6 PUFA-derived eicosanoid levels were observed to be increased, which stimulated breast cancer initiation, invasion and metastasis (54).
Cyclooxygenase (COX)-2 is an enzyme that serves an active role in prostaglandin synthesis, and increased levels are associated with inflammation in all subtypes of breast cancer (55–57). The COX and lipoxygenase (LOX) enzymes are key factors for the enzymatic production of PG and LT (58). ω-3 fatty acids compete with ω-6 fatty acids for COXs for the production of eicosanoids, and a suppressive effect on COX-2 expression has been observed (59–62). COX enzymes produce two-series prostanoids, including PG and TX, and four-series LT, including LTC4, LTD4, LTE4 and LTF4, while LOX enzymes produce hydroxyeicosatetraenoic acids (48,63). In a transgenic mouse model expressing human epidermal growth factor receptor 2 (HER2)/neu, treatment with dietary ω-3 PUFAs inhibited breast tumor cell proliferation and upregulated COX-2 expression (64).
In breast tissue, the metabolites of the arachidonate 5-lipoxygenase pathway are able to induce tumorigenesis and sustain breast cancer progression (65–68). A study on LOX genetic variants combined with ω-6 PUFAs revealed a significant increase of breast cancer risk (14). According to a study on the breast cancer cell line MCF-7, DHA upregulated syndecan-1 (a component of the extracellular matrix) expression and promoted apoptosis via downregulation of MEK/extracellular signal-regulated kinases (Erk)/Bad signaling (69). Another study indicated that ω-3 PUFA treatment reduced the effect of E2 on epidermal growth factor receptor (EGFR), Erk1/2 and AKT, and upregulated G protein-coupled estrogen receptor 1 (GPER1)-cyclic adenosine 5′-phosphate (cAMP)-protein kinase A (PKA) signaling (70). In MDA-MB-231 breast cancer cells, linoleic acid (LA) induces focal adhesion kinase (FAK) activation and cell migration by modulating a FAK-dependent pathway (33).
6. Regulation of gene expression
Evidence-based preclinical studies and epidemiologic data consistently support the anticancer effect of ω-3 PUFAs based on their capacity to target key genes altered in breast cancer (71–78). EGFR and HER2 are cell surface receptor tyrosine kinases, representing key therapeutic targets in breast cancer management (39,79,80). ω-3 PUFAs may represent a dietary approach for controlling growth factor-mediated carcinogenesis, by activating tyrosine kinase transduction pathways, p38 mitogen-activated protein kinase activation and apoptosis induction (71). Restoring EGFR signaling was observed in many breast cancer cases as being correlated with dietary habits (72), while an apoptotic effect of DHA from marine sources was found by targeting EGFR pathways in malignant breast tissue (38). In MCF-7 and T47D cells, ω-3 PUFA treatment may initiate pro-apoptotic signaling of estrogen by increasing the GPER1-cAMP-PKA signaling response, and inhibiting EGFR, Erk1/2 and AKT activity (70).
Overexpression of the tyrosine kinase receptor, ErbB2/HER2/neu, occurs in 25–30% of invasive breast cancer cases with poor prognosis (73). HER2/neu is an oncogene that is overexpressed in many types of cancer, with an important role in development, progression and chemosensitivity of tumors; studies have demonstrated that it is downregulated by ω-3 PUFAs (73,74).
As described in previous studies, ω-3 PUFA effects are also observed at the translational and post-translational level. In mammary cancer cell lines (MCF10A, MCF7, T47D and MDA-MB-231), ω-3 PUFAs may modulate the protein expression of the transcription regulator enhancer of zeste 2 polycomb repressive complex 2 subunit (36), while the activation of peroxisome proliferator-activated receptors (PPAR) was induced in the same type of cancer cells (75). PPARs (PPARα, PPARγ and PPARβ/δ) are ligand-activated transcription factors of the nuclear hormone receptor superfamily involved in glucose and fatty acid metabolism (76). This PUFA-mediated PPAR activation exerts an effect on several molecular mechanisms, including apoptosis and autophagy (36,81–84). PPARβ expression was reduced by a ω-3 PUFA-rich diet in mammary tumors, while in other circumstances, the expression of other PPAR mRNA was modulated, leading to the inhibition of breast cancer cell growth (35). In MCF-7 breast cancer cells, ω-3 PUFA ethanolamides, docosahexaenoyl ethanolamine (DHEA) and eicosapentaenoyl ethanolamine (EPEA), augmented the expression of PPARγ, inducing autophagy (77). At the same time, in MCF-7 and MDA-MB-231 cells, AA decreased the Erk1/2 phosphorylation level, and positively modulated PPARγ and PPARα expression (78). In MCF-7 cells, DHEA and EPEA stimulated the expression of PPARγ as well as PPAR response element-dependent transcription by upregulating phosphatase and tensin homolog (PTEN) expression, while inhibiting the AKT-mechanistic target of the syntetic agent rapamycin via mTOR pathway (77). In another study, MCF-7 cells treated with DHA from a cultured microalga demonstrated increased apoptosis via the upregulation of the B-cell lymphoma 2 (Bcl-2)-associated X protein/Bcl-2 ratio, and inhibition of cell growth (79). In a rat model of breast cancer, dietary ω-3 PUFAs increased the apoptotic index in tumor cells (80,81).
AA induces nuclear factor (NF)κB-DNA binding activity through a phosphoinositide 3-kinase- and AKT-dependent pathway (82). ω-3 PUFAs were demonstrated to modulate total AKT expression (83). In contrast with ω-6 PUFAs, ω-3 PUFAs reduced COX-2 and NFκB expression, decreasing the level of cell invasiveness (84). LA induces FAK and NFκB activation, migration and invasion in MCF10A human mammary epithelial cells (85).
Another fundamental process related to carcinogenesis is cell proliferation. Ki-67 is a nuclear protein used as a prognostic or predictive marker in breast cancer (86). Treatment with α-linolenic acid (ALA)-rich flaxseed oil in rats induced a decrease in tumor size, together with decreased Ki-67 levels (87,88). Proliferating cell nuclear antigen (PCNA) is also considered by researchers to be a potential prognostic marker in breast cancer (89). It has been demonstrated that diets rich in ω-3 PUFAs reduce the percentage of proliferating tumor cells by decreasing the expression levels of PCNA (90).
Studies have indicated that PUFAs have an effect on lipid metabolism in mammary tumors by modifying the expression levels of fatty acid binding protein 5, cluster of differentiation 36, FAS and ER genes (91). The alterations of gene expression levels were demonstrated to be time-dependent in MDA-MB-231 cells following ALA treatment, accompanied by low levels of ID1, and increased JUN, NME1 and thrombospondin 1 expression (92). A significant increase of Erk1/2 and AKT phosphorylation levels was observed in MCF-7 cells treated with a combination of tamoxifen and ω-3 PUFA, compared to ω-3 PUFA alone (93). Additionally, mammary tumor growth and Py230 cancer cell proliferation was inhibited by ω-3 PUFAs, independent of GPR120 signaling, suggesting that ω-3 PUFAs act in a Toll-like receptor 4-mediated fashion, or via peroxisome proliferator-activated receptors or other G protein-coupled receptors (94).
7. Antimetastatic and antiangiogenic activity
Different fatty acid compositions affect the affect the breast cancer carcinogenic mechanisms, in particular those associated with tumor growth, proangiogenic and metastatic capacities in breast cancer cells (95,96). In HT115 and MDA-MB-231, ω-6 PUFAs enhanced the expression of a metastasis-suppressor gene, nm-23 (97). Studies conducted on various animal models have demonstrated the effect of PUFAs in regards to cellular growth. A reduction in tumor growth and proliferative abilities caused by PUFAs was observed in an immunocompromised nude murine model of transplanted human breast cancer cells (98). A fish oil diet rich in EPA and DHA in a murine model of MDA-MB-231 human breast cancer cells led to the prevention of bone metastases (99).
The molecular mechanism by which the administration of PUFAs, alone or alongside other compounds, may affect the metastatic potential of tumors remains to be deciphered. The anti-proliferative and anti-invasion activity of DHA may be connected to alterations in the composition of fatty acids, which leads to damage to the membranes of tumor cells, and consequently reduction in metastatic potential (96,100,101).
A diet rich in fatty acids (DHA and EPA) of marine origin in patients with breast cancer was correlated with reduced mortality (102). ω-3 PUFAs lead to E-cadherin expression upregulation, while inhibiting the invasion mechanisms in mammary malignant cells (36), since the appropriate expression of E-cadherin is important in maintaining the integrity of intracellular adhesions (103).
In human breast tumor tissues, AA and cytosolic phospholipase A2 were demonstrated to be associated with the signaling activity of mTOR complex (C)1 and mTORC2, and with expression levels of vascular epithelial growth factor (68).
8. Regulation of miRNA expression
The mechanisms by which dietary factors modulate the expression of miRNA in breast cancer cells have not been completely elucidated (104–106). Experimental studies have suggested that some nutrients, including ω-3 PUFAs (107–109), have anticancer effects through the modulation of miRNA expression levels (110–113). Previous studies have demonstrated extensive interactions between ω-3 PUFAs and miRNA in cancer, lipid metabolism and inflammation (105). Recent findings indicated that DHA, which has anti-inflammatory and anticancer effects, is able to downregulate miRNA-21, causing an increase in tumor necrosis factor α mRNA expression levels and, subsequently, triggering apoptosis in human cancer cells (106,107). High expression of miRNA-21 is strongly correlated with poor prognosis in breast cancer, demonstrating a negative impact on overall survival and disease/recurrence-free survival (104,106,108). As part of its mechanism of action, DHA treatment promotes inhibition of receptor-interacting protein 1 kinase and AMP-activated protein kinase-α, resulting in nuclear accumulation of Foxo3a, which, in turn, binds to the miRNA-21 promoter causing its transcriptional repression (107). Other studies have demonstrated that expression levels of certain miRNA, including let-7a, miRNA-23b, miRNA-27a/b, miRNA-21, let-7 and miRNA-320b, in breast cancer cell exosomes have been increased by DHA treatment (47–109). An in vitro study on mammary cancer cell models indicated that DHA treatment inhibited the expression of colony stimulating factor 1 and miRNA-21, supporting the evidence found by an in vivo study (110). In MCF-7 and MDA-MB-231 breast cancer cell lines, the promoter of miRNA-21 contains a NFκB binding element, which, in association with the DHA treatment, leads to decreased miRNA-21 expression levels by inhibiting NFκB activity (105,110). Although the mechanisms by which ω-3 PUFAs contribute to the altered expression of these miRNA remain unclear, some authors suggest that its direct targets are involved, together with other associated proteins, including PTEN, Bcl-2, programmed cell death 4 and NFκB (110–112).
A PUFA-enriched diet correlates with changes in circulating miRNA (upregulated miRNA include miRNA-18a, −19b, −106a, −130b, −192, −486-5p and −769-5p; downregulated miRNA include miRNA-125a-5p, −221, −328 and −330-3p) that may serve an important role in the PUFA dietary systemic effect (113). In a rat model of inflammation, dietary ω-3 and ω-6 PUFAs may alter the miRNA expression profile (42). Functional analyses of these changes in miRNA expression profiles have indicated that dietary PUFAs are implicated in the maintenance of immune homeostasis via the expression of miRNA (43). In same study by Zheng et al (42), it was demonstrated that ω-3 PUFAs suppress inflammation in vivo by inhibiting the expression levels of miR-18-5p, −19b-3p and −146b-5p.
In addition to inflammation homeostasis, ω-3 PUFAs have also been implicated in the downregulation of miRNA-26a/b expression, promoting the upregulation of 15-hydroxyprostaglandin dehydrogenase, which catalyzes the oxidation of the pro-inflammatory lipid mediator, prostaglandin E2, leading to a decrease in cell proliferation (34). DHA may positively modulate expression levels of miRNA related to lipid metabolism, including miRNA-30c and −192, in cancer and obesity (114–117). Studies in breast cancer have demonstrated that miRNA-30c negatively regulates NFκB signaling and cell cycle progression (118), while miRNA-192 inhibits cell proliferation (119). Knockdown of DICER in enterocyte Caco-2 cells exposed to DHA lipid micelles revealed multiple genes regulating lipid metabolism that are modulated by miRNA-30c and miR-192 (117). miR-33a and miR-122 expression levels in the liver are upregulated in rats with cafeteria-diet induced dyslipidemia; however, these levels are counteracted by the presence of ω-3 PUFAs in vivo (120).
The beneficial effects of ω-3 PUFAs extend to its related metabolites, such as Resolvin D1 (RvD1), which is a well-known anti-inflammatory agent that induces upregulation of miRNA-208a and miR-219 in in vivo transgenic mice overexpressing N-formyl peptide receptor 2 (121). A study by Krishnamoorthy et al (121) indicated that this RvD1-induced upregulation of miRNA-208a promotes increased secretion levels of the anti-inflammatory cytokine, interleukin-10, in human macrophages.
9. Conclusion
Dietary factors, such as fatty acids, have been recognized as influential factors in the activation of carcinogenic events or disease progression, and have been associated with a direct connection to breast cancer prevention. PUFAs differentially inhibit mammary tumor development by inflicting modifications to the morphology of cell membranes, and influencing signaling pathways, gene expression and apoptosis. Observing the molecular mechanisms involved in the activity of dietary PUFAs on breast cancer development and progression suggests that dietary supplements, in combination with anticancer drugs, should be provided under medical supervision. The majority of studies recommend that patients consume a diet rich in ω-3 PUFAs, while reducing the intake of ω-6 PUFAs, particularly in the case of chemoprevention purposes. Therefore, modification of dietary habits, particularly regarding the choice and amounts of fats consumed, may be used as a strategy for breast cancer prevention. For better results in this field, additional clinical trials are required to evaluate the specific effects of PUFAs on breast cancer outcomes.
Acknowledgements
The present study was part of the research ‘New strategies for improving life quality and survival in cancer patients: Molecular and clinical studies of the tumor genome in deuterium-depleted water treatment augmentation-GenCanD’ (grant no. 128/2014; PN-II-PT-PCCA-2013-4-2166) and ‘Targeting the TGFβ pathway: An alternative for breast cancer therapy; POSCCE 709 BreastImpact’ (grant no. PN-II-RU-TE-2014-4-1464, 307 from 01/10/2015).
References
- 1.Fenga C. Occupational exposure and risk of breast cancer. Biomed Rep. 2016;4:282–292. doi: 10.3892/br.2016.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irimie A, Achimas-Cadariu P, Burz C, Puscas E. Multiple primary malignancies-epidemiological analysis at a single tertiary institution. J Gastrointestin Liver Dis. 2010;19:69–73. [PubMed] [Google Scholar]
- 3.Bougnoux P, Hajjaji N, Maheo K, Couet C, Chevalier S. Fatty acids and breast cancer: Sensitization to treatments and prevention of metastatic re-growth. Prog Lipid Res. 2010;49:76–86. doi: 10.1016/j.plipres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Tourassi G, Yoon HJ, Xu S. A novel web informatics approach for automated surveillance of cancer mortality trends. J Biomed Inform. 2016;61:110–118. doi: 10.1016/j.jbi.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay D, Kim SK, Taylor S, Abramowitz JS, Tolin D, Coles M, Timpano KR, Olatunji B. An examination of obsessive-compulsive symptoms and dimensions using profile analysis via multidimensional scaling (PAMS) J Anxiety Disord. 2014;28:352–357. doi: 10.1016/j.janxdis.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Snedeker SM. Chemical exposures in the workplace: Effect on breast cancer risk among women. AAOHN J. 2006;54:270–279. doi: 10.1177/216507990605400604. quiz 280–281. [DOI] [PubMed] [Google Scholar]
- 7.Braicu C, Berindan-Neagoe I, Pileczki V, Cojocneanu-Petric R, Pop LA, Puscas E, Irimie A, Buiga R. Breast tumor bank: An important resource for developing translational cancer research in Romania. Cancer Biomark. 2014;14:119–127. doi: 10.3233/CBM-130309. [DOI] [PubMed] [Google Scholar]
- 8.Carruba G, Cocciadiferro L, Di Cristina A, Granata OM, Dolcemascolo C, Campisi I, Zarcone M, Cinquegrani M, Traina A. Nutrition, aging and cancer: Lessons from dietary intervention studies. Immun Ageing. 2016;13:13. doi: 10.1186/s12979-016-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braicu C, Chiorean R, Irimie A, Chira S, Tomuleasa C, Neagoe E, Paradiso A, Achimas-Cadariu P, Lazar V, Berindan-Neagoe I. Novel insight into triple-negative breast cancers, the emerging role of angiogenesis, and antiangiogenic therapy. Expert Rev Mol Med. 2016;18:e18. doi: 10.1017/erm.2016.17. [DOI] [PubMed] [Google Scholar]
- 10.Irimie AI, Braicu C, Cojocneanu-Petric R, Berindan-Neagoe I, Campian RS. Novel technologies for oral squamous carcinoma biomarkers in diagnostics and prognostics. Acta Odontol Scand. 2015;73:161–168. doi: 10.3109/00016357.2014.986754. [DOI] [PubMed] [Google Scholar]
- 11.Braicu C, Cojocneanu-Petric R, Chira S, Truta A, Floares A, Petrut B, Achimas-Cadariu P, Berindan-Neagoe I. Clinical and pathological implications of miRNA in bladder cancer. Int J Nanomedicine. 2015;10:791–800. doi: 10.2147/IJN.S72904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitó M, Konstantinidou V. Nutritional genomics and the mediterranean diet's effects on human cardiovascular health. Nutrients. 2016;8:218. doi: 10.3390/nu8040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ordovas JM, Kaput J, Corella D. Nutrition in the genomics era: Cardiovascular disease risk and the Mediterranean diet. Mol Nutr Food Res. 2007;51:1293–1299. doi: 10.1002/mnfr.200700041. [DOI] [PubMed] [Google Scholar]
- 14.Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med (Maywood) 2010;235:785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- 15.Minich DM, Bland JS. Personalized Lifestyle Medicine: Relevance for nutrition and lifestyle recommendations. ScientificWorldJournal. 2013;2013:129841. doi: 10.1155/2013/129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.High olive oil consumption linked to lower breast cancer risk. Harv Womens Health Watch. 2015;23:8. [PubMed] [Google Scholar]
- 17.Filik L, Ozyilkan O. Olive-oil consumption and cancer risk. Eur J Clin Nutr. 2003;57:191. doi: 10.1038/sj.ejcn.1601497. [DOI] [PubMed] [Google Scholar]
- 18.Corsetto PA, Montorfano G, Zava S, Jovenitti IE, Cremona A, Berra B, Rizzo AM. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2011;10:73. doi: 10.1186/1476-511X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of Omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 21.Mansara PP, Deshpande RA, Vaidya MM, Kaul-Ghanekar R. Differential ratios of omega fatty acids (AA/EPA+DHA) modulate growth, lipid peroxidation and expression of tumor regulatory MARBPs in breast cancer cell lines MCF7 and MDA-MB-231. PLoS One. 2015;10:e0136542. doi: 10.1371/journal.pone.0136542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negi AK, Bhatnagar A, Agnihotri N. Fish oil augments celecoxib mediated alteration in apoptotic pathway in the initiation phase of 7,12-dimethylbenz(α) anthracene-induced mammary carcinogenesis. Biomed Pharmacother. 2016;79:9–16. doi: 10.1016/j.biopha.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Serini S, Fasano E, Piccioni E, Cittadini AR, Calviello G. Differential anti-cancer effects of purified EPA and DHA and possible mechanisms involved. Curr Med Chem. 2011;18:4065–4075. doi: 10.2174/092986711796957310. [DOI] [PubMed] [Google Scholar]
- 24.Merendino N, Costantini L, Manzi L, Molinari R, D'Eliseo D, Velotti F. Dietary ω-3 polyunsaturated fatty acid DHA: A potential adjuvant in the treatment of cancer. Biomed Res Int. 2013;2013:310186. doi: 10.1155/2013/310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simopoulos AP. An Increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Nair J, Linseisen J, Owen RW, Bartsch H. Lipid peroxidation and DNA adduct formation in lymphocytes of premenopausal women: Role of estrogen metabolites and fatty acid intake. Int J Cancer. 2012;131:1983–1990. doi: 10.1002/ijc.27479. [DOI] [PubMed] [Google Scholar]
- 27.Bassett JK, Hodge AM, English DR, MacInnis RJ, Giles GG. Plasma phospholipids fatty acids, dietary fatty acids, and breast cancer risk. Cancer Causes Control. 2016;27:759–773. doi: 10.1007/s10552-016-0753-2. [DOI] [PubMed] [Google Scholar]
- 28.Hidaka BH, Kimler BF, Fabian CJ, Carlson SE. An empirically derived dietary pattern associated with breast cancer risk is validated in a nested case-control cohort from a randomized primary prevention trial. Clin Nutr ESPEN. 2017;17:8–17. doi: 10.1016/j.clnesp.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Lim SY, Shin A, Sung MK, Ro J, Kang HS, Lee KS, Kim SW, Lee ES. Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: A case-control study. BMC cancer. 2009;9:216. doi: 10.1186/1471-2407-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyengar NM, Hudis CA, Gucalp A. Omega-3 fatty acids for prevention of breast cancer: An update and the state of the science. Curr Breast Cancer Rep. 2013;5:247–254. doi: 10.1007/s12609-013-0112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pender-Cudlip MC, Krag KJ, Martini D, Yu J, Guidi A, Skinner SS, Zhang Y, Qu X, He C, Xu Y, et al. Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue. Cancer Sci. 2013;104:760–764. doi: 10.1111/cas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serna-Marquez N, Villegas-Comonfort S, Galindo-Hernandez O, Navarro-Tito N, Millan A, Salazar EP. Role of LOXs and COX-2 on FAK activation and cell migration induced by linoleic acid in MDA-MB-231 breast cancer cells. Cell Oncol (Dordr) 2013;36:65–77. doi: 10.1007/s13402-012-0114-4. [DOI] [PubMed] [Google Scholar]
- 34.Yao L, Han C, Song K, Zhang J, Lim K, Wu T. Omega-3 polyunsaturated fatty acids upregulate 15-PGDH expression in cholangiocarcinoma cells by inhibiting miR-26a/b expression. Cancer Res. 2015;75:1388–1398. doi: 10.1158/0008-5472.CAN-14-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wannous R, Bon E, Mahéo K, Goupille C, Chamouton J, Bougnoux P, Roger S, Besson P, Chevalier S. PPARβ mRNA expression, reduced by n-3 PUFA diet in mammary tumor, controls breast cancer cell growth. Biochim Biophys Acta. 2013;1831:1618–1625. doi: 10.1016/j.bbalip.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, Dimri GP. Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis. 2010;31:489–495. doi: 10.1093/carcin/bgp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers KR, Kikawa KD, Mouradian M, Hernandez K, McKinnon KM, Ahwah SM, Pardini RS. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis. 2010;31:1523–1530. doi: 10.1093/carcin/bgq111. [DOI] [PubMed] [Google Scholar]
- 38.Corsetto PA, Montorfano G, Zava S, Jovenitti IE, Cremona A, Berra B, Rizzo AM. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2011;10:73. doi: 10.1186/1476-511X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merendino N, Costantini L, Manzi L, Molinari R, D'Eliseo D, Velotti F. Dietary ω-3 polyunsaturated fatty acid DHA: A potential adjuvant in the treatment of cancer. Biomed Res Int. 2013;2013:310186. doi: 10.1155/2013/310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Qian SY. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomedical J. 2014;37:112–119. doi: 10.4103/2319-4170.131378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose DP, Connolly JM. Regulation of tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr Cancer. 2000;37:119–127. doi: 10.1207/S15327914NC372_1. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Z, Ge Y, Zhang J, Xue M, Li Q, Lin D, Ma W. PUFA diets alter the microRNA expression profiles in an inflammation rat model. Mol Med Rep. 2015;11:4149–4157. doi: 10.3892/mmr.2015.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onisim A, Achimas-Cadariu A, Vlad C, Kubelac P, Achimas-Cadariu P. Current insights into the association of Nestin with tumor angiogenesis. J BUON. 2015;20:699–706. [PubMed] [Google Scholar]
- 44.Wassall SR, Stillwell W. Docosahexaenoic acid domains: The ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Belevych AE, Ho HT, Terentyeva R, Bonilla IM, Terentyev D, Carnes CA, Gyorke S, Billman GE. Dietary omega-3 fatty acids promote arrhythmogenic remodeling of cellular Ca2+ handling in a postinfarction model of sudden cardiac death. PLoS One. 2013;8:e78414. doi: 10.1371/journal.pone.0078414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen G, Wandall-Frostholm C, Sadda V, Oliván-Viguera A, Lloyd EE, Bryan RM, Jr, Simonsen U, Köhler R. Alterations of N-3 polyunsaturated fatty acid-activated K2P channels in hypoxia-induced pulmonary hypertension. Basic Clin Pharmacol Toxicol. 2013;113:250–258. doi: 10.1111/bcpt.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 49.Owen RW, Haubner R, Wurtele G, Hull E, Spiegelhalder B, Bartsch H. Olives and olive oil in cancer prevention. Eur J Cancer Prev. 2004;13:319–326. doi: 10.1097/01.cej.0000130221.19480.7e. [DOI] [PubMed] [Google Scholar]
- 50.Fabian CJ, Kimler BF. Marine-derived omega-3 fatty acids: Fishing for clues for cancer prevention. Am Soc Clin Oncol Educ Book. 2013:97–101. doi: 10.1200/EdBook_AM.2013.33.97. [DOI] [PubMed] [Google Scholar]
- 51.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 52.Wysoczański T, Sokoła-Wysoczańska E, Pękala J, Lochyński S, Czyż K, Bodkowski R, Herbinger G, Patkowska-Sokoła B, Librowski T. Omega-3 fatty acids and their role in central nervous system-a review. Curr Med Chem. 2016;23:816–831. doi: 10.2174/0929867323666160122114439. [DOI] [PubMed] [Google Scholar]
- 53.Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 54.Vona-Davis L, Rose DP. The obesity-inflammation-eicosanoid axis in breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:291–307. doi: 10.1007/s10911-013-9299-z. [DOI] [PubMed] [Google Scholar]
- 55.Miglietta A, Toselli M, Ravarino N, Vencia W, Chiecchio A, Bozzo F, Motta M, Torchio B, Bocca C. COX-2 expression in human breast carcinomas: Correlation with clinicopathological features and prognostic molecular markers. Expert Opin Ther Targets. 2010;14:655–664. doi: 10.1517/14728222.2010.486792. [DOI] [PubMed] [Google Scholar]
- 56.Harris RE, Casto BC, Harris ZM. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J Clin Oncol. 2014;5:677–692. doi: 10.5306/wjco.v5.i4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Ma D. The Role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients. 2014;6:5184–5223. doi: 10.3390/nu6115184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Culp BR, Titus BG, Lands WE. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med. 1979;3:269–278. doi: 10.1016/0161-4630(79)90068-5. [DOI] [PubMed] [Google Scholar]
- 60.Hamid R, Singh J, Reddy BS, Cohen LA. Inhibition by dietary menhaden oil of cyclooxygenase-1 and −2 in N-nitrosomethylurea-induced rat mammary tumors. Int J Oncol. 1999;14:523–528. doi: 10.3892/ijo.14.3.523. [DOI] [PubMed] [Google Scholar]
- 61.Ringbom T, Huss U, Stenholm Å, Flock S, Skattebøl L, Perera P, Bohlin L. COX-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod. 2001;64:745–749. doi: 10.1021/np000620d. [DOI] [PubMed] [Google Scholar]
- 62.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calder PC. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc Nutr Soc. 2013;72:326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 64.Yee LD, Agarwal D, Rosol TJ, Lehman A, Tian M, Hatton J, Heestand J, Belury MA, Clinton SK. The inhibition of early stages of HER-2/neu-mediated mammary carcinogenesis by dietary n-3 PUFAs. Mol Nutr Food Res. 2013;57:320–327. doi: 10.1002/mnfr.201200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett. 2007;258:31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Tavakoli-Yaraki M, Karami-Tehrani F, Salimi V, Sirati-Sabet M. Induction of apoptosis by Trichostatin A in human breast cancer cell lines: Involvement of 15-Lox-1. Tumor Biol. 2013;34:241–249. doi: 10.1007/s13277-012-0544-7. [DOI] [PubMed] [Google Scholar]
- 67.Moumen M, Chiche A, Decraene C, Petit V, Gandarillas A, Deugnier MA, Glukhova MA, Faraldo MM. Myc is required for β-catenin-mediated mammary stem cell amplification and tumorigenesis. Mol Cancer. 2013;12:132. doi: 10.1186/1476-4598-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen ZH, Su YC, Lai PL, Zhang Y, Xu YF, Zhao A, Yao GY, Jia CH, Lin J, Xu S, et al. Critical role of arachidonic acid-activated mTOR signaling in breast carcinogenesis and angiogenesis. Oncogene. 2013;32:160–170. doi: 10.1038/onc.2012.47. [DOI] [PubMed] [Google Scholar]
- 69.Sun H, Hu Y, Gu Z, Owens RT, Chen YQ, Edwards IJ. Omega-3 fatty acids induce apoptosis in human breast cancer cells and mouse mammary tissue through syndecan-1 inhibition of the MEK-Erk pathway. Carcinogenesis. 2011;32:1518–1524. doi: 10.1093/carcin/bgr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao W, Ma Z, Rasenick MM, Yeh S, Yu J. N-3 poly-unsaturated fatty acids shift estrogen signaling to inhibit human breast cancer cell growth. PLoS One. 2012;7:e52838. doi: 10.1371/journal.pone.0052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–553. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 72.Bhargava R, Gerald WL, Li AR, Pan Q, Lal P, Ladanyi M, Chen B. EGFR gene amplification in breast cancer: Correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol. 2005;18:1027–1033. doi: 10.1038/modpathol.3800438. [DOI] [PubMed] [Google Scholar]
- 73.Zou Z, Bellenger S, Massey KA, Nicolaou A, Geissler A, Bidu C, Bonnotte B, Pierre AS, Minville-Walz M, Rialland M, et al. Inhibition of the HER2 pathway by n-3 polyunsaturated fatty acids prevents breast cancer in fat-1 transgenic mice. J Lipid Res. 2013;54:3453–3463. doi: 10.1194/jlr.M042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menéndez JA, Vázquez-Martín A, Ropero S, Colomer R, Lupu R. HER2 (erbB-2)-targeted effects of the omega-3 polyunsaturated fatty acid, alpha-linolenic acid (ALA; 18:3n-3) in breast cancer cells: The ‘fat features’ of the ‘Mediterranean diet’ as an ‘anti-HER2 cocktail’. Clin Transl Oncol. 2006;8:812–820. doi: 10.1007/s12094-006-0137-2. [DOI] [PubMed] [Google Scholar]
- 75.Clay CE, Namen AM, Atsumi G, Willingham MC, High KP, Kute TE, Trimboli AJ, Fonteh AN, Dawson PA, Chilton FH. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 1999;20:1905–1911. doi: 10.1093/carcin/20.10.1905. [DOI] [PubMed] [Google Scholar]
- 76.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, Lanzino M, De Amicis F, Sisci D, Mauro L, et al. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J Cell Physiol. 2013;228:1314–1322. doi: 10.1002/jcp.24288. [DOI] [PubMed] [Google Scholar]
- 78.Bocca C, Bozzo F, Martinasso G, Canuto RA, Miglietta A. Involvement of PPARalpha in the growth inhibitory effect of arachidonic acid on breast cancer cells. Br J Nutr. 2008;100:739–750. doi: 10.1017/S0007114508942161. [DOI] [PubMed] [Google Scholar]
- 79.Chiu LC, Wong EY, Ooi VE. Docosahexaenoic acid from a cultured microalga inhibits cell growth and induces apoptosis by upregulating Bax/Bcl-2 ratio in human breast carcinoma MCF-7 cells. Ann N Y Acad Sci. 2004;1030:361–368. doi: 10.1196/annals.1329.045. [DOI] [PubMed] [Google Scholar]
- 80.Jiang W, Zhu Z, McGinley JN, El Bayoumy K, Manni A, Thompson HJ. Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary N-3 fatty acids. Cancer Res. 2012;72:3795–3806. doi: 10.1158/0008-5472.CAN-12-1047. [DOI] [PubMed] [Google Scholar]
- 81.Dozio E, Ruscica M, Passafaro L, Dogliotti G, Steffani L, Marthyn P, Pagani A, Demartini G, Esposti D, Fraschini F, Magni P. The natural antioxidant alpha-lipoic acid induces p27(Kip1)-dependent cell cycle arrest and apoptosis in MCF-7 human breast cancer cells. Eur J Pharmacol. 2010;641:29–34. doi: 10.1016/j.ejphar.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 82.Villegas-Comonfort S, Castillo-Sanchez R, Serna-Marquez N, Cortes-Reynosa P, Salazar EP. Arachidonic acid promotes migration and invasion through a PI3K/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2014;90:169–177. doi: 10.1016/j.plefa.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 83.Ravacci GR, Brentani MM, Tortelli T, Jr, Torrinhas RS, Saldanha T, Torres EA, Waitzberg DL. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J Nutr Biochem. 2013;24:505–515. doi: 10.1016/j.jnutbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Horia E, Watkins BA. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis. 2007;28:809–815. doi: 10.1093/carcin/bgl183. [DOI] [PubMed] [Google Scholar]
- 85.Espinosa-Neira R, Mejia-Rangel J, Cortes-Reynosa P, Salazar EP. Linoleic acid induces an EMT-like process in mammary epithelial cells MCF10A. Int J Biochem Cell Biol. 2011;43:1782–1791. doi: 10.1016/j.biocel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 86.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 87.Saggar JK, Chen J, Corey P, Thompson LU. Dietary flaxseed lignan or oil combined with tamoxifen treatment affects MCF-7 tumor growth through estrogen receptor- and growth factor-signaling pathways. Mol Nutr Food Res. 2010;54:415–425. doi: 10.1002/mnfr.200900068. [DOI] [PubMed] [Google Scholar]
- 88.Truan JS, Chen JM, Thompson LU. Flaxseed oil reduces the growth of human breast tumors (MCF-7) at high levels of circulating estrogen. Mol Nutr Food Res. 2010;54:1414–1421. doi: 10.1002/mnfr.200900521. [DOI] [PubMed] [Google Scholar]
- 89.Taftachi R, Ayhan A, Ekici S, Ergen A, Ozen H. Proliferating-cell nuclear antigen (PCNA) as an independent prognostic marker in patients after prostatectomy: A comparison of PCNA and Ki-67. BJU Int. 2005;95:650–654. doi: 10.1111/j.1464-410X.2005.05356.x. [DOI] [PubMed] [Google Scholar]
- 90.Olivo SE, Hilakivi-Clarke L. Opposing effects of prepubertal low- and high-fat n-3 polyunsaturated fatty acid diets on rat mammary tumorigenesis. Carcinogenesis. 2005;26:1563–1572. doi: 10.1093/carcin/bgi118. [DOI] [PubMed] [Google Scholar]
- 91.Zhang P, Kong J. Doxorubicin-tethered fluorescent silica nanoparticles for pH-responsive anticancer drug delivery. Talanta. 2015;134:501–507. doi: 10.1016/j.talanta.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 92.Wiggins AK, Mason JK, Thompson LU. Growth and gene expression differ over time in alpha-linolenic acid treated breast cancer cells. Exp Cell Res. 2015;333:147–154. doi: 10.1016/j.yexcr.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 93.Wu S, Guo Y, Wu Y, Zhu S, He Z, Chen YQ. Omega-3 free fatty acids inhibit tamoxifen-induced cell apoptosis. Biochem Biophys Res Commun. 2015;459:294–299. doi: 10.1016/j.bbrc.2015.02.103. [DOI] [PubMed] [Google Scholar]
- 94.Chung H, Lee YS, Mayoral R, Oh DY, Siu JT, Webster NJ, Sears DD, Olefsky JM, Ellies LG. Omega-3 fatty acids reduce obesity-induced tumor progression independent of GPR120 in a mouse model of postmenopausal breast cancer. Oncogene. 2015;34:3504–3513. doi: 10.1038/onc.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85:1743–1747. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 96.Rose DP, Connolly JM, Liu XH. Effects of linoleic acid and gamma-linolenic acid on the growth and metastasis of a human breast cancer cell line in nude mice and on its growth and invasive capacity in vitro. Nutr Cancer. 1995;24:33–45. doi: 10.1080/01635589509514391. [DOI] [PubMed] [Google Scholar]
- 97.Jiang WG, Hiscox S, Bryce RP, Horrobin DF, Mansel RE. The effects of n-6 polyunsaturated fatty acids on the expression of nm-23 in human cancer cells. Br J Cancer. 1998;77:731–738. doi: 10.1038/bjc.1998.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Senzaki H, Iwamoto S, Ogura E, Kiyozuka Y, Arita S, Kurebayashi J, Takada H, Hioki K, Tsubura A. Dietary effects of fatty acids on growth and metastasis of KPL-1 human breast cancer cells in vivo and in vitro. Anticancer Res. 1998;18:1621–1627. [PubMed] [Google Scholar]
- 99.Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2010;402:602–607. doi: 10.1016/j.bbrc.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kinoshita K, Noguchi M, Tanaka M. Effects of linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid on the growth and metastasis of MM48 mammary tumor transplants in mice. Int J Oncol. 1996;8:575–581. doi: 10.3892/ijo.8.3.575. [DOI] [PubMed] [Google Scholar]
- 101.Kimura Y, Sumiyoshi M. Antitumor and antimetastatic actions of eicosapentaenoic acid ethylester and its by-products formed during accelerated stability testing. Cancer Sci. 2005;96:441–450. doi: 10.1111/j.1349-7006.2005.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patterson RE, Flatt SW, Newman VA, Natarajan L, Rock CL, Thomson CA, Caan BJ, Parker BA, Pierce JP. Marine fatty acid intake is associated with breast cancer prognosis. J Nutr. 2011;141:201–206. doi: 10.3945/jn.110.128777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mareel M, Leroy A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev. 2003;83:337–376. doi: 10.1152/physrev.00024.2002. [DOI] [PubMed] [Google Scholar]
- 104.Berindan-Neagoe I, Calin GA. Molecular pathways: microRNAs, cancer cells, and microenvironment. Clin Cancer Res. 2014;20:6247–6253. doi: 10.1158/1078-0432.CCR-13-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berindan-Neagoe I, Pdel Monroig C, Pasculli B, Calin GA. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64:311–336. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin GA. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015;22:34–45. doi: 10.1038/cdd.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fluckiger A, Dumont A, Derangère V, Rébé C, de Rosny C, Causse S, Thomas C, Apetoh L, Hichami A, Ghiringhelli F, Rialland M. Inhibition of colon cancer growth by docosahexaenoic acid involves autocrine production of TNFα. Oncogene. 2016;35:4611–4622. doi: 10.1038/onc.2015.523. [DOI] [PubMed] [Google Scholar]
- 108.Tang Y, Zhou X, Ji J, Chen L, Cao J, Luo J, Zhang S. High expression levels of miR-21 and miR-210 predict unfavorable survival in breast cancer: A systemic review and meta-analysis. Int J Biol Markers. 2015;30:e347–e358. doi: 10.5301/jbm.5000160. [DOI] [PubMed] [Google Scholar]
- 109.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mandal CC, Ghosh-Choudhury T, Dey N, Choudhury GG, Ghosh-Choudhury N. miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis. 2012;33:1897–1908. doi: 10.1093/carcin/bgs198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shah MS, Davidson LA, Chapkin RS. Mechanistic insights into the role of microRNAs in cancer: Influence of nutrient crosstalk. Front Genet. 2012;3:305. doi: 10.3389/fgene.2012.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parasramka MA, Ho E, Williams DE, Dashwood RH. MicroRNAs, diet and cancer: New mechanistic insights on the epigenetic actions of phytochemicals. Mol Carcinog. 2012;51:213–230. doi: 10.1002/mc.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ortega FJ, Cardona-Alvarado MI, Mercader JM, Moreno-Navarrete JM, Moreno M, Sabater M, Fuentes-Batllevell N, Ramírez-Chávez E, Ricart W, Molina-Torres J, et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J Nutr Biochem. 2015;26:1095–1101. doi: 10.1016/j.jnutbio.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 114.Farago N, Fehér LZ, Kitajka K, Das UN, Puskás LG. MicroRNA profile of polyunsaturated fatty acid treated glioma cells reveal apoptosis-specific expression changes. Lipids Health Dis. 2011;10:173. doi: 10.1186/1476-511X-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chartoumpekis DV, Zaravinos A, Ziros PG, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, Habeos IG. Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS One. 2012;7:e34872. doi: 10.1371/journal.pone.0034872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077–2084. doi: 10.1093/carcin/bgp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gil-Zamorano J, Martin R, Daimiel L, Richardson K, Giordano E, Nicod N, García-Carrasco B, Soares SM, Iglesias-Gutiérrez E, Lasunción MA, et al. Docosahexaenoic acid modulates the enterocyte Caco-2 cell expression of microRNAs involved in lipid metabolism. J Nutr. 2014;144:575–585. doi: 10.3945/jn.113.189050. [DOI] [PubMed] [Google Scholar]
- 118.Shukla K, Sharma AK, Ward A, Will R, Hielscher T, Balwierz A, Breunig C, Münstermann E, König R, Keklikoglou I, Wiemann S. MicroRNA-30c-2-3p negatively regulates NF-κB signaling and cell cycle progression through downregulation of TRADD and CCNE1 in breast cancer. Mol Oncol. 2015;9:1106–1119. doi: 10.1016/j.molonc.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu F, Meng X, Tong Q, Liang L, Xiang R, Zhu T, Yang S. BMP-6 inhibits cell proliferation by targeting microRNA-192 in breast cancer. Biochim Biophys Acta. 2013;1832:2379–2390. doi: 10.1016/j.bbadis.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 120.Baselga-Escudero L, Arola-Arnal A, Pascual-Serrano A, Ribas-Latre A, Casanova E, Salvadó MJ, Arola L, Blade C. Chronic administration of proanthocyanidins or docosahexaenoic acid reverses the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS One. 2013;8:e69817. doi: 10.1371/journal.pone.0069817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hardman WE, Sun L, Short N, Cameron IL. Dietary omega-3 fatty acids and ionizing irradiation on human breast cancer xenograft growth and angiogenesis. Cancer Cell Int. 2005;5:12. doi: 10.1186/1475-2867-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]