Abstract
Background
Invasive group A Streptococcus (GAS) infections are associated with significant morbidity and mortality rates. We report the epidemiology and trends of invasive GAS over 8 years of surveillance.
Methods
From January 2005 through December 2012, we collected data from the Centers for Disease Control and Prevention’s Active Bacterial Core surveillance, a population-based network of 10 geographically diverse US sites (2012 population, 32.8 million). We defined invasive GAS as isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis (NF) or streptococcal toxic shock syndrome (STSS). Available isolates were emm typed. We calculated rates and made age- and race-adjusted national projections using census data.
Results
We identified 9557 cases (3.8 cases per 100 000 persons per year) with 1116 deaths (case-fatality rate, 11.7%). The case-fatality rates for septic shock, STSS, and NF were 45%, 38%, and 29%, respectively. The annual incidence was highest among persons aged ≥65 years (9.4/100 000) or <1 year (5.3) and among blacks (4.7/100 000). National rates remained steady over 8 years of surveillance. Factors independently associated with death included increasing age, residence in a nursing home, recent surgery, septic shock, NF, meningitis, isolated bacteremia, pneumonia, emm type 1 or 3, and underlying chronic illness or immunosuppression. An estimated 10 649–13 434 cases of invasive GAS infections occur in the United States annually, resulting in 1136–1607 deaths. In a 30-valent M-protein vaccine, emm types accounted for 91% of isolates.
Conclusions
The burden of invasive GAS infection in the United States remains substantial. Vaccines under development could have a considerable public health impact.
Keywords: group A Streptococcus, epidemiology, surveillance, vaccine, streptococcal toxic shock syndrome
Invasive infections caused by group A Streptococcus (GAS) include bacteremic pneumonia, sepsis, necrotizing fasciitis (NF), streptococcal toxic shock syndrome (STSS), and focal infections, such as arthritis and bacteremic skin and soft-tissue infections (SSTIs). GAS more commonly causes noninvasive infections such as pharyngitis, sometimes with suppurative (eg, otitis media) or nonsuppurative (eg, acute rheumatic fever, acute glomerulonephritis) complications. GAS infections are associated with substantial morbidity and mortality rates worldwide, with an estimated 500 000 deaths globally per year [1–4].
Management of invasive GAS infection relies heavily on timely diagnosis, appropriate antimicrobial therapy, and, in some cases (eg, NF), prompt surgical intervention [5]. There are few prevention tools other than recommendations for control of disease in nosocomial clusters and outbreaks in closed facilities [6, 7]. Several GAS vaccine candidates are currently under development [8–11], including a 30-valent vaccine covering M proteins common in North America and Europe and those considered “rheumatogenic” [12].
The Centers for Disease Control and Prevention (CDC) have conducted surveillance for invasive GAS infection since 1995 as part of the Active Bacterial Core surveillance (ABCs) of the Emerging Infections Programs Network [13]. We report the epidemiologic characteristics of invasive GAS infection in the United States for 2005–2012, evaluate trends in incidence since 2000, and estimate potential impact of the 30-valent vaccine [12] currently under development.
METHODS
Surveillance
Invasive GAS cases were identified through ABCs, a population-and laboratory-based surveillance system that tracks invasive GAS in 10 geographically disparate US sites, covering a population of 32.8 million (10.4% of the total US population) in 2012. ABCs defines an invasive GAS infection as isolation of GAS from a normally sterile body site (eg, blood or cerebrospinal fluid), or from a wound culture accompanied by NF or STSS, in a resident of the surveillance catchment population. The ABCs sites and counties in which surveillance has been consistent since 2005 include the areas around San Francisco Bay, California (3 counties), Denver, Colorado (5 counties), Atlanta, Georgia (20 counties), Baltimore, Maryland (6 counties), Portland, Oregon (3 counties), and Rochester and Albany, New York (15 counties); urban areas in Tennessee (11 counties); and the entire states of Minnesota, New Mexico, and Connecticut. These areas were included for incidence rate and trend calculations. In 2010, Tennessee expanded its surveillance area to 20 counties; this larger surveillance area was included in descriptive analyses only. In trend analyses that included surveillance from 2000 through 2004, the 2 most recently added ABCs sites were excluded (Colorado joined ABCs in 2001, New Mexico in 2004).
Descriptive Epidemiology
Descriptive analyses included all cases with culture dates from 1 January 2005 through 31 December 2012. Annual summary of overall disease rates are shared on the ABCs Web site [14]. For national rate and burden estimates, race- and age-specific disease rates were applied from the aggregate surveillance area to the age and racial distributions of the US population for each year. We used regional and national postcensal population estimates for each year as the denominator. All incidences are expressed as cases per 100 000 population per year. The Cochran-Armitage test was used to test the statistical significance of the linear trend in disease incidence from 2000 to 2012. Case patients with unknown race were distributed according to those with known race in each surveillance area and age category. Case-fatality rates (CFRs) were calculated using outcome at the time of discharge from hospital, emergency department, or clinic.
Case patients with GAS cultured from blood, but for whom no clinical syndrome was identified, were categorized as having bacteremia without a source. To allow comparisons with published ABCs GAS data from 1995–1999 [1] and 2000–2004 [2], we categorized clinical syndromes as described in previous reports, unless otherwise indicated. We compared clinical syndromes in children aged <10 years and adults aged ≥65 years with those in persons aged 10–64 years, using log binomial regression models. We also modeled the probability of death among case patients with a specific clinical syndrome using log binomial models and compared the CFR of the youngest and oldest age groups with that for patients aged 10–64 years.
Case patients who had undergone surgery in the 7 days preceding their first positive culture result were categorized as “recent surgery.” Any infection in a pregnant woman, whether specific to pregnancy (eg, endometritis, chorioamnionitis, septic abortion) or simply occurring during pregnancy, was considered “pregnancy related.” Hospital-onset infections were defined as infections in case patients hospitalized for ≥3 days before their positive GAS culture. Underlying conditions were grouped similarly to previously published data [1, 2]. Two variables added to the ABCs case report form in 2009 were analyzed separately: chronic skin breakdown and septic shock syndrome. We modeled the probability that a case patient had a specific underlying condition using a log binomial model with surveillance area as the only covariate. We compared the proportions of case patients with an underlying condition in each site with the mean proportion having the underlying condition across all ABCs sites.
GAS Typing
All available GAS isolates were typed at the CDC’s Streptococcus laboratory after they were confirmed to be GAS. A combination of emm sequence typing and restriction profiling was used to deduce emm types through 2007. Beginning in 2008, emm typing for all isolates was performed by sequencing the variable M serotype–specific region of the emm gene amplicons. The protocol for emm typing and a complete, downloadable listing of emm type sequences are available online [15, 16].
Estimates of Vaccine Benefit
To estimate the potential benefits of a multivalent vaccine, we used available emm typing results to calculate the proportions of cases and deaths by emm type in the proposed 30-valent vaccine (types 1, 2, 3, 4, 5, 6, 11, 12, 14, 18, 19, 22, 24, 28, 29, 44, 49, 58, 73, 75, 77, 78, 81, 82, 83, 87, 89, 92, 114, and 118) [12].
Predictors of Death
Predictors of death were evaluated using a logistic regression model. In this model we reclassified the clinical syndrome of case patients with multiple clinical syndromes; these cases were classified with the highest-severity syndrome, based on the CFR for case patients presenting with only 1 syndrome, and SSTIs (not including NF) were grouped into a single syndrome. Underlying illnesses and medical conditions known to increase risk for GAS infection were grouped into 4 categories: no underlying illness, skin condition (eg, burns, wounds, and chronic skin breakdown), chronic illness (eg, cirrhosis, obesity, diabetes, and chronic obstructive pulmonary disease), and immunosuppression (eg, sickle cell disease, nephrotic syndrome, human immunodeficiency virus [HIV]/AIDS, and cancer). A person with multiple underlying illnesses was placed in the category of highest severity based on the following decreasing levels of severity: immunosuppression, chronic illness, and skin condition.
The model included only case patients for whom information on all variables was available. We excluded case patients with an unknown outcome, those from a site that did not record information on HIV/AIDS status or collect GAS isolates, and those with no available isolate or with emm-nontypeable isolates. Variables associated with death (P < .20) in univariate analysis were entered in the multivariable model and retained if the P value was <.05. Collinearity and all 2-way interactions were evaluated. All analyses were conducted using SAS software, version 9.3.
RESULTS
Demographic Characteristics and Disease Rates
ABCs sites reported 9557 cases of invasive GAS infection during the 8-year surveillance period. GAS was most frequently isolated from blood (7837 cases; 82%), joint space (766 cases; 8%), pleural fluid and tissue (249 cases; 3%), bone (175 cases; 2%), muscle (172 cases; 2%), peritoneal fluid (134 cases; 1%), and cerebrospinal fluid (75 cases; 1%). For some patients, GAS was isolated from >1 site. The median age of case patients was 52 years (range, 0 days to 106 years); 54% were male. Sixty percent of patients were white; the remainder were black (16%), American Indian and Alaskan Native (4%), Asian and Pacific Islanders (3%), or unknown race (17%). The distribution of unknown race varied by site, ranging from 3% in Maryland to 41% in Oregon.
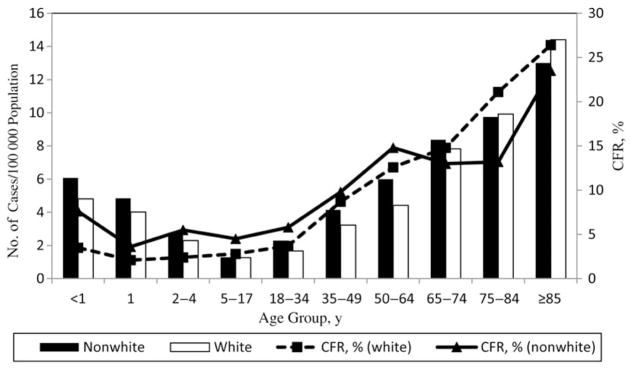
Most case patients (n = 8814; 92.5%) were hospitalized (data missing/unknown for 27 cases), and 1117 died (overall CFR, 11.7%). Compared with those not hospitalized, hospitalized case patients presented more frequently with pneumonia (16% vs 10%; P < .001), SSTI (42% vs 23%; P < .001), and NF (7% vs 1%; P < .001) and less frequently with bacteremia without focus (23% vs 48%; P < .001). Outcomes were unknown for 9 patients (0.1%). CFR was highest among persons aged ≥85 years (26.1%) and lower among nonwhite than among white patients (10.3% vs 12.2%; relative risk, 0.84; 95% confidence interval, .73–.98; Figure 1). Adjusting for US racial and age distributions, we estimate that 10 649–13 434 cases and 1136–1607 deaths due to invasive GAS occurred annually in the United States in 2005–2012.
Figure 1.
Incidence and case-fatality rates (CFRs) of invasive group A Streptococcus infections by age group and race in 2005–2012. Race and age group-specific rates of disease were taken from the surveillance areas in continuous use since 2005. Unknown race data were distributed among known values in the surveillance areas.
Incidence Rates
Invasive GAS rates were bimodal, with a peak among children age <2 years (incidence, 6.5/100 000 and 4.4/100 000 among nonwhites and whites, respectively) and a second, higher peak among those aged ≥50 years (8.1/100 000 and 7.2/100 000 among nonwhites and whites, respectively). Incidence rates were highest among patients aged >85 years (14.4/100 000), followed by adults aged 75–84 years (10.0/100 000), and then adults aged 65–74 years (8.1/100 000; Figure 1). The annual incidence was higher among non-white patients (4.5/100 000) than among white patients (3.6/100 000; relative risk, 1.2; 95% confidence interval, 1.2–1.3).
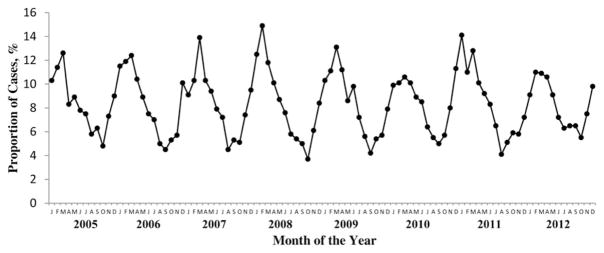
The average annual incidence for the 8 years of surveillance was 3.8 invasive GAS cases per 100 000 persons (range, 2.7–6.2/100 000; Table 1) and varied by site. Most sites had slight year-to-year variation. There was no statistically significant trend in rates among the 10 sites in continuous surveillance from 2005 to 2012 (P = .23) or among the 8 sites with continuous surveillance since 2000 (P = .57; Table 1). Most disease occurred in winter and early spring (Figure 2), a pattern consistent across syndromes and ABCs sites.
Table 1.
Incidence of Invasive Group A Streptococcus Disease, by Active Bacterial Core Surveillance Site and Year for 2005–2012a
| ABCs Site | Incidence, Cases/100 000 Persons (n = 9493) | 8-Year Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | ||
| NM | 4.77 | 6.45 | 5.58 | 7.00 | 6.07 | 5.15 | 7.97 | 6.66 | 6.22 |
| CO | 7.65 | 5.02 | 5.94 | 5.76 | 4.91 | 6.82 | 5.30 | 4.50 | 5.72 |
| MD | 5.63 | 6.13 | 5.42 | 4.85 | 5.60 | 3.57 | 4.51 | 3.07 | 4.84 |
| CA | 3.74 | 4.09 | 4.12 | 4.14 | 3.91 | 4.73 | 4.08 | 3.99 | 4.10 |
| OR | 3.57 | 3.76 | 3.25 | 3.82 | 2.42 | 3.29 | 4.08 | 3.61 | 3.47 |
| NY | 3.89 | 2.72 | 3.10 | 3.76 | 3.70 | 3.59 | 4.09 | 3.67 | 3.57 |
| CT | 2.79 | 2.77 | 3.77 | 3.28 | 2.53 | 4.22 | 4.05 | 3.09 | 3.32 |
| MN | 2.36 | 3.31 | 3.31 | 3.47 | 3.51 | 2.96 | 4.30 | 3.12 | 3.30 |
| TN | 2.90 | 2.86 | 2.97 | 3.13 | 3.42 | 4.28 | 3.05 | 2.56 | 3.15 |
| GA | 2.08 | 2.91 | 2.56 | 2.76 | 2.20 | 3.50 | 3.25 | 2.25 | 2.69 |
| All sites | 3.57 | 3.77 | 3.80 | 3.92 | 3.62 | 4.07 | 4.26 | 3.41 | 3.80 |
| Estimated US incidenceb | 3.59 | 3.80 | 3.81 | 3.92 | 3.63 | 4.09 | 4.31 | 3.41 | 3.82 |
Abbreviations: ABCs, Active Bacterial Core Surveillance; CA, California; CO, Colorado; CT, Connecticut; GA, Georgia; MD, Maryland; MN, Minnesota; NM, New Mexico; NY, New York; OR, Oregon; TN, Tennessee.
Among 8 sites with continuous surveillance since 2000 (P = .57), the rates per 100 000, by year, were 3.62 (2000), 3.78 (2001), 3.22 (2002), 4.11 (2003), 3.11 (2004), 3.13 (2005), 3.47 (2006), 3.47 (2007), 3.53 (2008), 3.32 (2009), 3.74 (2010), 3.88 (2011), and 3.06 (2012).
Surveillance rates were directly standardized to the age and racial distribution of the US population for each year.
Figure 2.
Seasonality of invasive group A Streptococcus infections in the United States, 2005–2012. Abbreviations on x-axis represent months (J, January; F, February; etc).
Underlying Disease
Information on underlying medical conditions known or suspected to increase risk of sporadic GAS infection was available for 9232 (97%) of the patients; 74% had ≥1 underlying condition, and 45% had ≥2. Most common were diabetes, acute skin breakdown, heart disease, and smoking (Table 2). Underlying conditions (particularly injection drug use and chronic skin breakdown) varied significantly by site.
Table 2.
Underlying Conditions Reported Among Case Patients With Invasive Group A Streptococcus Disease by Surveillance Site, 2005–2012
| Underlying Conditionb | ABCs Site, No. (%)a | Total (n = 9232) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CA (n = 1043) | CO (n = 1075) | CT (n = 934) | GA (n = 1075) | MD (n = 1011) | MN (n = 1278) | NM (n = 975) | NY (n = 607) | OR (n = 434) | TNc (n = 800) | ||
| Diabetes mellitus | 215 (20.6)d | 237 (22.1)d | 258 (27.6)d | 262 (24.4) | 265 (26.2) | 306 (23.9) | 270 (27.7)d | 160 (26.4) | 94 (21.7) | 237 (29.6)d | 2304 (25.0) |
| Acute skin breakdowne | 211 (20.2) | 180 (16.7)d | 183 (19.6) | 255 (23.7)d | 264 (26.1)d | 243 (19.0) | 222 (22.8)d | 108 (17.8) | 127 (29.3)d | 55 (6.9)d | 1848 (20.0) |
| Heart diseasef | 155 (14.9)d | 145 (13.5)d | 223 (23.9)d | 217 (20.2) | 214 (21.2)d | 231 (18.1) | 166 (17.0) | 165 (27.2)d | 60 (13.8)d | 189 (23.6)d | 1765 (19.1) |
| Smoker | 231 (22.2)d | 163 (15.2) | 132 (14.1) | 157 (14.6) | 279 (27.6)d | 150 (11.7)d | 160 (16.4) | 65 (10.7)d | 93 (21.4)d | 126 (15.8) | 1556 (16.3) |
| Cancerg | 113 (10.8) | 93 (8.7) | 129 (13.8)d | 125 (11.6)d | 116 (11.5)d | 142 (11.1)d | 69 (7.1)d | 31 (5.1)d | 44 (10.1) | 69 (8.6) | 931 (10.1) |
| Alcohol use | 131 (12.6)d | 105 (9.8)d | 60 (6.4) | 65 (6.1)d | 75 (7.4) | 85 (6.7) | 134 (13.7)d | 42 (6.9) | 38 (8.8) | 41 (5.1)d | 776 (8.4) |
| Immunosuppressionh | 78 (7.5) | 69 (6.4)d | 111 (11.9)d | 98 (9.1) | 81 (8.0) | 96 (7.5) | 37 (3.8)d | 55 (9.1) | 59 (13.6)d | 69 (8.6) | 753 (8.2) |
| Emphysema or COPD | 88 (8.4) | 79 (7.4) | 111 (11.9)d | 73 (6.8) | 88 (8.7) | 84 (6.6)d | 73 (7.5) | 52 (8.6) | 33 (7.6) | 73 (9.1) | 754 (8.1) |
| Renal failure/dialysis | 88 (8.4)d | 66 (6.1) | 61 (6.5) | 94 (8.7)d | 64 (6.3) | 96 (7.5) | 62 (6.4) | 56 (9.2)d | 15 (3.5)d | 65 (8.1) | 667 (7.2) |
| Injection drug use | 161 (15.4)d | 39 (3.6) | 40 (4.3) | 16 (1.5)d | 142 (14.1)d | 10 (0.8)d | 64 (6.6)d | 13 (2.1)d | 31 (7.1)d | 18 (2.3)d | 534 (5.8) |
| Chronic skin breakdowni | 71 (13.3)d | 6 (1.2)d | 127 (25.7)d | 59 (10.3) | 75 (17.1)d | 39 (5.5)d | 26 (4.9)d | 27 (8.4) | 49 (22.4)d | 20 (4.4)d | 499 (10.1) |
| No underlying illness | 245 (23.5) | 372 (34.6)d | 223 (23.9) | 280 (26.1) | 167 (16.5)d | 382 (29.9)d | 222 (22.8) | 157 (25.9) | 95 (21.9) | 245 (30.6)d | 2388 (25.9) |
| >1 Underlying illnessj | 534 (51.2)d | 395 (36.7)d | 468 (50.1)d | 471 (43.8) | 593 (58.7)d | 465 (36.4)d | 449 (46.1) | 253 (41.7) | 226 (52.1)d | 331 (41.4)d | 4185 (45.3) |
Abbreviations: ABCs, Active Bacterial Core Surveillance; CA, California; CO, Colorado; COPD, chronic obstructive pulmonary disease; CT, Connecticut; GA, Georgia; MD, Maryland; MN, Minnesota; NM, New Mexico; NY, New York; OR, Oregon; TN, Tennessee.
Data represent No. (%) of underlying conditions, unless otherwise indicated.
Some patients had >1 underlying condition. The underlying condition was unknown for 325 case patients (3.4%). Conditions that totaled <5% of cases are not presented here and include cerebrovascular accident (366 cases; 4.0%), human immunodeficiency virus infection/AIDS (218 cases; 2.4%), and cirrhosis/liver failure (326 cases; 3.5%).
Tennessee data are from 11 counties for 2005–2009 and 20 counties for 2010–2012.
P < .05 (variation across sites when each site was compared with the mean).
Includes burns, varicella, blunt or penetrating trauma, and surgical wounds.
Includes heart failure or atherosclerotic cardiovascular disease.
Includes leukemia, myeloma, Hodgkin disease, or other cancers.
Resulting from immunoglobulin deficiency, treatment of systemic lupus erythematosus, nephrotic syndrome, organ or bone marrow transplantation, sickle cell disease, asplenia, or receipt of immunosuppressive therapy.
The chronic skin condition variable was added in 2009 and includes the presence of a current chronic dermatologic condition in which the integrity of the skin is compromised, such as psoriasis, eczema, decubitus ulcer, or other chronic ulcers. Numerators are divided by state-specific denominators for cases with known underlying conditions (CA, n = 534; CO, n = 520; CT, n = 494; GA, n = 575; MD, n = 439; MN, n = 707; NM, n = 531; NY, n = 320; OR, n = 219; TN, n = 456; total, n = 4795).
Data represent No. (%) of case patients with >1 underlying condition.
Clinical Syndromes
The distribution of clinical syndromes, by age group and CFR, are presented in Table 3; 41% of patients presented with SSTIs, 25% with isolated bacteremia, and 16% with pneumonia. Compared with patients aged 10–64 years, those aged <10 years were more likely to present with meningitis and central nervous system infections, septic arthritis, osteomyelitis, abscesses, epiglottitis or otitis media, or isolated bacteremia. Patients aged ≥65 years were more likely to present with pneumonia, bacteremia, or SSTIs. Those with septic shock, STSS, NF, meningitis, or pneumonia had the highest CFRs.
Table 3.
Clinical Syndromes and Case-Fatality Rates in Case Patients With Invasive Group A Streptococcus Infections by Age Group, 2005–2012
| Syndromea | Case Patient Age Group | P Valueb | |||
|---|---|---|---|---|---|
|
| |||||
| All Ages (n = 9557) | <10 y (n = 955) | 10–64 y (n = 5752) (Reference) | ≥65 y (n = 2850) | ||
| Septic shockc | <.001 | ||||
| Case patients, No. (%) | 677 (13.7) | 22 (4.6) | 434 (14.8) | 221 (14.5) | |
| CFR (CFR for sole syndrome), %d | 33.8 (45.0) | 13.6 (60.0) | 29.7 (38.8) | 43.4 (55.8) | |
|
| |||||
| STSSe | <.001 | ||||
| Case patients, No. (%) | 381 (4.0) | 28 (2.9) | 265 (4.6) | 88 (3.1) | |
| CFR (CFR for sole syndrome), % | 25.7 (37.9) | 0 (0) | 23.4 (32.8) | 40.9 (70.0) | |
|
| |||||
| NFe | <.001 | ||||
| Case patients, No. (%) | 641 (6.7) | 7 (0.7) | 499 (8.7) | 135 (4.7) | |
| CFR (CFR for sole syndrome), % | 20.8 (29.0) | 0 (0) | 16.3 (21.9) | 38.5 (42.9) | |
|
| |||||
| Meningitis or CNS infection | <.001 | ||||
| Case patients, No. (%) | 110 (1.2) | 30 (3.1) | 62 (1.1) | 18 (0.6) | |
| CFR (CFR for sole syndrome), % | 23.6 (26.9) | 26.7 (35.3) | 21.0 (20.0) | 27.8 (30.0) | |
|
| |||||
| Pneumonia | <.001 | ||||
| Case patients, No. (%) | 1509 (15.8) | 150 (15.7) | 819 (14.2) | 540 (19.0) | |
| CFR (CFR for sole syndrome), % | 18.8 (17.7) | 4.7 (4.4) | 15.5 (13.8) | 27.8 (27.9) | |
|
| |||||
| Bacteremia | <.001 | ||||
| Case patients, No. (%) | 2383 (24.9) | 277 (29.0) | 1306 (22.7) | 800 (28.1) | |
| CFR (CFR for sole syndrome), % | 14.3 (14.4) | 4.7 (4.7) | 11.9 (11.9) | 21.9 (21.9) | |
|
| |||||
| Abdominal or peritoneal infection | <.001 | ||||
| Case patients, No. (%) | 190 (2.0) | 21 (2.2) | 151 (2.6) | 18 (0.6) | |
| CFR (CFR for sole syndrome), % | 14.7 (11.5) | 4.8 (8.3) | 15.9 (11.0) | 16.7 (18.2) | |
|
| |||||
| SSTIf | <.001 | ||||
| Case patients, No. (%) | 3890 (40.7) | 210 (22.0) | 2390 (41.6) | 1290 (45.3) | |
| CFR (CFR for sole syndrome), % | 7.3 (4.8) | 0 (0) | 5.5 (3.0) | 11.7 (8.3) | |
|
| |||||
| Septic arthritis | <.001 | ||||
| Case patients, No. (%) | 980 (10.3) | 138 (14.5) | 661 (11.5) | 181 (6.4) | |
| CFR (CFR for sole syndrome), % | 3.9 (3.5) | 0 (0) | 3.3 (3.5) | 8.8 (7.5) | |
|
| |||||
| Abscess | <.001 | ||||
| Case patients, No. (%) | 576 (6.0) | 109 (11.4) | 384 (6.7) | 83 (2.9) | |
| CFR (CFR for sole syndrome), % | 1.6 (0) | 0 (0) | 1.6 (0) | 3.6 (0) | |
|
| |||||
| Osteomyelitis | <.001 | ||||
| Case patients, No. (%) | 430 (4.5) | 72 (7.5) | 271 (4.7) | 87 (3.1) | |
| CFR (CFR for sole syndrome), % | 3.7 (1.3) | 0 (0) | 4.1 (2.1) | 5.8 (0) | |
|
| |||||
| Pregnancy-related infectiong | NA | ||||
| Case patients, No. (%) | NA | NA | 246 (20.1) | NA | |
| CFR (CFR for sole syndrome), % | NA | NA | 2.5 (1.1) | NA | |
|
| |||||
| Endocarditis or pericarditis | .02 | ||||
| Case patients, No. (%) | 131 (1.4) | 6 (0.6) | 93 (1.6) | 32 (1.1) | |
| CFR (CFR for sole syndrome), % | 7.6 (6.0) | 16.7 (25.0) | 4.3 (6.1) | 15.6 (0) | |
|
| |||||
| Epiglottitis or otitis media | <.001 | ||||
| Case patients, No. (%) | 128 (1.3) | 38 (4.0) | 61 (1.1) | 29 (1.0) | |
| CFR (CFR for sole syndrome), % | 3.9 (0) | 0 (0) | 4.9 (0) | 6.9 (0) | |
|
| |||||
| Otherh | <.001 | ||||
| Case patients, No. (%) | 39 (0.4) | 27 (2.8) | 12 (0.2) | 0 (0) | |
| CFR (CFR for sole syndrome), % | 2.6 (2.6) | 0 (0) | 8.3 (8.3) | NA | |
Abbreviations: CFR, case-fatality rate; CNS, central nervous system; NA, not applicable; NF, necrotizing fasciitis; SSTI, skin and soft-tissue infection; STSS, streptococcal toxic shock syndrome.
Case patients may have had >1 syndrome, with the exception of bacteremia without a source. Clinical syndromes, such as cutaneous and/or soft-tissue infection and pneumonia, had to be accompanied by recovery of an isolate from a normally sterile site or specimen, such as blood, to meet case definitions (except for NF and STSS). A total of 2214 case patients had >1 syndrome. For 9 patients, the outcome was missing, including 5 with bacteremia, 4 with cutaneous infection, and 1 each with NF, pneumonia, abscess, and osteomyelitis.
Comparison by age category of the proportion of invasive GAS infections attributable to a particular syndrome, determined with χ2 test, using the value for the 10–64-year age range as the referent and P < .05 the cutoff for significance.
The variable “septic shock” was added in 2009 (denominator, n = 4941).
Parenthetical values are CFRs for case patients in whom the specified syndrome was the only syndrome present.
A total of 73 case patients (0.8%) presented with both STSS and NF (combined CFR, 27.4%).
SSTI includes cellulitis, infected ulcers, erysipelas, wound infections, phlebitis/thrombophlebitis, lymphangitis, lymphadenitis, bursitis without a GAS-positive joint fluid culture, and gangrene; excludes NF.
For women aged 15–44 years; infections include endometritis (n = 82), septic abortion (n = 11), chorioamnionitis (n = 8), and puerperal sepsis (n = 33).
Other includes hemolytic uremic syndrome (n = 1), unknown syndromes (n = 14), and other syndromes (n = 24).
Among the 8921 case patients for whom information was available (93%), 315 (3.5%) had undergone recent surgery (CFR, 11.8%). Among women of childbearing age (aged 15–44 years), 246 (20.1%) of the invasive GAS infections were pregnancy related. Of case patients with available information, 358 (4.1%) had healthcare-associated disease, and 38 died (CFR, 10.6%). The distribution of syndromes and CFRs were similar in healthcare-onset and community-onset cases (CFR, 10.6 vs 11.7%; P = .57).
Emm Sequence Types and Potential Vaccine-Preventable Disease
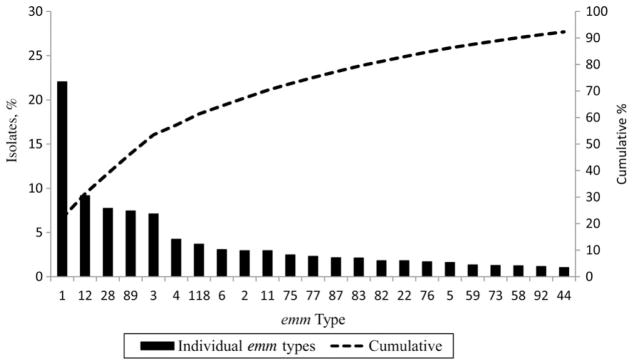
Of 9 states submitting isolates for evaluation, bacterial isolates were available for typing for 86% of cases; only 4 could not be typed. The distribution of emm types varied by site and by year. Although 99 unique emm types were identified during 2005–2012, the 23 most common accounted for 92% of cases (Figure 3). Most common were emm types 1 (22%), 12 (9%), 28 (8%), 89 (7%), and 3 (7%), which cumulatively accounted for 53% of isolates. The emm types in the proposed 30-valent vaccines accounted for 91% of all isolates, 92% of those with NF or STSS, and 96% of isolates among patients who died.
Figure 3.
Distribution of invasive group A streptococcal isolates from Active Bacterial Core surveillance among the 23 most common emm types found during 2005–2012; these included any emm type that comprised >1% of total isolates.
Predictors of Death
Increasing age, residence in a nursing home, recent surgery, presence of specific syndromes (septic shock, NF, meningitis, isolated bacteremia, or pneumonia), emm type 1 or 3, and either underlying chronic illness or immunosuppression were all independent predictors of death (Table 4). No statistically significant 2-way interactions were found.
Table 4.
Independent Risk Factors Associated With Death Due to Invasive Group A Streptococcus Infection for Cases Reported in 2005–2012a
| Variable | Odds Ratio (95% CI) |
|---|---|
| Age group, y | |
| <18 | 0.83 (.46–1.52) |
| 18–34 | Reference |
| 35–49 | 2.00 (1.30–3.08) |
| 50–64 | 2.80 (1.85–4.25) |
| 65–74 | 3.34 (2.15–5.17) |
| ≥75 | 5.41 (3.53–8.27) |
| Race | |
| White | Reference |
| Nonwhite | 0.91 (.74–1.12) |
| Sex | |
| Male | Reference |
| Female | 1.04 (.88–1.23) |
| Recent surgeryb | |
| Yes | 1.66 (1.06–2.61) |
| No | Reference |
| Nursing home residencec | |
| Yes | 1.84 (1.38–2.44) |
| No | Reference |
| Syndrome | |
| Syndrome other than those listed below | Reference |
| Septic shock | 9.11 (5.83–14.24) |
| Necrotizing fasciitis | 5.25 (3.15–8.77) |
| Meningitis or CNS infection | 4.12 (1.72–9.87) |
| Pneumonia | 3.44 (2.19–5.40) |
| Bacteremia without source | 3.20 (2.08–4.93) |
| SSTI | 0.92 (.58–1.48) |
| emm Type | |
| Not 1, 3, 12, 28, or 89 | Reference |
| 1 | 1.39 (1.12–1.72) |
| 3 | 1.75 (1.30–2.36) |
| 12 | 1.08 (.79–1.48) |
| 28 | 1.00 (.72–1.42) |
| 89 | 0.92 (.65–1.30) |
| Health status | |
| No underlying illness | Reference |
| Skin conditiond | 0.84 (.54–1.31) |
| Chronic illness | 1.37 (1.06–1.76) |
| Immunosuppression | 1.64 (1.24–2.18) |
Abbreviations: CI, confidence interval; CNS, central nervous system; SSTI, skin and soft-tissue infection.
Data from New York and Connecticut were excluded, because deaths were associated with human immunodeficiency virus infection/AIDS were not reported (New York) or isolates were not available to enable emm typing (Connecticut).
Surgery within 7 days before first positive culture.
Residence at time of first positive culture.
Includes penetrating and blunt trauma, burns, varicella, surgical wounds (within 7 days before first positive culture), and chronic skin condition.
DISCUSSION
Invasive GAS infections continue to have be associated with morbidity and mortality rates in the United States, with an estimated 10 649–13 434 cases resulting in 1136–1607 deaths each year. The estimated national incidence is similar to the incidence of invasive GAS in Canada (4.3/100 000) and many European countries (2–4/100 000) (CFR, 14%–19%) [17–21]. However, substantially higher rates of disease have been documented in other geographic areas, including Fiji (9.9/100 000), North Queensland, rural Kenya, and New Zealand (7.9/100 000) [22–25], as well as in European countries experiencing outbreaks, including Iceland (1.1–4.0/100 000), Sweden (6.1/100 000), and Ireland (0.8–2.7/100 000) [26–28].
Although annual incidence rates varied by site—with the lowest mean rates reported in Georgia (2.7/100 000) and the highest in New Mexico (6.2/100 000)—none of the 10 ABCs sites showed significant changes in incidence over time. Moreover, when limiting our analysis to the 8 ABCs sites in continuous surveillance since 2000, we found no significant change in rates of invasive GAS infections during a 13-year period. The stability of national incidence is remarkable and may only decline with use of an effective vaccine. Continued surveillance is needed to detect any future increase in invasive GAS infections due to potential emerging strains.
Prior publications have shown that the incidence of invasive GAS is higher in persons aged ≥65 years living in the community and in long-term-care facilities, in terms of both sporadic infections and outbreaks [29]. In our study, we further examined rates among the very elderly; incidence peaked at 14/100 000 and the CFR reached 25% among those aged ≥85 years. In addition, the CFRs for most syndromes increased with age and were dramatically higher among some: 70% of elderly patients presenting with STSS alone and 56% of those with NF as their only syndrome died of their infections. Given that the population of elderly individuals is growing in the United States [30], increased attention needs to be given to severe presentations in this population.
To reduce morbidity and mortality rates due to GAS infections, effective prevention tools are desperately needed. The current candidate GAS vaccines entering or nearing clinical study are the 30-valent N-terminal M-protein–based vaccine and the conserved M-protein vaccine (minimal epitope J8 vaccine) [31]. The 30-valent vaccine would cover 91% of GAS infections reported by ABCs surveillance. The United States and other high-income countries account for only a small proportion of the total burden of GAS disease, and significantly greater heterogeneity of emm types has been documented in low-income geographic regions [32]. Fortunately, the 30-valent vaccine has been shown to evoke cross-opsonic bactericidal antibodies against a variety of nonvaccine serotypes, potentially increasing vaccine efficacy in developing countries [12, 33]. The addition of these nonvaccine emm types could increase the potential vaccine coverage of invasive GAS infections in the United States to 96%. Furthermore, other vaccines in development are based on conserved epitopes.
ABCs surveillance began formally tracking septic shock as a distinct syndrome in 2009. Septic shock had the highest CFR both when found along with another syndrome as well as when it was the only syndrome present. A more detailed chart review is necessary to identify any important clinical differences between physician-diagnosed “septic shock” and STSS (see STSS case definition [34]). The frequency with which providers use the standard clinical criteria to define STSS is unknown but is probably low; measuring physician-diagnosed STSS alone may underestimate the burden of severe GAS infections significantly. Because there have been few new treatment options for severe GAS infections, approaches to both STSS and septic shock continue to rely on promoting routine infection control, prompt case identification, and prevention of secondary cases among postpartum and postsurgical patients, supportive care, antibiotics, intravenous immunoglobulin, and prompt surgical attention when needed [35].
Our identification of recent surgery as an independent predictor of death should further alert providers to consider the diagnosis of invasive GAS in these patients. Prompt recognition of GAS and institution of antimicrobial therapy may limit morbidity and mortality risks. Whereas the presence of “any skin condition” was not significantly associated with increased risk of death, this variable includes both chronic and acute skin conditions, which may obscure risks that attend specific skin conditions. Although most invasive GAS infections are sporadic, wounds (eg, surgical wounds and decubiti) have been recognized as a common risk factor for GAS infection in nosocomial outbreaks and outbreaks among persons living in nursing homes and other group settings [36–38]. Many severe infections have also been found to occur within 24–72 hours of nonpenetrating trauma that results in a deep hematoma [39].
Our study highlights a consistent seasonal pattern of invasive GAS infections with a peak in winter and early spring and a nadir in the late summer and fall. A recent review of data from 5 countries in Europe and a study in Sweden found similar seasonal patterns in all countries [18, 27]. One proposed mechanism for a seasonal relationship is concurrently circulating viral infections during high-incidence periods, along with effects of crowding and close contact due to increased time spent indoors. Crowding and exposure to children with pharyngitis—a major reservoir of GAS—have been recognized as significant risk factors for invasive GAS infections [40].
Rates of invasive GAS infection measured by ABCs are minimum estimates because ABCs captures only invasive infections with sterile-site isolates unless GAS cultures from wounds are accompanied by STSS or NF syndromes. Furthermore, standard ABCs methods probably underreport cases of STSS and NF because providers do not always use these specific terms to describe such cases in medical records [41].
In the United States, invasive GAS remains a serious infection associated with high mortality rates. With no new effective tools for disease prevention, rates have remained unchanged during the last 13 years. In this setting, more rapid recognition of severe GAS infections and prompt and appropriate treatments are needed. Given that the initial presentation of invasive GAS infections is often nonspecific and such infections can cause severe disease and death, vaccines are sorely needed. The emm types causing invasive GAS disease are the most prevalent causes of GAS pharyngitis [42]. Decreasing the impact of GAS pharyngitis alone in the United States through immunization with an M-protein vaccine would potentially be cost-effective and reduce the primary reservoir of invasive infections. In addition, vaccination may also reduce unnecessary antibiotic prescriptions for pharyngitis, leading to additional indirect benefits.
Acknowledgments
We thank the numerous persons in the Active Bacterial Core surveillance (ABCs) areas who maintain the ABCs system in all 10 sites. We also thank the multitude of laboratorians and technicians who isolate the ABCs pathogens and make it possible to track these infections, and the surveillance and laboratory personnel at the Centers for Disease Control and Prevention (CDC) for their careful work characterizing the isolates.
Financial support. Financial support for ABCs is provided by the CDC’s Emerging Infections Programs.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. All authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.O’Brien KL, Beall B, Barrett NL, et al. Epidemiology of invasive group A Streptococcus disease in the United States, 1995–1999. Clin Infect Dis. 2002;35:268–76. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- 2.O’Loughlin RE, Roberson A, Cieslak PR, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853–62. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 3.Department of Child and Adolescent Health and Development, World Health Organization. The current evidence for the burden of group A streptococcal diseases. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 4.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 5.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705–10. doi: 10.1086/511638. [DOI] [PubMed] [Google Scholar]
- 6.Prevention of Invasive Group ASIWP. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis. 2002;35:950–9. doi: 10.1086/342692. [DOI] [PubMed] [Google Scholar]
- 7.Public Health Agency of Canada. Guidelines for the Prevention and Control of Invasive Group A streptococcal disease. 32S2. CCDR; 2006. pp. 1–26. [Google Scholar]
- 8.McNeil SA, Halperin SA, Langley JM, et al. Safety and immunogenicity of 26-valent group A Streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41:1114–22. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 9.Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun. 2002;70:2171–7. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan D. StreptAvax (ID Biomedical) Curr Opin Investig Drugs. 2006;7:186–90. [PubMed] [Google Scholar]
- 11.Kotloff KL, Dale JB. Progress in group A streptococcal vaccine development. Pediatr Infect Dis J. 2004;23:765–6. doi: 10.1097/01.inf.0000136296.98686.c5. [DOI] [PubMed] [Google Scholar]
- 12.Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29:8175–8. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langley G, Schaffner W, Farley MM, et al. Twenty years of active bacterial core surveillance. Emerg Infect Dis. 2015;21:1520–8. doi: 10.3201/eid2109.141333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs) [Accessed February 2016];Surveillance reports. Available at: http://www.cdc.gov/abcs/reports-findings/surv-reports.html.
- 15.Centers for Disease Control and Prevention. [Accessed November 2014];Protocol for emm typing. Available at: http://www.cdc.gov/streplab/protocol-emm-type.html.
- 16.Centers for Disease Control and Prevention. Streptococcus pyogeness emm sequence database. 2011. [Google Scholar]
- 17.Laupland KB, Ross T, Church DL, Gregson DB. Population-based surveillance of invasive pyogenic streptococcal infection in a large Canadian region. Clin Microbiol Infect. 2006;12:224–30. doi: 10.1111/j.1469-0691.2005.01345.x. [DOI] [PubMed] [Google Scholar]
- 18.Lamagni TL, Darenberg J, Luca-Harari B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46:2359–67. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlaminckx BJ, van Pelt W, Schouls LM, et al. Long-term surveillance of invasive group A streptococcal disease in The Netherlands, 1994–2003. Clin Microbiol Infect. 2005;11:226–31. doi: 10.1111/j.1469-0691.2004.01068.x. [DOI] [PubMed] [Google Scholar]
- 20.Lepoutre A, Doloy A, Bidet P, et al. Epidemiology of invasive Streptococcus pyo-genes infections in France in 2007. J Clin Microbiol. 2011;49:4094–100. doi: 10.1128/JCM.00070-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montes M, Ardanuy C, Tamayo E, Domenech A, Linares J, Perez-Trallero E. Epidemiological and molecular analysis of Streptococcus pyogenes isolates causing invasive disease in Spain (1998–2009): comparison with non-invasive isolates. Eur J Clin Microbiol Infect Dis. 2011;30:1295–302. doi: 10.1007/s10096-011-1226-x. [DOI] [PubMed] [Google Scholar]
- 22.Steer AC, Jenney A, Kado J, et al. Prospective surveillance of invasive group A streptococcal disease, Fiji, 2005–2007. Emerg Infect Dis. 2009;15:216–22. doi: 10.3201/eid1502.080558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton R, Smith HV, Wood N, Siegbrecht E, Ross A, Ketheesan N. Invasive group A streptococcal disease in North Queensland (1996–2001) Indian J Med Res. 2004;119(suppl):148–51. [PubMed] [Google Scholar]
- 24.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 25.Williamson DA, Morgan J, Hope V, et al. Increasing incidence of invasive group A Streptococcus disease in New Zealand, 2002–2012: a national population-based study. J Infect. 2015;70:127–34. doi: 10.1016/j.jinf.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Olafsdottir LB, Erlendsdottir H, Melo-Cristino J, et al. Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Euro Surveill. 2014;19:5–14. [PubMed] [Google Scholar]
- 27.Darenberg J, Henriques-Normark B, Lepp T, Tegmark-Wisell K, Tegnell A, Widgren K. Increased incidence of invasive group A streptococcal infections in Sweden, January 2012–February 2013. Euro Surveill. 2013;18:20443. doi: 10.2807/1560-7917.es2013.18.14.20443. [DOI] [PubMed] [Google Scholar]
- 28.Meehan M, Murchan S, Bergin S, O’Flanagan D, Cunney R. Increased incidence of invasive group A streptococcal disease in Ireland, 2012 to 2013. Euro Surveill. 2013;18:20556. doi: 10.2807/1560-7917.es2013.18.33.20556. [DOI] [PubMed] [Google Scholar]
- 29.Thigpen MC, Richards CL, Jr, Lynfield R, et al. Invasive group A streptococcal infection in older adults in long-term care facilities and the community, United States, 1998–2003. Emerg Infect Dis. 2007;13:1852–9. doi: 10.3201/eid1312.070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent GK, Velkoff VA. Current population reports P25-1138. Washington, DC: US Census Bureau; 2010. The next four decades: the older population in the United States: 2010 to 2050. [Google Scholar]
- 31.Moreland NJ, Waddington CS, Williamson DA, et al. Working towards a group A streptococcal vaccine: report of a collaborative Trans-Tasman workshop. Vaccine. 2014;32:3713–20. doi: 10.1016/j.vaccine.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611–6. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 33.Dale JB, Penfound TA, Tamboura B, et al. Potential coverage of a multivalent M protein-based group A streptococcal vaccine. Vaccine. 2013;31:1576–81. doi: 10.1016/j.vaccine.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention National Notifiable Diseases Surveillance System (NNDSS) [Accessed November 2015];Streptococcal toxic shock syndrome (STSS) (Streptococcus pyogenes): 2010 case definition. Available at: http://wwwn.cdc.gov/nndss/conditions/streptococcal-toxic-shock-syndrome/case-definition/2010.
- 35.Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group A streptococcal infections. Clin Infect Dis. 2014;59:358–65. doi: 10.1093/cid/ciu304. [DOI] [PubMed] [Google Scholar]
- 36.Jordan HT, Richards CL, Jr, Burton DC, Thigpen MC, Van Beneden CA. Group A streptococcal disease in long-term care facilities: descriptive epidemiology and potential control measures. Clin Infect Dis. 2007;45:742–52. doi: 10.1086/520992. [DOI] [PubMed] [Google Scholar]
- 37.Beaudoin AL, Torso L, Richards K, et al. Invasive group A Streptococcus infections associated with liposuction surgery at outpatient facilities not subject to state or federal regulation. JAMA Intern Med. 2014;174:1136–42. doi: 10.1001/jamainternmed.2014.1875. [DOI] [PubMed] [Google Scholar]
- 38.Dooling KL, Crist MB, Nguyen DB, et al. Investigation of a prolonged group A streptococcal outbreak among residents of a skilled nursing facility, Georgia, 2009–2012. Clin Infect Dis. 2013;57:1562–7. doi: 10.1093/cid/cit558. [DOI] [PubMed] [Google Scholar]
- 39.Stevens DL. Invasive group A Streptococcus infections. Clin Infect Dis. 1992;14:2–11. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 40.Factor SH, Levine OS, Schwartz B, et al. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis. 2003;9:970–7. doi: 10.3201/eid0908.020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowgill KD, Van Beneden Chris, Wright C, Beall B, Schuchat A Active Bacterial Core Surveillance Team. Burden of streptococcal toxic shock syndrome and necrotizing fasciitis—United States, 2002 [abstract 238]. Program and abstracts of the 43rd Annual Meeting of the Infectious Diseases Society of America; San Diego California. Alexandria, VA: Infectious Diseases Society of America; 2003. p. 2002. [Google Scholar]
- 42.Shulman ST, Tanz RR, Dale JB, et al. Seven-year surveillance of North American pediatric group A streptococcal pharyngitis isolates. Clin Infect Dis. 2009;49:78–84. doi: 10.1086/599344. [DOI] [PubMed] [Google Scholar]