Abstract
Thalidomide [α-(N-phthalimido)glutarimide] (1) is a sedative and antiemetic drug originally introduced into the clinic in the 1950s for the treatment of morning sickness. Although marketed as entirely safe, more than 10,000 babies were born with severe birth defects. Thalidomide was banned and subsequently approved for the treatment of multiple myeloma and complications associated with leprosy. Although known for more than 5 decades, the mechanism of teratogenicity remains to be conclusively understood. Various theories have been proposed in the literature including DNA damage and ROS, inhibition of angiogenesis and inhibition of cereblon. All the theories have their merits and limitation. Although the recently proposed cereblon theory has gained wide acceptance, it fails to explain the metabolism and low dose requirement reported by a number of groups. Recently we have provided convincing structural evidence in support of the presences of arene oxide and the quinone reactive intermediates. However, the ability of these reactive intermediates to impart toxicity/teratogenicity needs investigation. Herein we report that the oxidative metabolite of thalidomide, di-hydroxythalidomide is responsible for generating ROS and DNA damage. We show using cell lines the formation of comet (DNA damage) and ROS. Using DNA cleavage assays we also show that catalase, radical scavengers and desferal is capable of inhibiting DNA damage. A mechanism of teratogenicity is proposed that not only explains the DNA damaging property but the metabolism, low concentration and species specificity requirements of thalidomide.
Keywords: Thalidomide, teratogenicity, DNA damage, Reactive intermediate
Introduction
Thalidomide [α-(N-phthalimido)glutarimide] (TD, 1) is a sedative and antiemetic drug originally introduced in the clinic in the 1950s for the treatment of morning sickness.1 Although marketed as entirely safe, more than 10,000 babies were born between 1957 and 1961 with severe birth defects that resulted in its withdrawal in the early 1960s.1, 2 However, owing to its clinical properties, TD was approved in 1998 for the treatment of lesions associated with leprosy and in 2006 for multiple myeloma.3, 4 In addition, TD is being tested for the treatment of many diseases including refractory esophageal Crohn’s disease, recurrent bleeding resulting from gastric angiodysplasia and hereditary hemorrhagic telangiectasia.5–8 The recent emergence of TD as a drug with clinical potential resulted in renewed interest in both its toxicity and pharmacological mechanisms, none of which are conclusively established. Moreover, prevention of inadvertent exposure of pregnant women to this drug is a continuing challenge, particularly in parts of the world where access to the drug is less restricted.
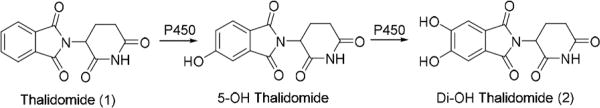
The teratogenicity of TD is very species-specific, being teratogenic in primates and rabbits but not in rats and mice.9 It was initially believed that the R isomer is sedative, whereas the S isomer is teratogenic; however, the two enantiomers are readily interconvertible.10 TD is metabolized in the liver to two major products, 5-hydroxythalidomide and 5´- hydroxythalidomide, by P450s.11,12 We have previously shown that P450 3A4 and 3A5 also oxidize TD to the 5-hydroxy and dihydroxy metabolites.13,14 The second oxidation step in the P450 3A4 pathway generates a reactive intermediate, possibly an arene oxide that can be trapped by the tripeptide glutathione (GSH) to give GSH adducts.3, 15, 16 The observation of GSH adduct formation with 5-hydroxythalidomide was confirmed in vivo in humanized mouse models.13 The dihydroxythalidomide (DHT, 2) product is further oxidized to the potentially toxic quinone intermediate that can also react with GSH to give the corresponding GSH adduct of DHT17. In both bacterial and AS52 cells thalidomide was found to be not genotoxic.18
Although TD has been known for more than half a century, its mechanism of teratogenicity remains to be convincingly established. More than 30 theories have been proposed including generation of reactive oxygen species (ROS), inhibition of angiogenesis, and non-covalent binding to the protein cereblon.2,19–22 However, none of the proposed theories are without limitations. For example, the ROS theory does not indicate the species responsible for ROS generation or the mechanism of ROS formation;22 the anti-angiogenesis theory proposes the involvement of a reactive intermediate but fails to present any direct evidence of any;21 and finally, the cereblon inhibition theory, although has recently gained wide acceptance is unable to explain the metabolism and low dose requirements (concentration of TD that is significantly higher than clinically relevant exposure levels were used in some of the reported experiments).23–27 Interestingly, the need for metabolism of TD for teratogenicity is consistently reported in the literature.20,28 Recently, we provided direct evidence for the presence of arene oxide and quinone reactive intermediates, using GSH as a trapping agent.15,17 However, the ability of these reactive intermediates to impart toxicity/teratogenicity is yet to be demonstrated. Herein we report the DNA damaging properties of the 5,6-dihydroxythalidomide (DHT, 2) metabolite that is readily oxidized to the corresponding quinone.
Experimental Procedures
Caution: Thalidomide and its metabolites are extremely teratogenic and toxic. Caution and care should be taken while handling these chemicals.
Materials
Unless otherwise mentioned all chemicals used are of the highest quality available and purchased from Sigma Aldrich (St. Louis, MO, USA). Agarose, agar, ethidium bromide, catalase, methanol, ethanol, isopropanol, DMSO, NaCl, EDTA, Tris-HCl, Triton-X 100, NaOH, paraformaldehyde, bovien serum albumin (BSA), biotin, histidine, ampicillin, oxoid nutrient broth No. 2, and protease inhibitor cocktail were obtained from HiMedia (Mumbai, India), sodium fluoride and orthovandate from MP Biomedicals, LLC (Solon Ohio, USA), CellROX Deep Red and Nuc Blue (Hoechst 33342) from ThermoFisher Scientific (Santa Clara, CA), Salmonella typhimurium TA100 from Microbial Type Culture Collection and Gene Bank (MTCC, Chandigarh, India), thalidomide and DMEDA from Tokyo Chemical Industry (Tokyo, Japan), nitrocellulose membranes from MDI Membrane Technologies (Ambala. India), LMPA and LE Agarose from Lonza (Rockland, ME, USA), γ-H2AX Alexa Fluor 488 from Biolegend (San Diego, CA, USA), and PAD-PARP antibody from Abcam (MA, USA). The 293T cell line was purchased from National Centre for Cell Culture (Pune, India) and authenticated by Short Tandem Repeat DNA profiling from Life Code Technology, Delhi, India. HepG2 cells were a generous gift from Dr. Soumya Sinha Roy at the CSIR Institute of Genomics and Integrative Biology, New Delhi. HUVEC was procured from HiMedia (Mumbai, India).
DNA Cleavage Assays
In a typical DNA cleavage reaction, plasmid DNA (pUC19, 1 µg) was incubated with dihydroxy thalidomide (DHT, 0.5–25 µM) in potassium phosphate buffer (1 mM, pH 7.4) at 37 °C for 12 h. For reactions containing an NADPH-generating system, 1 µl of it was added such that the final concentration of NADP+ is 250 µM. To prepare the NADPH-generating system, equal volumes of NADP+ (10 mM), and glucose 6-phosphate (100 mM) were premixed and glucose 6-phosphate dehydrogenase (1U) was added to it. Following incubations, reactions were subjected to DMEDA (100 mM) workup for 2 hours at 37 °C, quenched by addition of 5 µL of glycerol loading buffer, and then electrophoresed for 45 min at 80 V in 1% agarose gel (w/v) containing 0.5 µg/mL ethidium bromide. DNA was visualized and quantified using an AlphaImager (ProteinSimple, CA) gel documentation system. Strand breaks per plasmid DNA molecule (S) were calculated using the equation S=−ln f1, where f1 is the fraction of plasmid present as form I. For assays with radical scavengers, methanol (1 M), isopropanol (1 M), or DMSO (1 M) was used. For some reactions catalase was used at concentration of 250 µg/mL, ferrous sulfate at (0–10 mM) or desferal at (1 mM).
Comet Assay
In a typical assay, sub-confluent cells treated with DHT, thalidomide, 5-hydroxythalidomide or menadione (10 µM each) for 1.5, 3, or 12 h were sandwiched in 0.5% low melting agarose on pre-coated agarose (1%) slides. Sandwiched cells were incubated in lysis buffer (10 mM Tris-HCl buffer (pH 10) containing 2.5 M NaCl, 100 mM EDTA, and 1% TritonX-100 (v/v)) for 2 hours, followed by 30 min incubation in alkaline buffer (300 mM NaOH and 1 mM EDTA, pH>13). Slides were electrophoresed in the same buffer at 21 V/300 mA for 30 min. Neutralization was done in 400 mM Tris-HCl (pH 7.5) buffer and staining with 1µg/mL ethidium bromide. All steps were carried out at 4 °C. Fixing was done with absolute ethanol, and imaging was done using a Leica DFC450C microscope (Wetzlar, Germany). ImageJ plugin OpenComet software was used to quantify the DNA damage.29
Reactive Oxygen Species Detection
Sub-confluent cells were initially treated with 10 µM of DHT for 1.5 and 3 h, and then CellROX Deep Red (5 µM) reagent was added and incubated for 6 30 min. Following incubation cells were treated with Hoechst counter stain for another 10 min, washed with phosphate-buffered saline solution, and immediately taken for imaging. For a positive control, menadione was used at a concentration of 100 µM for 2 h.
Western Blot Analysis for PAR-PARP
Cells were treated with 10 µM DHT for 15, 45, 90, and 180 min and subsequently lysed using RIPA Lysis buffer (150 mM NaCl, 50 mM Tris, pH 8.0 and NP40 1%). Protease inhibitor cocktail along with NaF (500 mM) and orthovandate (100 mM) were added to the lysate and incubated on ice for 30 min, with a single brief vortex mixing of 5 s. After incubation, the lysate was centrifuged at 1,000 g for 10 min and supernatant taken. Quantification of protein was done using a Bradford assay.30 Proteins in the lysate were separated using 8% SDS-PAGE and transferred to a nitrocellulose membrane. PAD-PARP (Abcam, 1:500, v/v) was used to check for DNA damage.31 Equal loading was confirmed using β-actin. Imaging was done on a LICOR instrument.
Statistical Analysis
All data were analyzed using GraphPad Prism software (Prism 5.03). Data are expressed as mean ±SD or mean ±SEM. A P-value of <0.05 was considered statistically significant in all experiments and represented by asterix (*).
Results and Discussion
DNA Cleavage by DHT
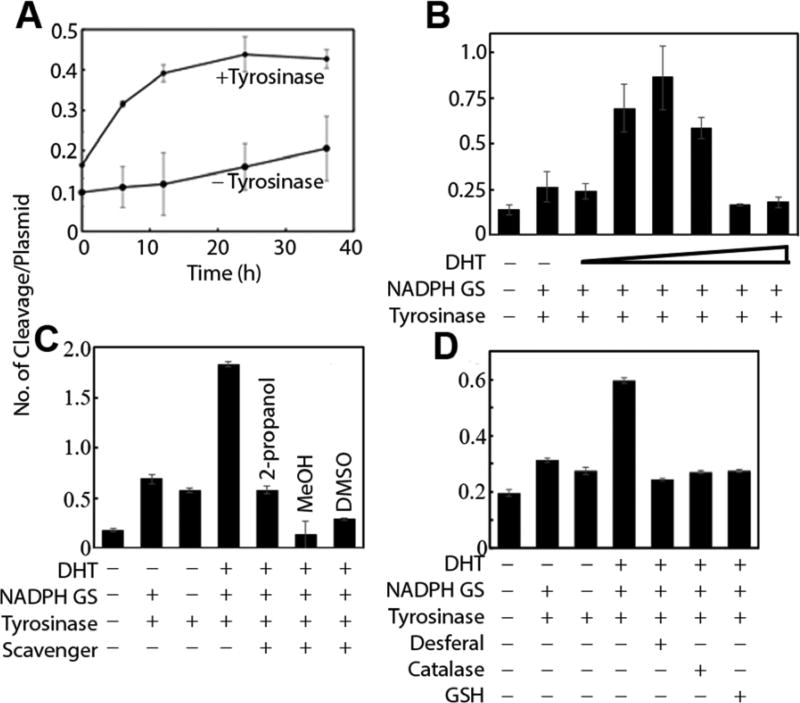
Because quinones are known to undergo redox cycling through the formation of semiquinones following one electron reductive activation, we tested the DNA cleaving ability of DHT (Figure 2A and Figure S1m supporting information).32 Plasmid DNA exist in the supercoiled form (I). Single-strand cleavage converts it to an open circular form (II) and double-strand cleavage makes it linearized (III). Each of the three forms have a distinct mobility in an agarose gel. Drugs causing DNA cleavage will convert supercoiled plasmid DNA into either form II or III depending on the nature of the cleavage. The extent and efficiency of this process can be determined and estimated by separating and quantitating each form in an agarose gel. Using this plasmid DNA cleavage assay, a low but reproducible amount of DNA cleavage was observed when DHT was treated alone. Addition of tyrosinase (known to oxidize DHT to the corresponding quinone) increased DNA cleavage.17 Addition of an NADPH-generating system along with tyrosinase further increased the DNA cleavage efficiency of DHT. This is surprising because NADPH is an obligate two-electron reducing agent and should not be involved in one electron reductive activation of DHT quinone. DHT undergoes slow but spontaneous oxidation to the quinone. The quinone comproportionates with DHT to generate the semiquinone.33 Addition of tyrosinase increased the concentration of the quinone while NADPH ensured steady level of the catechol necessary for semiquinone generation. Surprisingly, increasing the concentration of DHT resulted in an initial increase in cleavage efficiency up to 1 µM, followed by a gradual decrease (Figure 2B). This may be due to DHT acting as a radical scavenger or limiting NADPH concentration.
Figure 2.
Plasmid DNA cleavage assay A) Plasmid DNA cleavage by 10 µM DHT in the presence and absence of tyrosinase. B) DNA cleavage efficiency by DHT (100 nM-10 µM) in the presence of tyrosinase and NADPH. (Experiments are done in triplicates, error bars represent ±SEM, Lane 1 vs 3 *P=0.029, Lane 1 vs 4 **P=0.002, Lane 1 vs 5 **P=0.0021, Lane 1 vs 6 ***P=0.0003, Lane 5 vs 7 **P=0.0023, Lane 5 vs 8 **P=0.0027) C) Effect of radical scavengers iPrOH (1 M), MeOH (1 M) or DMSO (1 M). (Lane 1 vs 2 ***P<0.001, Lane 1 vs 3 ***P<0.0001, Lane 1 vs 4 ***P<0.0004, Lane 1 vs 5 **P=0.0033, Lane 1 vs 7 ***P=0.0004, Lane 4 vs 5 **P=0.0015, Lane 4 vs 6 ***P<0.0006, Lane 4 vs 7 ***P=0.0004) D) Effect of catalase (250 µg/mL), desferal (1 mM), or GSH (5 mM). (Lane 1 vs 2 **P=0.0013, Lane 1 vs 3 *P=0.018, Lane 1 vs 4 ***P=0.0001, Lane 1 vs 5 *P=0.021, Lane 1 vs 6 **P=0.0052, Lane 1 vs 7 **P=0.0038, Lane 4 vs 5 ***P<0.0001, Lane 4 vs 6 ***P<0.0001, Lane 4 vs 7 ***P<0.0001).
To understand the mechanism of DNA cleavage by DHT, we looked into its DNA cleaving ability in the presence of radical scavengers (iPrOH, MeOH, and DMSO), catalase, desferal, and GSH (Figures 2C and D).34 DNA cleavage was significantly inhibited in presence of all the agents used here, clearly indicating the involvement of H2O2, metals, radicals, and quinones. Quinones are known to undergo redox-cycling upon one-electron reductive activation to generate superoxide radical. The net outcome of this futile cycle is drug-mediated transfer of electrons from a reducing equivalent, to molecular oxygen. The superoxide radical anion is converted to H2O2, which then undergoes Fenton chemistry in the presence of metals to produce the well-known DNA damaging agent hydroxyl radical (•OH). The cytotoxic effect of H2O2 is mitigated by various enzymes including catalase, while desferal inhibits Fenton chemistry by chelating metals.34 The inhibition of DNA cleavage by 5 mM GSH is consistent with its ability to quench radicals and trap quinones, clearly suggesting the involvement of these species in DNA cleavage. The presence of GSH in cells at a concentration of about 5 mM also suggests a protective role against DNA damage by DHT in vivo as well as the ability of these metabolites to deplete GSH, as reported in the literature.
Comet assay
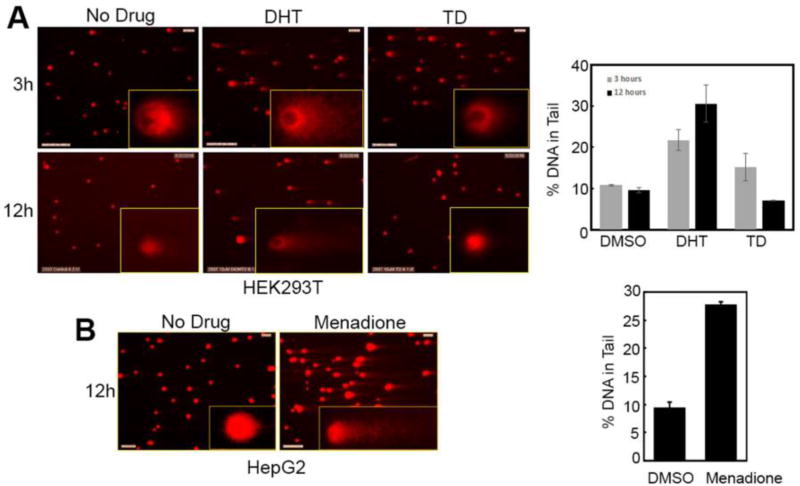
To determine if DHT will damage DNA in a cell that contains various protective pathways including GSH (~5 mM), we performed comet assays on three cell lines: HEK293T (embryonic), HepG2 (liver), and HUVEC (endothelial). The extent of DNA damage is represented by the length and intensity of the comet tail. Alkaline comet assays clearly showed significant DNA cleavage in HEK293T, HepG2, and HUVEC cells when treated with 10 µM DHT, a clinically relevant exposure levels, but not TD (Figure 3 and Figure S2, supporting information). This may be because TD is not metabolized to DHT in these cell lines. Although in theory HepG2 cells contain some of the P450s that might be involved in the two-step sequential oxidation of TD to DHT, albeit in lower amounts, an initial higher concentration of TD should result in preferential biotransformation of TD to the 5-hydroxy metabolite during the course of these assays. The extent of DNA cleavage by DHT is comparable to that caused by the prototypical redox cycling agent menadione (10 µM) in HepG2 cells. Interestingly, for the normal MCF10A cells, no comet was observed when treated with DHT. This may be due to the reduced oxidation of the DHT to the quinone, lack of a one-electron reductive system, or detoxification of the quinone by GSH. To address the tumor selectivity of DHT, we transformed MCF10A cells with the HER2 and H1047R PI3K oncogenes. MCF10A is a non-tumorigenic mammary epithelial cell line that is used as a popular model system for testing effects of oncogenes and tumor suppressors on cellular transformations.35 Here, we have genetically engineered MCF10A cells by stably expressing (through retroviral infection carrying oncogenes of interest followed by drug selection) two oncogenes HER2 (Human epidermal growth factor receptor 2) and H1047R mutant PI3K p110a subunit (H1047R PIK3CA). According to previously published report,36 introduction of these two oncogenes in MCF10A cell line dramatically altered its growth, serum/growth actor requirement, mobility, invasiveness as well as sensitivity toward HER2-targeting drugs. Thus, this dual oncogene expressing variant of MCF10A cell line can be considered as transformed. Consistent with the above observation, a comet was seen in this transformed cell line indicating the tumor selectivity of DHT (Figure S2, supporting information). However, the mechanism of this tumor selectivity needs further investigation.
Figure 3.
Comet assay with 293T cells. 293T cells in culture were treated with DHT (10 µM), TD (10 µM), or DMSO for 3 (DMSO vs DHT *P=0.0268) or 12 h (DMSO vs DHT *P=0.0224, DMSO vs TD *P=0.0262, DHT vs TD *P=0.016). Experiments ae done in duplicates with error bars representing ±SD. A) Cells showing presence of comet with the inset picture representing a magnified image showing comet formation in one cell. B) Quantitation of DNA cleavage measured as the amount of DNA in the tail of the comets formed. The comet image of menadione is shown in the supporting information (Figure S2).
Detection of ROS in cells using microscope
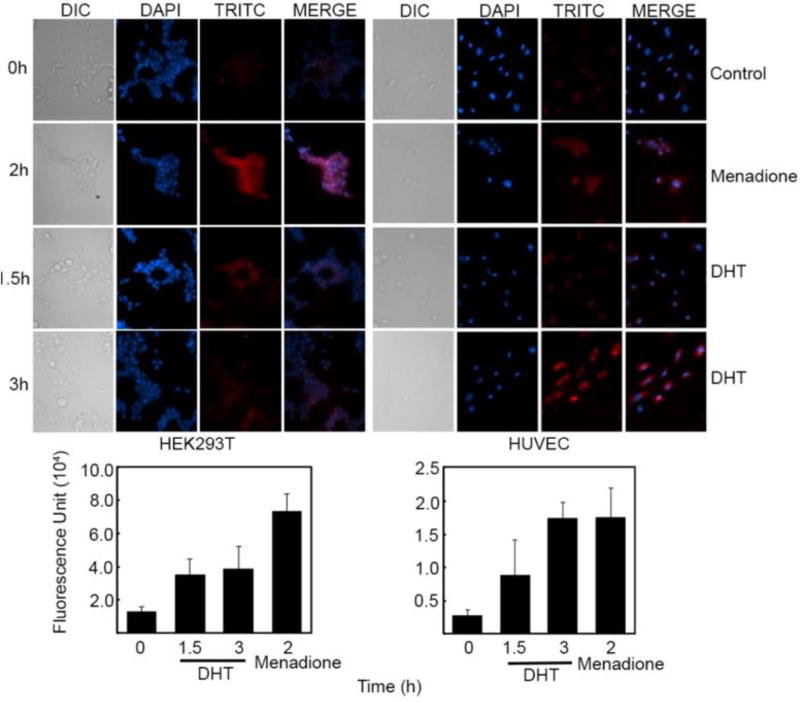
Because the DNA cleavage assays clearly indicated a typical quinone mechanism, we looked for the formation of ROS in cells using the CellROX deep red reagent (the dye used is tetramethylrhodamine, TRITC).37 The CellROX reagent is a cell-permeant non-specific dye (can detect any reactive oxygen species) that upon oxidation by ROS produces bright red fluorescence. When HEK293T, HUVEC, and HepG2 cells were treated with DHT for 1.5 and 3 h, ROS formation was detected. The ROS generation was particularly pronounced in HUVEC cells following 3 h drug treatment. These results are consistent with earlier report that TD causes oxidative stress.22 Our data provided direct biochemical evidence that TD, following metabolism, can cause oxidative damage.
Detection of single strand DNA cleavage
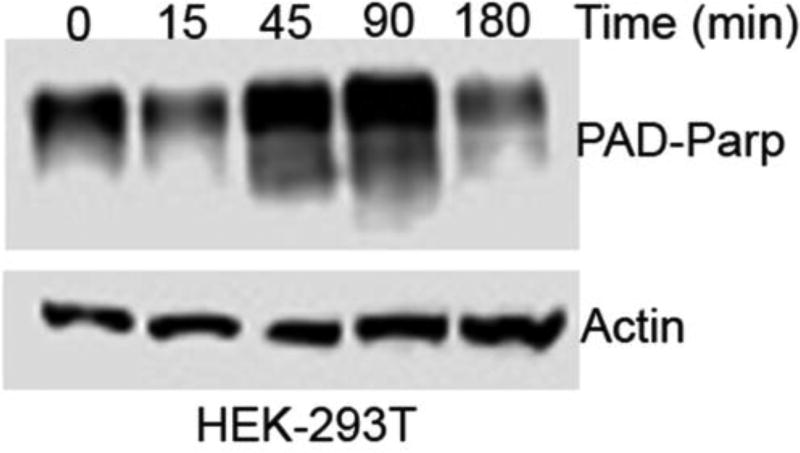
Although all of the evidence points towards DNA single strand breaks (SSB), it is important to gain additional evidence to propose a mechanism. Accordingly, we performed a PARP assay. PARP is a nuclear protein involved in detection and signaling of SSB to the cellular enzyme-machinery involved in SSB repair. PARP activation is an immediate cellular response to DNA SSB. After PARP detects SSB, it binds to DNA and begins the synthesis of a poly (ADP-ribose) chain (PAR) as a signal for DNA repair. Detection of PAR containing PARP is a clear indication of the presence of DNA SSB. Western blot (immunoblot) analysis using a PAR-PARP specific antibody revealed an increase in the level of PAR-PARP within 45 min of DHT treatment (Figure 5). This immediate response in the level of PAR-PARP clearly indicates that DHT produced DNA SSB in a cell. The decrease in the level of PAR-PARP within 3h of DHT treatment is probably due to degradation of DHT or the quinone intermediate.
Figure 5.
Western blot analysis showing the presence of Pad-Parp.
Mechanism of DNA cleavage by thalidomide
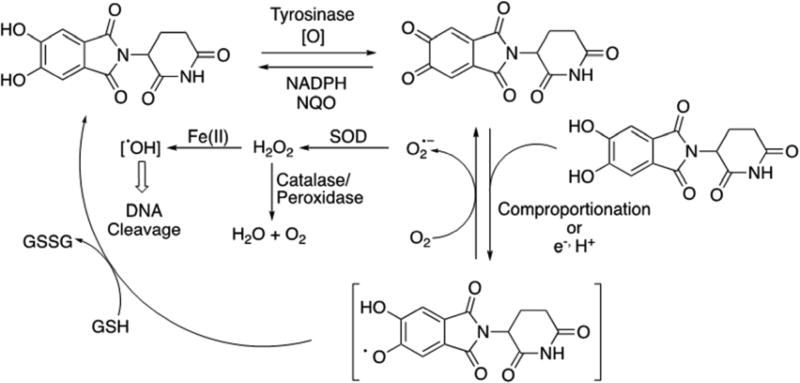
The data reported here is consistent with that typically seen for quinones.38, 39 Based on the observations made here a mechanism of DNA damage by DHT is proposed (Figure 6). According to the proposed mechanism, DHT is oxidized to the quinone, which undergoes comproportionation with the reduced DHT to generate the semiquinone radical intermediate. The semiquinone in the presence of oxygen is back oxidized to the quinone along with the formation of superoxide radical anion and finally H2O2. In the presence of metals, H2O2 undergoes Fenton chemistry to produce the well-known DNA damaging agent hydroxyl radical.
Figure 6.
Proposed mechanism of DNA damage by DHT.
According to the mechanism proposed here, the toxic effect of DHT is not dependent on the cellular concentration of DHT but on the opposing action of the reducing equivalents and the protective effects of catalase, quinone reductases, and GSH. Interestingly, the concentration of catalase is significantly lower in fetal liver compared to adult.40 Until gestation week 6 the level of catalase is undetectable.40 Because most organ development is complete by gestation week 6, the results presented here fit well with the observed teratogenic properties of TD, including the metabolism requirement, time sensitive window, and low dose. The ROS theory was initially proposed by Wells and coworkers and supported by others.22, 41 However, according to the results presented here TD is unable to generate ROS and cause DNA damage. The TD oxidation product DHT are the true species capable of generating ROS and causing DNA damage.
The mechanism proposed here for the teratogenicity of thalidomide clearly explains the metabolism and/or low dose requirements along with the GSH depletion reports. But the teratogenicity of TD is also species specific. Interestingly, mutant mice that are deficient in antioxidant enzymes or glutathione or are having inhibition of the glutathione peroxidase or reductase enzymes are sensitive to thalidomide embryopathy.42 According to the mechanism proposed here presence of these enzymes and GSH should provide a protection from oxidative damage by DHT and hence from teratogenicity. Mouse embryos having these agents should in principle be insensitive to the teratogenic effect of DHT. Thus, the mechanism proposed here not only provides a direct chemical basis for the in vivo oxidative DNA damage mediated teratogenicity of this drug but also explains the various observations made regarding the teratogenicity of TD. Although our proposed mechanism seems consistent with the various literature reports, it is quite possible that several of the mechanisms are working in parallel or synergistically.
Conclusion
In conclusion we have shown that the dihydroxy metabolite of thalidomide is capable of causing extensive redox activated DNA cleavage. The cleavage was also observed in comet assays. The DNA cleavage is radical mediated through the formation of ROS. The proposed mechanism is consistent with that of typical quinones and explains some of the unusual properties of this drug.
Supplementary Material
Figure 1.
Cytochrome P450 mediated biotransformation of thalidomide15,17
Figure 4.
Detection of ROS in HEK293T and HUVEC cells. Cells in culture were either treated with DHT (10 µM) for 1.5 and 3 h (HUVEC 0hr vs 3hr *P=0.0039, 0hr vs menadione *P=0.029 and HEK293T 0hr vs menadione *P=0.020) or menadione (200 µM) for 2 h. Presence of ROS was detected using the deep red CellRox reagent having tetramethylrhodamine (TRITC) as the fluorescent dye. Experiments are done in triplicates with error bars representing ±SEM. A) Microscopic images of cells showing formation of ROS B) Quantitation of ROS measured as the fluorescence intensity within the cell.
Acknowledgments
Funding Sources
This work was supported in part by the DBT, Ramalingaswami Re-entry fellowships BT/HRD/35/02/2006 (G.C.), & 102/IFD/SAN/1576/2014-15 (A.C.), SERB, DBT early career grant ECR/2015/000197 (G.C.) & ECR/2015/000198 to (A.C.), Shiv Nadar University, the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 17K08425 (H.Y.), and U. S. National Institute of Health Grant R01 GM118122 (F.P.G.).
Abbreviations
- BSA
Bovine Serum Albumin
- DAPI
4',6-diamidino-2-phenylindole
- DHT
Dihydoxy thalidomide
- DIC
Differential interference contrast
- DMEDA
N, N′-dimethylethylenediamine
- DMSO
Dimethyl sulfoxide
- EDTA
Ethylenediaminetetraacetic acid
- FITC
Fluorescein isothiocyanate
- GSH
Glutathione
- H1047R PI3K
H1047R mutant PI3K p110a subunit
- HEK293T
Human Embryonic kidney cell line
- HepG2
Human hepatoma G2 cell line
- HER2
Human epidermal growth factor receptor 2
- HUVEC
Human umbilical vein endothelial cell line
- LMPA
Low melting point agarose
- MCF10A
Michigan cancer foundation 10A cell line
- NaCl
Sodium Chloride
- NaOH
Sodium hydroxide
- NP40
Nonident P40
- P450
Cytochrome P450
- PARP
Poly (ADP-Ribose) polymerase
- ROS
Reactive oxygen species
- SDS-PAGE
Sodium dodecyl sulphate- poly acryl amide gel electrophoresis
- SSB
Single strand break
- TD
Thalidomide
- TRITC
Tetramethylrhodamine
Footnotes
The authors declare no competing financial interest.
DNA agarose gel, Comet assay, and ROS assay image data. This information is available free of charge via the internet at http://pubs.acs.org/.
References
- 1.Speirs A. Thalidomide and congenital abnormalities. Lancet. 1962;1:303–305. doi: 10.1016/s0140-6736(62)91248-5. [DOI] [PubMed] [Google Scholar]
- 2.Stephens TD, Bunde CJ, Fillmore BJ. Mechanism of action in thalidomide teratogenesis. Biochem. pharmacol. 2000;59:1489–1499. doi: 10.1016/s0006-2952(99)00388-3. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F, Moreau P, Waage A, Spencer A, Ludwig H, Boccadoro M, Harousseau J. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111:3968–3977. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- 4.Zangari M, Elice F, Tricot G. Immunomodulatory drugs in multiple myeloma. Expert Opin. Investig. Drugs. 2005;14:1411–1418. doi: 10.1517/13543784.14.11.1411. [DOI] [PubMed] [Google Scholar]
- 5.Barkin JA, Schonfeld WB, Deshpande AR. Successful use of thalidomide for refractory esophageal Crohn’s disease. Am. J. Gastroenterol. 2013;108:855–857. doi: 10.1038/ajg.2013.49. [DOI] [PubMed] [Google Scholar]
- 6.Bond A, Ahmed W. Thalidomide for the treatment of angiodysplasia in a patient with acute upper gastrointestinal haemorrhage. BMJ Case Reports. 2016;2016 doi: 10.1136/bcr-2015-213522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchini M, Lippi G. Thalidomide for hereditary haemorrhagic telangiectasia. The Lancet Haematology. 2015;2:e457–458. doi: 10.1016/S2352-3026(15)00222-7. [DOI] [PubMed] [Google Scholar]
- 8.Cervantes F, Martinez-Trillos A. Myelofibrosis: an update on current pharmacotherapy and future directions. Expert Opin. Pharmacother. 2013;14:873–884. doi: 10.1517/14656566.2013.783019. [DOI] [PubMed] [Google Scholar]
- 9.Tseng S, Pak G, Washenik K, Pomeranz MK, Shupack JL. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J. Am. Acad. Dermatol. 1996;35:969–979. doi: 10.1016/s0190-9622(96)90122-x. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Shibata N, Sukeguchi D, Takashima M, Nakamura S, Toru T, Matsunaga N, Hara H, Tanaka M, Obata T, Sasaki T. Synthesis, configurational stability and stereochemical biological evaluations of (S)- and (R)-5-hydroxythalidomides. Bioorg. Med. Chem. Lett. 2009;19:3973–3976. doi: 10.1016/j.bmcl.2009.02.108. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Helsby N, Palmer BD, Tingle M, Baguley BC, Kestell P, Ching LM. Metabolism of thalidomide in liver microsomes of mice, rabbits, and humans. J. Pharmacol. Exp. Ther. 2004;310 doi: 10.1124/jpet.104.067793. [DOI] [PubMed] [Google Scholar]
- 12.Ando Y, Fuse E, Figg WD. Thalidomide metabolism by the CYP2C subfamily. Clin. Cancer Res. 2002;8:1964–1973. [PubMed] [Google Scholar]
- 13.Yamazaki H, Suemizu H, Igaya S, Shimizu M, Shibata N, Nakamura M, Chowdhury G, Guengerich FP. In vivo formation of a glutathione conjugate derived from thalidomide in humanized uPA-NOG mice. Chem. Res. Toxicol. 2011;24:287–289. doi: 10.1021/tx200005g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki H, Suemizu H, Shimizu M, Igaya S, Shibata N, Nakamura M, Chowdhury G, Guengerich FP. In vivo formation of dihydroxylated and glutathione conjugate metabolites derived from thalidomide and 5-Hydroxythalidomide in humanized TK-NOG mice. Chem. Res. Toxicol. 2012;25:274–276. doi: 10.1021/tx300009j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury G, Murayama N, Okada Y, Uno Y, Shimizu M, Guengerich FP, Yamazaki H. Human liver microsomal cytochrome P450 3A enzymes involved in thalidomide 5-hydroxylation and formulation of a glutathione conjugate. Chem. Res. Toxicol. 2010;23:1018–1024. doi: 10.1021/tx900367p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon GB, Spielberg SP, Blake DA, Balasubramanian V. Thalidomide teratogenesis: evidence for a toxic arene oxide metabolite. Proc. Natl. Acad. Sci. USA. 1981;78:2545–2548. doi: 10.1073/pnas.78.4.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury G, Shibata N, Yamazaki H, Guengerich FP. Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analogue of thalidomide. Chem. Res. Toxicol. 2014;27:147–156. doi: 10.1021/tx4004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teo S, Morgan M, Stirling D, Thomas S. Assessment of the in vitro and in vivo genotoxicity of Thalomid (thalidomide) Teratog. Carcinog. and Mutagen. 2000;20:301–311. doi: 10.1002/1520-6866(2000)20:5<301::aid-tcm6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 20.Bauer KS, Dixon SC, Figg WD. Inhibition of angiogenesis by thalidomide requires metabolic activation, which is species dependent. Biochem. Pharmacol. 1998;55:1827–1834. doi: 10.1016/s0006-2952(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 21.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain PP, Lopez-Girona A, Miller K, Carmel G, Pagarigan B, Chie-Leon B, Rychak E, Corral LG, Ren YJ, Wang M, Riley M, Delker SL, Ito T, Ando H, Mori T, Hirano Y, Handa H, Hakoshima T, Daniel TO, Cathers BE. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 2014;21:803–809. doi: 10.1038/nsmb.2874. [DOI] [PubMed] [Google Scholar]
- 24.Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, Tichkule RB, Schebesta M, Forrester WC, Schirle M, Hassiepen U, Ottl J, Hild M, Beckwith RE, Harper JW, Jenkins JL, Thoma NH. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512:49–53. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA, Ebert BL. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science (New York, N.Y.) 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG., Jr The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science (New York, N.Y.) 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petzold G, Fischer ES, Thoma NH. Structural basis of lenalidomide-induced CK1alpha degradation by the CRL4(CRBN) ubiquitin ligase. Nature. 2016;532:127–130. doi: 10.1038/nature16979. [DOI] [PubMed] [Google Scholar]
- 28.Braun A, Harding F, Weinreb S. Teratogen metabolism: thalidomide activation is mediated by cytochrome P-450. Toxicol. Appl. Pharmacol. 1986;55:1827–1834. doi: 10.1016/0041-008x(86)90449-7. [DOI] [PubMed] [Google Scholar]
- 29.Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D, Clement MV. OpenComet: an automated tool for comet assay image analysis. Redox biology. 2014;2:457–465. doi: 10.1016/j.redox.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall T, Williams KM. Coomassie blue protein dye-binding assays measure formation of an insoluble protein-dye complex. Anal. Biochem. 1992;204:107–109. doi: 10.1016/0003-2697(92)90147-y. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Liu L, Yang S, Song N, Zhou X, Gao J, Yu N, Shan L, Wang Q, Liang J, Xuan C, Wang Y, Shang Y, Shi L. Histone demethylase KDM5B is a key regulator of genome stability. Proc. Natl. Acad. Sci. USA. 2014;111:7096–7101. doi: 10.1073/pnas.1324036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolton JL, Chang M. Quinoids as reactive intermediates in estrogen carcinogenesis. Adv. Exp. Med. Biol. 2001;500:497–507. doi: 10.1007/978-1-4615-0667-6_75. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Wagner BA, Lehmler H, Buettner GR. Semiquinone Radicals from Oxygenated Polychlorinated Biphenyls: Electron Paramagnetic Resonance Studies. Chem. Res. Toxicol. 2008;21:1359–1367. doi: 10.1021/tx8000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniels JS, Gates KS, Tronche C, Greenberg MM. Direct evidence for bimodal DNA damage induced by tirapazamine. Chem. Res. Toxicol. 1998;11:1254–1257. doi: 10.1021/tx980184j. [DOI] [PubMed] [Google Scholar]
- 35.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods (San Diego, Calif.) 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29:5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyman LM, Franz KJ. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 2012;256:2333–2356. doi: 10.1016/j.ccr.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 2004;17:55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- 39.Klaus V, Hartmann T, Gambini J, Graf P, Stahl W, Hartwig A, Klotz LO. 1,4-Naphthoquinones as inducers of oxidative damage and stress signaling in HaCaT human keratinocytes. Arch. Biochem. Biophys. 2010;496:93–100. doi: 10.1016/j.abb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Espeel M, Briere N, De Craemer D, Jauniaux E, Roels F. Catalase-negative peroxisomes in human embryonic liver. Cell & Tissue Res. 1993;272:89–92. doi: 10.1007/BF00323574. [DOI] [PubMed] [Google Scholar]
- 41.Sauer H, Gunther J, Hescheler J, Wartenberg M. Thalidomide inhibits angiogenesis in embryoid bodies by the generation of hydroxyl radicals. Am. J. Pathol. 2000;156:151–158. doi: 10.1016/S0002-9440(10)64714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells PG, Bhuller Y, Chen CS, Jeng W, Kasapinovic S, Kennedy JC, Kim PM, Laposa RR, McCallum GP, Nicol CJ, Parman T, Wiley MJ, Wong AW. Molecular and biochemical mechanisms in teratogenesis involving reactive oxygen species. Toxicol. Appl. Pharmacol. 2005;207:354–366. doi: 10.1016/j.taap.2005.01.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.