Introduction
Tolerance: the ability to endure what one cannot avoid; also referred to as an accepted dispensation to particular rules. When applied to orally-administrated antigen, the definition of tolerance represents the capacity of the immune system to adapt to innocuous dietary proteins and commensal bacteria because we are unable to avoid them. These antigens are detected in the gut epithelium and Lamina Propria within minutes after consumption (1) suggesting a critical role of the gastro-intestinal (GI) tract in oral tolerance development.. Currently, it is postulated that this state of unresponsiveness to ingested antigens is initiated by tolerogenic CD103 + DCs residing in the GI lamina propria (2). Following capture of luminal antigens, these cells migrate to the draining lymph node to present antigen-derived epitopes to induce antigen-specific regulatory CD4 + T cells. Failure to develop oral tolerance can lead to a cascade of adverse reactions such as IgE-mediated food allergies, celiac disease, autoimmune diseases, and infections. These immune-related disorders have emerged as major health problems worldwide because of the rapid increase in prevalence over the past decade (3). Food-induced allergic reactions are an immediate, adverse reaction triggered predominantly by cross-linking of food-derived antigen-specific IgE bound to the high-affinity IgE receptor FcεR on mast cells after re-exposure to allergen (4, 5). These can cause clinical symptoms ranging from mild mouth itching and abdominal pain to life-threatening anaphylaxis. Standard of care is allergen avoidance and prompt treatment of allergic reactions when they develop after accidental ingestion. One promising area of current investigation is the use of oral immunotherapy (OIT) as a method to eventually restore oral tolerance to food. Improving our understanding of oral tolerance mechanisms will aid in the reduction of food allergy prevalence rates through pre-exposure prophylaxis (due to natural tolerance development) and create new strategies for food allergy therapy (due to induced tolerance).
The Gastrointestinal mucosa: A unique place in oral tolerance development
The GI tract is exposed to a large array of non-self-antigen on a continual basis, including numerous commensal bacteria and well over 30 kg of food proteins per year (6). Yet, the GI immune system does not elicit cellular or humoral immune responses to these harmless antigens as it protects against pathogenic microbes. This phenomenon of balancing the immune response to commensal microbes and food has been termed “oral tolerance” and refers to local and systemic immune unresponsiveness to orally administered soluble antigens. It may develop naturally or be induced by allergen immunotherapy (AIT). The GI mucosa is the largest immunologic site in the body designed to distinguish between beneficial and harmful components in the gut to maintain systemic immune tolerance (7, 8). It is composed of three major compartments: the epithelial layer, the lamina propria (LP) and the gut-associated lymphoid tissue (GALT) where adaptive immune responses are initiated (9). Immune responses in the gut are efficiently induced in Mesenteric lymph nodes (mLN) and Peyer’s patches (PP), which are the main components of the organized gut-associated lymphoid tissue (GALT). Payer’s patches are lymphoid-cell accumulation areas found in the submucosa, primarily the small intestine, and consist of B-cell follicles and surrounding T-cell areas. Intestinal epithelial cells form a tight and selective barrier that allows highly controlled paracellular and transcellular transport of molecules or antigens necessary to the induction of appropriate immune responses in the gut (10). The barrier function of the GI tract is aided by the presence of a protective, hydrophobic mucus-coated surface that traps antigen and dimeric immunoglobulin A (IgA) that binds food proteins. Together this prevents absorption of antigen across the intestinal epithelium (11). However, an estimated 2% of gut luminal food proteins pass through the gut epithelium intact and are disseminated locally or systemically through the circulation or the lymphatic system (12). Tissue-resident T lymphocytes are abundant in the GI immune system and play important roles in mucosal immunity and oral tolerance (13). Their adaptation to these environments requires constant discrimination between natural stimulation coming from harmless microbiota and food, and pathogens that need to be cleared. Several factors are involved to ensure durable tolerance to harmless intestinally derived antigens, including the dose, nature and routes of antigen entry at sensitization. Physical barriers, digestion and composition of the intestinal flora are also thought to contribute to the ability to develop oral tolerance (14). The microbiome is a complex collection of resident bacteria in the gut and elsewhere that can profoundly impact immune responses in the GI tract. For instance, gut microbiota has been associated with increased production of Immunoglobulin A (IgA), that may protect against food allergy by neutralizing food antigens and limiting their access to the immune system (15, 16). Exposing children to a variety of food types from an early age might be an important contributing factor to induction of oral immune tolerance, thus preventing the development of severe food allergies(17)(18).
Mechanism of Oral Tolerance
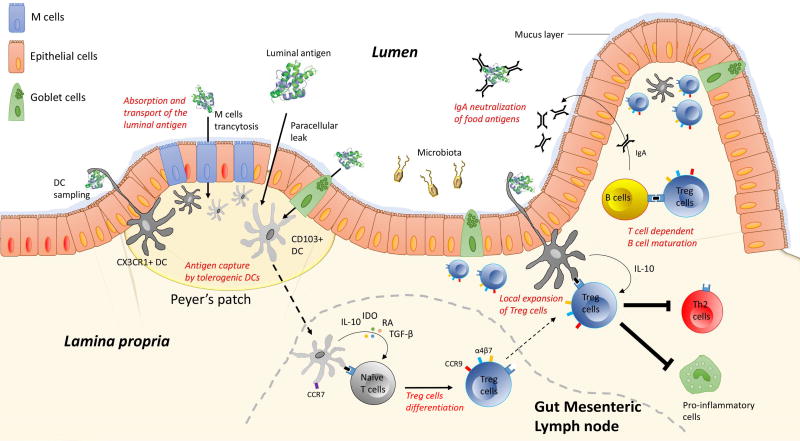
Despite a growing literature in the field, mechanisms of how ingested protein are normally rendered non-immunogenic through oral tolerance are not fully understood and largely rely on murine experimental models. The initial contact between immune cells and antigens from the lumen is a critical step in the induction of intestinal immune response. Hadis et al (19) recently proposed a model of stepwise oral tolerance induction comprising the generation of Treg cells in the gut-draining lymph nodes, followed by migration into the gut and subsequent local expansion of Treg cells driven by intestinal macrophages. Upon ingestion, immunogenic food proteins are denatured and degraded into small peptides by the digestive process in the gut. Absorption and transport of the luminal antigen involves complex processes, which might include transcytosis through intestinal epithelial cells, paracellular diffusion and endocytosis via microfold cells (M cells) thus enabling antigenic presentation to underlying immune cells. Dendritic cells (DC) that express the integrin CD11b and the chemokine receptor CX3CR1 can also directly sample the luminal content through the formation of trans-epithelial dendrites (20–22). In specific areas of the epithelium, called Peyer’s patches, internalized luminal antigens are captured by antigen-presenting cells (APCs), which then migrate into gut-draining mesenteric lymph nodes (mLN) where they initiate activation and differentiation of effector or regulatory T cells depending on cytokine milieu (23). In the lamina propria (LP), DCs that express the integrin CD103 and CD11c promote IgA production, imprint gut homing on lymphocytes and promote the differentiation of naïve T cells into regulatory T (Treg) cells (2). Intestinal mucin and cytokines produced by epithelial cells and innate lymphoid cells may also contribute to tolerance by modifying the phenotype of gastrointestinal dendritic cells.(16, 24). It is also postulated that a different type of epithelial cell, the goblet cell, preferentially delivers luminal antigen to tolerogenic DCs implying a key role for this cell in intestinal immune homeostasis (25). Compared to CD103+ DCs, antigen-loaded CX3CR1+ DCs do not express CCR7 and thus do not migrate to the mLN. Their role seems to be restricted to the local antigen-specific expansion of Treg cells in the lamina propria. Vitamin A metabolite retinoic acid, IDO, IL-10 and TGF-B (all produced by CD103+ DC) are thought to be critical determinants in defining the mLN tolerogenic environment (26, 27). Following activation, T cells differentiated in mLN by CD103+ DC then up-regulate the gut-homing molecules integrin α4β7 and the chemokine (C-C motif) receptor 9 (CCR9), which provides homing properties to the activated T cells to migrate back to the lamina propria (28, 29). Treg cells then undergo expansion under the influence of IL-10 produced by local antigen-loaded CX3CR1+DC cells (Figure 1). Although Treg-cells specific to food allergens are formed and localized in the intestine, they also can be found in circulation to maintain systemic tolerance (30).
Figure 1.
Schematic overview of the stepwise mechanisms involved in the development and maintenance of oral tolerance. Upon ingestion and digestive process, luminal antigen are absorbed and transported through intestinal epithelial cells thus enabling antigenic presentation to underlying immune cells. This complex process might include: 1) paracellular diffusion, 2) endocytosis via microfold cells and globlet cells, and 3) trans-epithelial DC sampling of the luminal antigen. In specific areas of the epithelium, called Peyer’s patches, luminal antigens are taken up by CD11c+CD103+DCs or CD11b+CX3CR1+DCs. While CX3CR1+DCs remain located in the lamina propria, antigen-loaded CD103+ DCs migrate to the mLN in a CCR7-dependent mechanism to promote the differentiation of naïve T cells into regulatory T (Treg) cells. After their generation in lymph nodes, Tregs up-regulate CCR9 and integrin α4β7 to home back to the lamina propria where they undergo local expansion to induce oral tolerance. In this process Treg cells play a major role in: 1) promoting B-cell class switching to produce the non-inflammatory IgA responses, 2) inducing T-cell anergy of effector cells, and 3) inhibiting downstream pro-inflammatory cells.
For many years, oral immune tolerance was envisioned as a multifaceted process involving clonal deletion, clonal anergy of reactive T cells and the active regulation by regulatory T cells. In this context, Weiner et al. (31, 32) suggested that the mechanism of oral tolerance was determined by the feeding regime used, with single high doses of antigen favoring clonal deletion or anergy, whereas multiple low doses of antigen were linked to T-cell-mediated suppression. However, transfer of CD25+ CD4+ T cells (which include Foxp3 + Tregs and activated effector cells) can induce oral tolerance to naïve animals (33). Similarly, depletion of antigen-specific FoxP3+ cells completely abolishes established functional oral tolerance, suggesting a dominant role of immunosuppressive FoxP3+ Treg cells in this process, especially Treg cells induced from conventional T cells in the periphery (pTreg cells) (34). While there are multiple subtypes of pTregs (8,9), both Foxp3+ and IL-10 producing Foxp3- pTregs are found in the GI tract. Their ability to produce pleiotropic immunoregulatory cytokines, such as transforming growth factor beta (TGF-β) and interleukin-10 (IL-10), plays a major role in promoting B-cell class switching to produce the non-inflammatory IgA responses. IL-10 also induces T-cell anergy of effector cells, sustains Treg populations and directly participates in inhibiting downstream pro-inflammatory cells, such as mast cells, basophils and eosinophils, leading to a state of unresponsiveness to ingested antigens or oral tolerance (5, 22).
Tolerance to intestinal bacteria and tolerance to food proteins differs by its effects on the immune system. However, it has been suggested that the presence of both diet- and microbiota-induced populations of pTreg cells may be required for complete tolerance to food antigens (35, 36). Using germ-free mice raised and bred on an elemental diet devoid of solid food dietary antigens, Kim et al. (37) recently demonstrated that the food antigens triggered FoxP3+ Treg cells to proliferate and accumulate in the small intestine lamina propria, whereas microbiota elicit pTreg cell generation in the colon, implying that a carefully controlled process regulates the local expansion of pTreg cells. In this elegant study the authors also found that pTreg cells that suppress immune responses to food are phenotypically and functionally distinct from microbiota-induced RORƳt+ pT reg cells generated in the colon. However, food antigen– induced pTreg cells appear to have a relatively short life span and it is unclear whether these food antigen–induced pTreg cells are also responsible for the systemic effects of oral tolerance. In experimental models of food allergy it has been shown that the depletion of macromolecules from the diet precludes the development of pTreg cells in the small intestine (38). In parallel, oral tolerance was maintained for several months after a single encounter with antigen suggesting that the generation of pTregs in response to intestinal antigens might be a continuously ongoing process (39).
Loss of Oral Tolerance
Food allergy and celiac disease are the most prevalent food induced pathology and are likely one of the consequences of a breakdown of oral tolerance characterized by elevated levels of IgE-positive myeloid cells (MC) in the small intestine(40). How oral tolerance might be disrupted in food allergies remains unclear, but a weakened ability to induce or regenerate pTreg-cells after exposure to food allergen may be a significant factor. For example, reduction in the number of pTreg-cells has been documented in children with food allergy (41). Failure to induce immunologic tolerance to food antigens is also thought to be affected by the timing of introduction to solid food, dose of antigen exposure and allergenic property of the food themselves acting as mucosal TH2 adjuvants (17). More recently, alterations in gut microflora composition have been implicated in the increase of allergy incidence. Gut microorganisms are the major source of natural antigens that continuously stimulate the GALT and induce mucosal immune tolerance to food proteins and molecular components of commensal bacteria (42). Disruption of the microbiome can occur via early antibiotic use in infants and in utero, even with very low doses (43). On occasion these effects can persist well into adulthood (44). A Western diet, typically high in fat but also low in fiber, may also be a critical determinant for intestinal flora diversity favoring or disfavoring Treg induction and epithelial integrity (45). In a recent study, Tan et al (46) established a role for dietary fiber in enhancing the tolerogenic CD103+ DC functions suggesting that their phenotype is highly dependent on environmental conditioning factors present locally in the small intestine.(47).
The general immune mechanism behind allergic sensitization to food and thus of breakdown of oral tolerance can be viewed as a stepwise process initiated by disturbances in antigen uptake, dissemination, and/or presentation of allergen-derived epitope. Indeed, those with food allergy are thought to have genetic predisposition factors for higher skin and gut permeability as well as increasing allergen penetrance (which can be further exacerbated by environmental insults)(48). It is also increasingly understood that infants can become sensitized to foods through skin contact alone, particularly in those individuals with eczema who demonstrate loss of function filaggrin mutations (49). Local disruption of the skin barrier integrity in the post-birth environment may condition mucosal DCs toward allergic sensitization versus the establishment of oral tolerance to ingested foods. For instance, presence of bacteria-induced barrier-protective response such as Clostridia in microbiota protects against sensitization by causing innate immune cells to produce high levels of interleukin-22 (IL-22), a signaling molecule known to decrease the permeability of the intestinal lining (15). Conversely, Intestinal inflammation can also abrogate the ability of CD103+ DCs to promote pTreg cell differentiation (50). Thus, one can argue that allergic sensitization to food antigens via a damaged GI tract may confer susceptibility to food allergy later in life. In susceptible individuals, local tissue perturbations lead to increased concentration of epithelial-derived cytokine such as IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) at mucosal surfaces (51). Acting through their respective receptors IL17RB, ST2 and TSLP-R, the resultant type 2-permissive microenvironment instruct TH2 cell-mediated allergic inflammation through effects on DCs and on tissue-resident ILC2 cells. Among TH2 cells, our group recently showed that allergen-specific TH2 cells represent a phenotypically distinct subset defined as CD4+ T cells that co-express the surface molecule CRTH2, CD161 and CD49d but lack CD27 and CD45RB expression (52). In contrast to conventional TH2 cells, this pro-allergic TH2 subset (confined to atopic individuals) co-expressed IL-33-R and IL-25R (52–54), providing evidence that food allergen-specific TH2 cell function may depend on tissue-derived cytokines at sites of tissue damage that regulate allergic immunity. Van Dyken et al. (55) further demonstrated that TH2 cells require stimulation by epithelial-derived cytokine in order to become functional TH2 effector cells in tissue, thereby comprising a checkpoint for effector cytokine acquisition. These findings validate the strategy of blocking tissue signals to treat allergic disease. Following allergic sensitization, pro-allergic TH2 cells migrate to a draining lymphoid node and prompt B cells to class switch, resulting in the production of IgE. IgE then binds to its high-affinity receptor FcεRI on the surface of mast cells and basophils, thereby arming these cells for activation (56). After the occurrence of allergic sensitization, endogenous intestinal IL-25 produced constitutively by tuft cells, one of the five intestinal epithelial cell lineages, may rapidly establish a positive feedback loop that increases ILC2s and TH2A effector functions. This in turn creates a TH2-permissive environment that dampens regulatory T cell production (57, 58). Atopic IL-4 signaling may also perpetuate allergic reactions to dietary proteins by inducing FcεR-expressing mast cell progenitors to develop into multi-functional IL-9producing Mucosal Mast Cells (MMC9s), which then expand greatly after repeated food ingestion (59). These cells function as type-2-promoting innate myeloid cells by generating prodigious amounts of the TH2 cytokines IL-9 and IL-13 and employing mast cell function (via the secretion of histamine and proteases). Thus, it is possible that local accumulation of IgE-bearing MMC9s will impose the breakdown of oral tolerance to ingested food antigens as recently reviewed by Yui-Hsi Wang (24).
Restoration of Oral tolerance during Immunotherapy
Evidence of a lack of oral tolerance in food allergic patients has increased the interest in utilizing oral immunotherapy (OIT) as a disease-modifying therapy via an effect on the gut immune system. The oral route of administration takes advantage of the unique properties of the intestinal immune system to induce tolerance. Such ability of continuous and gradual increase of orally administered antigen to restore oral tolerance to food allergens was first described in 1908 by A.T. Schofield (60). At present, OIT is one of the most actively investigated therapeutic approaches for food allergy. However, in most cases, only patients remaining on maintenance doses do not develop reactions and it is uncertain whether OIT leads to the development of oral tolerance or only to desensitization. Consumption of bacterial strains, such as Bifidobacterium longum 35624 and Clostridia, can result in increased numbers of intestinal Foxp3+ T regulatory cells that are capable of suppressing food allergy and colitis. (15, 61). However, in the Nagler et al study, reintroduction of another major group of intestinal bacteria Bacteroides, failed to alleviate sensitization. Thus, one would envision that opportunistic intestinal flora is involved in the process of cultivating oral tolerance.
Immunological changes accompanying oral desensitization include decreased reactivity of mast cells and basophils, increased food-specific IgG4 antibodies, and eventually decreased food-specific IgE antibodies (62). OIT is believed to restore oral tolerance in patients with food allergy through interaction of the allergen with mucosal DC that initiates downstream immune system modulation through development of Treg cells (63). For example, a higher frequency of milk allergen-specific Treg cells has been shown to correlate with a phenotype of mild clinical disease and favorable prognosis (64). Desensitization is the first change noted with the initiation of OIT. During this state, protection depends on the regular ingestion of the food allergen; when dosing is interrupted or discontinued, the protective effect may be lost or significantly decreased. By tracking ex vivo changes in peripheral peanut-reactive T cells in participants during OIT, our group recently observed a strong correlation between decrease of peanut-specific TH2 cells and achievement of peanut desensitization (52). Interestingly, remaining allergen-specific T cell subtypes, which share similar features with those seen in non-allergic individuals, remain unchanged. Similarly, a mechanistic study using single-cell sorting and transcriptional profiling of individual T cells collected throughout OIT showed that induction of anergic T cells along with decreased allergen-specific TH2 cells accompanied oral desensitization (65).
The ultimate goal of OIT is to restore permanent oral tolerance, which is established when food may be ingested without allergic symptoms, despite prolonged periods of avoidance. A phase 1 peanut-OIT study by Syed et al targeted such identification of specific immune mechanisms associated with a tolerant clinical phenotype (66). While they observed that serum IgE or IgG4 level along with basophil activation did not statistically differentiate between clinical “immune tolerance” and “immune desensitization”, epigenetic changes in antigen-induced Treg cells were shown to be predictive of long-lasting clinical benefit after the withdrawal of OIT. In murine experimental models of food allergy, it has been shown that cow’s milk OIT induced lamina propria pTreg cells that controlled the allergic reaction through the production of IL-10 and TGF-β (67). Interestingly, in their model, the adoptive transfer of FoxP3+ Tregresulted in pronounced disease exacerbation, thus confirming that Treg cells have an essential role in resolving food allergy (33). One possible mechanism to explain and integrate all these results into a cohesive schema is that restoration of permanent oral tolerance is a stepwise process which likely involves the initial anergy and deletion of allergen-specific TH2 cells (desensitization state) followed by the development of allergen-specific T cells with stable regulatory properties at later stages (tolerance state)(68). However, local immune mechanisms associated with OIT remain to be further investigated, most particularly with respect to cells involved in allergen uptake at the administration site.
SUMMARY
Oral tolerance to dietary antigens and commensal bacteria is crucial to prevent the development of food allergies, celiac disease and autoimmune diseases. The oral route of administration takes advantage of the unique set of immune cells and pathways involved in the induction of oral tolerance. Early microbial colonization and exposure to solid food plays an important role in promoting natural oral tolerance. OIT is believed to initiate desensitization through interaction of an allergen with mucosal DCs that initiate downstream immune system modulation through Tregs and effector T cells. Future in-depth understanding of the process involved in the restoration of oral tolerance through OIT will likely contribute to a more effective treatment of disease.
Key points.
-
-
Gastrointestinal tract has an abundant mucosal immune system to develop and maintain oral tolerance.
-
-
The oral route of administration takes advantage of the unique set of immune cells and pathways involved in the induction of oral tolerance.
-
-
Food allergy results from a loss of oral tolerance toward ingested antigens.
-
-
OIT is believed to initiate desensitization through interaction of an allergen with mucosal DCs that initiate downstream immune system modulation through Tregs and effector T cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. The Journal of experimental medicine. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J.Allergy Clin.Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Vickery BP, Scurlock AM, Jones SM, Burks AW. Mechanisms of immune tolerance relevant to food allergy. The Journal of allergy and clinical immunology. 2011;127:576–584. doi: 10.1016/j.jaci.2010.12.1116. quiz 585-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. The Journal of allergy and clinical immunology. 2016;137:984–997. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 7.Faria AMC, Reis BS, Mucida D. Tissue adaptation: Implications for gut immunity and tolerance. The Journal of experimental medicine. 2017;214:1211–1226. doi: 10.1084/jem.20162014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefrancois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu Rev Immunol. 2006;24:681–704. doi: 10.1146/annurev.immunol.24.021605.090650. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 10.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 11.Perrier C, Corthesy B. Gut permeability and food allergies. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41:20–28. doi: 10.1111/j.1365-2222.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 12.Warshaw AL, Walker WA, Isselbacher KJ. Protein uptake by the intestine: evidence for absorption of intact macromolecules. Gastroenterology. 1974;66:987–992. [PubMed] [Google Scholar]
- 13.Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol. 2013;23:R389–400. doi: 10.1016/j.cub.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature reviews. Immunology. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR, Antonopoulos DA, Zhou L, Chang EB, Fu YX, Nagler CR. Commensal bacteria protect against food allergen sensitization. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berin MC. Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin Immunopathol. 2012;34:633–642. doi: 10.1007/s00281-012-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G, Team LS. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, Brough HA, Santos AF, Harris KM, Radulovic S, Basting M, Turcanu V, Plaut M, Lack G, Immune Tolerance Network L-OST. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med. 2016;374:1435–1443. doi: 10.1056/NEJMoa1514209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Persson EK, Scott CL, Mowat AM, Agace WW. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. European journal of immunology. 2013;43:3098–3107. doi: 10.1002/eji.201343740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nature reviews. Immunology. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 22.Wawrzyniak M, O'Mahony L, Akdis M. Role of Regulatory Cells in Oral Tolerance. Allergy Asthma Immunol Res. 2017;9:107–115. doi: 10.4168/aair.2017.9.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nature reviews. Immunology. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YH. Developing food allergy: a potential immunologic pathway linking skin barrier to gut. F1000Res. 2016;5 doi: 10.12688/f1000research.9497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nature reviews. Immunology. 2006;6:682–692. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- 28.Johansson-Lindbom B, Agace WW. Vitamin A helps gut T cells find their way in the dark. Nature medicine. 2004;10:1300–1301. doi: 10.1038/nm1204-1300. [DOI] [PubMed] [Google Scholar]
- 29.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. The Journal of experimental medicine. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scurlock AM, Vickery BP, Hourihane JO, Burks AW. Pediatric food allergy and mucosal tolerance. Mucosal Immunol. 2010;3:345–354. doi: 10.1038/mi.2010.21. [DOI] [PubMed] [Google Scholar]
- 31.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 33.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102:3295–3301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]
- 34.Kim KS, Surh CD. Induction of Immune Tolerance to Dietary Antigens. Adv Exp Med Biol. 2015;850:93–118. doi: 10.1007/978-3-319-15774-0_8. [DOI] [PubMed] [Google Scholar]
- 35.Prince BT, Mandel MJ, Nadeau K, Singh AM. Gut Microbiome and the Development of Food Allergy and Allergic Disease. Pediatr Clin North Am. 2015;62:1479–1492. doi: 10.1016/j.pcl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nature medicine. 2016;22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 37.Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858–863. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 38.Strobel S, Ferguson A. Persistence of oral tolerance in mice fed ovalbumin is different for humoral and cell-mediated immune responses. Immunology. 1987;60:317–318. [PMC free article] [PubMed] [Google Scholar]
- 39.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelz BJ, Bryce PJ. Pathophysiology of Food Allergy. Pediatr Clin North Am. 2015;62:1363–1375. doi: 10.1016/j.pcl.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. The Journal of experimental medicine. 2004;199:1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chistiakov DA, Bobryshev YV, Kozarov E, Sobenin IA, Orekhov AN. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Front Microbiol. 2014;5:781. doi: 10.3389/fmicb.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zarate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J. 2014;13:61. doi: 10.1186/1475-2891-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016;15:2809–2824. doi: 10.1016/j.celrep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 47.Chewning JH, Weaver CT. Development and survival of Th17 cells within the intestines: the influence of microbiome- and diet-derived signals. Journal of immunology. 2014;193:4769–4777. doi: 10.4049/jimmunol.1401835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkataraman D, Soto-Ramirez N, Kurukulaaratchy RJ, Holloway JW, Karmaus W, Ewart SL, Arshad SH, Erlewyn-Lajeunesse M. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. The Journal of allergy and clinical immunology. 2014;134:876–882. e874. doi: 10.1016/j.jaci.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. European journal of immunology. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–268. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 52.Wambre E, Bajzik V, DeLong JH, O'Brien K, Nguyen QA, Speake C, Gersuk VH, DeBerg HA, Whalen E, Ni C, Farrington M, Jeong D, Robinson D, Linsley PS, Vickery BP, Kwok WW. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401) doi: 10.1126/scitranslmed.aam9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitson-Salazar A, Yin Y, Wansley DL, Young M, Bolan H, Arceo S, Ho N, Koh C, Milner JD, Stone KD, Wank SA, Prussin C. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human T(H)2 cell subpopulation with enhanced function. The Journal of allergy and clinical immunology. 2016;137:907–918. e909. doi: 10.1016/j.jaci.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Lam EP, Kariyawasam HH, Rana BM, Durham SR, McKenzie AN, Powell N, Orban N, Lennartz-Walker M, Hopkins C, Ying S, Rimmer J, Lund VJ, Cousins DJ, Till SJ. IL-25/IL-33-responsive TH2 cells characterize nasal polyps with a default TH17 signature in nasal mucosa. The Journal of allergy and clinical immunology. 2016;137:1514–1524. doi: 10.1016/j.jaci.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, Erle DJ, Locksley RM. A tissue checkpoint regulates type 2 immunity. Nature immunology. 2016;17:1381–1387. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends in immunology. 2014;35:69–78. doi: 10.1016/j.it.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 57.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JB, Chen CY, Liu B, Mugge L, Angkasekwinai P, Facchinetti V, Dong C, Liu YJ, Rothenberg ME, Hogan SP, Finkelman FD, Wang YH. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. The Journal of allergy and clinical immunology. 2016;137:1216–1225. e1211–1215. doi: 10.1016/j.jaci.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, Oettgen HC. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. The Journal of allergy and clinical immunology. 2011;127:795–805. e791–796. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schofield A. A Case of Egg Poisoning. The Lancet. 1908;171:716. [Google Scholar]
- 61.Konieczna P, Ferstl R, Ziegler M, Frei R, Nehrbass D, Lauener RP, Akdis CA, O'Mahony L. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS One. 2013;8:e62617. doi: 10.1371/journal.pone.0062617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perezabad L, Reche M, Valbuena T, Lopez-Fandino R, Molina E, Lopez-Exposito I. Oral Food Desensitization in Children With IgE-Mediated Cow's Milk Allergy: Immunological Changes Underlying Desensitization. Allergy Asthma Immunol Res. 2017;9:35–42. doi: 10.4168/aair.2017.9.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? The Journal of allergy and clinical immunology. 2013;131:14–22. doi: 10.1016/j.jaci.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. The Journal of allergy and clinical immunology. 2009;123:43–52. e47. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 65.Ryan JF, Hovde R, Glanville J, Lyu SC, Ji X, Gupta S, Tibshirani RJ, Jay DC, Boyd SD, Chinthrajah RS, Davis MM, Galli SJ, Maecker HT, Nadeau KC. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1286–1295. doi: 10.1073/pnas.1520180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O'Riordan G, Galli SJ, Nadeau KC. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) The Journal of allergy and clinical immunology. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smaldini PL, Orsini Delgado ML, Fossati CA, Docena GH. Orally-Induced Intestinal CD4+ CD25+ FoxP3+ Treg Controlled Undesired Responses towards Oral Antigens and Effectively Dampened Food Allergic Reactions. PLoS One. 2015;10:e0141116. doi: 10.1371/journal.pone.0141116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wambre E. Effect of allergen-specific immunotherapy on CD4+ T cells. Curr Opin Allergy Clin Immunol. 2015;15:581–587. doi: 10.1097/ACI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]