Abstract
Introduction
One of the greatest problems associated with continuous renal replacement therapy (CRRT) is the early clotting of filters. A literature search revealed three case reports of lipemic blood causing recurrent clotting and reduced CRRT circuit survival time in adult patients, but no reports of cases in children.
Diagnosis/treatment
A 23-month-old male infant with Martinez–Frias syndrome and multivisceral transplant was admitted to the hospital with severe sepsis and hemolytic anemia. He developed acute kidney injury, fluid overload and electrolyte imbalances requiring CRRT and was also administered total parenteral nutrition (TPN) and fat emulsion. The first circuit lasted 60 h before routine change was required. The second circuit showed acute clotting after only 18 h, and brownish-milky fluid was found in the circuit tubing layered between the clotted blood. The patient’s serum triglyceride levels were elevated at 988 mg/dL. The lipid infusion was stopped and CRRT restarted. Serum triglyceride levels improved to 363 mg/dL. The new circuit lasted 63 h before routine change was required.
Conclusion
Clotting of CRRT circuits due to elevated triglyceride levels is rare and has not been reported in the pediatric population. Physicians should be mindful of this risk in patients receiving TPN who have unexpected clotting of CRRT circuits.
Keywords: Child–Martinez, Frias syndrome, Continuous renal replacement therapy, Acute clotting
Case summary
Initial presentation
A 23-month-old male infant with Martinez–Frias syndrome (duodenal atresia, anemia, neonatal cholestasis, pancreatic endocrine and exocrine dysfunction) presented with a 2-day history of cough and rhinorrhea followed by a low-grade fever, up to 38.3 °C, and voluminous watery, non-bloody stools.
In the emergency department, he was tachycardic, febrile and ill in appearance. His creatinine level was slightly elevated at 0.6 (normal range 0.1–0.4) mg/dL, and urinalysis revealed a specific gravity of 1.016 (normal range 1.003–1.035) and no blood or protein. Due to concern for sepsis, the patient was started on vancomycin, piperacillin–tazobactam, intravenous fluids and stress doses of hydrocortisone and was transferred to the intensive care unit. His stool studies and respiratory viral panel were positive for Cryptosporidium and rhinovirus.
Past history
The patient underwent multivisceral transplantation (liver, stomach, small bowel, colon and pancreas) and splenectomy 15 months prior to presentation. He was maintained on tacrolimus and prednisone for immunosuppression. He had a history of elevated creatinine and intermittent hyperkalemia since undergoing transplantation, likely related to the tacrolimus regimen. He had hyperphosphatemia controlled with sevelamer. Baseline blood work prior to admission showed a creatinine level of 0.4 mg/dL with normal potassium, acid–base balance, and phosphorous while on Florinef and sevelamer, as well as a serum triglyceride level of 188 mg/dL.
Hospital course
At admission, he had a low hemoglobin count of 5.7 (normal range 10.5–13.5) mg/dL and diagnosed with autoimmune hemolytic anemia with warm and cold autoantibody positivity. A packed red blood cell (pRBC) transfusion was initially deferred due to the resultant difficulty with the crossmatch. His autoimmune hemolytic anemia was treated with methylprednisone 2 mg/kg/day and subsequent intravenous immunoglobulin. On hospital day 2, his hemoglobin count decreased to 3.9 g/dL, with a platelet count of 684 (normal range 150–400) thousand/μL. He developed shock with significant metabolic acidosis [venous pH of 6.92 (normal range 7.34–7.44), bicarbonate of 5 (normal range 20–26) mEq/L and base deficit of −24] and was resuscitated with emergent intubation, bicarbonate administration, bolus doses of epinephrine, and pRBC transfusion. Over the next 48 h, he developed worsening acute kidney injury due to a combination of shock-induced acute tubular necrosis and exposure to nephrotoxic medications. On day 4 of admission, he developed oliguria (urine output of 95 mL in the prior 24 h) with a creatinine level of 2.1 mg/dL, hyperkalemia with potassium up to 5.4 (normal range 4.1–5.4) mEq/L, persistent hypocalcemia with a serum calcium level of 7.1 (normal range 8.7–9.8) mg/dL, ionized calcium level of 0.88 (normal range 1–1.17) mmol/L and hyperphosphatemia with phosphorus level of 9 (normal range 3.8–6.5) mg/dL. The patient was started on continuous veno-venous hemodiafiltration (CVVHDF). Nutrition was solely intravenous fluids until day 6 of admission when total parenteral nutrition (TPN) was started at 25 mL/h. The TPN initially contained 12.5 % (75 g) dextrose, which was increased to 18 % (108 g) dextrose on day 7, and 2.5 g/kg/day amino acids and a 20 % fat emulsion with 2 g/kg/day of lipids.
Continuous renal replacement therapy
Indications for CVVHDF included fluid overload, anuria and rising serum potassium levels. A 7-Fr catheter was placed in the right external jugular vein for continuous renal replacement therapy (CRRT) access. Heparin was used for anticoagulation. A Prisma M60 dialyzer (Gambro, Lund, Sweden) was used, with a blood flow rate of 60 mL/min, dialysate flow rate of 1260 mL/min/1.73 m2 and replacement fluid rate of 1260 mL/min/1.73 m2 (pre-filter, pre-pump 840 mL/min/1.73 m2).
The first CRRT circuit lasted 60 h before routine change was required. While on the circuit, the patient received 2 units of pRBCs, 1 unit of fresh frozen plasma and 1 unit of cryoprecipitate. At the time of the circuit coming down, the activated clotting time (ACT) was 177 s, transmembrane pressure (TMP) was 75 mmHg, the filter pressure was 123 mmHg and the return line pressure was 71 mmHg.
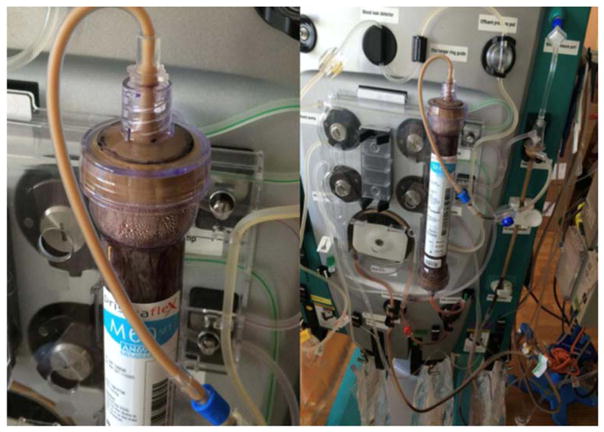
On day 8 of admission, after only 18 h on the second circuit, the bedside nurse noted very negative access pressures. The circuit was paused, and the catheter lines were power flushed with a 10-mL syringe of normal saline. However, the circuit acutely shut down due to clotting with brownish-milky fluid in the tubing layered between the clotted blood (Fig. 1). Prior to the circuit coming down, the ACT was 193 s, TMP was 57 mmHg, filter pressure was 134 mmHg and return line pressure was 87 mmHg. No blood products were given while the patient was on the second CRRT circuit.
Figure 1.
Presence of a brownish-milky fluid noted in the continuous renal replacement therapy circuit tubing at the time of circuit clotting
Questions
What are the various factors that increase risk of CRRT circuit clotting?
What are the consequences of premature clotting of CRRT circuits?
How would you proceed to evaluate and diagnose the etiology of clotting in this patient?
How would you proceed with management of this problem?
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.