Abstract
Background & Aims
The approval of all-oral direct-acting antiviral (DAA) regimens for the treatment of hepatitis C virus (HCV) has led to the expansion of therapy to include patients with cirrhosis who have hepatocellular carcinoma (HCC). Data on the use of DAAs in HCV+ patients with HCC is limited. The aim of this study was to assess the efficacy of all-oral-DAA regimens in HCV+ cirrhotic patients who have or had HCC compared to those without HCC.
Methods
A retrospective cohort study was conducted on all cirrhotic patients who were treated for HCV with DAAs at our institution between January 2014 and November 2015.
Results
A total of 421 HCV+ patients with cirrhosis were identified, of whom 33% had active or a history of HCC. Failure to achieve sustained virologic response (SVR) occurred in 21% of patients with HCC compared to 12% of patients without HCC (p = 0.009). Of the 29 patients with HCC who did not achieve SVR, 27 (93%) occurred when an active tumor was present. DAA therapy in the presence of an inactive tumor or after removal of tumor (resection/transplant) resulted in excellent SVR rates, similar to those without HCC (p <0.0001). In multivariable analysis, the primary predictor of DAA treatment failure was the presence of active HCC at the time of HCV treatment initiation (adjusted odds ratio = 8.5, 95% confidence interval = 3.90–18.49).
Conclusions
The presence of active HCC tumor at the initiation of HCV therapy is significantly associated with all-oral DAA treatment failure. HCV treatment after curative therapies for HCC resulted in excellent SVR.
Lay summary
The new medications for hepatitis C have excellent cure rates. However, our study shows that in patients with both liver cancer and hepatitis C, they do not achieve these cure rates. Patients with liver cancer are almost 8 times more likely to fail hepatitis C treatment than patients without liver cancer.
Keywords: Liver cancer, Sustained viral response, Cirrhosis, Treatment, Liver transplant, Hepatitis C virus, Hepatocellular carcinoma
Introduction
Hepatitis C virus (HCV) has been the leading cause of cirrhosis in the United States for several decades [1]. Until recently, the standard of care for HCV treatment was interferon based therapy (IFN), which was wrought with intolerable side effects, long treatment duration, and suboptimal sustained virologic response (SVR) rates of approximately 40% for patients with genotype 1 [2,3]. SVR rates were lower in patients with advanced fibrosis [4]. The approval of direct-acting antiviral agents (DAA) has subsequently revolutionized therapy for HCV. SVR rates are consistently over 90% for genotype 1 despite the presence of cirrhosis [5]. Furthermore, the all-oral DAA regimens are well tolerated and require a shorter length of therapy than the IFN-based therapies.
Cirrhosis in the setting of HCV is a leading risk factor for the development of hepatocellular carcinoma (HCC) with an annual incidence rate of 1–4% per year [6]. The availability of DAAs has led to a significant increase in the number of patients receiving HCV therapy, including those with cirrhosis and HCC. The impetus to treat HCV in patients with known HCC is similar to those without HCC: to ameliorate ongoing inflammation and the development of further fibrosis that ultimately can lead to the clinical consequences of portal hypertension. The registration trials that led to the approval of the DAAs excluded patients with HCC [7–10] or included only a few patients with HCC [11]. The only trial that was dedicated to patients with HCC was a phase II trial that examined the use of sofosbuvir and ribavirin pretransplant in patients with HCC listed for liver transplantation, 70% of whom achieved post liver transplantation SVR [12]. Initially patients received treatment for 24 weeks or until they underwent liver transplantation. However, after observing 3 pre-transplant virologic relapses in HCC patients who had received the standard 24 weeks of therapy, the trial was revised to continue treatment in all individuals for 48 weeks or until they underwent liver transplantation [12]. It should be noted that sofosbuvir and ribavirin has lower SVR rates in patients with genotype 1 than current regimens and is no longer recommended [13,14].
The efficacy of HCV therapy with contemporary highly effective, all-oral DAA regimens in patients with HCC is currently unknown. Patients with HCC may have lower rates of SVR as the tumor may serve as a viral reservoir, providing an immune tolerant environment for the HCV. The aim of our study was to determine if the presence or history of HCC impacted the likelihood of achieving SVR with highly effective, all-oral DAAs regimens.
Patients and methods
Patients
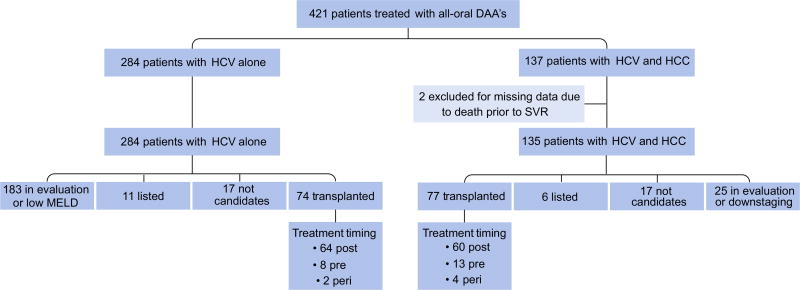
We performed a single center, retrospective cohort analysis of all patients with a history of cirrhosis in the setting of HCV who were initiated on all-oral DAA therapy at Northwestern Memorial Hospital between January 2014 and November 2015. Patients were identified through our specialty pharmacy database, and hepatology and transplantation clinic visits. During that time period, 421 patients with cirrhosis received all-oral DAA therapy. Of these, 284 (67%) had HCV without HCC and 137 (33%) had HCV with current or a history of HCC. Two patients (both with HCC) were excluded from the final analysis, because of missing treatment response data due to death. Of the remaining 419 patients, 284 (67%) were in the non-HCC group and 135 (33%) were in the HCC group (Fig. 1).
Fig. 1. Study population.
DAA, direct-acting antiviral; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease.
The presence of chronic HCV infection was defined as detectable HCV RNA by real-time polymerase chain reaction at the initiation of therapy. Genotype was confirmed and documented. Cirrhosis was defined by one of the following: liver biopsy, FibroScan® >12.5 kPa, acoustic radiation force impulse >2.0 m/s, magnetic resonance (MR) elastography >5 kPa, or FibroSURE™ testing indicating F4 disease. Patients were classified as being in the HCC group if they had radiographic evidence of a lesion on 4-phase multidetector computed tomography (CT) scan or dynamic contrast-enhanced MR imaging that had arterial hypervascularity and venous or delayed phase washout prior to HCV therapy initiation, if they underwent resection of HCC prior to antiviral therapy with DAAs, or if they underwent liver transplantation for HCC and were treated after transplantation with a DAA regimen. Tumor was confirmed on explant for all transplanted individuals.
For the non-HCC group, in patients who received a transplant, explant data was reviewed to ensure that patients documented as having HCV alone did not have histologic evidence of HCC. Patients who did not undergo transplantation underwent regular assessment for HCC with semiannual alpha fetoprotein (AFP) assessment and liver imaging with ultrasound or cross sectional imaging.
Data collection
Patient information was procured from a prospectively maintained electronic database, the Organ Transplant Tracking Record and the hospital system’s outpatient based electronic medical record.
Clinical characteristics for the patients were obtained at the time of HCV treatment initiation. Data collected included patient age, gender, race, ethnicity, body mass index (BMI), alcohol history, calculated model for end-stage liver disease (MELD) score, Child-Turcotte-Pugh (CTP) class, and AFP at the time of HCV treatment initiation. HCV specific data included baseline HCV viral load, prior treatment history, genotype, type of DAA used, and duration of DAA therapy. The particular DAA regimen used was determined at the discretion of individual physicians based on the HCV genotype and the regimens available at the time. Given the time period of the inclusion criteria, a few regimens used are no longer considered standard of care. For our analysis, we classified regimens as either adequate or inadequate based upon current HCV therapy guidelines. Inadequate regimens were defined as sofosbuvir and simeprevir (SOF/SIM) for 12 weeks, sofosbuvir and ledipasvir (SOF/LDV) for 12 weeks in treatment experienced patients, or sofosbuvir and ribavirin (SOF/RBV). We assessed response to DAA therapy by documenting the end of treatment (EOT) response and the SVR12. SVR12 was defined as an undetectable HCV RNA (HCV viral load <15), 12 weeks after completion of HCV therapy. Treatment failure was defined as the presence of detectable HCV RNA within 12 weeks of discontinuation of antiviral therapy.
Patients in the HCC group had additional data collected regarding their cancer history. Patients were considered part of the HCC group if they had a known diagnosis of HCC prior to the initiation of DAA treatment. For all patients with HCC, tumor size and AFP level were collected at the time of HCC diagnosis. Notation was made as to whether tumor was present at the time of all-oral DAA initiation. The presence of tumor was defined as a lesion on imaging delineated as HCC; this included individuals with previously treated lesions with radioembolization (Y90) or chemoembolization (TACE) who had evidence of radiographic tumor response with tumor necrosis. Patients that received a resection or transplant and subsequently started DAA were categorized in the HCC group, although were defined as having absent tumor. The presence of active tumor was also noted, defined as arterial enhancement and venous washout on tri-phasic CT or MRI imaging, or the existence of active tumor on explant pathology. All other data was collected at the time of HCV therapy initiation and included lesion size, number of lesions, Barcelona Clinic Liver Cancer (BCLC) stage, the presence of metastatic disease, and the type(s) of therapy for HCC. The study was approved by the Institutional Review Board of Northwestern University.
Clinical characteristics were described using frequency counts and percentages for categorical variables and means with standard deviations for continuous variables. Univariate comparisons of HCV treatment response or HCC status characteristics used t tests for continuous variables and ANOVA, Chi square or Fisher exact test for categorical variables. Multivariable logistic regression models were then used to assess the association of presence of HCC tumor on HCV treatment failure. Covariates in the multivariable model were chosen a priori for clinical importance. Potential confounders included age, gender, race, ethnicity, CTP class, platelet count, prior history of HCV treatment, HCV genotype, hepatitis B core antibody status, treatment regimen and treatment timing related to transplant. Patients who died prior to the EOT but did not have a post-treatment HCV RNA test were excluded from analysis (n = 2). Interaction terms were generated between HCC status and race, sex, age, and HCV treatment response. A p value <0.05 was considered statistically significant. Analyses were performed using STATA 9.4 (STATA institute, Cary, NC).
For further details regarding the materials used, please refer to the CTAT table.
Results
The baseline characteristics of the entire HCV positive population and comparison between patients in the HCC and non-HCC groups are shown in Table 1. Patients treated for HCV with DAAs had a mean age of 61 years and were predominantly Caucasian (63%) and male (68%). Most patients had HCV genotype 1 (86%), had previously been treated for HCV (60%), and were largely CTP class A (74%). Transplantation status, reasons for non-listing and the timing of HCV treatment relative to transplantation for all patients are shown in Fig. 1. Orthotopic liver transplantation (OLT) was performed in 151 patients, of whom 74 (49%) were in the non-HCC group and 77 (51%) were in the HCC group. Patients who did not undergo OLT were either too healthy to be listed, were listed and awaiting transplantation, were in the process of OLT evaluation, were too old for transplant (age >75), or had advanced HCC and thus, were ineligible for transplantation.
Table 1.
Baseline Characteristics of HCV+ patients who underwent treatment with all-oral DAA therapy stratified by HCC status.
| Characteristics | Entire cohort (n = 421) |
Non-HCC (n = 284) |
HCC (n = 137) |
p value* |
|---|---|---|---|---|
| Age, mean, years | 61, 8.3 | 60, 8.4 | 64, 7.4 | <0.0001 |
| Male, n (%) | 288 (68) | 185 (65) | 103 (75) | 0.04 |
| Race/Ethnicity, no. (%) | ||||
| Caucasian | 256 (65) | 165 (61) | 91 (67) | 0.81 |
| African American | 64 (16) | 45 (17) | 64 (14) | |
| Hispanic | 67 (16) | 47 (17) | 67 (15) | |
| Other | 22 (3) | 15 (5) | 22 (4) | |
| BMI (kg/m2), mean | 29, 5.3 | 29, 5.5 | 28, 4.9 | 0.21 |
| Former alcohol use, no. (%) | 100 (24) | 64 (23) | 36 (26) | 0.24 |
| Calculated MELD | 10, 4 | 11, 4.2 | 10, 3.6 | 0.15 |
| Child-Turcotte-Pugh Class, no. (%) | ||||
| A | 310 (74) | 201 (71) | 109 (81) | 0.03† |
| B | 107 (25) | 81 (29) | 26 (19) | |
| C | 3 (1) | 2 (0.1) | 1 (0.7) | |
| Ascites, no. (%) | 94 (22) | 75 (26) | 19 (14) | 0.004 |
| Hepatic encephalopathy, no. (%) | 69 (16) | 56 (20) | 13 (9) | 0.008 |
| AFP (ng/ml) | 31, 80.5 | 23, 45.6 | 50, 125.5 | 0.004 |
| Creatinine (mg/dl) | 1.09, 1.09 | 1.09, 1.28 | 1.07, 0.48 | 0.88 |
| Total bilirubin (mg/dl) | 1.46, 1.84 | 1.53, 1.62 | 1.33, 2.23 | 0.30 |
| Albumin (g/dl) | 3.65, 0.62 | 3.64, 0.63 | 3.68, 0.59 | 0.56 |
| INR | 1.18, 0.30 | 1.19, 0.30 | 1.15, 0.31 | 0.22 |
| AST (units/L) | 80, 59.6 | 80, 54.4 | 83, 69.3 | 0.60 |
| ALT (units/L) | 76, 69.5 | 73, 53.6 | 83, 94.1 | 0.17 |
| Platelets (µl) | 128, 80.2 | 126, 84.1 | 132, 71.6 | 0.47 |
| Sodium (mmol/L) | 137, 2.98 | 137, 2.94 | 138, 3.05 | 0.06 |
| HBsAg present, n (%) | 4 (1) | 3 (1) | 1 (0.7) | 0.76 |
| HBcIgG present, n (%) | 119 (30) | 68 (26) | 51 (39) | 0.02 |
| Log HCV viral load | 13.5, 5.8 | 13.5, 7.20 | 13.7, 6.14 | 0.79 |
| Treatment experienced, n (%) | 252 (60) | 173 (61) | 79 (58) | 0.52 |
| HCV genotype (%) | ||||
| 1 | 360 (86) | 244 (86) | 116 (85) | 0.16 |
| 2 | 22 (5) | 18 (6) | 4 (3) | |
| 3 | 35 (8) | 19 (7) | 16 (12) | |
| Other | 4 (0.01) | 3 (1) | 1 (0.07) | |
| Treatment classification**(%) | ||||
| Inadequate | 266 (63) | 187 (66) | 79 (58) | 0.12 |
| Adequate | 155 (37) | 97 (34) | 58 (42) |
Data are presented as mean, standard deviation or number (%).
HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HCV-HCC, individuals with both hepatitis C and hepatocellular carcinoma; n, number; BMI, body mass index; MELD, model for end-stage liver disease; AFP, alpha fetoprotein; INR, international normalized ratio; AST, aspartate transaminase; ALT, alanine transaminase; HBsAg, hepatitis B surface antigen; HBcIgG, hepatitis B core IgG.
t test for continuous variables and Chi square or Fisher exact test for categorical variables for the comparison between non-HCC and HCC groups.
Treatment classified as inadequate or adequate based on current HCV treatment standards. Inadequate treatment was defined as simeprevir/sofosbuvir 12 weeks orsofosbuvir/ledipasivir 12 weeks in treatment experienced patients or sofosbuvir/ribavirin regimens.
Comparisons between Child-Turcotte-Pugh A vs. B or C.
Compared to patients without HCC, patients with HCC were significantly more likely to be male (75% vs. 65%; p = 0.04), were older (64 vs. 60 years; p <0.0001), have a positive hepatitis B core antibody (39% vs. 26%; p = 0.006), and have higher baseline AFP levels (23 vs. 50 ng/ml; p = 0.004). Patients without HCC had more underlying liver dysfunction manifested by a higher prevalence of ascites (26% vs. 14%, p = 0.004), hepatic encephalopathy (20% vs. 9%, p = 0.008), and CTP B or C disease (29% vs. 19%, p = 0.03) compared to those with HCC. There was no significant difference between groups in terms of age, BMI, calculated MELD score, percentage who were treatment experienced, or HCV genotype. In order to isolate the effects of HCC on DAA treatment failure among those with active cirrhosis, a sensitivity analysis was performed excluding all patients who were treated with DAA therapy post-transplant. Baseline demographics of patients with cirrhosis at the time of DAA initiation is shown in Table S1.
HCC patient characteristics
There were 137 patients with a history of cirrhosis who had both HCV and HCC. The mean age was 64 years and patients were primarily Caucasian (67%). The average MELD score was 10 and the majority of patients had CTP A disease (81%). Average AFP at HCV treatment initiation was 50 ng/ml and 58% of patients were treatment experienced. Genotype 1 was again the predominant genotype (85%); 3% had genotype 2, and 12% had genotype 3.
Tumor characteristics of the HCC group are shown in Table 2. The average tumor size at HCC diagnosis and HCV treatment initiation were 33 ± 3 and 31 ± 3 mm, respectively. The mean AFP at the time of HCC diagnosis was 751.6 ± 665.5 ng/ml. At the time of HCV treatment initiation, 64 patients (47%) had tumor present on imaging. On further review of imaging and explant pathology of those with tumor present, 59 patients (43%) had active tumor at the time of treatment. The majority of patients with tumor present were BCLC stage A (64%). Out of the entire HCC cohort, only 37 patients (27%) had multifocal HCC at treatment initiation and 2 patients (1%) had metastatic disease.
Table 2.
Tumor characteristics of patients with HCC who underwent treatment with all-oral DAA therapy.
| HCC tumor characteristics | HCC group (n = 137) |
|---|---|
| HCC size (mm), mean, SD | |
| At diagnosis | 33.1, 2.9 |
| At treatment | 31.2, 2.8 |
| AFP (ng/ml) at diagnosis | 751.6, 665.6 |
| Tumor present at HCV treatment, n (%) | 64 (47) |
| Active tumor* present at HCV treatment, n (%) | 59 (43) |
| BCLC stage at HCV treatment, n (%) | |
| 0 | 3 (5) |
| A | 40 (63) |
| B | 14 (22) |
| C | 5 (8) |
| D | 1 (2) |
| Number of lesions, n (%) | |
| 0–1 | 120 (73) |
| 2–3 | 27 (20) |
| 4 or more | 8 (6) |
| Metastatic disease, n (%) | 2 (1) |
| Treatments received** | |
| Resection | 18 (13) |
| OLT | 77 (56) |
| Y90 | 83 (61) |
| TACE | 25 (18) |
HCC, hepatocellular carcinoma; AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Clinic; OLT, orthotopic liver transplant; Y90, radioembolization; TACE, transcatheter arterial chemoembolization.
Active tumor was defined as arterial enhancement and venous washout on tri-phasic CT, contrast-enhanced MRI imaging, or the existence of active tumor onexplant pathology.
Patients may have received multiple therapies.
Treatment characteristics for the 137 HCC patients are also shown in Table 2. Seventy-seven patients (56%) received a liver transplant, for which 60 (78%) patients were treated for HCV post-transplant, 13 (22%) pre-transplant, and 4 (7%) peri transplant. Hepatic resection was performed in 18 (13%) patients. In those who were resected, 3 initiated HCV treatment with tumor present and 15 were treated after resection. Radioembolization and TACE were carried out either as primary HCC therapy or in conjunction with surgical intervention as part of a down staging protocol in 61% and 18% of patients respectively. Out of the 84 patients who received radioembolization, HCV therapy was initiated in 25 patients prior to receiving radioembolization and 59 after. All 25 patients who received TACE began HCV therapy after receiving TACE.
DAA treatment outcomes
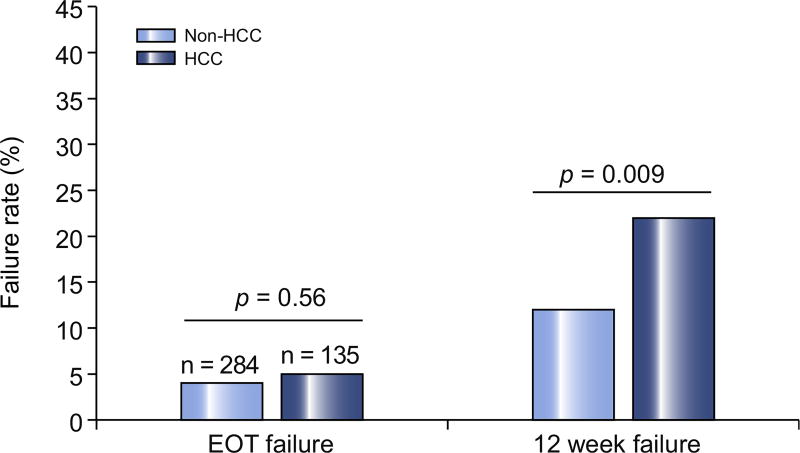
HCV treatment outcomes for both groups are shown in Fig. 2. The EOT failure rate in all patients was approximately 5%. EOT failure occurred in 12 (4%) patients without HCC compared to 7 (5%) patients with HCC (p = 0.56). There was a significant difference in DAA failure at 12 weeks following completion of HCV therapy (Fig. 2). A total of 62 patients (15%) failed HCV therapy at 12 weeks. Patients in the non-HCC group had a 12-week DAA failure rate of 12% compared to a failure rate of 21% in the HCC group (p = 0.009).
Fig. 2. Percentage of patients who failed hepatitis C therapy at the end of treatment and at 12 weeks post-treatment.
EOT, end of treatment; HCC, hepatocellular carcinoma. *t test used for statistical analysis.
In the population who underwent liver transplantation, 14 out of 151 patients (9%) failed treatment: 4 failures occurred in the post-transplantation setting, 2 of whom had a history of pretransplantation diagnosis of HCC. The other 10 failures occurred in the pre-transplantation setting in patients without HCC. Patients in the HCC group who were treated with DAAs after resection or OLT had similar failure rates to those that did not have HCC (3% vs. 2%, p = 0.65).
DAA treatment outcomes by regimen
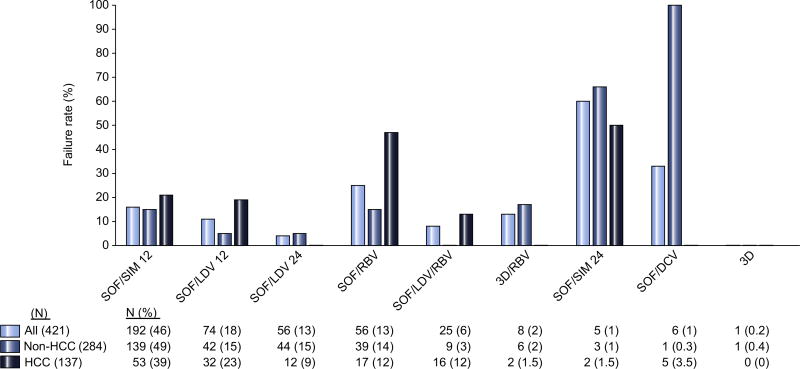
The HCV treatment regimens used in all patients are shown in Fig. 3. SOF/SIM for 12 weeks was the most common regimen (46%) followed by SOF/LDV for 12 (18%) and 24 weeks (13%), SOF/RBV for 24 weeks (13%), and SOF/LDV/RBV for 12 weeks (6%). The type of regimen predicted HCV treatment failure (p = 0.005). Regimens associated with the highest failure rate were ones that are classified as inadequate, including SOF/SIM for 12 weeks (60%), followed by SOF/RBV (25%, Fig. 3). In the entire cohort, 75% of those who failed DAA therapy received inadequate therapy compared to 62% who achieved SVR12 (p = 0.06). When examined by HCC status, patients with active tumor were more likely (52%) than patients without HCC (35%) or a history of treated HCC (22%) to have received adequate DAA therapy (p = 0.004).
Fig. 3. Failure rate of DAA therapy by treatment regimen received.
SOF, sofosbuvir; SIM, simeprevir; RBV, ribavirin; LDV, ledipasvir; DCV, declatasvir; 3D, ombitasvir/paritaprevir/ritonavir.
Predictors of DAA treatment failure
Univariate predictors of HCV treatment failures are shown in Table 3. Treatment failures had a higher international normalized ratio (INR) (1.3 vs. 1.2, p = 0.01), higher aspartate aminotransferase (AST) (98 vs. 78 units/L, p = 0.02), and a lower platelet count (103 vs. 133 µl, p = 0.007) compared to those who achieved SVR12. Failures were more likely to be Caucasian (17% vs. 11%, p = 0.02), have ascites (25% vs. 12%, p = 0.003), and have active tumor present (93% vs. 30%, p <0.0001) at the time of treatment. A higher percentage of CTP B and C patients failed treatment compared to those who achieved SVR12 (40% vs. 24%, p = 0.01). Patients who were not transplanted had higher failure rates than those who received a transplant (18% vs. 9%, p = 0.01). The majority of DAA treatment failures were genotype 1 (81%).
Table 3.
Univariate predictors of DAA treatment failure.
| Characteristics | SVR12 (n = 356) | Failure (n = 63) | p value* |
|---|---|---|---|
| Age, mean, years | 61, 8.5 | 61, 7.1 | 0.56 |
| Male, n (%) | 237 (67) | 48 (77) | 0.10 |
| Race/ethnicity, n (%) | |||
| Caucasian | 210 (62) | 44 (71) | 0.72 |
| African American | 57 (17) | 6 (10) | |
| Hispanic | 55 (16) | ||
| Other | |||
| BMI (kg/m2), mean | 29, 5.3 | 30, 5.6 | 0.24 |
| Former alcohol use, n (%) | 81 (23) | 17 (27) | 0.04 |
| Calculated MELD | 10, 4.1 | 11, 3.4 | 0.20 |
| Child-Turcotte-Pugh class, n (%) | |||
| A | 270 (76) | 37 (60) | 0.01 |
| B | 83 (23) | 23 (37) | |
| C | 1 (1) | 2 (3) | |
| Ascites, n (%) | 71 (20) | 23 (37) | 0.003 |
| Hepatic encephalopathy, n (%) | 54 (15) | 14 (23) | 0.15 |
| AFP (ng/ml) | 29.2, 82.5 | 41.3, 73.5 | 0.31 |
| Creatinine (mg/dl) | 1.13, 1.17 | 0.87, 0.27 | 0.09 |
| Total bilirubin (mg/dl) | 1.44, 1.95 | 1.63, 1.06 | 0.44 |
| INR | 1.16, 0.31 | 1.27, 0.22 | 0.01 |
| AST (units/L) | 77.9, 57.4 | 97.8, 69.9 | 0.01 |
| ALT (units/L) | 73.7, 60.8 | 89.7, 69.7 | 0.10 |
| Platelets (µl) | 132.8, 83.7 | 103.2, 53.1 | 0.01 |
| Sodium (mmol/L) | 137.3, 3.04 | 137.6, 2.57 | 0.55 |
| HBsAg present, n (%) | 4 (1) | 0 (0) | 0.41 |
| HBcIgG present, n (%) | 103 (31) | 14 (24) | 0.52 |
| Log HCV viral load | 13.7, 2.1 | 13.2, 2.6 | 0.12 |
| Treatment experienced, n (%) | 212 (60) | 39 (63) | 0.64 |
| Transplant status | 135 (38) | 13 (21) | 0.01 |
| HCV genotype | |||
| 1 | 307 (86) | 50 (81) | 0.004 |
| 2 | 22 (6) | 0 (0) | |
| 3 | 23 (7) | 11 (18) | |
| Other | 3 (1) | 1 (1) | |
| Regimen | |||
| SOF/SIM 12 weeks | 159 (45) | 31 (50) | 0.005 |
| SOF/LDV 12 weeks | 66 (19) | 8 (13) | |
| SOF/LDV 24 weeks | 53 (15) | 2 (3) | |
| SOF/RBV | 42 (12) | 14 (22) | |
| SOF/LDV/RBV | 23 (6) | 2 (3) | |
| SOF/SIM 24 weeks | 2 (0.6) | 3 (5) | |
| 3D/RBV | 7 (2) | 1 (2) | |
| 3D | 1 (0.3) | 0 (0) | |
| HCC status | |||
| History of HCC | 106 (30) | 29 (47) | 0.009 |
| Tumor present | 37 (10) | 27 (44) | <0.0001 |
| Active tumor | 31 (30) | 27 (93) | <0.0001 |
Data are presented as mean, standard deviation or number (%).
HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HCV-HCC, individuals with both hepatitis C and hepatocellular carcinoma; n, number; BMI, body mass index; MELD, model for end-stage liver disease; AFP, alpha fetoprotein; INR, international normalized ratio; AST, aspartate transaminase; ALT, alanine transaminase; HBsAg, hepatitis B surface antigen; HBcIgG, hepatitis B core IgG; SOF, sofosbuvir; SIM, simeprevir; LDV, ledipasvir; RBV, ribavirin; 3D, ombitasvir/paritaprevir/ritonavir.
t test for continuous variables and Chi-square or Fisher exact test for categorical variables.
DAA treatment failure characteristics by liver disease severity
In the non-HCC group, 95% of patients had genotype 1 (95%) and 5% were genotype 3. In the HCC group, 79% of patients had genotype 1 and 21% were genotype 3. The non-HCC group had a higher percentage of treatment experienced patients (76% vs. 50%) and more advanced liver disease, as represented by their CTP and MELD scores. The percentage of non-HCC patients with CTP stage A, B, and C were 52%, 45%, and 3% respectively, as opposed to those with HCC of which 71% had CTP A and 29% with CTP B disease. The average MELD score was 12 for patients in the non-HCC group compared to 9.6 in the HCC group who failed.
DAA treatment failure characteristics by tumor status
The presence of tumor impacted failure rates in the 135 patients with HCC who were treated with DAAs with tumor present vs. those who were treated after therapy with resection or transplantation. Failure rates for HCC patients who had tumor present (n = 64), were 42% compared to only 3% in patients who no longer had tumor present (n = 71, p <0.0001). In the 64 patients with tumor present on imaging, 58 (90%) had active tumor on either imaging (n = 38) or subsequent surgical explant pathology (n = 20). The failure rate for patients with active tumor present at the time of HCV treatment initiation (n = 58) was 48% vs. 0% among the 5 patients who had non-active tumor present at the time of HCV treatment initiation (p = 0.04). There was no significant difference in failure rates among patients with inactive tumor or those without tumor present, compared to patients without HCC (p = 0.54). These findings equated to significant differences across the HCC cohort that depended upon the presence of active tumor, with failure rates in patients with no tumor, inactive tumor, and active tumor of 2%, 0%, and 20% respectively (p <0.0001 for trend).
The 27 HCC patients who had tumor present and failed treatment primarily had BCLC stage A disease (59%), followed by stage B (29%), stage 0 (7%), and stage C (4%). Patients with single lesions had similar failure rates to patients with multifocal HCC; failure rates were 44% and 42% respectively. There was no statistically significant difference in DAA treatment regimen among patients with HCC who failed treatment (p for trend < 0.08). The timing of liver directed therapy was evaluated in patients that failed DAA treatment: 10 patients received liver directed therapy prior to HCV therapy, 14 post HCV therapy, and 3 patients received no liver directed therapy.
Multivariable predictors of DAA treatment failure
In the multivariable analysis, the primary predictor of DAA treatment failure was the presence of active HCC tumor at the time of HCV treatment initiation, even after controlling for age, gender, race, ethnicity, CTP score, platelet count, genotype, and DAA regimen used (adjusted odds ratio (AOR) 8.49; 95% confidence interval (CI) = 3.90–18.49, p <0.001). A secondary predictor of failure was use of an inadequate treatment regimen (AOR 2.85; 95% CI = 1.32–6.16, p = 0.008). Notably, CTP score and genotype did not predict treatment failure (Table 4). In sensitivity analyses excluding all individuals treated post-transplant, active tumor remained a significant predictor of treatment failure (AOR 5.80; 95% CI = 2.55–13.2, p <0.001; Table S2). In sensitivity analysis excluding patients with genotype 3 HCV, which is known to be difficult to treat, active tumor was still the primary predictor of treatment failure (AOR 7.38; 95% CI = 3.26–16.72, p <0.001; Table S3).
Table 4.
Multivariable Predictors of DAA treatment failure in the entire population.
| Covariate | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Age (per year) | 0.99 | 0.95–1.03 | 0.69 |
| Male gender | 1.38 | 0.67–2.82 | 0.39 |
| White race | 0.71 | 0.28–1.77 | 0.46 |
| Hispanic ethnicity | 1.57 | 0.52–4.77 | 0.42 |
| Child-Turcotte Pugh class (A vs. B and C) | 1.64 | 0.83–3.21 | 0.15 |
| Platelets (per 1 µl) | 0.99 | 0.99–1.00 | 0.09 |
| Genotype 3 (vs. 1 or 4) | 1.69 | 0.64–4.42 | |
| HbCIgG present | 0.59 | 0.29–1.22 | 0.16 |
| Inadequate regimen* | 2.85 | 1.32–6.16 | 0.008 |
| Active tumor | 8.49 | 3.90–18.49 | <0.001 |
HBcIgG, Hepatitis B Core IgG.
Treatment classified as inadequate or adequate based on current HCV treatment standards. Inadequate treatment was defined as simeprevir/sofosbuvir 12 weeks or sofosbuvir/ledipasivir 12 weeks in treatment experienced patients or sofosbuvir/ribavirin regimens.
Discussion
We have shown that presence of active HCC tumor at the time of HCV treatment initiation is associated with treatment failure using an all-oral DAA regimen, independent of traditional predictors of HCV treatment failure. These observational findings raise important questions about the optimal treatment timing of HCV among patients with HCC.
Our results show that the presence of active HCC had a negative impact on achieving SVR. Overall failure in our HCC cohort was 21% compared to only 12% in those without HCC. Furthermore, of the 64 patients initiated on therapy with tumor present, 27 patients (43%) failed. All 27 patients had confirmed active tumor on either imaging or explant. There were 5 individuals with tumor present on imaging, but who had no evidence of viable tumor post liver directed therapy (LDT). None of these 5 individuals failed HCV treatment and they had no significant difference in HCV treatment outcomes compared to an individual without HCC. There were 71 patients who had HCC but were successfully treated with resection or transplantation, of whom only 2 patients (3%) failed HCV therapy. These data all suggest that it is not merely a history of HCC that influences treatment failure, but rather the presence of active tumor. On multivariate analysis, patients with active HCC present at the time of DAA initiation had eight-fold increased risk of failing HCV therapy compared to those without HCC. The presence of active HCC was the greatest predictor of treatment failure. Traditional factors that have correlated with a lower likelihood of HCV treatment response, such as genotype 3, African American race, and poor liver synthetic function, as shown by high MELD or CTP scores, did not appear to explain the difference in SVR between the 2 groups. Sensitivity analysis excluding patients with genotype 3 shows that active tumor remains the primary predictor of treatment failure. Additionally, patients without HCC tended to have more advanced liver disease at therapy initiation and had a higher proportion of treatment experienced patients than those with HCC.
Looking at failure rates across treatment regimens, patients who failed DAA therapy were more likely to have received an inadequate regimen (75% vs. 62%, p = 0.06). On multivariate analysis, as one might expect, inadequate regimens were predictive of treatment failure. However, patients with active HCC were less likely to receive inadequate therapy and still had higher failure rates than patients without HCC or a history of HCC treated by resection or transplantation. Active HCC was a much stronger predictor of treatment failure than regimen on multivariate analysis (AOR 8.49 vs. 2.85). Regimens that are now considered the standard of care, such as SOF/LDV 12 or 24 weeks or SOF/LDV/ RBV had lower overall failure rates than the first generation of DAAs (11%, 4%, and 8% respectively). Failure rates of 11% and 8% are higher than noted in the registry trials. All patients were monitored by our specialty pharmacy and were felt to have excellent compliance. We feel that these higher failure rates on these standard regimens occurred primarily in treatment naive patients with HCC, who had a failure rate of 19% for SOV/LDV 12 weeks and 13% for SOF/LDV/RBV.
The biological explanation for the diminished SVR in patients with active HCC is not entirely clear. The vast majority of HCV treatment failures were not seen at the end of treatment, but were only observed 12 weeks post-treatment (5% EOT failure vs. 21% 12-week failure). HCC may serve as a sanctuary for HCV, where virus particles may evade DAA therapy. HCC has been implicated as a viral reservoir for hepatitis B (HBV). One study identified HBV cccDNA in HCC cells of individuals who had post-transplantation recurrence of HBV and HCC [15]. The amount of tumor present in our cohort, did not predict treatment failure, as most failures were in patients with BCLC stage A and were solitary tumors.
Another putative explanation may be related to the effects of LDT. It is possible that DAAs may penetrate and distribute unequally within areas that have been treated with LDT due to decreased blood flow. LDT, particularly radioembolization, may induce local hepatic fibrosis, a traditional risk factor for HCV treatment failure [16]. While the timing of initiation of HCV therapy relative to timing of Y90 did not impact SVR, these numbers are small and the time frame was short between Y90 and initiation of HCV therapy.
In contrast to patients who had tumor present at the time of HCC therapy, patients with history of HCC treated with DAAs after resection or OLT had similar failure rates to patients that never had HCC (3% vs. 2%). Treatment post-transplantation for all patients was also found to be an independent predictor of achieving SVR on multivariate analysis. This finding is likely due to improved synthetic function in patients post-OLT. While not specifically examined in this study, it is possible that patients treated post-OLT receive longer treatment duration or the addition of ribavirin. Based on our observations, this could have significant impact on the timing of HCV therapy in patients with HCC listed for OLT. Patients who fail HCV therapy prior to transplant may have fewer options for post-transplant HCV treatments and be at risk for developing resistance-associated variants.
Our study has several limitations, mainly that it is retrospective and observational in nature. Several patients in our cohort also received first generation all-oral DAA regimens. Future prospective studies with the newer DAA therapies will be needed to confirm the findings. Additionally, this study is a single center study. We have aggressively treated HCV in our patients, including patients with more advanced tumor. The findings may not be generalizable to all institutions that only treat patients eligible for definitive HCC therapy. Finally, the only population for which HCC status did not impact treatment outcomes was the 22 individuals with genotype 2. This may be because this subpopulation only had 4 individuals with HCC. Our results at this time are only generalizable to the other genotypes.
In conclusion, our data demonstrate that the presence of active HCC at the initiation of HCV therapy is significantly associated with DAA treatment failure. In contrast, DAA therapy in the presence of inactive tumor or post removal of tumor (resection/ transplant) resulted in excellent SVR rates, similar to patients without HCC. Additional studies are needed to elucidate the complex interaction between tumor status, HCV and the immune system response to DAA therapy. Increased knowledge of these mechanisms will improve understanding of the ideal timing for treatment of HCV in patients with concomitant HCC. There may be a trade-off in optimizing HCV clearance and risk of recurrent HCC depending upon the immunologic properties of an individual tumor.
Supplementary Material
Acknowledgments
We would like to thank Colleen O’Brien and Mary Welti for their assistance in identifying patients for medical record review. We also thank Dyanna Gregory for her assistance with the statistical analysis.
Financial support
Dr. VanWagner is supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
Prenner: Nothing to disclose. VanWagner: Research: Novartis; Speaker’s Bureau: Salix. Flamm: Research: Gilead, Abbvie; Speaker’s Bureau: Gilead, Abbvie, Merck. Advisory Boards: Gilead, Abbvie, Merck. Salem: Consultant: BTG, Merit, Boston Scientific, Amgen, Terumo. Lewandowski: Advisory Board: BTG, Boston Scientific. Consultant: Cook Medical. Kulik: Advisory Boards: Gilead, Baylor, Salix. For full details, please see the supplementary disclosures file.
Authors’ contributions
Stacey Prenner: Hypothesis, data collection, analytic plan, results interpretation, manuscript drafting, submission preparation and editing. Lisa B. VanWagner: Analytic plan, performed analyses, results interpretation, manuscript editing. Steven Flamm: results interpretation, manuscript editing. Riad Salem: Results interpretation, submission editing. Robert Lewandowski: Results interpretation, submission editing. Laura Kulik: Hypothesis, analytic plan, results interpretation, manuscript drafting, submission editing. Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2017.01.020.
References
- 1.Khullar V, Firpi RJ. Hepatitis C cirrhosis: New perspectives for diagnosis and treatment. World J Hepatol. 2015;7:1843–1855. doi: 10.4254/wjh.v7.i14.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516 e501. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Bailly F, Pradat P, Virlogeux V, Zoulim F. Antiviral therapy in patients with hepatitis C virus-induced cirrhosis. Dig Dis. 2015;33:613–623. doi: 10.1159/000375359. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43:1276–1292. doi: 10.1111/apt.13633. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767–1775. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 8.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 9.Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2) Hepatology. 2016;64:360–369. doi: 10.1002/hep.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, et al. Efficacy and safety of 8 weeks vs. 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (CWORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1087–1097. doi: 10.1016/S0140-6736(14)61793-1. [DOI] [PubMed] [Google Scholar]
- 11.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–1505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry MP, Forns X, Chung RT, Terrault NA, Brown R, Jr, Fenkel JM, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148:100–107 e101. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312:353–361. doi: 10.1001/jama.2014.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 15.Faria LC, Gigou M, Roque-Afonso AM, Sebagh M, Roche B, Fallot G, Ferrari TC, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008;134:1890–1899. doi: 10.1053/j.gastro.2008.02.064. [Quiz 2155] [DOI] [PubMed] [Google Scholar]
- 16.Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4:198. doi: 10.3389/fonc.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.