Abstract
Objective
To describe the prevalence of insomnia symptoms in women with perimenopause and to examine differences in self-reported insomnia symptoms at different stages of perimenopause over 10 years.
Design
A secondary analysis of self-reported sleep symptoms and clinical variables using 10 years of publicly available data from the Study of Women Across the Nation (SWAN).
Setting
The dataset of women’s insomnia symptoms was obtained from the publicly available data from the SWAN. The parent study settings included Detroit, Michigan, Northern New Jersey, Los Angeles, California, Boston, Massachusetts, Chicago, Illinois, and Pittsburgh, Pennsylvania.
Participants
Multiethnic midlife women with a mean age of 46 (N=3302) categorized as pre-and perimenopausal at baseline.
Methods
Dependent variables included self-reported insomnia symptoms: difficulty falling asleep (sleep latency), wake after sleep onset, early morning awakenings, and sleep quality. Descriptive analysis was completed for each 1-year study interval. Repeated measures logistic regression was used to identify if insomnia symptoms changed over time by stage of perimenopause.
Results
Insomnia symptoms were present in 31%-42% of perimenopausal women at any 1-year study interval. Insomnia symptoms were more prevalent in the late stage of perimenopause than the early stage (p<0.001). The odds of having any insomnia symptoms were 1.3 times greater for women in the late stage of perimenopause than in early stage of perimenopause (95% CI [1.2, 1.5]; p<0.001).
Conclusions
Insomnia symptoms are prevalent in women who are transitioning to menopause, and stage of perimenopause may heighten the risk of developing symptoms of insomnia disorder, which is associated with negative cardiometabolic outcomes.
Keywords: perimenopause, insomnia, women, sleep disturbances, menopause, climacteric
Sleep disorders are present in 50-70 million people (Institute of Medicine [IOM], 2006), and insomnia is the most prevalent sleep disorder among the general population (Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008). Although insomnia is a distinctly defined sleep disorder with specific diagnostic criteria, insomnia symptoms are often examined in isolation. It is important to recognize insomnia symptoms in women during perimenopause through health care screenings and patient education for an accurate diagnosis of insomnia disorder. The Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) identifies the criteria for insomnia disorder as any one symptom of prolonged sleep latency, increased wake after sleep onset, and/or the occurrence of early morning awakenings, that is present for three or more nights per week, for three or more consecutive months, along with daytime impairment and sufficient opportunity for sleep (American Psychiatric Association, 2013).
Within the next decade, it is projected that 500 million women in the United States will be within the estimated age range (35-55 years) to transition to perimenopause (United States Census Bureau, 2010). During perimenopause, women begin to experience variations in the menstrual cycle because of the fluctuation of estradiol, progesterone, and follicle stimulating hormones. They commonly experience symptoms that include hot flashes, night sweats, sleep disruption, and an overall decrease in quality of life (Freeman et al., 2007). Early perimenopause is distinctly different than late perimenopause and is defined as having a menstrual cycle in the preceding three months but with irregularity of cycles. On the other hand, late perimenopause is defined as having a menstrual cycle in the last 12 months but not in the preceding three months (Harlow et al., 2012). Chronic insomnia is a significant risk factor for new onset medical and psychiatry morbidity, and when left untreated is costly to the individual (up to $5000 per annum) and costly to society in the form of poor work productivity and absenteeism (up to $150 billion per annum in direct and indirect costs) (Daley, Morin, LeBlanc, Grégoire, & Savard, 2009; Ozminkowski, Wang, & Walsh, 2007). Though the evidence to date consistently identifies insomnia symptoms and chronic insomnia diagnoses are associated with individual and societal costs and untoward general population outcomes, less is known about insomnia symptoms in women during the perimenopause.
Women have a greater risk than men of developing chronic insomnia during their lifetimes (Ozminkowski et al., 2007); a lifetime prevalence rate of 63% was reported among women aged 18 and older (N=511) in a U. S. study (National Sleep Foundation [NSF], 2002). The prevalence of insomnia symptoms increases in women as they enter perimenopause (Ensrud, Larson, Guthrie, & Joffe, 2011; Freeman et al., 2007), and nearly 40% of women between the ages of 40 and 55 years reported insomnia symptoms (Kravitz et al., 2003). According to the 2007 Sleep in America Poll, 59% of women with perimenopause reported experiencing insomnia symptoms at least a few nights per week (NSF, 2002) and women who experience other symptoms of perimenopause, such as hot flashes and depression, were more likely to also report insomnia symptoms (Arakane et al., 2011; Blümel et al., 2012; Pien, Sammel, Freeman, Lin, & DeBlasis, 2008; Woods & Mitchell, 2010). The alteration of hormones that occurs during the natural progression of perimenopause has the potential to increase the risk of developing insomnia because of the following factors: vasomotor symptoms that disrupt the sleep cycle, coexistent psychological disorders such as anxiety and depression that contribute to impaired sleep, and the altered central neural control of sleep-wake states and thermal threshold associated with hormonal alterations (Freedman, 2005a, 2005b).
Untreated insomnia is a public health concern. In the general U. S. population, the cost of insomnia treatment, loss of productivity at work, and decreased quality of life confer a tremendous burden (Daley, Morin, LeBlanc, Gregoire, & Savard, 2009; Hatoum, Kong, Kania, Wong, & Mendelson, 1998; Simon & VonKorff, 1997).
Poor sleep quality in mid-life women was associated with primary sleep disorders, anxiety, and depression (Joffe, Massler, & Sharkey, 2010) and hypertension (Vgontzas, Liao, Bixler, Chrousos, & Vela-Bueno, 2009). Since the number of perimenopausal women will significantly increase during the next several decades in the United States, it is critically important to gain insight into the prevalence of diagnosable insomnia in this high-risk group. Furthermore, little is known about the prevalence of insomnia symptoms across the stages of early and late perimenopause, and such information will potentially support recommendations for the timing of insomnia screening and intervention for prevention and treatment.
The overall purpose of our study was to describe the prevalence of insomnia symptoms in perimenopausal women and to determine the prevalence of insomnia symptoms by perimenopausal stage in mid-life women over ten years. The following study questions were addressed: 1) What is the prevalence of nighttime insomnia symptoms measured annually in perimenopausal women; 2) What is the prevalence of insomnia symptoms by perimenopausal stage in women; and 3) Is there a difference in reported insomnia symptoms by perimenopausal stage?
Methods
Design
Our study was a secondary and longitudinal analysis of publicly-available data from the Study of Women’s Health Across the Nation (SWAN), a multisite, longitudinal study of the natural history of women transitioning to menopause (Sowers, Crawford, Sternfeld, & Kelsey, 2000) funded by the National Institutes of Health’s National Institute on Aging, National Institute of Nursing Research, and Office of Research on Women’s Health. The objective of the SWAN Study was to describe the chronology of the biological and psychological characteristics of the menopausal transition on health and risk factors for age-related chronic illnesses (Sowers et al., 2000). The SWAN study had both a cross-sectional and longitudinal component with an emphasis on including racially/ethnically diverse women.
Setting and Sample
SWAN employed a random population sampling plan using telephone numbers generated from random digit dialing lists and list-based frames such as city census, electrical utility customer lists, health maintenance organization enrollment lists, and an enrollment list from an earlier study (Sowers et al., 2000). A total of 202,985 sampling units were screened for potential participation in the SWAN cross-sectional study resulting in 34,446 eligible women. The study included seven sites; Boston (45% African American, 55% Caucasian), Chicago (55% African American, 45% Caucasian), Detroit (66% African American, 33% Caucasian), Los Angeles (55% Japanese, 45% Caucasian), Newark (66% Hispanic, 33% Caucasian), Oakland (55% Chinese, 45% Caucasian), and Pittsburgh (33% African American, 66% Caucasian). Of the cross-sectional participants, 6521 women were eligible for the SWAN longitudinal study based on the following inclusion criteria: 1) aged 42-52 years, 2) no surgical removal of the uterus and/or both ovaries prior to study enrollment, 3) not currently using exogenous hormone preparations affecting ovarian functions, 4) reporting at least one menstrual period in the past three months, and 5) self-identification with the site specific racial/ethnic strata. A total of 3302 women were included in the longitudinal study resulting in an overall response rate of 50.7% (Sowers et al., 2000). SWAN study methods are discussed elsewhere in more detail (Sowers et al., 2000).
Procedure
Our secondary analysis of SWAN data was conducted using 10-years of publicly-available data from the Inter-University Consortium for Political and Social Research (ICPSR). Data from perimenopause through one year after menopause were used to examine the differences in the insomnia symptom prevalence between perimenopausal stages. Pre-perimenopausal women were included in the baseline sample with the expectation that during the ten years of data collection, the participants would likely progress through the perimenopausal stages. An a priori power analysis determined that a sample size of 2400 participants would provide 85% power to detect an odds ratio of 1.6 between two comparison groups (such as early vs. late perimenopause) with respect to the individual insomnia symptoms. This calculation assumed an overall α=0.05, 2-sided test statistic, and 25% missing data. Participants were included if they had complete data for perimenopausal stage and all insomnia symptom data. The actual data missing was 5.4%. Data from menopausal women, defined as the absence of menses for greater than one year, were excluded from analysis since the primary focus of the study was perimenopausal women.
Measures
Insomnia symptoms
The primary dependent variables were self-reported insomnia symptoms measured by the following annual survey items: Over the past two weeks, 1) did you have trouble falling asleep; 2) did you wake up several times a night; 3) did you wake up earlier than you planned to, and were you unable to fall asleep again; and 4) average sleep quality. The first three survey items were rated on a five point Likert scale; 1) no, not in the past 2 weeks; 2) yes, less than once a week; 3) yes, 1 or 2 times per week; 4) yes, 3-4 times per week; and 5) yes, 5 or more times per week. Sleep quality was assessed on a four point Likert scale; 1) restful; 2) average quality; 3) restless; and 4) very restless. These four questions were adapted from the 5-item Women’s Health Initiative Insomnia Rating Scale from the Women’s Health Initiative Project (Troxel, Buysse, Hall, & Matthews, 2009) by the investigators of the parent study. For the purposes of our analysis, the self-reported sleep disturbances were categorized as follows: Item 1, sleep latency; item 2, wake after sleep onset; item 3, early morning awakenings, and item 4, sleep quality.
The frequency of sleep latency, wake after sleep onset, and early morning awakenings were dichotomized as “no” (< 3 times per week) and “yes” (≥ 3 times per week), consistent with the DSM-5 criteria for insomnia symptoms (American Psychiatric Association, 2013). Overall, insomnia symptoms were defined as any of the three symptoms being reported as “yes.” Sleep quality was dichotomized as “overall good” sleep quality (self-reported ratings of restful or average quality) and “overall poor” sleep quality (self-reported ratings of restless or very restless). Insomnia disorder is established based on symptoms but also requires daytime impairment assessment; because such data were not collected in the parent study, we only report on insomnia symptoms, not insomnia disorder. Insomnia symptom criteria were defined as any one symptom of prolonged sleep latency, increased wake after sleep onset, and/or the occurrence of early morning awakenings, that was self-reported as present for three or more nights per week, for three or more consecutive months.
Stage of menopause
Menopause status was defined using the Stages of Reproductive Workshop + 10 (STRAW) criteria (Harlow et al., 2012). Irregular menstrual bleeding was used to distinguish between categories of menopause including postmenopausal, late perimenopausal, early perimenopausal, and premenopausal. Postmenopause was defined as no menses for 12 months. Late perimenopause was defined as no menses for the last three to 12 months. Early perimenopause was defined as a menses in the last three months but menstruation was not predictable. Premenopause was defined as menses occurring in the last three months with no decrease in predictability of menstruation (Harlow et al., 2012).
Analysis
Descriptive statistics were used to assess the distribution of all study variables (e.g. frequencies, means, and standard deviations) for baseline and annual data. Descriptive analyses with frequencies (n [%]) are reported for each individual symptom for the entire perimenopausal sample, as well as the overall rate of insomnia symptoms. Frequencies were examined by study interval (Year [Y] 0-Y1, Y1-Y2, Y2-3; etc.) and across all intervals to describe the overall rate of symptoms of insomnia in perimenopausal women and by perimenopausal stage (early and late).
Repeated measures logistic regression was used to identify if symptoms of insomnia (sleep latency, wake after sleep onset, early morning awakenings, sleep quality) change over time by perimenopausal stage. Changes in self-reported symptoms of insomnia were analyzed annually, from baseline until the participant was considered menopausal, taking into account the menopausal stage (early or late perimenopause). With the identification of any significant difference between stages (p< 0.05), post-hoc analyses were conducted to identify which rates were significantly different. All analyses were conducted using SAS version 9.2. Significance level for this study was identified at p< 0.05 for all analyses.
Results
Sample Description
At baseline, the study sample (N=3302) was predominantly White (47%), early perimenopausal (45%) and pre-perimenopausal (55%), married (65%) women. The majority of the sample completed some college (74%) and was employed outside of the home (80%). Fifty-six percent of participants reported less than one day of feeling depressed per week at baseline, most were sedentary (72.2%) and overweight (BMI 28.27 kg/m2 ± 7.2). According to the 2010 US Census bureau survey, the study sample is representative of the female population by age, sex, race, and Hispanic origin (United States Census Bureau, 2010). In year one, the majority of the sample included early perimenopausal women (n=1721 [52%]) compared to pre-perimenopausal women (n=1457; [44%]) and late perimenopausal women (n=124; [4%]). Across the 10-year study period, forty five percent of the participants transitioned from pre-perimenopause to early or late perimenopause and 55% transitioned to menopause. With each annual data set evaluated, menopausal women were excluded from the data set which accounts for a diminishing sample size (Table 1). In year 10, the early and late perimenopausal groups were similar in size (n=155, n=128 respectively). Additional descriptive characteristics of the participants are displayed in Table 2.
Table 1.
Sample Size By Annual Data Collection Points
| Baseline | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Year 7 | Year 8 | Year 9 | Year 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Reproductive Status | |||||||||||
| Surgical Menopause | 0 | 32 | 62 | 91 | 121 | 146 | 171 | 184 | 195 | 205 | 209 |
| Post-Menopausal | 0 | 56 | 164 | 348 | 567 | 820 | 1021 | 1185 | 1360 | 1535 | 1681 |
| Late Perimenopause | 0 | 126 | 207 | 240 | 286 | 282 | 240 | 237 | 212 | 172 | 136 |
| Early Perimenopause | 1486 | 1732 | 1562 | 1408 | 1197 | 996 | 740 | 562 | 399 | 268 | 168 |
| Pre-perimenopause | 1816 | 719 | 450 | 307 | 194 | 120 | 80 | 48 | 28 | 15 | 8 |
|
| |||||||||||
| Total n per Year | 3302 | 2881 | 2748 | 2710 | 2679 | 2617 | 2448 | 2327 | 2278 | 2255 | 2245 |
Table 2.
Sample Description at Baseline (N=3302)
| Characteristic | Frequency (n[%]) or Mean (±SD) |
|---|---|
| Age (years) | 45.85 (± 2.69) |
| Menopause Status | |
| Early Perimenopausal | 1486 (45%) |
| Pre-perimenopausal | 1816 (55%) |
| Race/Ethnicity | |
| White | 1551 (47%) |
| Black or African American | 933 (28%) |
| Chinese or Chinese American | 246 (7%) |
| Japanese or Japanese American | 281 (9%) |
| Hispanic | 286 (9%) |
| Marital Status | |
| Single | 439 (13%) |
| Married/Living as Married | 2149 (65%) |
| Separated | 156 (5%) |
| Widowed | 65 (2%) |
| Divorced | 435 (13%) |
| Education | |
| Less than high school | 238 (7%) |
| High school | 585 (18%) |
| Some college/technical school | 1045 (32%) |
| College graduate | 662 (20%) |
| Post graduate | 737 (22%) |
| Family Income | |
| Less than $19,999 | 490 (14.8%) |
| $20,000 to $49,999 | 1088 (32.9%) |
| $50,000 to $99,999 | 1162 (35.2%) |
| $100,000 or more | 472 (14.3%) |
| Employed | |
| Yes | 2643 (80%) |
| No | 649 (19.7%) |
| BMI (kg/m2) | 28.27 (±7.2) |
| Depressed in past week | |
| <1 Day | 1849 (56%) |
| 1-2 Days | 891 (27%) |
| 3-4 Days | 372 (11.3%) |
| 5-7 Days | 185 (5.6%) |
| Vasomotor Symptomsa | |
| Hot Flashes | |
| Yes | 878 (26.6%) |
| No | 2407 (72.9%) |
| Cold Sweats | |
| Yes | 365 (11.1%) |
| No | 2841 (86%) |
| Night Sweats | |
| Yes | 963 (29.2%) |
| No | 2321 (70.3%) |
Frequency rating range over the last 2 weeks: not at all, 1-5 days, 6-8 days, 9-13 days, every day.
Incidence of Symptoms of Insomnia
Of the 3302 women included in this study, 31% (n=578) at year one and 42% (n=216) at year 10 had symptoms of insomnia according to the DSM-5 criteria (American Psychiatric Association, 2013). Additionally, the individual symptoms of insomnia, Wake after sleep onset, sleep latency and early morning awakenings, remained prevalent and steadily increased over the decade of data collection (Tables 3-5). The symptom most prevalent for perimenopausal women in the study was wake after sleep onset (Figure 1). Thirty one percent (n=525) of the early perimenopausal women had insomnia symptoms in year one of the study; the occurrence increased to 39% (n=60) by year 10. In the late perimenopausal group, insomnia symptoms were present in 32% (n=39) (Year1) and 48% (n=61, Year10).
Table 3.
Wake After Sleep Onset By Annual Data Collection Points
| Year | No < 3 nights per week n(%) | Yes ≥ 3 nights per week n(%) | Sample (N) |
|---|---|---|---|
| 1 | 1389 (73.5) | 489 (25.9) | 1890 |
| 2 | 1261 (8.9) | 539 (29.5) | 1829 |
| 3 | 1159 (66.7) | 522 (30.0) | 1739 |
| 4 | 1011 (63.0) | 508 (31.7) | 1604 |
| 5 | 904 (63.5) | 402 (28.2) | 1424 |
| 6 | 660 (57.3) | 368 (32.0) | 1151 |
| 7 | 566 (57.6) | 335 (34.1) | 983 |
| 8 | 459 (57.0) | 287 (35.6) | 806 |
| 9 | 356 (55.2) | 226 (35.0) | 645 |
| 10 | 285 (55.6) | 185 (36.1) | 513 |
Table 5.
Early Morning Awakenings By Annual Data Collection Points
| Year | No < 3 nights per week n(%) | Yes ≥ 3 nights per week n(%) | Sample (N) |
|---|---|---|---|
| 1 | 1634 (86.5) | 245 (13.0) | 1890 |
| 2 | 1524 (83.3) | 276 (15.1) | 1829 |
| 3 | 1414 (81.3) | 266 (15.3) | 1739 |
| 4 | 1267 (79.0) | 251 (15.6) | 1604 |
| 5 | 1114 (78.2) | 191 (13.4) | 1424 |
| 6 | 858 (74.5) | 171 (14.9) | 1151 |
| 7 | 737 (75.0) | 165 (16.8) | 983 |
| 8 | 616 (76.4) | 130 (16.1) | 806 |
| 9 | 486 (75.4) | 96 (14.9) | 645 |
| 10 | 386 (75.2) | 84 (16.4) | 513 |
Figure 1.
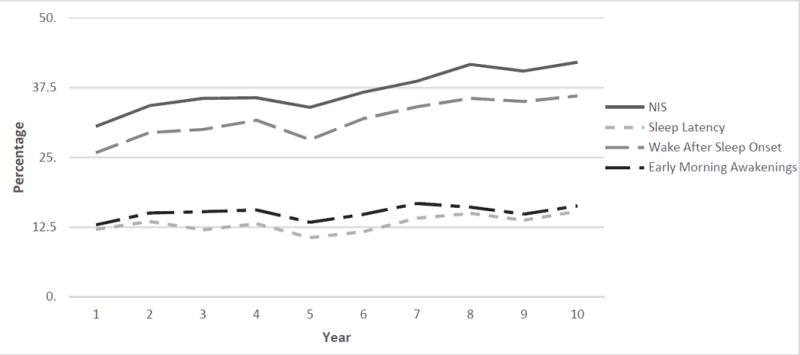
Nighttime Insomnia Symptoms (NIS) in Perimenopausal Women across Ten Years of Data
Symptom Differences by Perimenopausal Stage
Overall insomnia symptoms were more prevalent in the late stage of perimenopause than early stage of perimenopause (Figure 3). The adjusted odds of having any one insomnia symptom was 1.3 times greater for those in late perimenopausal stage versus early stage of perimenopause (X2=26.01, [df1]; 95% CI 1.2, 1.5; p<0.001) demonstrating an association based on perimenopausal stage. Similarly, perimenopausal women categorized as being in the late stage of perimenopause had increased odds of each self-reported insomnia symptom. Sleep latency was associated with late stage perimenopause with an adjusted OR 1.3: X2=12.17 [df2]; 95% CI 1.1, 1.5; p=0.001when compared to the early stage perimenopause. Wake after sleep onset was associated with late stage perimenopause with an adjusted OR 1.4: X2=30.57 [df2]; 95% CI 1.2, 1.5; p<0.001 when compared to the early stage perimenopause. Early morning awakenings in late stage perimenopausal women had an adjusted OR 1.3: X2=9.05 [df2]; 95% CI 1.1, 1.5; p=0.003 when compared to early stage perimenopausal women demonstrating an association with perimenopausal stage. Sleep quality in late stage perimenopausal women demonstrated an association with an adjusted OR 1.3: X2=19.83 [df2]; 95% CI 1.2, 1.5; p<0.001 when compared to early stage perimenopausal women (Table 6).
Table 6.
Difference in Insomnia Symptoms by Perimenopausal Stage
| Late versus Early Perimenopause | ||||
|---|---|---|---|---|
|
| ||||
| Outcome Variable | Adjusted Odds Ratio | 95% CI | Df2 | p value |
| Sleep Latency (SL) | 1.3 | 1.1,1.5 | 12.17 | 0.0005 |
| WASO | 1.4 | 1.2,1.5 | 30.57 | <0.001 |
| Early Morning Awakenings (EMA) | 1.3 | 1.1,1.5 | 9.05 | 0.003 |
| Sleep Quality (SQ) | 1.3 | 1.2,1.5 | 19.83 | <0.001 |
Notes. Dichotomized values operationalized by Diagnostic and Statistical Manual of Mental Disorders 5 criteria for diagnosable insomnia with symptoms reported ≥ 3 times per week over 2 week period. Adjusted odds ratio; adjusted for baseline insomnia symptom value.
Discussion
The purpose of our secondary longitudinal analysis was to describe the prevalence of insomnia symptoms in women during perimenopause and to determine the prevalence of insomnia symptoms in women by perimenopausal stage, early versus late. A major finding of our study was that 31% to 42% of all women in the sample had symptoms of insomnia during perimenopause. In addition, late perimenopausal women had significantly more insomnia symptoms compared to early perimenopausal women. These results suggest that as women transition through the perimenopausal period, a higher incidence of insomnia symptoms is possible.
Insomnia Symptoms During Perimenopause
Based on the study findings, women during perimenopause have exceedingly higher rates of insomnia symptoms than the general North American population, which is estimated at 15% to 20% and specifically among women across the lifespan to average 16% (Morin, LeBlanc, et al., 2011). This finding suggests a high likelihood of insomnia symptoms during perimenopause. At any time across the ten-year study period, at least 18% (n=280) of perimenopausal women categorized their sleep quality as poor. Yet, wake after sleep onset and early morning awakenings increased over the study period as perimenopausal women progressed through perimenopause while difficulty falling asleep was not as frequently reported. This could indicate that the single insomnia symptom of difficulty falling asleep is closely associated with sleep quality while other insomnia symptoms are not recognized by perimenopausal women as insomnia-specific.
Perimenopausal women may not recognize their insomnia symptoms as an indicator of a sleep disorder, such as insomnia disorder. Perimenopausal women may accept sleep disturbances as normal and therefore may be unlikely to seek treatment (Nosek, Kennedy, & Gudmundsdottir, 2012). In a Gallop poll performed in the United States it was reported that only 5% of insomniacs ever seek treatment from a healthcare professional (1991). By delaying or not seeking treatment of insomnia symptoms, the likelihood of chronic insomnia increases (Ohayon, 2006). This is likely the result of behavioral changes such as napping, sleeping in late on weekends or going to bed early, and rumination about sleep perpetuating the cycle of insomnia (Perlis, Gehrman, & Ellis, 2011).
Insomnia Prevalence By Perimenopausal Stages
In prior published studies, researchers found that approximately half of all perimenopausal women experienced sleep disturbances (Kravitz et al., 2003; National Sleep Foundation [NSF], 2007; Woods & Mitchell, 2005). It is unclear from the extant literature if there is a stage of perimenopause when screening for insomnia disorder should be initiated.
For any reported symptom in our sample, women in the late perimenopausal stage had a higher frequency of self-reported insomnia symptoms than early perimenopausal women. As women progressed from early to late perimenopause, the adjusted odds of poor sleep quality were 1.3 times that of early perimenopause. These findings extend and support the work of Kravitz et al (2008) who reported sleep disturbances are associated with perimenopause and are more prevalent in the late perimenopausal stage (Kravitz & Joffe, 2011). This is not unique to the United States. In Australia, it was reported that sleep problems increased as women progressed from late reproductive to early menopausal years in a 9-year longitudinal study; however, this was measured by asking participants about trouble sleeping which is not a measurable insomnia symptom (Dennerstein, Lehert, Guthrie, & Burger, 2007). Woods and Mitchell (2010) reported that nighttime awakenings worsened in the late perimenopause to postmenopausal stages, further supporting our findings.
The transition from early to late perimenopause is therefore likely to be a major precipitating factor in the development of insomnia disorder as indicated by the high prevalence of insomnia symptoms that emerge during perimenopause. This precipitating factor is associated with fluctuations in estradiol, follicle stimulating hormone, and inhibin B, all measures of ovarian aging (Freedman, 2005b; Freeman et al., 2007). Hot flashes triggered by slight increases in core body temperature, a reduced thermoneutral zone in the brain, and fluctuations in endogenous hormones as women transition to late perimenopause influence the development of insomnia disorder in perimenopausal women (Freedman, 2005b, 2005a). As perimenopause and in particular late perimenopause is caused by biophysiologic changes that also influence sleep regulation and sleep continuity, perimenopausal women should be considered a high-risk group for insomnia disorders.
Implications
Our findings suggest that assessment of sleep in perimenopausal women is clinically necessary. It is important for nurses, primary care providers, as well as women’s health providers to initiate screenings for insomnia symptoms in this high-risk population. Nurses are in an opportune position to initiate screenings regarding sleep and educate women on simple changes to improve their sleep patterns. During routine health visits, questions related to insomnia symptoms and sleep satisfaction should be employed. Screenings should at a minimum consist of the four sleep questions used in the SWAN study (Table 4); alternatively, validated insomnia instruments such as the Insomnia Severity Index (Morin, Belleville, et al., 2011) or the Pittsburgh Sleep Quality Index (Morin, Belleville, et al., 2011) could be employed to identify perimenopausal women at risk for insomnia disorder. Such an approach will support the much-needed conversation among providers and mid-life women about the risk and negative health outcomes associated with insomnia disorder.
Table 4.
Sleep Latency By Annual Data Collection Points
| Year | No < 3 nights per week n(%) | Yes ≥ 3 nights per week n(%) | Sample (N) |
|---|---|---|---|
| 1 | 1647 (87.1) | 230 (12.2) | 1890 |
| 2 | 1553 (84.9) | 248 (13.6) | 1829 |
| 3 | 1471 (84.6) | 210 (12.1) | 1739 |
| 4 | 1307 (81.5) | 211 (13.2) | 1604 |
| 5 | 1154 (81.0) | 152 (10.7) | 1424 |
| 6 | 894 (77.7) | 135 (11.7) | 1151 |
| 7 | 763 (77.6) | 139 (14.1) | 983 |
| 8 | 625 (77.5) | 121 (15.0) | 806 |
| 9 | 493 (76.4) | 89 (13.8) | 645 |
| 10 | 391 (76.2) | 79 (15.4) | 513 |
Insomnia disorder has a negative effect on health outcomes, confers cardiometabolic consequences, and increases the risk of depression and hypertension (Joffe et al., 2010; Matthews et al., 2014; Serrano & Warnock, 2007). Grandner, Jackson, Pak and Gehrman (2012) reported a significant risk for obesity, diabetes, myocardial infarction and coronary artery disease among adults reporting insomnia disorder. Health care related costs associated with insomnia disorder are estimated at $15 billion annually (Daley et al., 2009). Based on the current study findings that perimenopausal women have a high prevalence of insomnia symptoms which may be indicative of insomnia disorder, or may be predisposing factors for the development of insomnia disorder, it is imperative to recognize insomnia symptoms among middle-aged women by conducting regular insomnia risk screening. In addition, practitioners and researchers in the fields of women’s health and sleep need to prioritize insomnia disorder as a potentially under-recognized and under-diagnosed sleep disorder in perimenopausal women.
Limitations
According to DSM-5, a diagnosis of insomnia disorder must include both nighttime and daytime impairment in the presence of ample opportunity for sleep to occur (American Psychiatric Association, 2013). A limitation of our study was the absence of SWAN data to permit examination of daytime impairment; thereby, diagnosable insomnia disorder was not possible. Our study results, however, suggested perimenopausal women do experience insomnia symptoms, which may indicate a high prevalence of insomnia disorder. An additional limitation was that sleep wake disorders such as obstructive sleep apnea, restless leg syndrome, and circadian sleep-wake disorders, as well as the use of sleep aids were not accounted for due to the absence of these data in the SWAN data set. This is an important limitation to note as other sleep wake disorders and use of sleep aids (pharmacological and non-pharmacological) are potential extraneous variables. Additionally, our results were not adjusted for age which could be a confounder for age-related sleep changes (Dijk, Duffy, & Czeisler, 2000).
Our study was based on self-reported sleep data, a commonly employed method used in sleep research. However, the use of both subjective and objective sleep data would further strengthen study results by permitting validation of subjective sleep data. Using sleep diaries in studies of this phenomenon will potentially provide insights to patterns of sleep in perimenopausal women; such a measure also reduces risk of recall bias, a limitation in the current study. The use of actigraphy, or accelerometry, in addition to the subjective sleep measures would also strengthen subsequent studies.
Finally, the secondary analysis design of our study did not permit for controlling all confounding factors, such as sedentary lifestyle, obesity, normal aging, and use of sleep medications. Future researchers who focus on the phenomenon of insomnia in perimenopausal women may consider a more complete and robust sleep data collection approach to validate the findings of the current study and extend knowledge about this phenomenon.
Recommendations for Research
To date, it is unclear why perimenopausal women are predisposed to insomnia symptoms. It is hypothesized that fluctuating hormone levels as the ovaries age precipitate vasomotor symptoms that contribute to sleep disturbances and self-reported insomnia symptoms. Future investigators of insomnia symptoms in perimenopausal women should include physiologic measures, such as estradiol, follicle stimulating hormone, and inhibin B, and objective measures of sleep, such as polysomography and actigraphy. These are important methodological considerations to better understand the trajectory of hormonal and sleep changes during the transition to menopause.
Conclusion
Understanding the prevalence of insomnia symptoms in perimenopausal women is a critical consideration given that 500 million women will be entering perimenopause in the next decade (United States Census Bureau, 2010) and the negative health outcomes associated with insomnia disorder (Grandner et al., 2012; Matthews et al., 2014; Terauchi et al., 2012). Women during perimenopause experience higher rates of insomnia symptoms than rates reported for the general adult population and insomnia symptoms are increasingly frequent in late perimenopause as compared to early perimenopause. Screening for insomnia symptoms by healthcare providers is necessary to identify perimenopausal women at risk for insomnia disorder which may reduce the burden of insomnia disorder among women during perimenopause. Intervention research is warranted to improve sleep and long-term health outcomes in perimenopausal women.
Table 7.
Clinical Screening for Insomnia Symptoms
| Clinical Question | |
|---|---|
| 1. | In the last 2 weeks have you had trouble falling asleep? |
| 2 | In the last 2 weeks did you wake up several times a night? |
| 3. | In the last 2 weeks have you woken up earlier than planned to and were not able to go back to sleep again? |
| 4 | What is your average sleep quality? |
Callouts.
Symptoms of insomnia confer negative cardiometabolic consequences and increase the risk of depression and hypertension. (Place after introduction)
Women report a higher prevalence of insomnia symptoms than men; however, the prevalence of insomnia symptoms among women nearly doubles during perimenopause. (Place after results section)
The transition from early to late perimenopause may be a major precipitating factor in the development of symptoms of insomnia. (Place late in the manuscript)
Biographies
Colleen Ciano, PhD, CRNP-C, is a member of the New Courtland Center for Transitions and Health, University of Pennsylvania, School of Nursing, Philadelphia, PA and a family nurse practitioner, Pinnacle Health Medical Group, Harrisburg, PA.
Tonya S. King, PhD, is professor of biostatistics in the Department of Public Health Sciences, Pennsylvania State University, College of Medicine, Hershey, PA.
Robin Redmon Wright, PhD, is an associate professor and program coordinator for Lifelong Learning and Adult Education, Pennsylvania State University, Capital Campus, Harrisburg, PA.
Michael Perlis, PhD, is an associate professor in the Behavioral Sleep Medicine Program, University of Pennsylvania, Philadelphia, PA.
Amy M. Sawyer, PhD, RN, is an assistant professor in the College of Nursing, Pennsylvania State University, University Park, PA and an adjunct assistant professor in the School of Nursing, University of Pennsylvania, Philadelphia, PA.
Footnotes
Disclosure: The authors report no conflict of interest or relevant financial relationships.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (fifth edition) 2013 Retrieved from http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596.
- Arakane M, Castillo C, Rosero MF, Peñafiel R, Pérez-López FR, Chedraui P. Factors relating to insomnia during the menopausal transition as evaluated by the Insomnia Severity Index. Maturitas. 2011;69(2):157–161. doi: 10.1016/j.maturitas.2011.02.015. https://doi.org/10.1016/j.maturitas.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Blümel JE, Cano A, Mezones-Holguín E, Barón G, Bencosme A, Benítez Z, Chedraui P, et al. A multinational study of sleep disorders during female mid-life. Maturitas. 2012;72(4):359–366. doi: 10.1016/j.maturitas.2012.05.011. https://doi.org/10.1016/j.maturitas.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Daley M, Morin CM, LeBlanc M, Grégoire J-P, Savard J. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- Dennerstein L, Lehert P, Guthrie JR, Burger HG. Modeling women’s health during the menopausal transition: A longitudinal analysis. Menopause. 2007;14(1):53–62. doi: 10.1097/01.gme.0000229574.67376.ba. https://doi.org/10.1097/01.gme.0000229574.67376.ba. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiology International. 2000;17(3):285–311. doi: 10.1081/cbi-100101049. https://doi.org/10.1081/CBI-100101049. [DOI] [PubMed] [Google Scholar]
- Ensrud K, Larson J, Guthrie K, Joffe H. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy menopausal women with hot flashes: A randomized controlled trial in the MsFLASH Network. Menopause. 2011;18(12):848–855. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR. Hot flashes: behavioral treatments, mechanisms, and relation to sleep. The American Journal of Medicine. 2005a;118(12, Supplement 2):124–130. doi: 10.1016/j.amjmed.2005.09.046. https://doi.org/10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Freedman RR. Pathophysiology and Treatment of Menopausal Hot Flashes. Seminars in Reproductive Medicine. 2005b;23(02):117–125. doi: 10.1055/s-2005-869479. https://doi.org/10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, Sheng L. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstetrics and Gynecology. 2007;110(2 Pt 1):230–240. doi: 10.1097/01.AOG.0000270153.59102.40. https://doi.org/10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders: Sleep disturbance and cardiometabolic disorders. Journal of Sleep Research. 2012;21(4):427–433. doi: 10.1111/j.1365-2869.2011.00990.x. https://doi.org/10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19(4):387–395. doi: 10.1097/gme.0b013e31824d8f40. https://doi.org/10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum HT, Kong SX, Kania CM, Wong JM, Mendelson WB. Insomnia, health-related quality of life and healthcare resource consumption. A study of managed-care organisation enrollees. PharmacoEconomics. 1998;14(6):629–637. doi: 10.2165/00019053-199814060-00004. [DOI] [PubMed] [Google Scholar]
- Insomnia.net. The high cost of insomnia in the United States. 2017 Retrieved from http://www.insomnia.net/research/cost/
- Institute of Medicine. Sleep disorders and sleep deprivation: An unmet public health problem. 2006 Retrieved from https://www.iom.edu:443/Repports/2006/Sleep-Disorders-and-Sleep-Deprivation-An-Unmet-Public-Health-Problem.aspx. [PubMed]
- Joffe H, Massler A, Sharkey K. Evaluation and management of sleep disturbance during the menopause transition. Seminars in Reproductive Medicine. 2010;28(05):404–421. doi: 10.1055/s-0030-1262900. https://doi.org/10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. doi: 10.1097/00042192-200310010-00005. https://doi.org/10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- Kravitz HM, Joffe H. Sleep During the Perimenopause: A SWAN Story. Obstetrics and Gynecology Clinics of North America. 2011;38(3):567–586. doi: 10.1016/j.ogc.2011.06.002. https://doi.org/10.1016/j.ogc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Chang Y, Kravitz HM, Bromberger JT, Owens JF, Buysse DJ, Hall MH. Sleep and risk for high blood pressure and hypertension in midlife women: the SWAN (Study of Women’s Health Across the Nation) Sleep Study. Sleep Medicine. 2014;15(2):203–208. doi: 10.1016/j.sleep.2013.11.002. https://doi.org/10.1016/j.sleep.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, LeBlanc M, Bélanger L, Ivers H, Mérette C, Savard J. Prevalence of insomnia and its treatment in Canada. Canadian Journal of Psychiatry. 2011;56(9):540–548. doi: 10.1177/070674371105600905. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Sleep in America poll 2002. Washington, DC: National Sleep Foundation; 2002. Retrieved from https://doi.org/10.1016/j.sleh.2015.04.001. [Google Scholar]
- National Sleep Foundation. 2007 sleep in America poll: Women and sleep. 2007 Retrieved from http://www.sleepfoundation.org/article/sleep-america-polls/2007-women-and-sleep.
- Nosek M, Kennedy H, Gudmundsdottir M. Distress during the menopause transition: A rich contextual analysis of midlife women’s narratives. SAGE Open. 2012;2:1–10. [Google Scholar]
- Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- Perlis M, Gehrman P, Ellis J. The natural history of insomnia: What we know, don’t know and need to know. Sleep Medicine Research. 2011;2:79–88. [Google Scholar]
- Pien GW, Sammel M, Freeman E, Lin H, DeBlasis B. Predictors of Sleep Quality in Women in the Menopausal Transition. Sleep. 2008;31(7):991–999. [PMC free article] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical Guideline for the Evaluation and Management of Chronic Insomnia in Adults. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine. 2008;4(5):487. [PMC free article] [PubMed] [Google Scholar]
- Serrano E, Warnock J, Jill K. Depressive disorders related to female reproductive transitions. Journal of Pharmacy Practice. 2007;20(5):385–391. https://doi.org/10.1177/0897190007304984. [Google Scholar]
- Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. American Journal of Psychiatry. 1997;154(10):1417. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- Sowers M, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Kelsey J, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and pathobiology. 1. San Diego, CA: Academic Press; 2000. pp. 175–188. [Google Scholar]
- Terauchi M, Hiramitsu S, Akiyoshi M, Owa Y, Kato K, Obayashi S, Kubota T. Associations between anxiety, depression and insomnia in peri- and post-menopausal women. Maturitas. 2012;72(1):61–65. doi: 10.1016/j.maturitas.2012.01.014. https://doi.org/10.1016/j.maturitas.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Buysse DJ, Hall M, Matthews KA. Marital happiness and sleep disturbances in a multi-ethnic sample of middle-aged women. Behavioral Sleep Medicine. 2009;7(1):2–19. doi: 10.1080/15402000802577736. https://doi.org/10.1080/15402000802577736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. World midyear population by age and sex for 2010. 2010 Retrieved from http://www.census.gov/population/international/data/idb/worldpop.php.
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with Objective Short Sleep Duration is Associated with a High Risk for Hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. The American Journal of Medicine. 2005;118(12):14–24. doi: 10.1016/j.amjmed.2005.09.031. https://doi.org/10.1016/j.amjmed.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES. Sleep Symptoms During the Menopausal Transition and Early Postmenopause: Observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]


