Abstract
Introduction
The chemokine receptor CCR5 has garnered significant attention in recent years as a target to treat HIV infection largely due to the approval and success of the drug Maraviroc. The side effects and inefficiencies with other first generation agents led to failed clinical trials, prompting the development of newer CCR5 antagonists.
Areas covered
This review aims to survey the current status of ‘next generation’ CCR5 antagonists in the preclinical pipeline with an emphasis on emerging agents for the treatment of HIV infection. These efforts have culminated in the identification of advanced second-generation agents to reach the clinic and the dual CCR5/CCR2 antagonist Cenicriviroc as the most advanced currently in phase II clinical studies.
Expert opinion
The clinical success of CCR5 inhibitors for treatment of HIV infection has rested largely on studies of Maraviroc and a second-generation dual CCR5/CCR2 antagonist Cenicriviroc. Although research efforts identified several promising preclinical candidates, these were dropped during early clinical studies. Despite patient access to Maraviroc, there is insufficient enthusiasm surrounding its use as front-line therapy for treatment of HIV. The non-HIV infection related development activities for Maraviroc and Cenicriviroc may help drive future interests.
Keywords: CCR5, CCR2, CXCR4, Maraviroc, HIV, entry inhibitors, antagonists, Cenicriviroc, chemokine receptors
1. Introduction
Since its discovery in 1983, the human immunodeficiency virus (HIV-1) has eluded broad-spectrum vaccination efforts and remains an imminent threat to public health with 35 million infections worldwide [1]. The development of highly active antiretroviral therapy (HAART) has significantly curbed the mortality rate previously associated with HIV-1 and has transformed the diagnosis from a death sentence to a chronic, manageable condition for many [2,3]. HAART consists of a personalized regimen of three or more antiviral drugs that interfere with various aspects of the viral lifecycle. To date, over 25 agents have been clinically approved for the treatment of HIV-1; however, the development of new antiviral agents is driven by the acquisition of drug resistance to preexisting regimens, simplified dosing schedules, and reduced pill burden to enhance patient compliance [4]. The classes of HAART medicines include both nucleoside and non-nucleoside reverse-transcriptase inhibitors (NNRTIs), HIV-1 protease inhibitors (PIs), HIV-1 integrase inhibitors, and viral–host entry inhibitors [5]. At the time of this writing, Enfuvirtide (Fuzeon®, Genetech CA, USA) and Maraviroc (MVC) are the only two clinically approved antiviral drugs that block HIV-1 entry [6]. Fuzeon® is an injectable fusion inhibitor that binds gp41 and prevents its insertion into the plasma membrane, but this drug is inconvenient to administer and produces significant inflammation at the injection site that eventually causes patient noncompliance precluding further use. MVC is an orally administered, potent CCR5 antagonist that prevents the HIV-1 envelope protein gp120 from binding to the host-based chemokine and CD4 receptor, making this the only mammalian-host target-based HIV medication (all references made to HIV in this review will refer to the HIV-1 form of the virus).
2. CCR5 biology and chemokine receptor-based HIV tropism
CCR5 is a promiscuous seven-transmembrane G-Protein Coupled Receptor (GPCR) that binds several endogenous chemokines to mediate receptor activation [7]. Macrophage inflammatory protein 1α (MIP-1α, CCL3) and 1β (MIP-1β, CCL4) and Regulated on Activation, Normal T cell Expressed and Secreted (RANTES) (CCL5) are potent agonists, whereas CCL8 and CCL13 are partial agonists that exhibit a spectrum of CCR5 activation. CCL7 has also been identified as a ligand for CCR5; however, CCL7 does not appear to induce receptor activation and is believed to be a natural antagonist [7]. Binding of CCR5 to an agonistic chemokine triggers a signaling cascade via intracellular G-coupled proteins located at the C-terminus to coordinate leukocyte trafficking [8,9] and recruit immune effector cells to inflamed or damaged tissues to prime the adaptive immune response [10]. The inhibition of CCR5 with MVC significantly reduces T-cell migration in peripheral blood mononuclear cells (PBMCs) in a dose-dependent manner and arrests T-cell proliferation at higher concentrations (100 μM) in vitro [11]. Similar reductions in chemotaxis were also noted for monocytes, macrophages, and monocyte-derived dendritic cells toward their cognate ligands in the presence of MVC (0.1–10 μM) [12]. These intrinsic immunomodulatory effects associated with CCR5 antagonism are highly desirable for the treatment of inflammatory and autoimmune conditions including graft-versus-host disease [13], multiple sclerosis [14], and ischemic stroke [15]. However, CCR5 has also been heavily implicated in the development of CD8+ T cells [16–18] which play a critical role in the clearance of virus-infected cells and in the pathogenesis of HIV infection [19,20]. Interestingly, the introduction of deleterious mutations or deletions within the CCR5 gene does not appear to induce dire immunological consequences beyond predisposition to West Nile virus [21,22], tickborne encephalitis [23], Toxoplasma gondii [24], and a handful of other central nervous system-localized infections [25,26].
CCR5 is intimately involved in HIV entry and renders several immune cells permissible to infection including memory/effector T cells, dendritic cells, macrophages, monocytes, B cells, and natural killer cells [27–29]. Entry is initiated when the viral envelope protein gp120 binds to CD4 on the target cell surface and subsequently anchors itself to a co-receptor, either CCR5 or CXCR4, which is essential for virion fusion to the plasma membrane. Inhibition of CCR5 and CXCR4 alone is sufficient to prevent HIV entry. Viral strains that bind CCR5 are classified as R5-tropic (R5), strains that interact with CXCR4 are X4-tropic (X4), and dual/mixed tropic viruses use either CCR5 or CXCR4 to mediate entry [30]. This is problematic for CCR5 and/or CXCR4 antagonists in that inhibition of one chemokine may cause a tropism shift, potentially exacerbating disease progression. Several lines of evidence have linked X4-tropic HIV with pronounced T-cell depletion, immunosuppression, and rapid progression to AIDS [31]. In contrast, CCR5-tropic virions generally produce a relatively mild asymptomatic infection over several years before compromising the host. One may speculate that inhibition of CXCR4 would lead to a less pathogenic infection; however, there is a clear preference for HIV to use CCR5 over CXCR4, rendering CCR5 as the more desirable drug target.
A natural 32-base pair deletion within the CCR5 gene (CCR5Δ32) has been extensively documented in 10–20% of the Caucasian population that confers resistance to HIV [32,33]. Δ32 heterozygotes express both functional and truncated CCR5 which confers a certain degree of protection from R5-HIV; however, these individuals are still permissive to infection [34]. Only individuals who express both CCR5Δ32 alleles (1% to 2% of the Caucasian population) completely lack surface expression of CCR5 and are immune to R5 HIV [35]. The curative potential of permanent CCR5 deficiency in HIV-infected persons was realized in two landmark studies demonstrating the first and only reported sterilizing cure for HIV infection following a stem-cell transplant of homozygous CCR5Δ32/CCR5Δ32 donor progenitor cells to an HIV-positive patient suffering from acute myeloid leukemia [36,37]. As of 2016, the so-called ‘Berlin patient’ remains HIV-free in the absence of antiretroviral therapy with no signs of disease progression or viral rebound. However, this procedure failed to cure a 27-year-old HIV-positive patient suffering from T-cell lymphoma and led to a shift in viral tropism (CCR5 to CXCR4) that resulted in death [38]. These findings combined with the low prevalence of homozygous CCR5Δ32 donor stem cells and a significant mortality (40–55%) [39,40] make stem-cell transplantation ill-suited for widespread clinical applications and encourages pharmaceutical intervention through a CCR5-based HAART-type regimen.
3. First-generation CCR5 antagonists
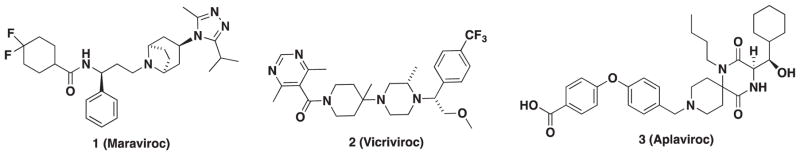
The area covering the potential of first-generation CCR5 antagonists for HIV treatment was previously reviewed before all clinical outcomes were known. Since then, MVC (1, Figure 1), an orally administered and potent CCR5 antagonist, received Food and Drug Administration (FDA) approval in 2007. This drug demonstrates a long plasma half-life (t1/2 15–23 h) [41] to support a convenient once-a-day dosing schedule [42,43] and exhibits broad-spectrum antiviral activity across several R5 isolates including 200 clinically derived HIV envelope pseudo-viruses with an average IC90 of approximately 14 nM [21]. Data collected from clinical trials (MOTIVATE (Maraviroc Therapy in Antiretroviral Treatment-Experienced HIV-1-Infected Patients) 1 and 2) indicate that MVC is generally well tolerated and significantly repressed viral load in R5-infected HAART-treatment-experienced patients; however, MVC is also hepatotoxic and is not recommended for treatment-naive patients or individuals infected with X4- or dual-tropic R5X4 virus [44–46]. The M/R5-tropic requirement of prequalification for MVC administration led the drug sponsor to develop a suitable test to determine viral population tropism within patients. This test, which now is known under the name TROFILE (Monogram/Quest Biosciences, San Francisco, CA) is currently used by clinicians [47]. MVC is the only tropism-dependent HIV drug and is designated as a backup regimen partly due to this prescreening requirement. Although this test is not approved by the FDA, it is required to ensure R5-only tropism and drug treatment success. Adoption of this practice was supported in the clinical trials of MVC, where a first-generation version of TROFILE test proved to be inaccurate allowing patients with mixed R5/X4 virus populations to take MVC. Significant drug failure was observed in these individuals [48]. The CCR5 to CXCR4 tropism shifts in patients taking MVC is currently unknown; however, observations of tropism shifts toward X4 using virus have been observed in naive and HAART (non-MVC)-treated individuals. Shifts also occur naturally during viral evolution as the X4-tropic virus is the main driver in the development of AIDS [49,50]. Thus, the tropism test is both a prequalifier as well as a limitation to CCR5 antagonists in the HIV setting since no other HIV medication requires this pretest. However, it is worth noting that current tropism assays have increased sensitivity to reliably detect X4-tropic HIV with rapid turnaround and at a low cost.
Figure 1.
First generation CCR5 antagonists.
Despite the need for the viral tropism test and initial setbacks involving implementation, there have been several clinically significant developments involving MVC showing its adoption into the mainstream of HIV treatments with some unexpected benefits [51]. Adding MVC to an antiviral regimen consisting of raltegravir/tenofovir/emtricitabine resulted in faster reduction of 2-LTR+ (2-long term repeat) newly infected cells and recovery of CD4+ T-cell counts and a modest reduction in total reservoir size after 48 weeks of treatment [52]. In another study, MVC was compared with efavirenz both in combination with zidovudine/lamivudine (MERIT [Maraviroc versus Efavirenz in Treatment-Naive Patients] trial) in HIV-infected patients, as it has been demonstrated that patients on HAART therapy are at increased risk for developing metabolic abnormalities that include elevated levels of serum triglycerides, low-density lipoprotein cholesterol (LDL-c), and reduced levels of high-density lipoprotein cholesterol [53]. In this study, MVC was not associated with elevations in total cholesterol, LDL-c, or triglycerides and showed beneficial effects on lipid profiles of dyslipidemic patients [54].
MVC has also been evaluated as a preexposure prophylaxis agent alone and in combination with reverse-transcriptase inhibitors. Preliminary studies with macaques have demonstrated that oral administration of MVC (44 mg/kg) alone is insufficient to prevent HIV infection despite high drug concentration in rectal tissues 24 h prior to inoculation [55]. Nonetheless, MVC has been pushed to human trials in the NEXT-PrEP (Novel Exploration of Therapeutics for PreExposure Prophylaxis) study although no data on the outcome of the study are available at the time of this writing [56].
In 2001, scientists at Schering-Plough (Kenilworth, NJ) identified the clinical candidate Vicriviroc (2, VVC, Figure 1) as a noncompetitive allosteric CCR5 antagonist [57,58]. VVC reached phase II trials for treatment-naive patients; however, these efforts were halted after patients experienced viral rebound with continued treatment [59]. Subsequent studies in HIV-infected treatment-experienced patients revealed that VVC demonstrated potent virologic suppression; however, this was accompanied by an increase in liver malignancies that further stalled the development of VVC [60]. Around the same time, GSK’s (Brentford, UK) CCR5 program identified spirodike-topiperazine derivative Aplaviroc (AVC) (AK-602 and GSK-873140). AVC (3, Figure 1) exhibited excellent activity against HIV-1Ba-L with an IC50 value of 0.4 nM and demonstrated a favorable pharmacokinetic profile in monkeys with an oral bioavailability of 30% [61]. Unfortunately, phase II clinical trials were terminated due to severe hepatotoxicity observed in infected patients.
The success of MVC and the failures of VVC and AVC have recently been scrutinized in reference to lack of druglikeness in these compounds as the potential reasons for failure in the clinic and setting the stage for future innovation [62]. Furthermore, the side effects and insufficiencies associated with VVC and AVC versus the excellent profile of MVC became the focus of ensuing research that would expand to a number of follow-ups and include compounds with activity against a second chemokine as a desirable approach. This review aims to survey the current status of ‘next-generation’ CCR5 antagonists in the early-stage pipeline with an emphasis on emerging agents and strategies as treatments for HIV infection.
4. Second-generation small-molecule CCR5 antagonists
The design of new CCR5 antagonists involves a number of different strategies including de novo drug design, high-throughput virtual screening, scaffold hopping, and other unique approaches such as drug–antibody conjugates. Many of the medicinal chemistry efforts have modified the scaffolds of MVC, VVC, and AVC to acquire novelty, increase potency, or enhance ADME (Absorption/Distribution/Metabolism/Excretion) properties. Few candidates reached early preclinical/clinical evaluation, and some were found to be superior to MVC.
In vitro bioassays are the first line of assessment for the activity of small-molecule CCR5 antagonists. The RANTES-binding assay utilizes radiolabeled chemokines of CCR5 such as [125I]-RANTES, [125I]-MIP-1α, and [125I]-MIP-1β to measure the competitive displacement of the radiolabeled ligand by the small molecule at the receptor site. In terms of chemokine receptor functional response, the calcium mobilization assay can be used to determine whether HIV has engaged with the co-receptor CCR5 or CXCR4, which would trigger a measurable flux of intracellular calcium. These assays can be used to measure responses to all relevant chemokine receptors including CCR5, CCR2, and CXCR4.
A second pivotal assay for small-molecule assessments is the anti-HIV assay, otherwise described as an anti-infectivity or antiviral assay, which is the main measure of the small molecule’s capacity to inhibit the replication of HIV in cells. Often PBMCs are the medium in which the assay is conducted as HIV primarily targets these cells, but often a more specific cell line such as peripheral blood lymphocyte (PBL) cells can be used. Inhibition of viral replication is measured directly by amount of viral RNA transcription or reverse-transcriptase enzyme activity. The IC50 values extrapolate directly to the clinical efficacy to suppress HIV infection and replication. These assays are not mechanistically sensitive to the type of antiviral activity and are used to measure activity against a multitude of different HIV targets including cell entry, reverse transcriptase, protease, and integrase.
Several surrogates to the PBM/LC (Peripheral Blood Mononuclear/Lymphocyte Cells) assays can also be used to detect viral infection and offer advantages of mechanistic bias or altered biochemical readouts. The CCR5 fusion assay offers a mechanistic bias for entry inhibitors in that it measures the ability to block the HIV entry by preventing the binding of gp120 or gp160:CD4 complex to the co-receptor CCR5. Another assay that can be useful in the initial characterization of anti-HIV compounds is the reporter gene-based multinuclear activation of a beta-galactosidase indicator (MAGI) assay usually conducted in receptor-transfected CEM (Human T-lymphoblastoid Leukemia) or human osteosarcoma (HOS) cells.
Promising preclinical candidates were met with a potency criterion in either one or several of the in vitro assays at low nanomolar levels of inhibition. However, their poor pharmacokinetic profile in animal studies, unsafe levels of inhibition to the human ether-à-go-go-related gene (hERG), or inhibition of the metabolic enzyme CYP2D6 halted their advancement into the clinic. Newer scaffolds identified from alternative techniques lie at a more nascent stage. The most advanced approach seems to involve incorporating dual chemokine activity. We will describe these efforts in this section.
4.1. Piperidine amide compounds
4.1.1. MVC-inspired/tropane second-generation compounds
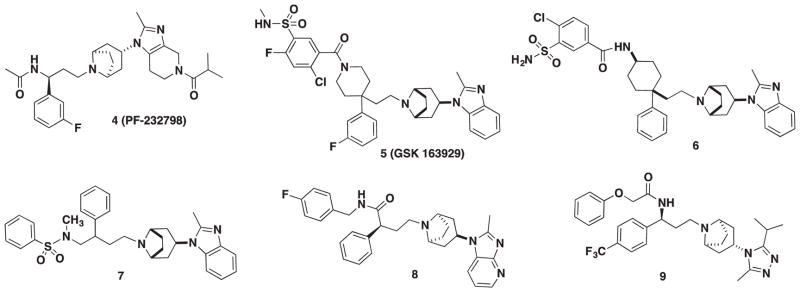
In 2011, Pfizer (New York, NY) reported a second-generation oral CCR5 antagonist imidazopiperidine drug candidate PF-232798 (4, Figure 2) during a follow-up program with the intention of improving the oral absorption of MVC [63]. Twenty-two compounds were synthesized, and lead candidate PF-232798 was identified after an in vivo pharmacokinetic screening in both rats and dogs. PF-232798 demonstrated potent anti-HIV activity (IC50 = 2.0 nM, HIV-1Ba-L) and modest hERG activity (IC50 = 12 μM) that was comparable to MVC and exhibited substantial oral absorption in rats and dogs. Stability studies in human liver microsomes indicated that PF-232798 was metabolically stable and not a suitable substrate for CYP34A, which could translate to a reduction in dosing frequency and a prolonged half-life. In addition, the compound retained antiviral activity against B-clade MVC-resistant HIV-1 isolate strain CC185. Phase II clinical trials were completed in August of 2013 with no adverse safety reactions reported at levels up to 250 mg; however, no other information about the status of PF-232798 (4) has been reported [64].
Figure 2.
Tropane-based CCR5 antagonists.
GSK 163929 (5) is a 4,4-disubstituted piperidine scaffold that was discovered at GlaxoSmithKline through a modular, high-throughput chemistry approach [65]. GSK 163929 had good potency with IC50 values of 4.26 and 3.47 nM against HIV-1Ba-L-infected HOS and PBL cells, respectively [66]. Excellent pharmacokinetic properties were observed, and a 7-day safety assessment in rats and dogs displayed no adverse effects; however, toxicity concerns halted the progression and development of 5 into the clinic [67]. In an attempt to attenuate toxicity, modifications of the carboxamide moiety were investigated but did not produce fruitful results [68]. Furthermore, drug clearance remained high in rats.
GSK also probed the utility of the nitrogen atom of the piperidine ring and the length of the carbon linker attaching the piperidine ring to the tropane moiety in the 4,4-disubstituted scaffold [69]. A cyclohexylamine scaffold emerged from this study with compound 6 as the most potent derivative (HOS, IC50 = 58 nM). Structure–activity relationship (SAR) studies suggested that the preferred stereochemistry for the cyclohexyl ring was cis and that a 2-carbon linker was the optimal length.
Two novel series were investigated by GSK – DAB (2-phenyl-1,4-diaminobutane) and MDAB (2-methyl-2-phenyl-1,4-diaminobutane), which differ by a methyl group at the 2-position [70]. The objective was to determine the influence of the tropane moiety on the antiviral properties of the scaffold, and it was concluded that the MDAB series attached to an endo-tropane motif led to higher potency in PBL cells, while the same motif for the DAB series did not. The study revealed two potent compounds (7) from the DAB series that showed excellent anti-HIV potency in both HOS and PBL cells (IC50 = 8 and 60 nM, respectively) as well as moderate drug clearance in rats, but poor bioavailability.
In 2010, Zhang and coworkers reported a new scaffold which was structurally related to MVC [71]. The strategy behind their SAR was to identify a bioisostere for MVC’s amide group that could be easily manipulated into other functional groups; thus, a carboxamide moiety was selected. Eleven compounds were synthesized in the study with the most potent compound 8 (Figure 2) with an IC50 = 14.4 nM in the RANTES-binding assay. Another new core scaffold based on the selection and assembly of privileged fragments from known CCR5 antagonists, particularly MVC, was reported. The scaffold features a novel propane-1,3-diamino bridge illuminated by compound 9 (Figure 2) that was found to have excellent activity against CCR5 in a RANTES-binding assay with an IC50 = 0.253 nM [72].
4.1.2. Monocyclic piperidine amides
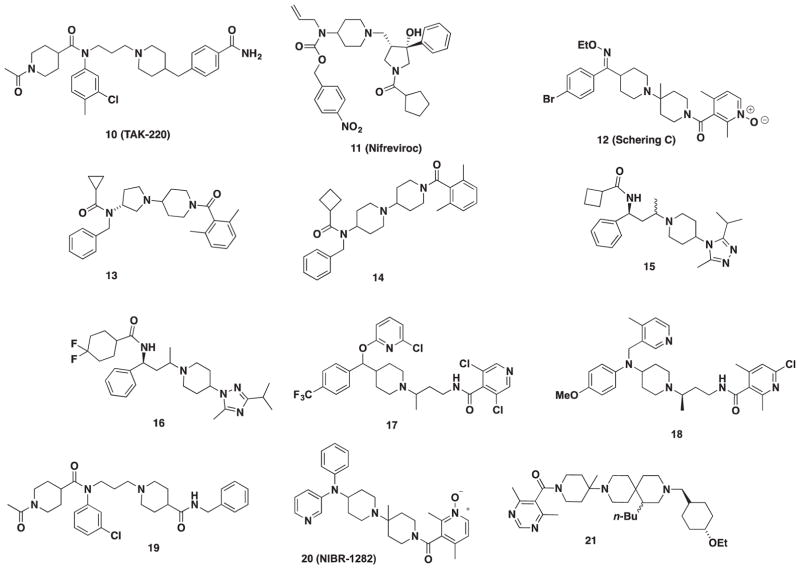
TAK-220 (10, Figure 3) was developed from a lead compound discovered through a high-throughput screening at Takeda (Osaka, Japan). The piperidine-4-carboxamide moiety of TAK-220 (10) was found to be important for HIV activity and conferred metabolic stability that led to the clinical development of 10 [73]. TAK-220 was licensed to Tobira Therapeutics (San Francisco, CA) and reached phase I clinical trials as of 2008, but no further progress has been reported [74].
Figure 3.
Piperidine-amide CCR5 antagonists.
Inspired by Merck’s (Kenilworth, NJ)1,3,4-trisubstituted pyrrolidines, a series of 1,3,3,4-tetrasubstituted pyrrolidine compounds were reported in 2007 to improve the poor oral bioavailability of Merck’s compounds [75]. This was accomplished by incorporating a hydroxylgroup at the 3-position on the pyrrolidine ring to furnish nifreviroc (11, TD-0232, Figure 3). Nifreviroc displayed an IC50 = 2.9 nM in the RANTES-binding assay and possessed a favorable pharmacokinetic profile in rats and dogs. The nitro moiety of 11 was considered a possible mutagenic liability, and subsequent optimization (TD-0680, 24) will be discussed later [76].
Pryde and coworkers were inspired by both the amide functionality of MVC and the piperidine amide moiety in Schering C (12, SCH-C, Figure 3) which led to SAR exploration. [77]. Thirty compounds were synthesized in the first round of SAR with the lead compound displaying a modest IC50 of 145 nM in a gp160 cell–cell fusion assay. A second round of synthesis based around their lead resulted in two additional hits: compound 13 and 14 (Figure 3) with an IC50 of 1 and 3 nM, respectively. The authors note that compounds 13 and 14 are moderately lipophilic and may be a source of poor metabolic stability in liver microsomes. However, high permeability and weak hERG activity identify these compounds as promising starting points for preclinical development.
Barber and coworkers designed the 1-amido-1-phenyl-3-piperdinylbutane series in search for an MVC-like compound without the tropane core to improve oral absorption [78]. The authors shifted toward a scaffold containing 4-heterocyclic substituted piperidines as bioisosteres of the amido group for improved permeability. Their initial lead compound 15 (Figure 3) (fusion assay, IC50 of 2.0 nM) contained a 1,3,4-triazole moiety that afforded higher potency and stability. Further optimization of the 4-heterocyclic-substituted piperidine series led to 1,2,4-triazole substitution with lead compound 16 (fusion assay, IC50 of 3.08 nM) that exhibited high oral bioavailability in rats, but did not extend the drug’s half-life [79].
In 2011, compound 17 (Figure 3), a pyridine-2-yloxymethylpiperidin-1-ylbutyl amide, was discovered by Skerlj and coworkers [80]. Their strategy toward this new scaffold was to modify SCH-C (12) by opening the lower piperidine ring and finding a substitute for the oxime moiety. Compound 17 was their lead compound, which had strong inhibition against R5 HIV-1 replication in PBMCs (IC50 = 9 nM), but oral bioavailability in dogs was extremely poor (F = 6%). The group decided to move away from this series and to focus on modifications around the aniline to create a novel pyridine-2-ylmethylaminopiperidin-1-ylbuytl amide series [81]. Compound 18 (Figure 3) emerged as the lead compound with an IC50 = 0.2 nM in CCR5 fusion and 2.2 nM in PBMC assay. Compound 18 achieved a favorable in vitro and in vivo profile but had unfavorable hERG activity (IC50 = 0.25 μM) [81]. Consequently, the series was abandoned due to cardiovascular concerns, and future works to mitigate hERG liabilities are ongoing.
A Y-shaped pharmacophore model that interacted with amino acids essential for CCR5 antagonism was used as the basis for a novel piperidine series. A patent disclosed in 2009 motivated additional SAR around the ‘Y’-shaped model that led to the design of novel piperidine-4-carboxamide derivatives [83]. Piperidine 19 (Figure 3) was subsequently discovered and displayed excellent inhibition against CCR5 (calcium mobilization assay, IC50 = 25.73 nM) and anti-HIV activity (IC50 = 73.01 nM). Further pharmacokinetic properties were examined in a rat models that demonstrated 15% oral bioavailability. Compound 19 also exhibited modest inhibition of the hERG channel (15% at 3 μM).
A novel piperidine scaffold [84] previously reported by Novartis (Basel, Switzerland) was found to have adverse cardiovascular side effects at submicromolar concentrations. In an attempt to dial out these undesirable properties, modifications to the diphenyl moiety were conducted which led to compound 20 (NIBR-1282, Figure 3) [85]. Compound 20 had an IC50 value of 5.1 nM in the CCR5-binding assay with no cardiovascular complications in animal models.
The diazaspiro[5.5]undeca-2-one compounds were derived from a 1-oxa-3,9-diazaspiro scaffold previously investigated by Roche [86,87]. A total of 27 derivatives were synthesized, and diazaspiro 21 (Figure 3) was identified as the most potent antagonist with an IC50 = 30 nM in the RANTES-binding assay. Compound 21 had an attractive pharmacokinetic profile with bioavailability at 66% and a half-life of 4.19 h (PO [oral administration]) in rats and displayed no significant CYP or hERG inhibition.
4.1.3. Cyclic and acyclic urea-piperidines
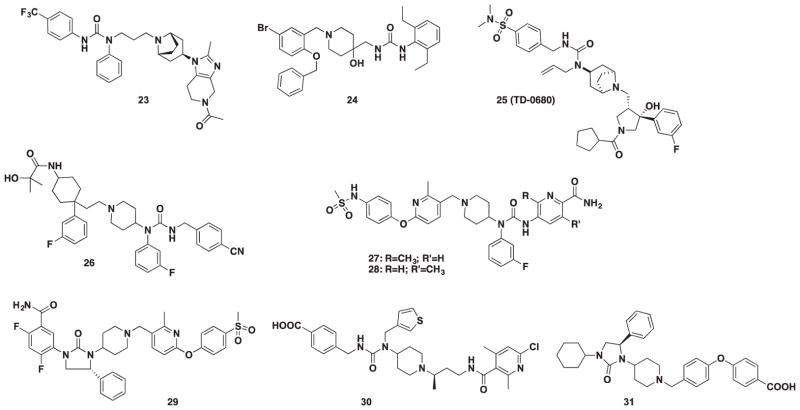
Ernst and coworkers designed a novel imidazopiperidinetropane scaffold by merging the attractive features of MVC with other known CCR5 antagonists. The western portion of the molecule was converted from a cyclohexylamide to a urea moiety that greatly improved anti-HIV activity. Considerable SAR led to the identification of urea 22 (Figure 4) which potently inhibited MIP-1β binding at IC50 = 1 nM with 52% bioavailability and a terminal life of 10.7 h [88].
Figure 4.
Piperidine-urea CCR5 antagonists.
A high-throughput screening of the internal library at Berlex Biosciences (San Pablo, CA) uncovered a guanylhydrazone derivative (37, Figure 6) as an initial starting point for a novel series of urea derivatives [89]. In a second report, continued SAR around scaffold 37 (Figure 6) revealed that a 4-hydroxy piperidine analog had an improved level of potency against MIP-1α binding to human CCR5/CD4-transfected HEK-293 (Human Embryonic Kidney-293) cells (IC50 = 49 nM) [90]. The addition of a urea group at the 4-position on the piperidine led to the acyclic urea derivative 23 (Figure 4) as their most potent lead. Unfortunately, poor pharmacokinetic properties led to the abandonment of this series.
Figure 6.
Other classes of CCR5 antagonists.
Building around the SAR that was successfully parlayed for Nifreviroc (11, TD-0232), significant tuning of the N-substituents led to a urea-based derivative containing a methyl sulfonamide moiety in place of the nitro group as a potent CCR5 antagonist. Further modifications included the incorporation of an exotropane and meta-fluoro moiety on the phenyl ring that led to 24 (TD-0680, Figure 4) [76]. TD-0680 (24) showed excellent antiviral activity against eight different HIV-1 isolates in the PBMC assay and significantly inhibited replication at sub-nanomolar concentrations in cell–cell fusion assays mediated by R5-tropic envelopes of three major subtypes.
In 2010, GSK scientists revisited their 4,4-disubstituted scaffold to modulate the amine moiety, and a urea-based group was found to be the most active with promising pharmacokinetic properties in rats [91]. Additional SAR was elaborated in a subsequent publication at the acyl position to improve pharmacokinetics [92]. Compound 25 (Figure 4) emerged as a preclinical candidate with excellent antiviral activity (HOS, IC50 = 8 nM) and 53% bioavailability in rats. Compound 25 advanced to PK (pharmacokinetic) studies in dogs and monkeys resulting in high bioavailability with low clearance in dogs, but low bioavailability (14%) and high clearance in monkeys.
Once GSK 163929 (5) was found to harbor toxicity, a significant redesign of the tropane benzoimidazole was initiated and led to a p-cyanobenzyl urea derivative. Additional work with scientists at Ono Pharmaceuticals (Osaka, Japan) resulted in the discovery of a novel pyridyl carboxamide series [67]. The effort began after a patent from ONO disclosed a CCR5 compound containing a urea moiety that was structurally similar to the urea moiety of 25. The compounds were designed to preserve the urea scaffold while modifying the carboxamide region to afford a new pyridyl carboxamide series. Two compounds 26 and 27 (Figure 4) emerged as preclinical leads with an improved PK profile in rats.
A novel urea series reported in 2011 was found to have potent anti-HIV activity in HOS and PBL cell assays [93]. The original acyclic urea series was found to be unstable in fasted simulated intestinal fluid due to the acidic urea-nitrogen proton. Therefore, a cyclic urea series was developed to improve stability. Compound 28 (Figure 4) resulted from these efforts and demonstrated favorable potency (HOS and PBL, IC50 = 4 and 1.0 nM, respectively) and exhibited good in vivo PK properties in both dogs and monkeys (%F = 2.1 and 20.2 mL/min/kg, respectively).
Lastly, scientists at Genzyme (Cambridge, MA) applied a combination of mutagenesis, molecular modeling, and medicinal chemistry to uncover two structurally diverse acyclic and cyclic urea derivatives. These compounds, shown as 29 and 30 (Figure 4), had nanomolar IC50 activity with no cytotoxicity or hERG activity [94].
4.2. VVC- and AVC-inspired piperazines and diketopiperazines
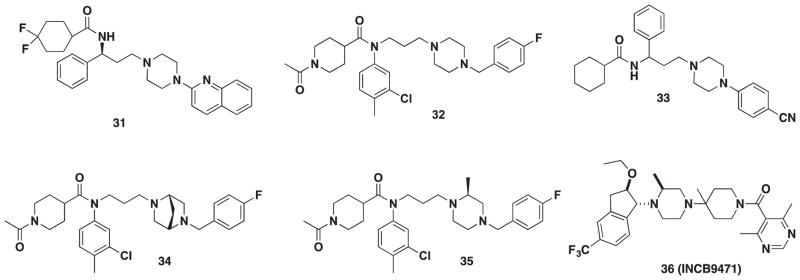
In 2008, a novel scaffold was created from the 4-(piperazin-1-yl)quinolone moiety of a lead compound discovered via virtual screening and the amide moiety of MVC [95]. Twelve derivatives were synthesized around this scaffold. Of these 12 compounds, 31 (Figure 5) yielded an IC50 of 0.233 μM, but this result was roughly 90-fold less potent than MVC. Despite this loss in potency, biological data obtained from strategic SAR modifications correlated with in silico experiments, which could provide insight into the future development of this scaffold.
Figure 5.
Piperizine CCR5 antagonists.
In 2012, Dong and coworkers synthesized 32 (Figure 5), which was inspired by the structure of TAK-220 (10) and an octahydropyrrole[3,4-c]pyrrole moiety published in a patent by Roche [96]. Their objective was to replace the 4-benzylpiperidine moiety with an isosteric piperazine. Eighteen derivatives were synthesized using TAK-220 as a template; however, 32 emerged as the lead compound and demonstrated potent anti-HIV-1 activity (IC50 = 6.17 nM), low cytotoxicity (CC50 > 100), and high oral bioavailability (%F = 56), but a short half-life (0.76 h) [97].
In 2013, Liu and coworkers devised a Y-shaped pharmacophore model previously used by Hu in the synthesis of piperidine 19. The Y-shaped model was used to design a novel piperazine series and was devised from previous work that elucidated the binding mode of both MVC and TAK-220 (10) [82]. The most potent lead was compound 33 (Figure 5) with an IC50 of 6.29 μM in a CCR5-mediated fusion assay and an IC50 of 0.44 μM in an anti-HIV assay.
From the same group, a 2015 report examined a fragment assembly-based strategy to develop a 2-methylpiperazin scaffold derived from the carboxamide moiety of TAK-220 (10) and the piperazine moiety from 33 [98]. The series resulted in compounds 34 and 35 (Figure 5) with nanomolar activity in CCR5 calcium mobilization assay (IC50 = 88 and 3.0 nM, respectively) as well as moderate antiviral activity (IC50 = 31.4 and 75.1 nM, respectively). In addition, no cytotoxicity was observed up to 10 μM and thus provided an excellent starting point for further optimization.
INCB9471 (36), developed by Incyte (Wilmington, DE), was inspired by Schering-Plough’s CCR5 antagonist VVC (2) [99]. Considerable SAR was conducted around the trifluoro-benzyl moiety, which was known to interact strongly with the receptor. An indane was chosen after conformational analysis revealed it to be a suitable replacement that provided enough rigidity while maintaining the correct orientation. INCB9471 is comparable in potency to SCH-D and inhibited R5 HIV-1 strains at IC50 values of 0.36 and 0.16 nM (ADA and Ba-L, respectively). INCB9471 exhibited excellent in vivo properties characterized by a drug half-life, low systemic clearance, and high volume of distribution in rats and dogs. Oral bioavailability was at maximum in rats and near maximum in dogs (% F = 100 and 95). INCB9471 advanced to phase I and II clinical trials and was found to be safe, but development was halted for other business priorities.
4.3. Miscellaneous scaffolds and approaches
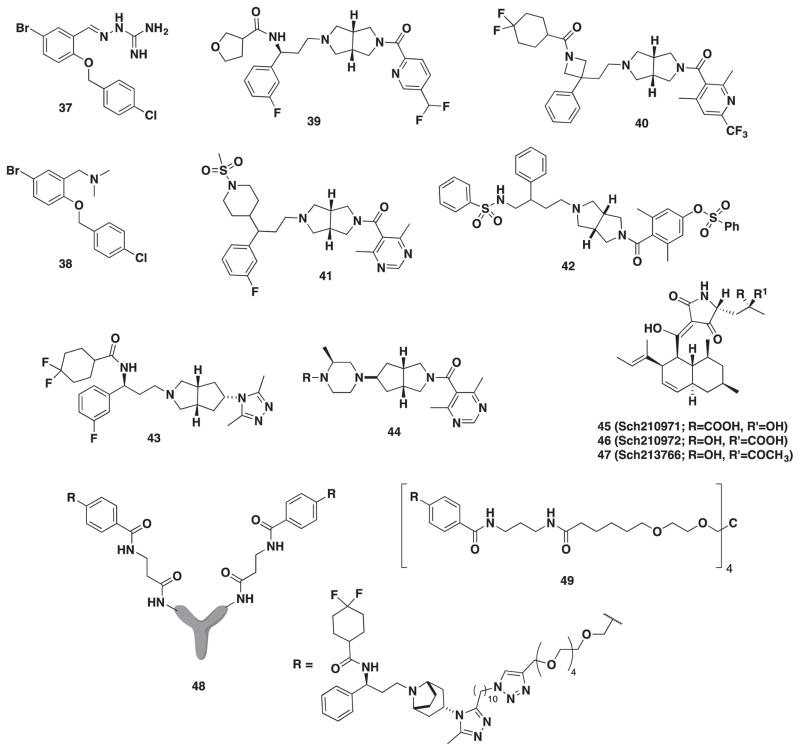
In 2007, Berlex Biosciences conducted a high-throughput screening of their internal library through a 125I-MIP-1α-binding assay on human CCR5/CD4 and found compound 37 (Figure 6) to have an IC50 = 2.2 μM in Ca2+ flux assay [89]. The study described the synthesis of 47 derivatives where the moiety at the R-position was replaced with a benzyl group, aryl group, or guanylhydrazone. It was discovered through the course of their study that the guanylhydrazone moiety of 37 was not required for potency, but rather a tertiary amine such as 38 (IC50 = 0.6 μM) was well tolerated. Compound 38 was selected for further exploration of the PK profile [90].
A new hexahydropyrrol[3,4-c]pyrrole moiety was identified through a high-throughput screening from a library of compounds at Roche [100]. The hexahydropyrrol[3,4-c]pyrrole moiety was considered to serve as a bioisostere for the tropane unit of MVC. The lead compound demonstrated excellent antiviral activity (IC50 = 7 nM) but was plagued by metabolic instability. In a subsequent report, detailed SAR around the N-substituted pyrazole subunit was conducted [101]. Efforts to optimize antiviral activity and metabolism led to compound 39 (Figure 6, antiviral IC50 = 11 nM). The authors remarked on the inability to improve in vitro metabolic stability while maintaining good antiviral activity, which ultimately lead to the abandonment of this series. In addition, two parallel series were examined where significant SAR around the secondary amide was probed to improve membrane permeability. Compounds 40 and 41 (Figure 6, antiviral IC50 = 1.2 and 17 nM, respectively) were identified as lead targets [102,103].
Compound 42 (Figure 6) was discovered during an investigation of two novel series called DAB (2-phenyl-1,4-diaminobutane) and MDAB (2-methyl-2-phenyl-1,4-diaminobutane) by scientists at GSK [70]. The study revealed compound 42 from the DAB series as containing excellent potency in both HOS and PBL cells (IC50 = 8 and 1 nM, respectively) as well as moderate drug clearance in rats, but poor bioavailability.
Lemoine and coworkers broadened the scope of the hexahydropyrrol[3,4-c]pyrrole motif and synthesized an endo 5-amino-3-azabicyclo[3.3.0]octane moiety. The 5-amino-3-azabicyclo[3.3.0]octane was explored as a replacement both for the tropane subunit of MVC and for the 4-aminopiperidine subunit in both VVC (2) and INCB9471 (36) to furnish 43 and substructure 44 (Figure 6) [104,105]. Each derivative was examined in a [128I]-RANTES-binding assay and an R5 antiviral assay, and the [3,4-c]pyrrole motif was found to be a suitable replacement in MVC, VVC, and INCB9471.
Many companies and research groups have broadened their search for novel scaffolds to target CCR5. Yang and coworkers in 2006 screened secondary metabolites from the microbial extracts of fungi, actinomycetes, or bacteria [106]. A high-throughput screening utilizing a radioactive CCR5-binding assay that inhibited RANTES binding was conducted on the extracts and led to the discovery of two tetramic acids Sch210971 (45, Figure 6) and Sch210972 (46, Figure 6) with an IC50 of 1.2 μM and 79 nM, respectively. The following year, the same group identified a third metabolite Sch213766 (47, Figure 6) that had an IC50 of 8.6 μM [107]. Tetramic acid compounds are known antimicrobials, but it is the first time that it has been described to have CCR5 activity.
Drug compliance continues to be a challenge for many patients bound to lifelong antiviral therapy. A drug candidate that demonstrates low clearance and a relatively long biological half-life has the potential to reduce dosing frequency and improve compliance. One in particular is to attach a small-molecule drug to an antibody creating chemically programmed antibodies (cpAbs). To produce a cpAbs, one can utilize the lysine residue of murine catalytic monoclonal antibody 38C2, which is capable of aldolase activity, and selectively label it with N-acyl-β-lactams. Another process is to attach a polyethylene glycol (PEG) linker to the small molecule in a process called PEGylation to improve properties. Asano explored both methods using MVC as the small molecule to improve properties [108]. The cpAbs 48 and PEGylated 49 (Figure 6) both had comparable IC50 values in the neutralization assay at 7.7 and 5.6 nM, respectively. Most importantly, both compounds were stable and held activity for up to 10 days compared to the parent drug MVC in human serum. Toxicology studies of the conjugates are unknown and thus warranted further study.
5. Dual chemokine CCR5 antagonists
5.1. Dual CCR5/CCR2 antagonists
The finding that MVC had a dyslipidemic effect in HIV patients spurred the interest that the multiple chemokines that bind to CCR5 may play a role in regulating the immune system in ways that should be extremely beneficial to HIV-infected patients. The promiscuous chemokine ligands that bind CCR5 (CCL 3, 4, 5, and 8) have commonality with other chemokine receptors including CCR1 (CCL 3, 4, and 5), CCR2 (CCL8), CCR3 (CCL5), CCR4 (CCL3 and 5), and CCR8 (CCL4) [109]. This redundancy among chemokines and their receptors also increases the likelihood of finding CCR5-based small molecules that bind to the other CCR receptors in this list. The first such reported multiple chemokine approach in the HIV setting has been the serendipitous finding of dual CCR5–CCR2 activity.
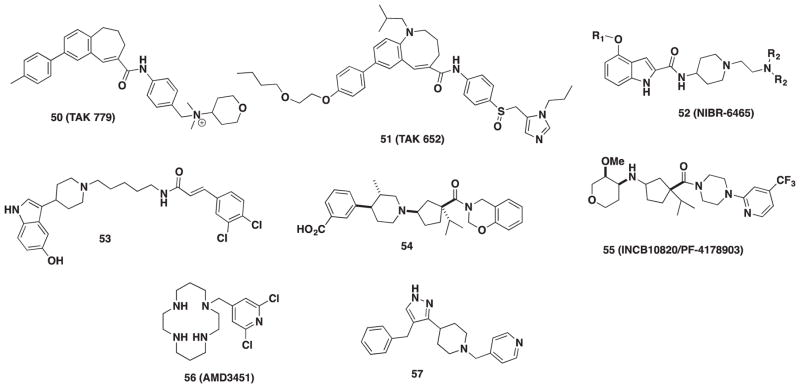
TAK-779 (50, Figure 7) was the first non-peptide CCR5 antagonist reported in 1999 [110]. A high-throughput screening of Takeda’s chemical library using both [125I]-RANTES and CHO/CCR5 assay identified this quaternary ammonium salt as one of their leads and a new CCR5 antagonist [111]. Optimization of TAK-779 structure generated a diverse series of benzoxapines, dihydronapthalenes, and benzocycloheptene derivatives. From these derivatives, the benzocycloheptene scaffold in 50 was the most selective toward the CCR5 receptor with nanomolar affinity but displayed activity toward CCR2 (IC50 = 27 μM) as well. The in vitro anti-HIV replication assays showed that 50 had strong potency with IC50 values against MAGI cells and PBMCs at 1.2 and 3.7 nM, respectively. Although TAK-779 became the lead compound, intrinsic issues with oral absorption stemming from the quaternary ammonium moiety required additional optimization to the core structure. TAK-652 (Cenicriviroc, 51, Figure 7) arose after optimizing the quaternary ammonium moiety to a sulfoxide functional group and a ring expansion of the [6,7]-fused 1-benzazepine to a [6,8]-fused -1-benzazocine [112]. A 10-fold increase in potency in the anti-HIV replication assay was observed for TAK-652 against six R5-clinical strains in PBMCs with a mean IC50 value of 0.25 nM. Inhibition of both receptors CCR2 and CCR5 was found to be similar with TAK-652 at 5.9 and 3.1 nM, respectively, and good oral absorption in rats, dogs, and monkeys was also observed. With high potency and improved oral bioavailability relative to TAK-779, TAK-652 was selected for further study and is currently in phase IIb clinical trials for treatment of HIV infection. This compound, now known as Cenicriviroc, is the most advanced CCR5 antagonist currently in clinical trials. Although the observation that MVC reduces dyslipidemia in HIV patients undergoing HAART treatment, the additional clinical benefits of blocking CCR2 with TAK-652 versus CCR5 alone are unknown at this time as CCR2 is not involved in HIV infection.
Figure 7.
Dual CCR5/CCCR2 and CCR5/CXCR4 compounds.
Novartis disclosed a series of indole derivatives that are dual CCR2/CCR5 inhibitors in a patent application [113]. Their initial hit compound was found via a high-throughput assay against CCR2 antagonists of Novartis’ chemical library [114]. Lead compound NIBR-6465 (52, Figure 7) exhibited high potency in both human and rat CCR2/CCR5 receptor assays [115]. In 2010, clinical trials with NIBR-6465 for HIV were disclosed, and 52 has successfully completed phase I studies [114,116].
Other dual CCR2/CCR5 inhibitors have been reported, but many have been pursued as anti-inflammatory agents. Several small molecules have been evaluated to target both receptors simultaneously, but evaluation of HIV activity was not performed, and no HIV data were provided. A high-throughput screening of the SmithKline Beecham Library identified an indole compound that was later optimized to 53 (Figure 7) with Ki values of 50 and 4.3 nM against CCR2 and CCR5, respectively [117]. Merck also investigated 54 (Figure 7) to combat various autoimmune diseases such as rheumatoid arthritis and multiple sclerosis [118]. The IC50 of 54 toward CCR2 was 4 nM and also inhibited CCR5 at 10 nM. Lastly, a collaboration between Incyte Corporation and Pfizer led to the discovery of dual CCR2 and CCR5 antagonist INCB10820/PF-4178903 (55, Figure 7) as a therapeutic agent to curb the recruitment of monocytes to sites of inflammation [119]. The IC50 for binding of 55 to CCR2 and CCR5 was 3.0 and 5.3 nM, respectively.
5.2. Dual CCR5/CXCR4 receptor compounds and approaches
Targeting both CCR5 and CXCR4 has potential to simultaneously thwart both X4- and R5-tropic viruses and would obviate the need for a tropism test; however, this concept has garnered sparse attention. Since tropism shifts occur in both treatment-experienced and treatment-naive HIV-infected patients, targeting both R5- and X4-tropic forms of the virus would provide a drug of more broad utility. Initial studies have demonstrated that this concept could work in practice. A substantial decrease (93%) in viral replication when an excess of CCL5 (ligand for CCR5) and CXCL12 (ligand for CXCR4) is present in cell cultures infected with dual-tropic HIV [120]. Furthermore, it was also shown that a combination of small-molecule antagonists (MVC and AMD3100) also abrogated replication of the same dual-tropic HIV strain. This work validated the idea that combination CCR5/CXCR4 therapy has the capacity to block HIV entry and prevent replication and could target both viruses simultaneously.
The search for a dual CCR5/CXCR4 HIV entry inhibitor has been reduced to practice, and the N-pyridinylmethyl cyclam AMD3451 (56, Figure 7) was reported as the first dual-tropic inhibitor in 2004 [121]. It is interesting to note that AMD3451 is structurally similar to AMD3100, which binds CXCR4 but not CCR5. AMD3451 was moderately active against X4 HIV-1 strains such as IIIB and NL4.3 with an IC50 of 1.2 and 1.8 μM, respectively, and also against the R5-tropic strain Ba-L with an IC50 value of 23.9 μM. Furthermore, dual-tropic HIV strains were inhibited by AMD3451 with an IC50 of 26.5 μM. Although AMD3451 does not have suitable potency for clinical use, its discovery serves as a proof-of-concept toward the future development of novel dual-tropic CCR5/CXCR4 antagonists.
A pyrazolo-piperidine series with CCR5/CXCR4 activity were recently discovered at Emory University through a combination of virtual high-throughput screening and Bayesian statistical partitioning [122]. The screening revealed a substructure with weak HIV inhibition (25 μM, MAGI assay) that consisted of a structural pyrazolo-piperidine core. A secondary screen led to compound 57, which demonstrated low micromolar HIV activity (MAGI assay). Subsequently, a cell–cell fusion assay established that 57 inhibited fusion similar to TAK-779 against R5-tropic HIV. However, 57 did not displace radioligand [125I]-CXCL12 from CXCR4. Interestingly, 57 exhibited activity in Ca2+ flux GPCR signaling which raises the possibility of negative allosteric modulation of CXCR4. Furthermore, 57 also displayed non-nucleoside reverse-transcriptase inhibitory activity both in time-of-addition studies and in an isolated HIV-1 reverse-transcriptase enzyme assay (IC50 = 9.0 μM). Additional studies with 57 revealed that its NNRTI activity is the primary mechanism of action; however, current efforts are in motion to enhance dual CCR5/CXCR4 inhibition while dialing out NNRTI activity.
6. Potential challenges for CCR5 antagonists: resistance mechanisms
Clinical experience has repeatedly demonstrated that HIV is prone to drug resistance. Studies with VVC [123–126], MVC [127,128], AVC [129], and AD101 [130] have concluded that a shift in co-receptor usage (CCR5 → CXCR4) is not the operative mechanism behind resistance to CCR5 antagonists in vitro or in vivo. Rather, adaptive changes in the gp120 V3 loop allows HIV to tighten its grip on CCR5 to use the drug-bound form of the receptor [124–126,131–133]. In several cases, inhibition is restored with monoclonal antibodies, RANTES, or other bulky CCR5 ligands that completely blockade HIV from approaching the active site of the receptor. In 2012, a novel mechanism of resistance was reported with MVC [134]. In that study, resistance to MVC was dependent on a single mutation in the C4 region of gp120 (N425K), which is known to interact with F43 within the D1 domain of CD4, not CCR5. Subsequent docking and modeling studies reveal that the K425 mutant generates new cation-π interactions with F43 on CD4 and suggest that enhanced interactions with CD4 circumvents the need for CCR5 binding which may be the source of MVC resistance. Sequence changes in the fusion peptide gp41 have also been reported to confer resistance to VVC [135,136], and other strain-specific V3 loop mutations contribute to MVC resistance in vivo [137]. Recently, the more advanced dual CCR5/CCR2 antagonist TAK-652 was the subject of resistance studies (R5 HIV-1 isolate KK) showing an escape virus with 200,000-fold reduction in activity [138]. Furthermore, the TAK-652-resistant virus was incapable of using CXCR4 as a co-receptor, confirming that no tropism shift had occurred during viral evolution. Taken together, it is clear that CCR5 antagonists are not immune to selective forces and are susceptible to unprecedented mechanisms of resistance, which do not include tropism shifts to X4 but rather HIV using drug-occupied CCR5 receptor.
A single report describing one strategy to overcome this resistance mechanism shows potential. The CCR5 antagonist TD-0680 (25) was reported to have retained efficacy against a dual TAK-779/MVC-resistant variant of HIV [139]. The authors show that TD-0680 had increased efficacy against HIV-1JR-FL and HIV-1Ba-L viruses in cell fusion and PBMC assays versus MVC and TAK-779. Furthermore, they show that TD-0680 retained activity against the dual TAK-779/MVC-resistant strain HIV-1Ba-L5.6r. They proposed that TD-0680 had increased interactions with the ECL2 (extra-cellular loop-2) region of CCR5, resulting in a more effective disruption of gp120 binding than other CCR5 antagonists. However, the failure of a CCR5 antagonist in the clinic from resistance development in patients during drug administration remains to be seen.
7. Expert opinion
The success of MVC in patients has set a high standard for next-generation CCR5 entry inhibitors as both VVC and AVC failed in the clinic mainly due to the lack of efficacy. However, the inadequacies of hepatotoxicity and use in only treatment-experienced patients place CCR5 antagonists in a secondary role in the treatment of HIV. The ongoing experimental and clinical research on CCR5 antagonists for HIV treatment has resulted in several significant second-generation agents with novel structural features and dual chemokine activity. These follow-up leads based on MVC and the other second-generation CCR5 compounds have failed to produce a clinical candidate or failed to reach advanced-stage clinical trials (Table 1, PF-232897, TAK-220, and INCB9471) with MVC itself remaining superior. Surprisingly, the only CCR5 antagonist currently in advanced clinical trials for the treatment of chronic HIV infection is Cenicriviroc (TAK-652, 51). In addition to CCR5, Cenicriviroc also binds to CCR2 and may confer immunologic, cardiovascular, and metabolic benefits in HIV-infected individuals who experience low levels of immune activation during prolonged HAART treatment. MVC has been observed to provide metabolic benefits, but this effect is mediated solely through the CCR5 receptor, as no intrinsic CCR2 activity has been reported with this drug. The additional physiologic benefit these drugs may bring is unlike any other medication in HAART as they are the only type of therapy to interact with a mammalian target and as such could be a game changer elevating the status of these drugs. However, it is unknown if the dual CCR5/CCR2 activity of Cenicriviroc will be significantly different or superior to MVC at this time. Unfortunately, MVC and Cenicriviroc still require the TROFILE test to prequalify patients that harbor only R5 virus. The development of a dual CCR5/CXCR4 HIV entry agent or coadministration of MVC with another suitable CXCR4 antagonist will circumvent the need for the TROFILE test in the future and could elevate such drugs and combinations into a first-line treatment option in drug-naive patients. Dual chemokine-based agents (CCR5/CXCR4 and/ or CCR5/CCR2) are the entry inhibitors of tomorrow and have the potential to provide benefits in comparison to MVC such as curbing inflammation associated with chronic HIV infection in treatment-experienced patients with undetectable viral loads and eliminating the need for tropism testing. Furthermore, due to the chemokine ligand redundancies, it is likely that other CCR receptors could be added to this approach.
Table 1.
Summary of clinical studies on CCR5 antagonists.
| Drug name/ sponsor | Clinical stages and trials | Outcomes and significant findings |
|---|---|---|
| Maraviroc (MVC) Pfizer/ViiV Healthcare | Approved by the Food and Drug Administration in 2007 for treatment-experienced patients with only CCR5 using virus (MOTIVATE 1 and 2). Treatment-naive patients in comparison to tenofovir/FTC. MVC versus efavirenz in combination with zidovudine–lamivudine in treatment-naive patients (MERIT). PrEP therapy in MSM. Microbicide Trials Network: phase I, MVC, dapivirine, and dapivirine–MVC combination vaginal ring Phase I/II. Allogeneic bone marrow transplantation for leukemia. |
Lower-dose favored (150 mg) as hepatotoxicity occurs with the 300 mg dose. TROFILE tropism testing pre-requirement. MVC had inferior performance: smaller reduction in viral load. Faster recovery of CD4+ T cells. Reduced metabolic abnormalities and dyslipidemia. Some lack of efficacy in MVC arm. Recruiting. MVC ring did not meet drug concentration end points. Reduction in graft rejection in host-vs.-graft disease. |
| Vicriviroc (VVC) Schering | Phase II/III in treatment-experienced patients. Halted/abandoned (VICTOR E1, 3, and 4) | Liver malignancies. Viral rebound. Viral reduction end points not met in phase III trials. |
| Aplaviroc (APL) GlaxoSmithKline | Phase I/II in treatment-experienced patients. Halted/abandoned. | Severe hepatotoxicity. Poor/under effective efficacy. |
| INCB9471 Incyte | Phase I/II. Halted. | Discontinued for business reasons – portfolio switch. Seeking development partner to continue. |
| PF-232798 Pfizer/ViiV Healthcare | Phase I in healthy volunteers 250 mg well tolerated. Phase II discontinued. | Drug was discontinued due to the success of MVC. |
| TAK-220 Tobira/Takeda | Reported in phase I. No conformation or data available. | Likely drug was put on hold and development shifted to TAK-652. |
| Cenicriviroc (CVC, TAK-652) Tobira/Takeda | Phase I – completed. Phase IIB. Ongoing, includes with FTC/TDF with Sustiva/Truvada with dolutegravir, midazolam HIV-neurocognitive impairment Phase IIB – HIV-infected and HIV-non-infected patients: For nonalcoholic steatohepatitis in adults with liver fibrosis (CENTAUR) For primary sclerosing cholangitis (PERSEUS) |
Drug was well tolerated, and no patient withdrawals were noted. Terminated. Completed. Results not reported. Completed. Results not reported. Recruiting patients. Ongoing. Enrolled non-HIV- and HIV-infected patients. Recruiting patients. |
FTC: 5-fluoro-3′-thia-2′,3′-dideoxycytadine; MSM: men having sex with men; VICTOR: Vicriviroc in Combination Treatment With Optimized ART Regimen; TDF: Tenofovir Disoproxyl Fumurate; CENTAUR: Efficacy and Safety Study of Cenicriviroc for the Treatment of Nonalcoholic Steatohepatitis (NASH); PERSEUS: Preliminary Efficacy and Safety of Cenicriviroc in Adult Subjects With Primary Sclerosing Cholangitis.
In closing, the potential advantages of reduced resistance profile and synergism with other HIV agents have yet to be realized. Furthermore, the prophylactic potential of MVC and other CCR5 antagonists against HIV remains to be seen in human trials. Lastly, there are other factors that are significant for the future development of CCR5 antagonists but lie outside the context of this review. One is the development of the CCR5 antibody PRO140, which requires infrequent dosing (mono or biweekly subcutaneous injections) due to the long half-life (3.4 days) in humans [140]. The successful development of this protein for use in the HIV setting may stimulate further interest in approaches such as the antibody/macromolecule/MVC conjugates (48 and 49) presented in this review or other technology (e.g. extended-release formulations) that greatly increase drug half-lives. The other significant findings are the use of MVC in prevention of host-versus-graft disease in stem-cell transplant patients increasing the clinical use of this agent outside of HIV [141] and trials of Cenicriviroc in patients with liver disease (non-alcoholic steatohepatitis and primary sclerosing choangitis [PCS]) to reduce fibrosis [142]. The favorable outcome of these efforts may also resurrect the therapeutic and commercial interest in research and development activities to find newer and more efficacious CCR5 antagonists with expanded utility.
Article highlights.
A brief background is provided on CCR5 receptor biology and pharmacology, the dual-tropism behavior of HIV (R5/X4), and the significance of the δ-32 deletion leading into the introduction of CCR5 entry inhibitors.
A short review on the clinical outcomes of the first generation CCR5 antagonists is presented with a focus on the only FDA approved drug in this class - Maraviroc.
A thorough review of the drug discovery and research efforts on second-generation CCR5 antagonists is provided identifying various drug scaffolds, preclinical and clinical candidates including PF-232798 and INCB9471.
The discovery of dual-chemokine (CCR5 and CCR2 or CXCR4) antagonists is discussed culminating with the identification of the dual CCR5/CCR2 antagonist TAK-652/Cenicriviroc as the most advanced candidate currently in Phase II trials.
A final examination of the upside potential, challenges and future for CCR5 antagonist development for HIV is provided. This includes the expanded role of Maraviroc in curbing immunologic and metabolic disorders, the challenges with the tropism test, the emergence of dual chemokine receptor pharmacology, drug-bound receptor resistance development, and use in non-HIV applications.
This box summarizes key points contained in the article.
Acknowledgments
Funding
This paper was not funded.
Footnotes
Declaration of interest
K. Giesler has received funding support from The Emory University. M. Kim, L. Wilson, D. Liotta, Y. Tahirovic and V. Truax have received postdoctoral support from Bristol-Myers Squibb. D. Liotta has also received NIH funding. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.World Health Organization Statistics on HIV/AIDS. Global Health Observatory Data on HIV/AIDS - World Health Organization. [cited 2016 Mar 1]; Available from: http://www.who.int/gho/hiv/en/
- 2.Kaufmann GR, Bloch M, Zaunders JJ, et al. Long-term immunological response in HIV-1-infected subjects receiving potent antiretroviral therapy. Aids. 2000 May 26;14(8):959–969. doi: 10.1097/00002030-200005260-00007. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Mehellou Y, De Clercq E. Twenty-six years of anti-HIV drug discovery: where do we stand and where do we go? J Med Chem. 2010 Jan 28;53(2):521–538. doi: 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- 5.Flexner C. HIV drug development: the next 25 years. Nat Rev Drug Discov. 2007 Dec;6(12):959–966. doi: 10.1038/nrd2336. [DOI] [PubMed] [Google Scholar]
- 6.Henrich TJ, Kuritzkes DR. HIV-1 entry inhibitors: recent development and clinical use. Curr Opin Virol. 2013 Feb;3(1):51–57. doi: 10.1016/j.coviro.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanpain C, Migeotte I, Lee B, et al. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999 Sep 15;94(6):1899–1905. [PubMed] [Google Scholar]
- 8.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998 Apr 9;392(6676):565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 9.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001 Feb;2(2):123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 10.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 11.Arberas H, Guardo AC, Bargalló ME, et al. In vitro effects of the CCR5 inhibitor maraviroc on human T cell function. J Antimicrob Chemother. 2013 Mar;68(3):577–586. doi: 10.1093/jac/dks432. [DOI] [PubMed] [Google Scholar]
- 12.Rossi R, Lichtner M, De Rosa A, et al. In vitro effect of anti-human immunodeficiency virus CCR5 antagonist maraviroc on chemotactic activity of monocytes, macrophages and dendritic cells. Clin Exp Immunol. 2011 Nov;166(2):184–190. doi: 10.1111/j.1365-2249.2011.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012 Jul 12;367(2):135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni J, Zhu YN, Zhong XG, et al. The chemokine receptor antagonist, TAK-779, decreased experimental autoimmune encephalomyelitis by reducing inflammatory cell migration into the central nervous system, without affecting T cell function. Br J Pharmacol. 2009 Dec;158(8):2046–2056. doi: 10.1111/j.1476-5381.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirabelli-Badenier M, Braunersreuther V, Viviani GL, et al. CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost. 2011 Mar;105(3):409–420. doi: 10.1160/TH10-10-0662. [DOI] [PubMed] [Google Scholar]
- 16.Kohlmeier JE, Miller SC, Smith J, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008 Jul 18;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohlmeier JE, Reiley WW, Perona-Wright G, et al. Inflammatory chemokine receptors regulate CD8(+) T cell contraction and memory generation following infection. J Exp Med. 2011 Aug 1;208( 8):1621–1634. doi: 10.1084/jem.20102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellino F, Huang AY, Altan-Bonnet G, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006 Apr 13;440( 7086):890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 19.Buckheit RW, Siliciano RF, Blankson JN. Primary CD8(+) T cells from elite suppressors effectively eliminate non-productively HIV-1 infected resting and activated CD4(+) T cells. Retrovirology. 2013 Jul;1:10. doi: 10.1186/1742-4690-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benito JM, López M, Soriano V. The role of CD8+T-cell response in HIV infection. AIDS Rev. 2004 Apr-Jun;6(2):79–88. [PubMed] [Google Scholar]
- 21.Lim JK, Murphy PM. Chemokine control of West Nile virus infection. Exp Cell Res. 2011 Mar 10;317(5):569–574. doi: 10.1016/j.yexcr.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006 Jan 23;203(1):35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindberg E, Mickiene A, Ax C, et al. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tickborne encephalitis. J Infect Dis. 2008 Jan 15;197(2):266–269. doi: 10.1086/524709. [DOI] [PubMed] [Google Scholar]
- 24.Khan IA, Thomas SY, Moretto MM, et al. CCR5 is essential for NK cell trafficking and host survival following infection. PLoS Pathog. 2006;2(6):e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larena M, Regner M, Lobigs M. The chemokine receptor CCR5, a therapeutic target for HIV/AIDS antagonists, is critical for recovery in a mouse model of Japanese encephalitis. PLoS One. 2012 Sep 21;7(9):e44834. doi: 10.1371/journal.pone.0044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Blondel G, Brassat D, Bauer J, et al. CCR5 blockade for neuroinflammatory diseases – beyond control of HIV. Nat Rev Neurol. 2016 Feb;12(2):95–105. doi: 10.1038/nrneurol.2015.248. [DOI] [PubMed] [Google Scholar]
- 27.Choe H, Farzan M, Sun Y, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85(7):1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 28.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185(9):1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosier DE. How HIV changes its tropism: evolution and adaptation? Curr Opin HIV AIDS. 2009 Mar;4(2):125–130. doi: 10.1097/COH.0b013e3283223d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daar ES, Kesler KL, Petropoulos CJ, et al. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin Infect Dis. 2007 Sep 1;45(5):643–649. doi: 10.1086/520650. [DOI] [PubMed] [Google Scholar]
- 32.Martinson JJ, Chapman NH, Rees DC, et al. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet. 1997 May;16( 1):100–103. doi: 10.1038/ng0597-100. [DOI] [PubMed] [Google Scholar]
- 33.Novembre J, Galvani AP, Slatkin M. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol. 2005 Nov;3(11):e339. doi: 10.1371/journal.pbio.0030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996 Sep 27;273(5283):1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 35.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996 Aug 9;86(3):367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 36.Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011 Mar 10;117(10):2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 37.Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009 Feb 12;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 38.Kordelas L, Verheyen J, Esser S. Shift of HIV tropism in stem-cell transplantation with CCR5 delta32 mutation. N Engl J Med. 2014;371(9):880–882. doi: 10.1056/NEJMc1405805. [DOI] [PubMed] [Google Scholar]
- 39.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31(8):667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 40.Sabloff M, Sobecks RM, Ahn KW, et al. Does total body irradiation conditioning improve outcomes of myeloablative human leukocyte antigen-identical sibling transplantations for chronic lymphocytic leukemia? Biol Blood Marrow Transplant. 2014;20(3):421–424. doi: 10.1016/j.bbmt.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fätkenheuer G, Pozniak AL, Johnson MA, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005 Nov;11( 11):1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 42•.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005 Nov;49(11):4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. First disclosure of Maraviroc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008 Oct 2;359(14):1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Lelyveld SF, Wensing AM, Hoepelman AI. The MOTIVATE trials: maraviroc therapy in antiretroviral treatment-experienced HIV-1-infected patients. Expert Rev Anti Infect Ther. 2012 Nov;10( 11):1241–1247. doi: 10.1586/eri.12.114. [DOI] [PubMed] [Google Scholar]
- 45.Sierra-Madero J, Di Perri G, Wood R, et al. Efficacy and safety of maraviroc versus efavirenz, both with zidovudine/lamivudine: 96-week results from the MERIT study. HIV Clin Trials. 2010 Jun 01;11(3):125–132. doi: 10.1310/hct1103-125. [DOI] [PubMed] [Google Scholar]
- 46••.Lieberman-Blum SS, Fung HB, Bandres JC. Maraviroc: a CCR5-receptor antagonist for the treatment of HIV-1 infection. Clin Ther. 2008 Jul;30(7):1228–1250. doi: 10.1016/s0149-2918(08)80048-3. First disclosure of Maraviroc clinical trials. [DOI] [PubMed] [Google Scholar]
- 47.Low AJ, McGovern RA, Harrigan PR. Trofile HIV co-receptor usage assay. Expert Opin Med Diagn. 2009 Mar;3(2):181–191. doi: 10.1517/17530050802708981. [DOI] [PubMed] [Google Scholar]
- 48.Rossetti B, Bianco C, Bellazzi LI, et al. Virological and immunological response to antiretroviral regimens containing maraviroc in HIV type 1-infected patients in clinical practice: role of different tropism testing results and of concomitant treatments. AIDS Res Hum Retroviruses. 2014 Jan;30(1):17–24. doi: 10.1089/aid.2012.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon B, Grabmeier-Pfistershammer K, Rieger A, et al. HIV coreceptor tropism in antiretroviral treatment-naive patients newly diagnosed at a late stage of HIV infection. Aids. 2010 Aug 24;24( 13):2051–2058. doi: 10.1097/QAD.0b013e32833c93e6. [DOI] [PubMed] [Google Scholar]
- 50.Weiser B, Philpott S, Klimkait T, et al. HIV-1 coreceptor usage and CXCR4-specific viral load predict clinical disease progression during combination antiretroviral therapy. AIDS. 2008 Feb 19;22( 4):469–479. doi: 10.1097/QAD.0b013e3282f4196c. [DOI] [PubMed] [Google Scholar]
- 51•.Parra J, Portilla J, Pulido F. Clinical utility of maraviroc. Clin Drug Investig. 2011;31(8):527–542. doi: 10.2165/11590700-000000000-00000. Most recent review of clinical trials of Maraviroc. [DOI] [PubMed] [Google Scholar]
- 52.Puertas MC, Massanella M, Llibre JM, et al. Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. Aids. 2014 Jan 28;28(3):325–334. doi: 10.1097/QAD.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 53.Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008 Sep 1;49(Suppl 2):S79–85. doi: 10.1097/QAI.0b013e318186519c. [DOI] [PubMed] [Google Scholar]
- 54•.MacInnes A, Lazzarin A, Di Perri G, et al. Maraviroc can improve lipid profiles in dyslipidemic patients with HIV: results from the MERIT trial. HIV Clin Trials. 2011 Jan-Feb;12(1):24–36. doi: 10.1310/hct1201-24. Trials showing that Maraviroc can improve the dyslipidemia caused by HIV and HAART. [DOI] [PubMed] [Google Scholar]
- 55.Massud I, Aung W, Martin A, et al. Lack of prophylactic efficacy of oral Maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol. 2013 Aug 15;87(16):8952–8961. doi: 10.1128/JVI.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.HTPN 069. A Phase II randomized, double-blind, study of the safety and tolerability of maraviroc (MVC), maraviroc + emtricitabine (MVC+FTC), maraviroc + tenofovir disoproxil fumarate (MVC+TDF), or Tenofovir disoproxil fumarate + Emtricitabine (TDF+FTC) for Pre-Exposure Prophylaxis (PrEP) to Prevent HIV Transmission in At-Risk Men Who Have Sex with Men and in At-Risk Women. HIV Prevention Trials Network. 2014 [cited 2016 Mar 25]; Available from: http://www.hptn.org/research_studies/hptn069.aspAnnouncement of PrEP study with Maraviroc and other HIV medications.
- 57.McCombie SW, Tagat JR, Vice SF, et al. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. III: synthesis, antiviral and pharmacokinetic profiles of symmetrical heteroaryl carboxamides. Bioorg Med Chem Lett. 2003 Feb 10;13(3):567–571. doi: 10.1016/s0960-894x(02)00918-6. [DOI] [PubMed] [Google Scholar]
- 58••.Tagat JR, McCombie SW, Nazareno D, et al. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]-4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]-4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagonist. J Med Chem. 2004 May 6;47(10):2405–2408. doi: 10.1021/jm0304515. First disclosure of Vicriviroc. [DOI] [PubMed] [Google Scholar]
- 59•.Alcorn K. Second company halts CCR5 inhibitor study in another blow to new drug class. 2005 [cited 2016 Mar 25]; Available from: http://www.aidsmap.com/Second-company-halts-CCR5-inhibitor-study-in-another-blow-to-new-drug-class/page/1422234/Announcement of the termination of Vicriviroc clinical trials.
- 60.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007 Jul 15;196(2):304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 61••.Adkison KK, Shachoy-Clark A, Fang L, et al. Pharmacokinetics and short-term safety of 873140, a novel CCR5 antagonist, in healthy adult subjects. Antimicrob Agents Chemother. 2005 Jul;49( 7):2802–2806. doi: 10.1128/AAC.49.7.2802-2806.2005. Disclosure of AVC in phase I clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62••.Leeson PD, Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov. 2007 Nov;6(11):881–890. doi: 10.1038/nrd2445. Reference comparing drug-like features of CCR5 antagonists under development. [DOI] [PubMed] [Google Scholar]
- 63•.Stupple PA, Batchelor DV, Corless M, et al. An imidazopiperidine series of CCR5 antagonists for the treatment of HIV: the discovery of N-{(1S)-1-(3-fluorophenyl)-3-[(3-endo)-3-(5-isobutyryl-2-methyl-4,5,6,7-tetrahydr o-1H-imidazo[4,5-c]pyridin-1-yl)-8-azabicyclo [3.2.1]oct-8-yl]propyl}acetamide (PF-232798) J Med Chem. 2011 Jan 13;54(1):67–77. doi: 10.1021/jm100978n. Discovery of PF-232798. [DOI] [PubMed] [Google Scholar]
- 64.Anti-HIV agents. Is PF-232798 a possible successor to maraviroc? TreatmentUpdate. 2008 Mar;20(2):8. [PubMed] [Google Scholar]
- 65.Kazmierski WM, Aquino C, Chauder BA, et al. Discovery of bioavailable 4,4-disubstituted piperidines as potent ligands of the chemokine receptor 5 and inhibitors of the human immunodeficiency virus-1. J Med Chem. 2008 Oct 23;51(20):6538–6546. doi: 10.1021/jm800598a. [DOI] [PubMed] [Google Scholar]
- 66.Kazmierski WM, Anderson DL, Aquino C, et al. Novel 4,4-disubstituted piperidine-based C–C chemokine receptor-5 inhibitors with high potency against human immunodeficiency virus-1 and an improved human ether-a-go-go related gene (hERG) profile. J Med Chem. 2011 Jun 09;54(11):3756–3767. doi: 10.1021/jm200279v. [DOI] [PubMed] [Google Scholar]
- 67.Duan M, Kazmierski WM, Chong PY, et al. Discovery of novel pyridyl carboxamides as potent CCR5 antagonists and optimization of their pharmacokinetic profile in rats. Bioorg Med Chem Lett. 2011 Nov 1;21(21):6470–6475. doi: 10.1016/j.bmcl.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 68.Duan M, Aquino C, Ferris R, et al. [2-(4-Phenyl-4-piperidinyl)ethyl] amine based CCR5 antagonists: derivatizations at the N-terminal of the piperidine ring. Bioorg Med Chem Lett. 2009 Mar 15;19( 6):1610–1613. doi: 10.1016/j.bmcl.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Duan M, Aquino C, Dorsey GF, Jr, et al. 4,4-Disubstituted cyclohexylamine based CCR5 chemokine receptor antagonists as anti-HIV-1 agents. Bioorg Med Chem Lett. 2009 Sep 1;19(17):4988–4992. doi: 10.1016/j.bmcl.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 70.Tallant MD, Duan M, Freeman GA, et al. Synthesis and evaluation of 2-phenyl-1,4-butanediamine-based CCR5 antagonists for the treatment of HIV-1. Bioorg Med Chem Lett. 2011 Mar 1;21( 5):1394–1398. doi: 10.1016/j.bmcl.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 71.Zhang HS, Feng DZ, Chen L, et al. Discovery of novel (S)-alpha-phenyl-gamma-amino butanamide containing CCR5 antagonists via functionality inversion approach. Bioorg Med Chem Lett. 2010 Apr 1;20(7):2219–2223. doi: 10.1016/j.bmcl.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 72.Fan X, Zhang H-S, Chen L, et al. Efficient synthesis and identification of novel propane-1,3-diamino bridged CCR5 antagonists with variation on the basic center carrier. Eur J Med Chem. 2010;45(7):2827–2840. doi: 10.1016/j.ejmech.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 73•.Imamura S, Ichikawa T, Nishikawa Y, et al. Discovery of a piperidine-4-carboxamide CCR5 antagonist (TAK-220) with highly potent anti-HIV-1 activity. J Med Chem. 2006 May 4;49( 9):2784–2793. doi: 10.1021/jm051034q. Disclosure of TAK-220. [DOI] [PubMed] [Google Scholar]
- 74.Drugs in clinical development for HIV: summary and table. Springer International Publishing. Pharm Med. 2015;29:105. [Google Scholar]
- 75•.Ma D, Yu S, Li B, et al. Synthesis and biological evaluation of 1,3,3,4-tetrasubstituted pyrrolidine CCR5 receptor antagonists. Discovery of a potent and orally bioavailable anti-HIV agent. ChemMedChem. 2007 Feb;2(2):187–193. doi: 10.1002/cmdc.200600182. Disclosure of nifreviroc. [DOI] [PubMed] [Google Scholar]
- 76.Li B, Jones ED, Zhou E, et al. Studies on the structure-activity relationship of 1,3,3,4-tetra-substituted pyrrolidine embodied CCR5 receptor antagonists. Part 2: discovery of highly potent anti-HIV agents. Bioorg Med Chem Lett. 2010 Sep 1;20( 17):5334–5336. doi: 10.1016/j.bmcl.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 77.Pryde DC, Corless M, Fenwick DR, et al. The design and discovery of novel amide CCR5 antagonists. Bioorg Med Chem Lett. 2009 Feb 15;19(4):1084–1088. doi: 10.1016/j.bmcl.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 78.Barber CG, Blakemore DC, Chiva JY, et al. 1-Amido-1-phenyl-3-piperidinylbutanes – CCR5 antagonists for the treatment of HIV. Part 1. Bioorg Med Chem Lett. 2009 Feb 15;19( 4):1075–1079. doi: 10.1016/j.bmcl.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Barber CG, Blakemore DC, Chiva JY, et al. 1-Amido-1-phenyl-3-piperidinylbutanes – CCR5 antagonists for the treatment of HIV: part 2. Bioorg Med Chem Lett. 2009 Mar 1;19(5):1499–1503. doi: 10.1016/j.bmcl.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Skerlj R, Bridger G, Zhou Y, et al. Design and synthesis of pyridin-2-yloxymethylpiperidin-1-ylbutyl amide CCR5 antagonists that are potent inhibitors of M-tropic (R5) HIV-1 replication. Bioorg Med Chem Lett. 2011 Apr 15;21(8):2450–2455. doi: 10.1016/j.bmcl.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 81.Skerlj R, Bridger G, Zhou Y, et al. Design and synthesis of pyridin-2-ylmethylaminopiperidin-1-ylbutyl amide CCR5 antagonists that are potent inhibitors of M-tropic (R5) HIV-1 replication. Bioorg Med Chem Lett. 2011 Dec 1;21(23):6950–6954. doi: 10.1016/j.bmcl.2011.09.133. [DOI] [PubMed] [Google Scholar]
- 82.Liu T, Weng Z, Dong X, et al. Design, synthesis and biological evaluation of novel piperazine derivatives as CCR5 antagonists. PLoS One. 2013;8(1):e53636. doi: 10.1371/journal.pone.0053636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu S, Gu Q, Wang Z, et al. Design, synthesis, and biological evaluation of novel piperidine-4-carboxamide derivatives as potent CCR5 inhibitors. Eur J Med Chem. 2014;71:259–266. doi: 10.1016/j.ejmech.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 84.Thoma G, Nuninger F, Schaefer M, et al. Orally bioavailable competitive CCR5 antagonists. J Med Chem. 2004 Apr 8;47( 8):1939–1955. doi: 10.1021/jm031046g. [DOI] [PubMed] [Google Scholar]
- 85.Thoma G, Beerli C, Bigaud M, et al. Reduced cardiac side-effect potential by introduction of polar groups: discovery of NIBR-1282, an orally bioavailable CCR5 antagonist which is active in vivo. Bioorg Med Chem Lett. 2008 Mar 15;18(6):2000–2005. doi: 10.1016/j.bmcl.2008.01.108. [DOI] [PubMed] [Google Scholar]
- 86.Yang H, Lin XF, Padilla F, et al. Discovery of a potent, selective and orally bioavailable 3,9-diazaspiro[5.5]undeca-2-one CCR5 antagonist. Bioorg Med Chem Lett. 2009 Jan 1;19(1):209–213. doi: 10.1016/j.bmcl.2008.10.115. [DOI] [PubMed] [Google Scholar]
- 87.Gabriel S, Rotstein D, inventors. Heterocyclic antiviral compounds patent. 20050176703 A1. US. 2005
- 88.Ernst J, Dahl R, Lum C, et al. Anti-HIV-1 entry optimization of novel imidazopiperidine-tropane CCR5 antagonists. Bioorg Med Chem Lett. 2008 Feb 15;18(4):1498–1501. doi: 10.1016/j.bmcl.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 89.Wei RG, Arnaiz DO, Chou YL, et al. CCR5 receptor antagonists: discovery and SAR study of guanylhydrazone derivatives. Bioorg Med Chem Lett. 2007 Jan 1;17(1):231–234. doi: 10.1016/j.bmcl.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 90.Lu SF, Chen B, Davey D, et al. CCR5 receptor antagonists: discovery and SAR of novel 4-hydroxypiperidine derivatives. Bioorg Med Chem Lett. 2007 Apr 1;17(7):1883–1887. doi: 10.1016/j.bmcl.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 91.Duan M, Peckham J, Edelstein M, et al. Discovery of N-benzyl-N ′-(4-pipyridinyl)urea CCR5 antagonists as anti-HIV-1 agents (I): optimization of the amine portion. Bioorg Med Chem Lett. 2010 Dec 15;20(24):7397–7400. doi: 10.1016/j.bmcl.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 92.Duan M, Peckham J, Edelstein M, et al. Discovery of N-benzyl-N ′-(4-pipyridinyl)urea CCR5 antagonists as anti-HIV-1 agents (II): modification of the acyl portion. Bioorg Med Chem Lett. 2010 Dec 15;20(24):7401–7404. doi: 10.1016/j.bmcl.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 93.Duan M, Kazmierski WM, Tallant M, et al. Discovery of a novel series of cyclic urea as potent CCR5 antagonists. Bioorg Med Chem Lett. 2011 Nov 1;21(21):6381–6385. doi: 10.1016/j.bmcl.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 94.Metz M, Bourque E, Labrecque J, et al. Prospective CCR5 small molecule antagonist compound design using a combined mutagenesis/modeling approach. J Am Chem Soc. 2011 Oct 19;133( 41):16477–16485. doi: 10.1021/ja2043722. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y, Zhou E, Yu K, et al. Discovery of a novel CCR5 antagonist lead compound through fragment assembly. Molecules. 2008;13( 10):2426–2441. doi: 10.3390/molecules13102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee EK, Melville CR, Rotstein DM, inventors. Octahydro-pyrrolo [3,4-c] derivatives and their use as antiviral compounds patent. 2005121145 A3. WO. 2006
- 97.Dong MX, Lu L, Li H, et al. Design, synthesis, and biological activity of novel 1,4-disubstituted piperidine/piperazine derivatives as CCR5 antagonist-based HIV-1 entry inhibitors. Bioorg Med Chem Lett. 2012 May 1;22(9):3284–3286. doi: 10.1016/j.bmcl.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Hu S, Wang Z, Hou T, et al. Design, synthesis, and biological evaluation of novel 2-methylpiperazine derivatives as potent CCR5 antagonists. Bioorg Med Chem. 2015 Mar 1;23(5):1157–1168. doi: 10.1016/j.bmc.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 99•.Xue CB, Chen L, Cao G, et al. Discovery of INCB9471, a potent, selective, and orally bioavailable CCR5 antagonist with potent anti-HIV-1 activity. ACS Med Chem Lett. 2010;1:483–487. doi: 10.1021/ml1001536. Disclosure of INCB9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rotstein DM, Melville CR, Padilla F, et al. Novel hexahydropyrrolo [3,4-c]pyrrole CCR5 antagonists. Bioorg Med Chem Lett. 2010 May 15;20(10):3116–3119. doi: 10.1016/j.bmcl.2010.03.095. [DOI] [PubMed] [Google Scholar]
- 101.Lemoine RC, Petersen AC, Setti L, et al. Exploration of a new series of CCR5 antagonists: multi-dimensional optimization of a sub-series containing N-substituted pyrazoles. Bioorg Med Chem Lett. 2010 Aug 15;20(16):4753–4756. doi: 10.1016/j.bmcl.2010.06.135. [DOI] [PubMed] [Google Scholar]
- 102.Lemoine RC, Petersen AC, Setti L, et al. Evaluation of secondary amide replacements in a series of CCR5 antagonists as a means to increase intrinsic membrane permeability. Part 1: optimization of gem-disubstituted azacycles. Bioorg Med Chem Lett. 2010 Jan 15;20( 2):704–708. doi: 10.1016/j.bmcl.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 103.Wanner J, Chen L, Lemoine RC, et al. Evaluation of amide replacements in CCR5 antagonists as a means to increase intrinsic permeability. Part 2: SAR optimization and pharmacokinetic profile of a homologous azacyle series. Bioorg Med Chem Lett. 2010 Nov 15;20( 22):6802–6807. doi: 10.1016/j.bmcl.2010.08.118. [DOI] [PubMed] [Google Scholar]
- 104.Lemoine RC, Petersen AC, Setti L, et al. Evaluation of a 3-amino-8-azabicyclo[3.2.1]octane replacement in the CCR5 antagonist maraviroc. Bioorg Med Chem Lett. 2010 Mar 1;20(5):1674–1676. doi: 10.1016/j.bmcl.2010.01.080. [DOI] [PubMed] [Google Scholar]
- 105.Lemoine RC, Petersen AC, Setti L, et al. Evaluation of a 4-aminopiperidine replacement in several series of CCR5 antagonists. Bioorg Med Chem Lett. 2010 Mar 15;20(6):1830–1833. doi: 10.1016/j.bmcl.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Yang SW, Mierzwa R, Terracciano J, et al. Chemokine receptor CCR-5 inhibitors produced by Chaetomium globosum. J Nat Prod. 2006 Jul;69(7):1025–1028. doi: 10.1021/np060121y. [DOI] [PubMed] [Google Scholar]
- 107.Yang SW, Mierzwa R, Terracciano J, et al. Sch 213766, a novel chemokine receptor CCR-5 inhibitor from Chaetomium globosum. J Antibiot (Tokyo) 2007 Aug;60(8):524–528. doi: 10.1038/ja.2007.67. [DOI] [PubMed] [Google Scholar]
- 108.Asano S, Gavrilyuk J, Burton DR, et al. Preparation and activities of macromolecule conjugates of the CCR5 antagonist maraviroc. ACS Med Chem Lett. 2014 Feb 13;5(2):133–137. [Google Scholar]
- 109.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 110.Baba M, Nishimura O, Kanzaki N, et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A. 1999 May 11;96(10):5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shiraishi M, Aramaki Y, Seto M, et al. Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J Med Chem. 2000 May 18;43(10):2049–2063. doi: 10.1021/jm9906264. [DOI] [PubMed] [Google Scholar]
- 112••.Seto M, Aikawa K, Miyamoto N, et al. Highly potent and orally active CCR5 antagonists as anti-HIV-1 agents: synthesis and biological activities of 1-benzazocine derivatives containing a sulfoxide moiety. J Med Chem. 2006 Mar 23;49(6):2037–2048. doi: 10.1021/jm0509703. Disclosure of TAK-652. [DOI] [PubMed] [Google Scholar]
- 113.Hersperger RJP, Pfenninger E, Wuethrich HJ, et al. Inventor chemokine receptor antagonists patent. 20070155721A1. US. 2007
- 114.Pease J, Horuk R. Chemokine receptor antagonists. J Med Chem. 2012 Nov 26;55(22):9363–9392. doi: 10.1021/jm300682j. [DOI] [PubMed] [Google Scholar]
- 115.Miltz W. A new approach for the treatment of autoimmune diseases. 235th National Meeting of the American Chemical Society; New Orleans (LA). 2008. cited Apr 6–10. [Google Scholar]
- 116.Cole PVS, Vasiliou S. Highlights from the 50th interscience conference on anti-microbial agents and chemotherapy (ICAAC) Drugs Future. 2010;35:1045–1067. [Google Scholar]
- 117.Forbes IT, Cooper DG, Dodds EK, et al. CCR2B receptor antagonists: conversion of a weak HTS hit to a potent lead compound. Bioorg Med Chem Lett. 2000 Aug 21;10(16):1803–1806. doi: 10.1016/s0960-894x(00)00347-4. [DOI] [PubMed] [Google Scholar]
- 118.Pasternak A, Marino D, Vicario PP, et al. Novel, orally bioavailable gamma-aminoamide CC chemokine receptor 2 (CCR2) antagonists. J Med Chem. 2006 Aug 10;49(16):4801–4804. doi: 10.1021/jm060439n. [DOI] [PubMed] [Google Scholar]
- 119.Zheng C, Cao G, Xia M, et al. Discovery of INCB10820/PF-4178903, a potent, selective, and orally bioavailable dual CCR2 and CCR5 antagonist. Bioorg Med Chem Lett. 2011 Mar 1;21(5):1442–1446. doi: 10.1016/j.bmcl.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 120.Gouwy M, Struyf S, Berghmans N, et al. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur J Immunol. 2011 Apr;41( 4):963–973. doi: 10.1002/eji.201041178. [DOI] [PubMed] [Google Scholar]
- 121•.Princen K, Hatse S, Vermeire K, et al. Inhibition of human immunodeficiency virus replication by a dual CCR5/CXCR4 antagonist. J Virol. 2004 Dec;78(23):12996–13006. doi: 10.1128/JVI.78.23.12996-13006.2004. Discovery of the first dual CCR5/CXCR4 antagonist AMD3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cox BD, Prosser AR, Sun Y, et al. Pyrazolo-piperidines exhibit dual inhibition of CCR5/CXCR4 HIV entry and reverse transcriptase. ACS Med Chem Lett. 2015 Jul 9;6(7):753–757. doi: 10.1021/acsmedchemlett.5b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pugach P, Marozsan AJ, Ketas TJ, et al. HIV-1 clones resistant to a small molecule CCR5 inhibitor use the inhibitor-bound form of CCR5 for entry. Virology. 2007 Apr 25;361(1):212–228. doi: 10.1016/j.virol.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ogert RA, Hou Y, Ba L, et al. Clinical resistance to vicriviroc through adaptive V3 loop mutations in HIV-1 subtype D gp120 that alter interactions with the N-terminus and ECL2 of CCR5. Virology. 2010 Apr 25;400(1):145–155. doi: 10.1016/j.virol.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 125.Tsibris AM, Sagar M, Gulick RM, et al. In vivo emergence of vicriviroc resistance in a human immunodeficiency virus type 1 subtype C-infected subject. J Virol. 2008 Aug;82(16):8210–8214. doi: 10.1128/JVI.00444-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ogert RA, Wojcik L, Buontempo C, et al. Mapping resistance to the CCR5 co-receptor antagonist vicriviroc using heterologous chimeric HIV-1 envelope genes reveals key determinants in the C2-V5 domain of gp120. Virology. 2008 Apr 10;373(2):387–399. doi: 10.1016/j.virol.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 127•.Westby M, Smith-Burchnell C, Mori J, et al. Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J Virol. 2007 Mar;81(5):2359–23 71. doi: 10.1128/JVI.02006-06. Study showing unique mechanism of resistance development of HIV to Maraviroc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roche M, Jakobsen MR, Ellett A, et al. HIV-1 predisposed to acquiring resistance to maraviroc (MVC) and other CCR5 antagonists in vitro has an inherent, low-level ability to utilize MVC-bound CCR5 for entry. Retrovirology. 2011;8:89. doi: 10.1186/1742-4690-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tilton JC, Amrine-Madsen H, Miamidian JL, et al. HIV type 1 from a patient with baseline resistance to CCR5 antagonists uses drug-bound receptor for entry. AIDS Res Hum Retroviruses. 2010 Jan;26(1):13–24. doi: 10.1089/aid.2009.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trkola A, Kuhmann SE, Strizki JM, et al. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):395–400. doi: 10.1073/pnas.012519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tilton JC, Wilen CB, Didigu CA, et al. A maraviroc-resistant HIV-1 with narrow cross-resistance to other CCR5 antagonists depends on both N-terminal and extracellular loop domains of drug-bound CCR5. J Virol. 2010 Oct;84(20):10863–10876. doi: 10.1128/JVI.01109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pfaff JM, Wilen CB, Harrison JE, et al. HIV-1 resistance to CCR5 antagonists associated with highly efficient use of CCR5 and altered tropism on primary CD4+ T cells. J Virol. 2010 Jul;84( 13):6505–6514. doi: 10.1128/JVI.00374-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuhmann SE, Pugach P, Kunstman KJ, et al. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J Virol. 2004;78(6):2790–2807. doi: 10.1128/JVI.78.6.2790-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ratcliff AN, Shi W, Arts EJ. HIV-1 resistance to maraviroc conferred by a CD4 binding site mutation in the envelope glycoprotein gp120. J Virol. 2013 Jan;87(2):923–934. doi: 10.1128/JVI.01863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anastassopoulou CG, Ketas TJ, Klasse PJ, et al. Resistance to CCR5 inhibitors caused by sequence changes in the fusion peptide of HIV-1 gp41. Proc Natl Acad Sci USA. 2009 Mar 31;106( 13):5318–5323. doi: 10.1073/pnas.0811713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anastassopoulou CG, Ketas TJ, Sanders RW, et al. Effects of sequence changes in the HIV-1 gp41 fusion peptide on CCR5 inhibitor resistance. Virology. 2012 Jul 5;428(2):86–97. doi: 10.1016/j.virol.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roche M, Salimi H, Duncan R, et al. A common mechanism of clinical HIV-1 resistance to the CCR5 antagonist maraviroc despite divergent resistance levels and lack of common gp120 resistance mutations. Retrovirology. 2013;10:43. doi: 10.1186/1742-4690-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138•.Baba M, Miyake H, Wang X, et al. Isolation and characterization of human immunodeficiency virus type 1 resistant to the small-molecule CCR5 antagonist TAK-652. Antimicrob Agents Chemother. 2007 Feb;51(2):707–715. doi: 10.1128/AAC.01079-06. Study showing mechanism of HIV resistance to TAK-652 is similar to Maraviroc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kang Y, Wu Z, Lau TCK. CCR5 antagonist TD-0680 uses a novel mechanism for enhanced potency against HIV-1 entry, cell-mediated infection, and a resistant variant. J Biol Chem. 2012;287:16499–16509. doi: 10.1074/jbc.M112.354084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140•.Biswas P, Tambussi G, Lazzarin A. Access denied? The status of co-receptor inhibition to counter HIV entry. Expert Opin Pharmacother. 2007 May;8(7):923–933. doi: 10.1517/14656566.8.7.923. Review of the clinical use of the CCR5 antibody PRO140 for HIV treatment. [DOI] [PubMed] [Google Scholar]
- 141•.Reshef R, Mangan JK, Luger SM, et al. Extended CCR5 blockade in graft-versus-host disease prophylaxis – a phase II study. Blood. 2014 Dec 06;124(21):2491–91. Secondary use of Maraviroc beyond HIV treatment. [Google Scholar]
- 142.CENTAUR: efficacy and safety study of cenicriviroc for the treatment of NASH in adult subjects with liver fibrosis – trial NCT02217475; and PERSEUS: preliminary efficacy and safety of cenicriviroc in adult subjects with primary sclerosing cholangitis – trial NCT02653625. [cited 8 Jan 2016]. Available from: www.clinicaltrials.gov.