Abstract
Nitric Oxide (NO), a potent vasodilator and vital signaling molecule, has been shown to contribute to the regulation of glomerular ultrafiltration. However, whether changes in NO occur in podocytes during the pathogenesis of salt-sensitive hypertension has not yet been thoroughly examined. We showed here that podocytes produce NO, and further hypothesized that hypertensive animals would exhibit reduced NO production in these cells in response to various paracrine factors, which might contribute to the damage of glomeruli filtration barrier and development of proteinuria. To test this, we isolated glomeruli from the kidneys of Dahl salt-sensitive (SS) rats fed a low salt (LS; 0.4% NaCl) or high salt (HS; 4% NaCl, 3 weeks) diets and loaded podocytes with either a combination of NO and Ca2+ fluorophores (DAF-FM and Fura Red, respectively) or DAF-FM alone. Changes in fluorescence were observed with confocal microscopy in response to adenosine triphosphate (ATP), angiotensin II (Ang II), and hydrogen peroxide (H2O2). Application of Ang II resulted in activation of both NO and intracellular calcium ([Ca2+]i) transients. In contrast, ATP promoted [Ca2+]i transients, but did not have any effects on NO production. SS rats fed a HS diet for 3 weeks demonstrated impaired NO production: the response to Ang II or H2O2 in podocytes of glomeruli isolated from SS rats fed a HS diet was significantly reduced compared to rats fed a LS diet. Therefore, glomerular podocytes from hypertensive rats showed a diminished NO release in response to Ang II or oxidative stress, suggesting that podocytic NO signaling is dysfunctional in this condition and likely contributes to the development of kidney injury.
Keywords: DAF FM, nitric oxide, hypertension, angiotensin II, hydrogen peroxide, Dahl salt-sensitive rat
Graphical Abstarct
1. Introduction
Nitric Oxide (NO) is a major signaling molecule in the kidney, where it plays a vital role in the regulation of glomerular filtration. It was reported that deficiency of eNOS (endothelial Nitric Oxide Synthase) results in elevated blood pressure [1], exacerbates renal injury, and accelerates development of proteinuria and glomerulosclerosis [2; 3; 4]. In a mouse model of diabetic nephropathy (DN), absence of eNOS was shown to be critical in the development of kidney injury, and specifically glomerular damage. eNOS−/− mice that were backcrossed to db/db mice exhibited pronounced albuminuria, increased glomerular basement membrane thickness, mesangial expansion, mesangiolysis, and focal segmental and early nodular glomerulosclerosis [5]. From these studies, it seems likely that significant changes in glomerular NO production, which can be triggered by inflammation, oxidative stress or other factors, can lead to glomerular epithelial cell (podocyte) damage and subsequent proteinuria in hypertensive conditions.
The role of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide, and NO in the pathophysiology of renal disease, specifically glomerular dysfunction, has been extensively studied in the past [6; 7; 8; 9]. Synthesis of ROS, including NO, in glomeruli can significantly change in various forms of glomerular injury [7; 10; 11; 12]. Oxidative stress due to increased production of ROS in the glomeruli of diabetic and hypertensive rats often correlates directly with increased albuminuria in early stages of these diseases [8; 13]. A recent study by Dolinina et at [14] suggested that the permeability of the glomerular filtration barrier is regulated by a balance between the bioavailability of NO and that of ROS; the authors elegantly showed that in healthy Wistar rats in vivo NOS inhibition causes a rapid increase in glomerular vascular permeability, and this phenomenon is dependent upon ROS.
In the kidney, NO can be released by the glomerular and tubular cells in response to a variety of physiological stimuli. It was shown that tubuloglomerular feedback (TGF) mechanisms, triggered by increased tubular perfusion, results in NO production by the macula densa cells [15]. Shear stress [16], endothelin-1 [17; 18], and insulin [19] can stimulate NO generation in the collecting duct. Moreover, increased Na+ concentration or luminal flow [20; 21] and angiotensin II (Ang II) [22] can increase NO production in the thick ascending limb. Ang II also stimulates release of NO in afferent arterioles [23]. Multiple studies have shown that NO level in the renal microvasculature affects glomerular permeability: for instance, there is greater abundance of NOS at the efferent versus afferent arterioles [24]; thus, reduced NO at the efferent endothelium may predispose to higher levels of glomerular capillary pressure, podocyte injury and glomerulosclerosis. Furthermore, a more recent study featuring microCT-based analyses of vasculature in the renal cortex revealed a loss of perfusable arterioles and glomeruli in eNOS−/− mice [25]. Altogether, the release of NO from the endothelium plays a crucial role in the maintenance of normal barrier permeability, and these effects should always be taken into considerations when studying the glomerulus as a whole [26].
Extracellular nucleotides stimulate an increase in glomerular albumin permeability due, in part, to glomerular NO production and actin reorganization in podocytes [27]. It was also shown that adenosine triphosphate (ATP) stimulates NO production in a number of cell types including endothelial cells [28], which play a crucial role in the TGF mechanism [29]. Furthermore, we and others previously reported that purinergic signaling is directly involved in the regulation of intracellular calcium ([Ca2+]i) in podocytes [30; 31] and glomerular albumin permeability [27], which can ultimately have effects on eNOS activation. Collectively, these studies suggest a complex regulatory network of NO and glomerular function.
Interdependent regulation of NO and calcium signaling is an important mechanism, which plays a critical role in many cell types. The majority of data for NO-Ca2+ interaction in the renal tissue is reported in the vasculature. Thus, recent reports showed that in skeletal muscle arterioles chronic NO deficit induced by L-NAME treatment results in the upregulation of T-type calcium channels [32; 33]. In vascular endothelial cells regulation of eNOS was reported to be calcium-dependent [34]. Additionally, stimulation of calcium-sensing receptors in vascular smooth muscle cells was shown to induce endothelium-dependent vasorelaxation, in part via a pathway involving production of NO, which resulted in activation of BK (large Ca2+-activated K+) channels [35]. Calcium-NO interaction in other cell types rather than vascular is less studied.
Yuen et al. performed an elegant study where they addressed the role of eNOS in podocytes of a diabetic model. Importantly, the authors noted that the development of acute hyperglycemia in eNOS−/− mice leads to podocyte injury, which could be prevented by inhibiting the Renin-Angiotensin-Aldosterone System (RAAS) with captopril or losartan [36]. These findings suggest that NO-mediated podocytopathy may be caused by paracrine (and possibly autocrine) mechanisms, and that RAAS plays an important role in this pathology. Considering the contribution of NO signaling in blood pressure control [1], identification of the factors causing podocyte dysfunction can reveal novel insight into the pathogenesis of hypertension. We propose that activation of specific signaling pathways result in changes in [Ca2+]i as well as generation of NO by podocytes. We further hypothesized that various paracrine factors compromised during salt-sensitive hypertension can trigger reduced NO production in podocytes and potentially contribute to damage of these cells.
2. Materials and Methods
2.1. Animals
Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the IACUC of the Medical College of Wisconsin. Either 8 (for the protocol with diet change) or 12 weeks old male Dahl salt-sensitive (SS) rats (SS/JrHsdMcwi) were used for experiments. Water was provided ad libitum and the salt content of each diet was 0.4% NaCl AIN-76 diet (#113755; Dyets, Bethlehem, PA) from weaning up to 8 weeks of age. At 8 weeks of age, the salt content of the chow was either maintained at 0.4% NaCl in the group fed a normal diet or increased to 4.0% NaCl AIN-76 diet (#113756; Dyets) and the rats were maintained on these diets for additional 3 weeks as previously described [37].
2.2. Isolation of the rat glomeruli and intracellular calcium and NO imaging
Experimental procedures were performed as described in our earlier publications [38; 39; 40]. Briefly, kidneys of 12 weeks old SS rats were cleared from the blood, excised and decapsulated; the cortex was isolated and minced using a singled edge razor blade. The minced tissue was sequentially pushed through a steel 100 mesh sieve and then pipetted through a 140 mesh sieve (04-881-5Z and 04-881-5X; Fisher Sci) using the culture medium solution RPMI1640 (Invitrogen, Inc) with 5% BSA. This tissue homogenate was then pipetted onto a 200 mesh sieve (S4145; Sigma) leaving the glomeruli on the top surface. The glomeruli were rinsed using the RPMI-BSA solution into a 15 ml conical tube and settled on ice. After sedimentation the excess of RPMI storage solution was removed and the isolated decapsulated glomeruli were used for confocal microscopy experiments. Isolated glomeruli were allowed to adhere onto 5 × 5 mm coverglass coated with poly-L-lysine (P4707; Sigma). Glomeruli were subjected to confocal measurements immediately after preparations in solution containing (in mM): 145 NaCl, 4.5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, pH 7.35). Fluorescence intensities were monitored by confocal laser scanning microscope system Nikon A1-R, detected using an oil immersed Plan Apo 60x/NA 1.4 Oil objective (Nikon) with the argon laser synchronous excitation at 488 nm and selective transmission of light trough the emission filters 520/25 and 680/25 for 4-amino-5 methylamino-2′,7′-difluorescein (DAF-FM, 5 μM; Cat. #D23844, Invitrogen) and Fura Red (2.5 μM; Cat. #F3021, Invitrogen), respectively. Fluorescence images were collected with 0.25 Hz frequency and processed with open source software Fiji (ImageJ 1.47v, National Institute of Health, USA).
2.3. Glomerular volume dynamics assay
For the measurements of the glomerular volume changes Dahl SS rats kidneys were harvested as described earlier, then glomeruli were isolated by differential sieving and stored on ice in a 5% BSA/RPMI solution with glomeruli non-permeable 150 kDa TRITC labeled dextran (TdB Consultancy AB, Uppsala, Sweden) as recently described [41; 42]. Then, glomeruli were attached to poly-L-lysine covered glass coverslips for imaging. Fluorescence intensity detected with the TRITC filter were monitored by confocal laser scanning microscope system Nikon A1-R, and represented the outer glomeruli space. A Z-stack of 27 consecutive focal planes (total slice thickness of ~72.5 μm) was collected every 2 minutes, which allowed reconstructing glomeruli volume using Fiji image processing package (ImageJ 1.47v, National Institute of Health, USA) and Origin Pro 9.0 (OriginLab, Northampton, MA). Volume changes created by the oncotic pressure induced by switching the surrounding medium from 5% into 1% BSA (dialyzed overnight through 50kDa filter) was monitored by 3D imaging throughout the experiment. For glomeruli volume reconstitution, TRITC signal was inverted, and each focal plane area (0.7984 pixel/micron) was processed by the Analyze Particles module (Fiji). Finally, glomerular volume was calculated by the integration of the obtained focal planes using OriginPro software. The details of this specific approach can be found in our recent publications [41; 42].
2.4. Statistical analysis
Data are presented as mean ± SEM. The values of intracellular calcium ion or NO production concentration at every moment of time for individual cells were averaged by the number of regions registered in the experiment (n = 15–20). Data were compared using the one-way ANOVA, and P < 0.05 is considered significant.
3. Results
3.1. Loading of glomeruli
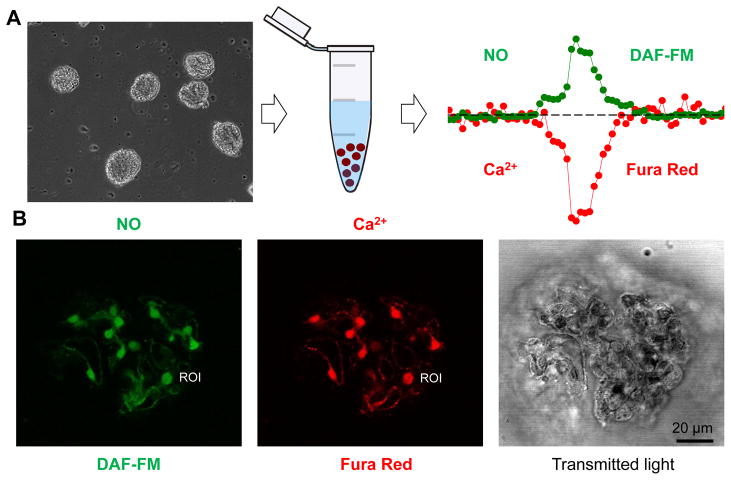
We have previously described an approach that can be used to monitor [Ca2+]i level in freshly isolated murine glomeruli [38]. Here we applied a similar technique, in which isolated decapsulated glomeruli were loaded with a calcium dye, Fura Red. In addition to [Ca2+]i measurements, we used the fluorescent indicator DAF-FM to detect NO production, either separately or together with Fura Red. Shown in Fig. 1 is a simplified schematic of the protocol and representative images of an isolated glomerulus loaded with DAF-FM and Fura Red.
Fig. 1.
Method of glomeruli isolation and confocal imaging. (A) The kidneys are excised and glomeruli are isolated from renal cortex by differential sieving as reported previously [38; 49]. Shown is an image of freshly isolated decapsulated glomeruli, which are subsequently loaded with fluorescent calcium (Fura Red) and NO (DAF-FM) dyes to perform confocal imaging. Scale bar is 80 μm. (B) Representative images of a rat glomerulus stained with DAF-FM (green pseudocolor) and Fura Red (red pseudocolor). Image of the same glomerulus taken with transmitted light is also shown. ROI – single Region of Interest (one podocyte) used for analysis.
3.2. Ang II and H2O2 evoke NO production in podocytes of isolated glomeruli
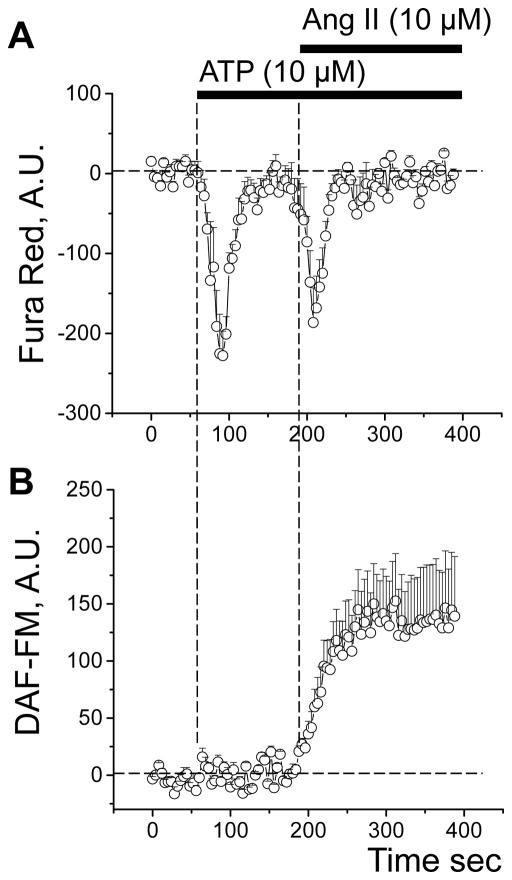
Our previous studies revealed that Ang II and ATP have profound effects on podocyte [Ca2+]i levels [30; 39; 40]. Since NO-Ca2+ interaction is established, we tested the effects of Ang II and ATP on NO production and [Ca2+]i transients in glomerular podocytes. To do this, confocal fluorescence imaging was done on isolated and decapsulated glomeruli from SS rats loaded with two dyes: DAF-FM for the detection of NO production and Fura Red for monitoring [Ca2+]i transients. Both Ang II and ATP stimulated [Ca2+]i release in glomeruli podocytes consistent with previous results [30; 39; 40] (Fig. 2A). However, only Ang II caused a significant increase in production of NO in these cells (Fig. 2B).
Fig. 2.
Ang II evokes NO production and an increase in [Ca2+]i in glomerular podocytes of SS rats. (A) Representative transients of [Ca2+]i dynamics in the podocytes of the Fura Red loaded SS rat glomeruli in response to application of ATP and Ang II. (B) DAF-FM transient increase demonstrating NO production in response to the same agents. ATP application produces a Ca2+ transient without affecting NO production in glomeruli podocytes. In contrast, application of Ang II promotes both Ca2+ and NO level elevation (note that a decrease in Fura Red signal shows elevation of intracellular Ca2+).
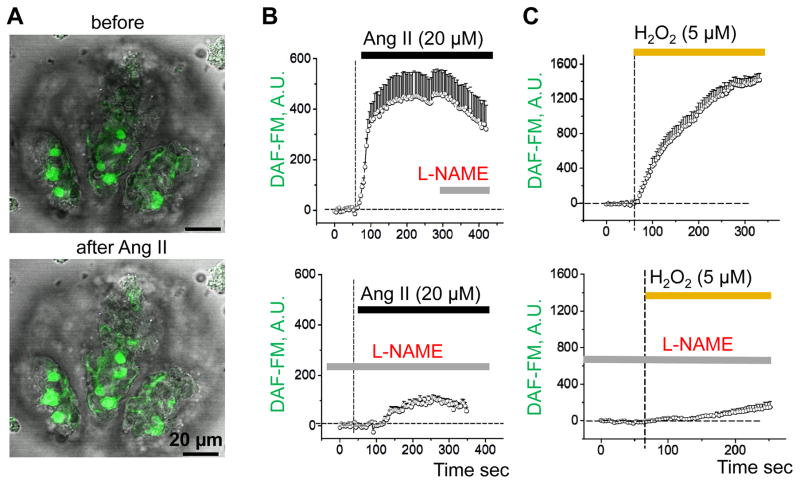
We further characterized the effects of Ang II and H2O2 on NO production. In these experiments, we used L-NAME to inhibit NOS in order to demonstrate the specificity and sensitivity of the detected response. Shown in Fig. 3A are representative images of an isolated glomerulus loaded with DAF-FM before and after application of Ang II. As summarized in Fig. 3B, Ang II acutely stimulated NO production in this preparation. Addition of L-NAME post-Ang II treatment caused a slight reduction in NO (top graph), whereas pretreatment with L-NAME almost completely abolished the effect of Ang II (bottom graph). Application of H2O2 similarly resulted in an immediate elevation of NO levels that was mostly abolished when glomeruli were pretreated with L-NAME (Fig. 3C). It should be noted that the presence of both an intracellular NO source and H2O2 may overestimate the level of NO production due to the DAF-FM dye properties, as previously described [43]. However, the observed changes in fluorescence are likely accurate similar to previous reports [44], since this effect was dramatically decreased by pretreatment with L-NAME (Fig. 3C).
Fig. 3.
Ang II and H2O2 evoke NO production in glomeruli podocytes. (A) Confocal imaging of glomeruli before (upper panel) and after (lower panel) Ang II application (merged with transmitted light). Scale bars are shown. DAF-FM transient increase demonstrating elevated levels of NO in response to 20 μM Ang II (B) or 5 μM H2O2 (C). Both Ang II- and H2O2-mediated NO production significantly decreased after pre-treatment of glomeruli with L-NAME (10 mM).
3.3. NO production in podocytes in response to Ang II and H2O2 upon development of salt-sensitive hypertension
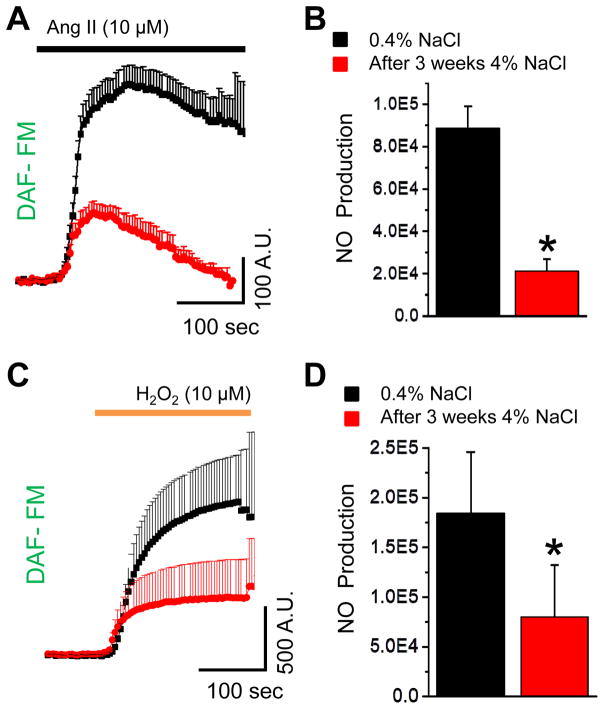
Experiments shown in Figs. 2 and 3 were performed using glomeruli isolated from SS rats fed a 0.4% NaCl diet. Following studies were designed to determine whether NO production in response to these stimuli was altered under hypertensive conditions. To test this, we took advantage of the Dahl SS hypertensive rat model, which is a well-characterized model of salt-induced hypertension [37; 45; 46; 47]. Importantly, SS rats fed a high salt diet develop kidney injury that mimics conditions observed in human salt-sensitive hypertension, including focal segmental glomerulosclerosis (FSGS) [48]. Glomeruli were isolated from the SS rats fed either a low salt (0.4% NaCl) of high salt (HS; 4% NaCl, 3 weeks) diet. As shown in Fig. 3, addition of Ang II and H2O2 caused a rapid increase of NO level in glomeruli isolated from rats fed a low salt diet. In rats fed a HS diet for 3 weeks, responses to Ang II- and H2O2 were significantly attenuated indicating that NO signaling pathways were disrupted during hypertensive conditions (Fig. 4A and 4B).
Fig. 4.
Impaired NO signaling in glomeruli podocytes of SS rats upon the development of salt-sensitive hypertension. (A) Changes in podocytes DAF-FM fluorescence in response to Ang II (10 μM) application on glomeruli of SS rats fed a LS (0.4% NaCl; black) and HS (4% NaCl, 3 weeks; red) diets. (B) Total NO production in response to Ang II stimulation calculated as an integral of DAF-FM transient for the 300 sec time interval (N≥5; n≥38 for each group; *P<0.05). (C) Changes in podocytes DAF-FM fluorescence in response to H2O2 (10 μM) in SS rats fed LS or HS diets. (D) Graph summarizing changes in H2O2-mediated NO production in glomeruli podocytes (N≥4; n≥30 for each group; *P<0.05).
3.4. The effects of NO donor on glomerular volume dynamics and permeability in the Dahl SS rats
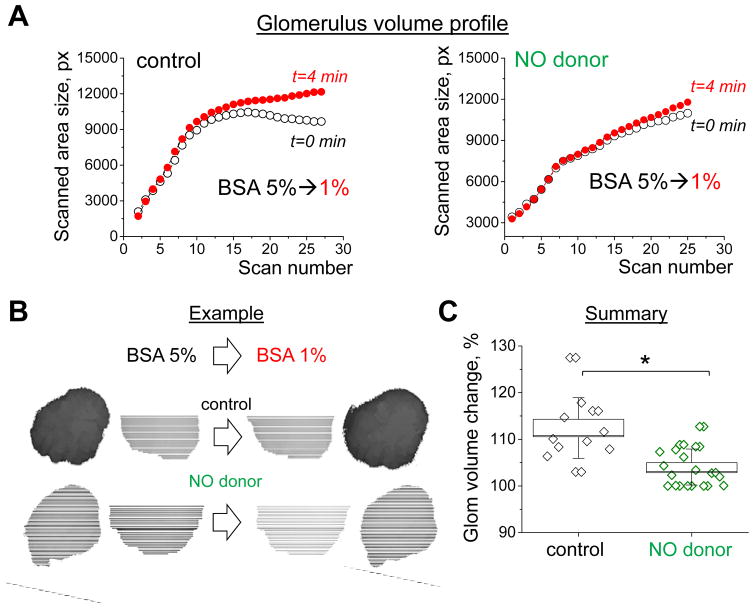
To further test the effects of NO bioavailability on the changes in glomerular filtration barrier permeability we conducted experiments designed to detect glomerular volume dynamics in response to changes in oncotic pressure (technique initially introduced by Savin et al. [49] and adapted by us in our recent manuscript [41]). Freshly isolated glomeruli were pretreated for 40 min with NO donor - DETA NONOate (50 μM) and placed in bath solution used for NO imaging in podocytes. Fast xyzt confocal scan was applied before and after introducing colloid osmotic pressure (exerted by the changes in bath solution albumin concentration from 5% to 1%). Obtained confocal images of glomerular profiles at certain Z-positions were recalculated into volume values (see Fig. 5); final mean glomerular volume changes were 112.4±1.9 vs 104.1±0.9 % for control and DETA NONOate pretreated groups, correspondingly. This suggests that the albumin reflection coefficient was lower (indicating greater permeability) when NO bioavailability is increased.
Fig. 5.
Changes in glomerular volume and permeability during the activation of NO signaling cascades in freshly isolated rat glomeruli. (A) Glomerular volume dynamics in response to changes in osmotic pressure (BSA changes from 5 to 1%) in control (left) or DETA NONOate pretreated (right) glomeruli. The line/symbol represents glomerular volume profile (each symbol is the size of a certain z-scan) before (black; t=0) and after (red; t=4min) change in osmotic pressure. (B) An example of glomerular volume change observed by confocal microscopy (shown are images in 5% BSA and after solution change to 1% BSA). (C) A summary of glomerular volumes (which reversibly correlates with the permeability of glomeruli filtration barrier) in control (vehicle) and DETA NONOate pretreated groups (N≥4; n≥12 for each group; *P<0.05).
4. Discussion
NO is a major paracrine and autocrine signaling molecule. In the kidney, it can be released by the glomerular and tubular cells, and it is also known to regulate renal hemodynamics and other mechanisms. Changes in glomerular NO production, which can be triggered by activation of RAAS, oxidative stress or other factors, can lead to damage of podocytes along with endothelial and tubular cells, and cause subsequent alterations in glomerular filtration rate resulting in severe proteinuria. For, instance, Li et al. demonstrated that exposure of isolated glomeruli to NO donors SNAP, DETA-NONOate and sodium nitroprusside resulted in compromised integrity of the glomerular filtration barrier and increased albumin permeability [50]. The importance of NO role in the regulation of renal microvasculature function cannot be overemphasized: multiple studies of vascular barrier function over the years have clearly demonstrated an increase in vascular permeability after NOS inhibition [51], which could be reversed by introducing NO donors into the system [52; 53; 54]. However, as stated in the recent manuscript by Dolinina et al. [14], which studied the role of NO-ROS antagonism in glomerular permeability and focused majorly on the effects this process has on microvasculature, “podocytes are important for the barrier function … through their interactions with the rest of the GFB, …, and modify its function without gross changes in cell shape”. Therefore, in this study we focused primarily on the podocyte as the source and effector of NO production.
The ability to detect NO production in podocytes upon activation of specific signaling pathways is important for studying glomerular disease pathogenesis. There are several approaches to measure the concentrations of ROS, including NO, in cells, tissues, and biological fluids [55]. Regarded as an acceptable fluorophore for measuring NO, the aromatic vicinal diamines of diaminofluoresceins (DAFs) have been designed to react with NO in the presence of dioxygen [56]. The large Stokes shift of a calcium dye Fura Red allows for the ability to measure many emission spectra using a single excitation wavelength [57]; this property allowed us to simultaneously measure DAF-FM and Fura Red in response to application of either ATP or Ang II. Combining these two fluorescent probes can provide insight into the cell signaling pathways or processes that may be occurring concurrently. As an example, similar approach has been shown to be useful for detecting NO with DAF-FM-DA probe in combination with a CellTracker Red CMTPX probe [58].
Currently the interest in the interdependent regulation of NO and calcium signaling in various types of cells and tissues is rapidly growing. A recent report by Kim et al. [59] demonstrated an indirect correlation between the levels of NO and calcium ions in the kidney using a dual microsensor inserted in a living rat kidney. The crosstalk between NO and calcium signaling is very complex; according to existing reports, NO is able to both induce or reduce [Ca2+]i concentration, and exert its action directly or indirectly, depending on the cell type or pathophysiological context [60]. Many calcium channels were shown to be regulated by NO either indirectly, when NO could modulate the channels via the generation of cGMP, cyclic ADP ribose, and/or induce protein kinases or directly, in which case NO might regulate channel activity through a post-translational modification (S-nitrosylation) [61; 62; 63]. Additionally, NO can affect Ca2+ pumps, including those located in sarcoplasmic/endoplasmic reticulum and plasma membrane, which results in cytosolic Ca2+-removal [64]. An interesting recent study reported that blockade of calcium channels or Ang II type 1 receptors (AT1R) in humans opposed the renal effects of NOS inhibition by counteracting oxidative stress responses to acutely impaired renal NO bioavailability [65].
Our results indicated that application of Ang II and H2O2 caused a rapid increase in NO generation in glomeruli podocytes. Interestingly, ATP had a profound effect on [Ca2+]i but did not elicit any changes in NO production. It was reported previously that eNOS can be regulated independently from [Ca2+]i but it usually results in low NO production [66]. This was likely the case in our experiments, and we suggest that the increase in [Ca2+]i in response to ATP by itself was insufficient to cause changes in NO. Previous studies by Jankowski and colleagues revealed that extracellular ATP may increase the filtration surface of the glomeruli via activation of P2Y receptors with the subsequent activation of the eNOS and soluble guanylyl cyclase [67] and that extracellular nucleotides stimulate an increase of glomerular albumin permeability via NOS pathway [27]. We were not able to detect any effects of ATP on NO production in the podocytes in our preparation. It is likely, however, that ATP and other purines mediate their effect on glomerular permeability via effects on other glomerular cells, or NO generation, for this process, simply requires more time. The current study focused on the role of NO production in the podocytes in response to Ang II, a peptide hormone of the RAAS.
The role of NO and its regulation by Ang II in salt-sensitive hypertension has been well described [68; 69; 70]. For instance, it was shown in normotensive humans who consumed a high salt diet, as well as in salt-sensitive individuals, that salt sensitivity could be mediated, in part, via a reduction in NO bioavailability [71; 72]. It was also reported that podocyte dysfunction and the development of proteinuria could be associated with a NO deficiency [73]. Genome-wide association study identified a hypertension susceptibility locus located in the promoter region of the eNOS gene [74]. For our experiments we used the Dahl SS rat, a naturally occurring model of salt-sensitive hypertension, which recapitulates many aspects of progressive human hypertension and has provided crucial insights into the mechanisms underlying salt sensitivity and renal injury [37; 75; 76; 77]. This model was used in previous studies revealing the contribution of NO to the development of salt-induced hypertension. About 25 years ago Chen and Sanders published a seminal study showing that L-arginine, the substrate for NO synthesis, abrogates salt-sensitive hypertension in Dahl SS rats [78]. Several groups further confirmed this important observation [79; 80; 81; 82; 83; 84]. Clinical studies also revealed that L-arginine infusion lowered blood pressure in salt-sensitive patients [85; 86]. As summarized in a recent review by Feng et al., endothelial responses to high salt intake affected arteriolar vasodilation and blood pressure [69]. Consistent with this, it was shown that glomerular production of NO is enhanced by increasing dietary sodium and it was proposed that endothelial NOS mediates this response [87]. Most of these studies have focused on the endothelial-podocyte crosstalk, suggesting that endothelial injury contributes to the development of kidney diseases. Our experiments revealed that podocytes themselves are able to produce NO, and that the Dahl SS hypertensive rat exhibit impaired podocyte NO production in response to Ang II and H2O2. Our data emphasizes the potential effects and importance of NO generation by podocytes and its necessary role in regulating renal function.
Interestingly, rapid increase in NO bioavailability in whole glomeruli introduced by an NO donor resulted in compromised integrity of glomeruli filtration barrier and an increase in permeability; on the other hand, NO production in podocytes was impaired with the development of hypertension. These effects suggest that podocytes are presumably working to compensate reduction in glomerular permeability by decreasing NO production during the development of hypertension. However, such compensatory action may be not enough to protect podocytes from yet other damage, such as that mediated by the [Ca2+]i increase and consequent cell apoptosis.
Supplementary Material
Highlights.
Application of Ang II results in a rapid elevation of NO and [Ca2+]i in the podocytes.
Effect of Ang II on NO production is specific, since ATP, a well-known activator of [Ca2+]i signaling, does not affect NO level.
Dahl SS rats fed a HS diet for the 3 weeks demonstrated impaired NO production in response to Ang II.
Acknowledgments
Source of funding
This research was supported by the National Institutes of Health grants R35 HL135749 (to A. Staruschenko), DP2 OD008396 (to A. Geurts), K99 DK105160 (to D. Ilatovskaya) and DRTC P30 DK020595 Pilot & Feasibility project (O. Palygin), American Heart Association 17SDG33660149 (O. Palygin), American Diabetes Association 1-15-BS-172, and Juvenile Diabetes Research Foundation 1-INO-2016-223-A-N (to A. Staruschenko).
Footnotes
Conflicts of interest
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daumerie G, Bridges L, Yancey S, Davis W, Huang P, Loscalzo J, Pointer MA. The effect of salt on renal damage in eNOS-deficient mice. Hypertens Res. 2010;33:170–6. doi: 10.1038/hr.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeringa P, van Goor H, Itoh-Lindstrom Y, Maeda N, Falk RJ, Assmann KJ, Kallenberg CG, Jennette JC. Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2000;156:879–88. doi: 10.1016/S0002-9440(10)64957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama T, Sato W, Kosugi T, Zhang L, Campbell-Thompson M, Yoshimura A, Croker BP, Johnson RJ, Nakagawa T. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am J Physiol Renal Physiol. 2009;296:F317–27. doi: 10.1152/ajprenal.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–9. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley AW, Jr, Abe M, Mori T, O’Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol. 2015;308:F179–97. doi: 10.1152/ajprenal.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25:119–46. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 9.Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am. 2017;101:169–193. doi: 10.1016/j.mcna.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, Vergely C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther. 2013;140:239–57. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Cattell V. Nitric oxide and glomerulonephritis. Kidney Int. 2002;61:816–21. doi: 10.1046/j.1523-1755.2002.00226.x. [DOI] [PubMed] [Google Scholar]
- 12.Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism. 1995;44:363–8. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 13.Tomohiro T, Kumai T, Sato T, Takeba Y, Kobayashi S, Kimura K. Hypertension aggravates glomerular dysfunction with oxidative stress in a rat model of diabetic nephropathy. Life Sci. 2007;80:1364–72. doi: 10.1016/j.lfs.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 14.Dolinina J, Sverrisson K, Rippe A, Oberg CM, Rippe B. Nitric oxide synthase inhibition causes acute increases in glomerular permeability in vivo, dependent upon reactive oxygen species. Am J Physiol Renal Physiol. 2016;311:F984–90. doi: 10.1152/ajprenal.00152.2016. [DOI] [PubMed] [Google Scholar]
- 15.Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD, Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol. 2010;298:F1465–71. doi: 10.1152/ajprenal.00650.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyndman KA, Bugaj V, Mironova E, Stockand JD, Pollock JS. NOS1-dependent negative feedback regulation of the epithelial sodium channel in the collecting duct. Am J Physiol Renal Physiol. 2015;308:F244–51. doi: 10.1152/ajprenal.00596.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol. 2006;290:F1315–9. doi: 10.1152/ajprenal.00450.2005. [DOI] [PubMed] [Google Scholar]
- 18.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension. 2008;51:1605–1610. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey G, Makhija E, George N, Chakravarti B, Godbole MM, Ecelbarger CM, Tiwari S. Insulin regulates nitric oxide production in the kidney collecting duct cells. J Biol Chem. 2015;290:5582–91. doi: 10.1074/jbc.M114.592741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabral PD, Hong NJ, Garvin JL. ATP mediates flow-induced NO production in thick ascending limbs. Am J Physiol Renal Physiol. 2012;303:F194–200. doi: 10.1152/ajprenal.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe M, O’Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW., Jr Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol. 2006;291:F350–F357. doi: 10.1152/ajprenal.00407.2005. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Vicente A, Saikumar JH, Massey KJ, Hong NJ, Dominici FP, Carretero OA, Garvin JL. Angiotensin II stimulates superoxide production by nitric oxide synthase in thick ascending limbs. Physiol Rep. 2016;4:e12697. doi: 10.14814/phy2.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patzak A, Lai EY, Mrowka R, Steege A, Persson PB, Persson AE. AT1 receptors mediate angiotensin II-induced release of nitric oxide in afferent arterioles. Kidney Int. 2004;66:1949–58. doi: 10.1111/j.1523-1755.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol. 1995;268:F885–98. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- 25.Perrien DS, Saleh MA, Takahashi K, Madhur MS, Harrison DG, Harris RC, Takahashi T. Novel methods for microCT-based analyses of vasculature in the renal cortex reveal a loss of perfusable arterioles and glomeruli in eNOS−/− mice. BMC Nephrol. 2016;17:24. doi: 10.1186/s12882-016-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feletou M, Kohler R, Vanhoutte PM. Nitric oxide: orchestrator of endothelium-dependent responses. Ann Med. 2012;44:694–716. doi: 10.3109/07853890.2011.585658. [DOI] [PubMed] [Google Scholar]
- 27.Kasztan M, Piwkowska A, Kreft E, Rogacka D, Audzeyenka I, Szczepanska-Konkel M, Jankowski M. Extracellular purines’ action on glomerular albumin permeability in isolated rat glomeruli: insights into the pathogenesis of albuminuria. Am J Physiol Renal Physiol. 2016;311:F103–11. doi: 10.1152/ajprenal.00567.2015. [DOI] [PubMed] [Google Scholar]
- 28.Lockwood SY, Erkal JL, Spence DM. Endothelium-derived nitric oxide production is increased by ATP released from red blood cells incubated with hydroxyurea. Nitric Oxide. 2014;38:1–7. doi: 10.1016/j.niox.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–29. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 30.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Physiol Cell Physiol. 2013;305:C1050–9. doi: 10.1152/ajpcell.00138.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roshanravan H, Dryer SE. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol. 2014;306:F1088–97. doi: 10.1152/ajprenal.00661.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howitt L, Matthaei KI, Drummond GR, Hill CE. Nox1 upregulates the function of vascular T-type calcium channels following chronic nitric oxide deficit. Pflugers Arch. 2015;467:727–35. doi: 10.1007/s00424-014-1548-5. [DOI] [PubMed] [Google Scholar]
- 33.Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE. Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium-channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res. 2013;98:449–57. doi: 10.1093/cvr/cvt043. [DOI] [PubMed] [Google Scholar]
- 34.Haines RJ, Corbin KD, Pendleton LC, Eichler DC. Protein kinase Calpha phosphorylates a novel argininosuccinate synthase site at serine 328 during calcium-dependent stimulation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2012;287:26168–76. doi: 10.1074/jbc.M112.378794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg HZ, Shi J, Jahan KS, Martinucci MC, Gilbert SJ, Vanessa Ho WS, Albert AP. Stimulation of calcium-sensing receptors induces endothelium-dependent vasorelaxations via nitric oxide production and activation of IKCa channels. Vascul Pharmacol. 2016;80:75–84. doi: 10.1016/j.vph.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuen DA, Stead BE, Zhang Y, White KE, Kabir MG, Thai K, Advani SL, Connelly KA, Takano T, Zhu L, Cox AJ, Kelly DJ, Gibson IW, Takahashi T, Harris RC, Advani A. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol. 2012;23:1810–23. doi: 10.1681/ASN.2011121170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlov TS, Levchenko V, O’Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JH, Cowley AW, Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol. 2013;24:1053–62. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Single-channel analysis and calcium imaging in the podocytes of the freshly isolated glomeruli. J Vis Exp. 2015;100:e52850. doi: 10.3791/52850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II - dependent activation of TRPC channels. Sci Rep. 2015;5:17637. doi: 10.1038/srep17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, Staruschenko A. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 2014;86:506–14. doi: 10.1038/ki.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilatovskaya DV, Palygin O, Levchenko V, Endres BT, Staruschenko A. The role of angiotensin II in glomerular volume dynamics and podocyte calcium handling. Sci Rep. 2017;7:299. doi: 10.1038/s41598-017-00406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endres BT, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Kamocka MM, McDermott-Roe C, Staruschenko A, Molitoris BA, Geurts AM, Palygin O. Intravital imaging of the kidney in a rat model of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2017;313:F163–F173. doi: 10.1152/ajprenal.00466.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balcerczyk A, Soszynski M, Bartosz G. On the specificity of 4-amino-5-methylamino-2′,7′-difluorofluorescein as a probe for nitric oxide. Free Radic Biol Med. 2005;39:327–35. doi: 10.1016/j.freeradbiomed.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–24. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 45.Miller B, Palygin O, Rufanova VA, Chong A, Lazar J, Jacob HJ, Mattson D, Roman RJ, Williams JM, Cowley AW, Jr, Geurts AM, Staruschenko A, Imig JD, Sorokin A. p66Shc regulates renal vascular tone in hypertension-induced nephropathy. J Clin Invest. 2016;126:2533–46. doi: 10.1172/JCI75079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowley AW, Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the importance of Nox4 in production of hypertension in Dahl salt-sensitive rats. Hypertension. 2016;67:440–50. doi: 10.1161/HYPERTENSIONAHA.115.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 2015;65:447–55. doi: 10.1161/HYPERTENSIONAHA.114.04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirano T, Ebara T, Furukawa S, Nagano S, Takahashi T. Mechanism of hypertriglyceridemia in Dahl salt-sensitive rats, an animal model of spontaneous nephrotic syndrome. Metabolism. 1994;43:248–256. doi: 10.1016/0026-0495(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 49.Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol. 1992;3:1260–9. doi: 10.1681/ASN.V361260. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Yao J, Morioka T, Oite T. Nitric oxide increases albumin permeability of isolated rat glomeruli via a phosphorylation-dependent mechanism. J Am Soc Nephrol. 2001;12:2616–24. doi: 10.1681/ASN.V12122616. [DOI] [PubMed] [Google Scholar]
- 51.Kubes P. Nitric oxide affects microvascular permeability in the intact and inflamed vasculature. Microcirculation. 1995;2:235–44. doi: 10.3109/10739689509146769. [DOI] [PubMed] [Google Scholar]
- 52.Suttorp N, Hippenstiel S, Fuhrmann M, Krull M, Podzuweit T. Role of nitric oxide and phosphodiesterase isoenzyme II for reduction of endothelial hyperpermeability. Am J Physiol. 1996;270:C778–85. doi: 10.1152/ajpcell.1996.270.3.C778. [DOI] [PubMed] [Google Scholar]
- 53.Holschermann H, Noll T, Hempel A, Piper HM. Dual role of cGMP in modulation of macromolecule permeability of aortic endothelial cells. Am J Physiol. 1997;272:H91–8. doi: 10.1152/ajpheart.1997.272.1.H91. [DOI] [PubMed] [Google Scholar]
- 54.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–81. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 55.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A S American Heart Association Council on Basic Cardiovascular Sciences. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res. 2016;119:e39–75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–53. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 57.Lund-Johansen F, Olweus J. Signal transduction in monocytes and granulocytes measured by multiparameter flow cytometry. Cytometry. 1992;13:693–702. doi: 10.1002/cyto.990130705. [DOI] [PubMed] [Google Scholar]
- 58.Nott A, Robinson JD, Riccio A. DAF-FM detection of nitric oxide in embryonic cortical neurons. Protocol Exchange. 2008 doi: 10.1038/nprot.2008.235. [DOI] [Google Scholar]
- 59.Kim YS, Ha Y, Sim J, Suh M, Lee Y. Location-dependent sensing of nitric oxide and calcium ions in living rat kidney using an amperometric/potentiometric dual microsensor. Analyst. 2016;141:297–304. doi: 10.1039/c5an01804h. [DOI] [PubMed] [Google Scholar]
- 60.Jeandroz S, Lamotte O, Astier J, Rasul S, Trapet P, Besson-Bard A, Bourque S, Nicolas-Frances V, Ma W, Berkowitz GA, Wendehenne D. There’s more to the picture than meets the eye: nitric oxide cross talk with Ca2+ signaling. Plant Physiol. 2013;163:459–70. doi: 10.1104/pp.113.220624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 62.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–66. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 63.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–83. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 64.Yao X, Huang Y. From nitric oxide to endothelial cytosolic Ca2+: a negative feedback control. Trends Pharmacol Sci. 2003;24:263–6. doi: 10.1016/S0165-6147(03)00122-6. [DOI] [PubMed] [Google Scholar]
- 65.Montanari A, Lazzeroni D, Pela G, Crocamo A, Lytvyn Y, Musiari L, Cabassi A, Cherney DZI. Calcium channel blockade blunts the renal effects of acute nitric oxide synthase inhibition in healthy humans. Am J Physiol Renal Physiol. 2017;312:F870–8. doi: 10.1152/ajprenal.00568.2016. [DOI] [PubMed] [Google Scholar]
- 66.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 67.Jankowski M, Szczepanska-Konkel M, Kalinowski L, Angielski S. Cyclic GMP-dependent relaxation of isolated rat renal glomeruli induced by extracellular ATP. J Physiol. 2001;530:123–30. doi: 10.1111/j.1469-7793.2001.0123m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29:415–24. doi: 10.1097/HJH.0b013e328341d19e. [DOI] [PubMed] [Google Scholar]
- 69.Feng W, Dell’Italia LJ, Sanders PW. Novel paradigms of salt and hypertension. J Am Soc Nephrol. 2017;28:1362–9. doi: 10.1681/ASN.2016080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou AP, Cowley AW., Jr Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep. 1999;1:178–86. doi: 10.1007/s11906-999-0016-7. [DOI] [PubMed] [Google Scholar]
- 71.Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension. 1999;33:1008–12. doi: 10.1161/01.hyp.33.4.1008. [DOI] [PubMed] [Google Scholar]
- 72.Cubeddu LX, Alfieri AB, Hoffmann IS, Jimenez E, Roa CM, Cubeddu R, Palermo C, Baldonedo RM. Nitric oxide and salt sensitivity. Am J Hypertens. 2000;13:973–9. doi: 10.1016/s0895-7061(00)00283-1. [DOI] [PubMed] [Google Scholar]
- 73.Attia DM, Ni ZN, Boer P, Attia MA, Goldschmeding R, Koomans HA, Vaziri ND, Joles JA. Proteinuria is preceded by decreased nitric oxide synthesis and prevented by a NO donor in cholesterol-fed rats. Kidney Int. 2002;61:1776–87. doi: 10.1046/j.1523-1755.2002.00313.x. [DOI] [PubMed] [Google Scholar]
- 74.Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, Arima H, Hoggart C, Tichet J, Nikitin YP, Conti C, Seidlerova J, Tikhonoff V, Stolarz-Skrzypek K, Johnson T, Devos N, Zagato L, Guarrera S, Zaninello R, Calabria A, Stancanelli B, Troffa C, Thijs L, Rizzi F, Simonova G, Lupoli S, Argiolas G, Braga D, D’Alessio MC, Ortu MF, Ricceri F, Mercurio M, Descombes P, Marconi M, Chalmers J, Harrap S, Filipovsky J, Bochud M, Iacoviello L, Ellis J, Stanton AV, Laan M, Padmanabhan S, Dominiczak AF, Samani NJ, Melander O, Jeunemaitre X, Manunta P, Shabo A, Vineis P, Cappuccio FP, Caulfield MJ, Matullo G, Rivolta C, Munroe PB, Barlassina C, Staessen JA, Beckmann JS, Cusi D. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension. 2012;59:248–55. doi: 10.1161/HYPERTENSIONAHA.111.181990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zicha J, Dobesova Z, Vokurkova M, Rauchova H, Hojna S, Kadlecova M, Behuliak M, Vaneckova I, Kunes J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res. 2012;61:S35–87. doi: 10.33549/physiolres.932363. [DOI] [PubMed] [Google Scholar]
- 76.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW., Jr Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 2015;65:447–55. doi: 10.1161/HYPERTENSIONAHA.114.04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW., Jr Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol. 2008;295:F837–42. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–67. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel A, Layne S, Watts D, Kirchner KA. L-arginine administration normalizes pressure natriuresis in hypertensive Dahl rats. Hypertension. 1993;22:863–9. doi: 10.1161/01.hyp.22.6.863. [DOI] [PubMed] [Google Scholar]
- 80.Kakoki M, Kim HS, Arendshorst WJ, Mattson DL. L-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1478–85. doi: 10.1152/ajpregu.00386.2004. [DOI] [PubMed] [Google Scholar]
- 81.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–818. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 82.Nakanishi K, Mattson DL, Cowley AW., Jr Role of renal medullary blood flow in the development of L-NAME hypertension in rats. Am J Physiol. 1995;268:R317–23. doi: 10.1152/ajpregu.1995.268.2.R317. [DOI] [PubMed] [Google Scholar]
- 83.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW., Jr Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266:H1918–26. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 84.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993;91:642–50. doi: 10.1172/JCI116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856–61. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 86.Campese VM, Amar M, Anjali C, Medhat T, Wurgaft A. Effect of L-arginine on systemic and renal haemodynamics in salt-sensitive patients with essential hypertension. J Hum Hypertens. 1997;11:527–32. doi: 10.1038/sj.jhh.1000485. [DOI] [PubMed] [Google Scholar]
- 87.Ying WZ, Sanders PW. Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-β1. Am J Physiol Renal Physiol. 1998;275:F18–24. doi: 10.1152/ajprenal.1998.275.1.F18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.