Abstract
Chronic over-nutrition and obesity induces low-grade inflammation throughout the body. Termed “metainflammation,” this chronic state of inflammation is mediated by macrophages located within the colon, liver, muscle and adipose tissue. A sentinel orchestrator of immune activity and homeostasis, macrophages adopt variable states of activation as a function of time and environmental cues. Metainflammation phenotypically skews these polarization states and has been linked to numerous metabolic disorders. The past decade has revealed several key regulators of macrophage polarization, including the Signal Transducer and Activator of Transcription (STAT) family, the peroxisome proliferator-activated receptor gamma (PPARγ), the CCAAT-enhancer-binding proteins (C/EBP) family and the Interferon regulatory factors (IRFs). Recent studies have also suggested that microRNAs (miRNAs) and long noncoding RNA (lncRNA) influence macrophage polarization. The pathogenic alteration of macrophage polarization in metainflammation is regulated by both extracellular and intracellular cues, resulting in distinct secretome profiles. Metainflammation-altered macrophage polarization has been linked to insulin insensitivity, atherosclerosis, inflammatory bowel disease, cancer and autoimmunity. Thus, further mechanistic exploration into the skewing of macrophage polarization promises to have profound impacts on improving global health.
Key terms: obesity, metainflammation, macrophage polarization
Introduction
Chronic over nutrition disturbs the body’s homeostasis and associates with physiologic disturbances such as high blood pressure, hyperglycemia, hyperlipidemia, and obesity. The constellation of these pathologies is termed metabolic syndrome, and it is affecting a growing percentage of the world’s population. Metabolic syndrome is associated with comorbidities such as diabetes, cardiovascular disease, osteoarthritis and cancer, greatly impacting mortality and morbidity (1). The inciting trigger of metabolic dysfunction has yet to be mechanistically elucidated, however chronic over-nutrition and resultant obesity are recognized as a cause of chronic, low-grade inflammation, historically described as “Syndrome X” (2), “The Deadly Quartet” (3), and “Insulin-Resistance Syndrome” (4). The unresolvable immune activation occurs in the absence of overt infection or frank autoimmune disease, but is recognized to not only affect local tissues, but systemic physiology through chronic, low-grade inflammation induced by obesity(5) (6) that is termed metainflammation.
The link between adipose tissue and inflammation was first substantiated in the 1990s, when tumor necrosis factor α (TNFα) mRNA was isolated from adipose tissue of obese rodents and discovered to attenuate phosphorylation events during insulin signaling (7) (8). Transcriptome analysis in obese mice then revealed a positive correlation between macrophage-related genes and increased body mass in 2003 (9). When compared to other tissues, the upregulation of these macrophage genes was observed earliest and in the greatest magnitude within white adipose tissue (10).
Macrophages represent an integral compartment of the innate immune system and are critical regulators of both normal homeostasis as well as pathology. Derived from hematopoietic progenitors, macrophages are crucial for proper tissue remodeling during fetal development (11). Furthermore, macrophages are equipped with the capacity to sense and respond to homeostatic alterations in adults (12). Macrophages are seeded throughout the body where they play important homeostatic roles. Key examples include within osteoclasts in bone, lung alveolar macrophages, and Kupffer cells in the liver (13). Within these organs, macrophages protectively remove dead cells, debris and lipoproteins from their environment (12). Beyond maintenance of homeostasis, macrophages also play critical roles in the repair of injured tissue (14) (15). Critical to this reparative function is the capacity for macrophages to sense tissue damage, then orchestrate a localized induction, then resolution of inflammation. Metainflammation conversely, is characterized by the unrelenting, chronic activation of macrophages which significantly, if not indefinitely, alters the body’s balance of inflammatory cues.
Since obesity and inflammation were first associated, much has been uncovered with respect to how macrophages take residence within specific tissues and assume differential states of activation under the influence of metainflammation. This review will cover these topics, with special emphasis on how macrophages maintain a chronic, meta-inflamed environment and how this environment instigates an imbalance of physiology, ultimately resulting in development of metabolic syndrome.
Macrophage Distribution and Tissue-residence in Metainflammation
This review will focus on the effects of obesity-associated meta-inflammation on macrophages at the sentinel site of nutrient absorption, gastrointestinal tract, as well as within metabolically active organs such as adipose tissue, the liver, and muscle.
a. Kinetics of Macrophage Localization
Macrophage residency is established through two primary mechanisms: (i) prenatally, or (ii) during adulthood from circulating monocytes (11) (13). (i) Seminal fate-mapping work using state-of-the-art genetic mouse models has established key characteristics of prenatally derived macrophage populations, which include liver Kupffer cells, skin Langerhans cells, and lung alveolar macrophages. These macrophages are generated from HSC without an intermediary monocyte phenotype and if absent, result in significant growth retardation, or mortality (16) (17). Once established prenatally, these macrophage populations are further self-sustaining in the physiologic steady state, expanding to fill their tissue resident niche with minimal input from circulating monocytes.(18) (19) (20).
During adulthood, bone marrow HSCs yield both myeloid and lymphoid progenitors, but it is from the myeloid progenitor pool that macrophages can be derived. HSC differentiate into monocytes, which then egress from the bone marrow via C-C Motif Chemokine Receptor 2 (CCR2). Within circulation, monocytes can be broadly categorized into Ly6Chigh or Ly6Clow populations. It is thought that Ly6Clow populations patrol and survey for intravascular damage, while Ly6Chigh populations take residence within peripheral tissues (21). While there is some debate as to the plasticity of these initial cell surface phenotypes in relation to their terminal fate, the dogma of “classical” monocyte-inflammatory, tissue resident macrophage is based on Ly6Chigh monocytes (22) (23) (13).
b. Adipose Tissue Macrophages
Originally characterized as an inert tissue depot of triglycerides, adipose tissue has since been recognized dynamic organ that orchestrates metabolic, endocrine and immune responses (24). Adipose tissue serves as a reservoir of fatty energy during times of fasting, but in settings of obesity, it releases serum factors such as adipokines, cytokines and chemokines to elicit recruitment of immune cells. In adipose tissue, infiltrating immune cells, such as macrophages, B cells, T cells, are the second largest population after adipocytes and play critical roles in regulating tissue function and homeostasis (25). Being a major portion of adipose tissue immune cells, the activity of adipose tissue macrophages (ATM) are closely correlated with inflammatory responses under obesity stress.
Adipose tissue can be classified into three categories based on function and appearance: White, Brown, and Beige.
1) White Adipose
White adipose tissue (WAT) comprises the majority of “fatty tissue” and is capable of releasing free fatty acids into the circulation when circulating glucose levels are low. WAT also secretes factors that modulate insulin sensitivity, and it was within WAT that an association between obesity and immune cell activation was first conceived (26). In comparing lean to obese mice, macrophage infiltration of adipose tissue was demonstrated to increase 10-fold – from 5% to up to 50% after reaching the state of obesity (9). Studies using obese animal models suggested a positive correlation of increased C-C Motif Chemokine Ligand 2 (CCL2, or monocyte chemoattractant protein-1, MCP-1) with increased adipose tissue mass. This link was substantiated by dominant-negative CCL2 expression mitigating insulin resistance and macrophage infiltration associated with high-fat diet and genetically-induced diabetes (27). More recently, the mRNA upregulation of the alarmin S100 calcium-binding protein A8 (S100A8) has been associated with early stages of high fat diet feeding, precipitating macrophage chemotaxis in both in vitro assays using RAW264.7 and 3T3-L1 adipocytes, and in vivo tracking models using LysMEGFP and intravital adipose tissue imaging (28). Though there has long been a clear association between inflammation and insulin resistance, the mechanistic steps linking these two events was largely unknown until 2011. Through time course analyses of macrophage content and insulin sensitivity of wildtype versus immunocompromised mice, it was elucidated that macrophage-induced inflammation was necessary for the sequelae of long-term insulin resistance upon high fat feeding (29).
Within expanding WAT, macrophages, both resident and infiltrating, localize to dying adipocytes and form crown-like structures (CLS) fusing into pro-inflammatory, multinucleated giant cells(30) (31). These CLS have been reported to account for over ninety percent of infiltrating macrophages and are a histologic hallmark of inflammation within adipose tissue (31) (32). In addition to causing adipose hypertrophy, obesity has also been associated with excessive free fatty acids (FFA). These FFA have been shown to activate Toll-like receptor 4 (TLR4) signaling from the 3T3-L1 adipocyte cell line and adipose tissue harvested from diabetic mouse models, further substantiating a link between inflammatory macrophage activation and insulin resistance (33).
2) Brown and Beige Adipose
In contrast to white adipose tissue, brown adipose tissue (BAT) possess high levels of a mitochondrial uncoupling protein and specializes in thermogenesis (34). Specifically, uncoupling protein-1 (UCP1) allows for the energy of ATP to be expended as heat. In contrast to white adipose, brown fat is also richly innervated, vascularized and shares a progenitor with muscle tissue (34). Similar to BAT, “beige” adipocytes can express high levels of UCP-1. Its alternative names including “brite” (“Br”own and wh”ite”) tissue, or inducible “brown-like” adipocytes highlight its phylogenetic link to WAT, but nevertheless functional similarity to BAT. While there is debate regarding what beige tissue is induced, it is largely believed that it basally expresses low levels of UCP1 that can be upregulated upon appropriate environmental cues (35) (36) (37) (38)
Promoting the beiging of WAT has served as a therapeutic target since genetic upregulation of “beige” genes have been linked to obesity-resistance in mouse models (39). Recent studies have suggested that macrophages play an active role in the beiging process, or development of BAT. In 2011, it was reported that mice exposed to low temperatures would upregulate their non-shivering thermogenesis genes in a macrophage-dependent manner. Through the genetic, in vivo manipulation of cytokines required for alternative M2 macrophage polarization, it was concluded that alternatively activated macrophages were required for the orchestration of thermogenic responses to cold through upregulation of catecholamine synthesis pathway (40) (41) (42). This notion of macrophage-mediated release of catecholamines to promote the browning of adipose tissue, however, has since been directly contradicted. In a recent study spanning six institutions, Fischer et al demonstrated that administration of IL-4 had no effect on either energy expenditure, or the browning of adipose tissues. Furthermore, the catecholamine synthesis enzyme implicated in the previous studies (tyrosine hydroxylase; Th) was absent in their rdTomato reporter mice, with no detectable levels of Th mRNA within macrophages isolated from BAT or inducible WAT (43). While the influence of alternatively activated macrophages being able to promote the browning of tissue is under debate, several reports have suggested that macrophage-induced inflammation may actively suppress adipose browning (38) (44) (45) (46). Thus, the true physiologic significance of macrophages in the beiging process or development of BAT has yet to be completely elucidated (35), but remains a critical gap in the literature.
c. Colon
The GI tract houses the largest total number of macrophages, and is the first organ to encounter nutrient excess (47). Besides playing a critical role in pathogen clearance, colonic macrophages also regulate inflammatory responses, local homeostasis and insulin sensitivity (48) (49). Intestinal macrophages play critical roles in chronic inflammation in the gut following obesity induced dysbiosis and intestinal barrier deficiency. Obesity in mice is associated with altered microbial composition in the gut (50, 51). Similarly, alterations in intestinal bacterial gene landscape correlates with obesity and its associated metabolic syndrome in humans (52) (53) (54). A consequence of altered microbiota composition in the gut is the increased intestinal permeability, which allows infiltration of bacteria and their degradative products, such as LPS, into lamina propria (55) (56) (57). Bacterial products trigger the activation of lamina propria macrophages in the intestine, resulting in chronic inflammation that triggers the development of metabolic diseases (55) (56) (57). In addition, in Inflammatory Bowel Disease (IBD), macrophages have been observed to take on a foamy appearance within lamina propria and to further potentiate inflammation, paralleling their well-known role in the pathophysiology of atherosclerosis.
d. Liver
Macrophages comprise a large proportion of the liver (20–40%) of the liver, a critical site of metabolic conversion (58). For instance, the liver is responsible for maintaining and regulating the body’s supply and excretion of cholesterol and has been demonstrated to be responsive to, and perpetuating of systemic energy excess. Notably, the gene Mgl2 which encodes the protein CD301A, a prototypical “M2” marker” has been recently implicated in guiding the maintenance of systemic energy surplus (59). Through inducible diptheria toxin depletion of Mgl2 in mononuclear phagocytes (MNP), Kumamoto et al showed that Mgl2 depletion surprisingly tracked with weight loss, improved insulin sensitivity, and decreased serum cholesterol levels. Central to this conclusion was that CD301+ MNPs secreted Relmα, which altered metabolic liver enzymes, including ones that regulate cholesterol elimination from the body via bile acids (60). Beyond responses to macrophage signals, the liver is also a directly affected by chronic fat overload. Nonalcoholic fatty liver disease (NAFLD) is a disease that affects almost a third of the Western population, and is associated with hepatocyte death, release of DAMPS, and resultant macrophage activation (58). Though both the liver and adipose tissue are capable of lipogenesis, the large majority of organismal fatty acids are conferred through dietary consumption(61) However, in obese states, human metabolic studies have shown that hepatic lipogenesis is increased, perhaps contributing to excessive fat mass(62). Within mice, proteomic and lipidomic analysis of obese versus lean hepatic endoplasmic reticulum demonstrated that obese mice had a higher phosphatidylcholine (PC) to phosphatidylethoanolamine (PE) ratio that disrupted the sarco/endoplasmic reticulum calcium-ATPase (SERCA), leading to ER stress that further stimulates lipid secretion from the liver, creating a vicious cycle linking obesity with insulin resistance and type 2 diabetes(63). During the progression of NAFLD, liver macrophages were found to enhance hepatic lipid accumulation (64, 65) and release of TNF-α, IL-1b and CCL2 (66) (67, 68). These cytokines not only triggered tissue damage, but they also lead to further activation of tissue residing macrophages, which promotes downstream liver fibrosis through production of TGF-β and PDGF (13, 69).
e. Muscle
As muscle is a major site of energy expenditure, it has a crucial role in overall energy homeostasis and whole-body metabolism. Under normal physiologic conditions, it helps normalize the body’s stores of glucose upon insulin stimulation (70). Similar to adipose tissue, muscle experiences increased macrophage infiltration in the setting of obesity and Type 2 Diabetes (70) (71). Histological studies have revealed that macrophages infiltrate intermuscular and perimuscular fat depots, albeit in markedly reduced total percentage in comparison to liver and adipose tissue. Nevertheless, it is hypothesized that skeletal muscle macrophages do indeed affect whole-body insulin resistance. Differentially activated macrophages conduct varying functions in muscle healing after injury (72). Additionally, macrophages also regulate cardiac tissue regeneration, maintain cardiac homeostasis and modulate the electric conduction of cardiomyocytes (73) (74).
Regulatory Mechanisms of Macrophage Polarization
In the 1980s, interferon gamma (IFNγ) was identified as an activator of macrophage phagocytic function (75) (76) (77). Since then, much has been discovered with regard to the molecular pathways involved in the macrophage priming process as well as the diversity of phenotypes that macrophages can adopt. In the 2000’s, substantial work seemed to elucidate a clear paradigm of macrophage polarization mirroring the opposing phenotypes of, for example, Th1 and Th2 cells. Once tissue resident, macrophages adopted either a proinflammatory M1 phenotype, or an anti-inflammatory M2 phenotype. They are generally accepted as transient, reversible, and occurring along a spectrum (5). M1 represents the “classically-activated” proinflammatory phenotype of macrophages capable of eliminating pathogens and cells infected by viruses or that have become transformed (5). They produce cytokines and inducible nitric-oxide synthase (iNOS), which provides effector molecules for microbicidal activities that oxidative stress that can inhibit proliferation of nearby cells (6, 78, 79). M2 represents an “alternatively” activated phenotype with anti-inflammatory activities that can promote wound healing (7).
Macrophage polarization state was first implicated in obesity when Lumeng et al. uncovered a propensity for M1 inflammatory gene expression over that of M2 after high-fat feeding(80). This skewing depended on expanding adipose tissue in obese mice expressing the macrophage chemoattractant CCL2, whereas lean mice, in the absence of CCL2 expression, had a predominate M2 gene profile (8) (81). The extent of how these differential macrophage phenotypes influence the browning of fat, or how fat might conversely influence macrophage polarization status, is of current debate. Groups had previously proposed that thermogenic BAT is sustained by M2 macrophages in response to cold or exercise (41) (82). However, more recent studies suggest that while there are intrinsic properties to white and brown adipose tissue that might sustain macrophage polarization (83), the contribution of M2 macrophages to brown adipose maintenance is minimal (43). However, the link between inflammation and suppression of beige fat, or suppression of UCP1 expression in brown fat has been consistently shown (80) (84).
a. Regulatory pathways of macrophage polarization
Within the past decade, several key regulators of macrophage polarization have been elucidated. Interferons have longed been recognized to prime inflammatory macrophage and members of the Signal Transducer and Activator of Transcription (STAT) family have been identified as key mediators of these responses. In addition, regulators of lipid metabolism, transcription factor families, microRNAs (miRNAs), and long non-coding RNAs (lncRNAs) have been shown in both in vitro and or in vivo models to be key regulators of macrophage polarization.
1) STAT family
The Janus Kinase (JAK)-STAT pathway is known to promote M1-like macrophages. In particular, STAT1 dimerizes in response to interferon-gamma (IFNγ) and induces M1-associated genes (85). In mice, STAT1 deficiency abolishes responsiveness to IFNγ and IFNα (86). Lipopolysaccharide (LPS), another well-known M1 inducer, functions through induction of IFNβ, which promotes the formation of STAT1-STAT2 heterodimers that mediates the induction of M1-associated genes by forming the IFN-stimulated gene factor 3 (ISGF3) complex (87).
In contrast to STAT1/2, STAT6 is associated with M2 macrophage polarization. IL-4 and IL-13 have both been shown in vitro and in vivo studies to induce M2 polarization (88) (89) (90). STAT6 mediates IL-4a signaling and regulates many M2 signature genes (88) (91). STAT6 signaling is further mediated by MCP-1-induced protein (MCPIP) by inducing reactive oxygen species, endoplasmic reticulum stress, and autophagy (92).
2) PPARγ and LXR signaling mediated polarization
The lipid metabolism regulator Peroxisome Proliferator-Activated Receptor gamma (PPARγ) is a negative regulator of pro-inflammatory genes (93) (94). PPARγ knockout in murine myeloid cells reduces M2-like activation and induces susceptibility to obesity, insulin resistance, and glucose intolerance (95). Evidence suggests that IL-4 and IL-13 acts upstream of PPARγ and regulates its expression in murine thioglycollate-elicited macrophages and human peripheral blood monocytes (96).
PPARγ has also been shown to bind the promoter region of miR223, a micro RNA implicated in myeloid differentiation (97) (98) (see section on miRNA for more detail on this mechanism). Interestingly, interactions between PPARγ and STAT6 have been shown in cultured mouse primary macrophages, in which STAT6 acts as a cofactor, facilitating the induction of PPARγ-regulated genes (99).
Additionally, the liver X receptors (LXRs), which, similar to PPARγ, are nuclear transcription factors that heterodimerize with the retinoid X receptor (RXRα), have been association with suppression of inflammatory pathways in macrophages (100) (101, 102). LXRs are largely associated with retrograde cholesterol transport and their activation has recently been implicated with the M2-promoting transcription factor MafB, which is downregulated by miR155 and miR33(103). While LXR activation has historically been tied to amelioration of autoimmune diseases and regression of atherosclerotic plaques, their role in metainflammation is an area that will surely be explored in the future.
3) The CREB-C/EBP pathways
Several members of the CCAAT-enhancer-binding proteins (C/EBP) family play important roles in macrophage activation. C/EBPβ mediates toll-like receptor-induced expression of arginase1 (ARG1), a signature gene of the M2 phenotype. C/EBPβ is also reported to promote expression of several M2 specific genes (such as Arg1 and mannose receptor c-type 1: Mrc1)), which itself is induced by the transcription factor cAMP-responsive element-binding protein (CREB). Deletion of CREB binding sites in the promoter region of C/EBPβ impaired muscle tissue repair in mice and inhibited expression of M2 related genes: Macrophage Scavenger Receptor 1 (MSR1), IL-10, IL-13 Receptor subunit receptor α1 (IL-13RA1), and ARG1 within macrophages, with no effect on the transcription of inflammatory, M1-associated genes (104). Upon LPS stimulation, CREB inhibited expression of M1-associated genes through p38 mediated induction of IL-10 and dual specificity protein phosphatase 1 (DUSP1) (105) (106). Another C/EBP family member, C/EBPσ was shown to induce M1-like pro-inflammatory responses in mouse bone marrow-derived macrophages, and its activity was inhibited by microRNA Let-7c (107). Another member of this family, C/EBPα, was required for both M1 and M2 activation of mouse macrophages (108). Altogether, CREB-C/EBP signaling plays a central role in the regulation of both M1- and M2-like macrophage polarization.
4) The interferon regulatory factors
Interferon regulatory factors (IRFs) have long been classified as regulators of type I interferons (IFN), and increasing evidence suggests that they also play important roles in regulating macrophage functions (109).
Knockout of IRF1 or IRF2 abolished pro-inflammatory responses in murine macrophages in response to LPS or IFNγ stimulation (110). Another member of the IRF family, IRF5 was suggested to promote M1 polarization while inhibiting M2-associated markers in human peripheral blood macrophages (111), and IRF6 was recently implicated in the negative regulation of M2 polarization of murine bone-marrow-derived macrophage (BMDM) through PPARγ inhibition (112). In contrast, some other IRFs mediated the anti-inflammatory type I Interferon responses. For example, IRF3 mediated anti-inflammatory signaling and contributed to M2 activation of human microglia (113) and IRF4 mediated IL4 induced M2 activation of murine BMDM (114). Further, studies on murine primary macrophages demonstrated a role for Lysine Demethylase 6B (KDM6B, or Jmjd3)-mediated histone demethylation of the Irf4 gene during the induction of M2 macrophage activation in response to parasites or fungi (115). In addition, IRF9 was suggested to be involved in the anti-inflammatory and M2-promoting effects of interferon tau in murine BMDM (116).
b. microRNA and lncRNA regulated macrophage polarization
In recent years there has been increasing evidence suggesting that microRNAs (miRNAs) and long noncoding RNA (lncRNA) play critical roles in regulating macrophage polarization, often through binding interactions with several key transcription factors.
1) miR223
As mentioned above, PPARγ is a crucial regulator of macrophage activation. Upon stimulation with Th2 cytokines (IL-4 or IL13), PPARγ activation in murine macrophages induced miR223 expression by binding upstream of miR223 which in turn inhibited expression of Nuclear Factor of Activated T-Cells 5 (NFATt5) and RAS p21 Protein Activator 1 (RASA1), promoting the development of an anti-inflammatory M2-like phenotype. This is consistent with the observation in mice with ablation of miR223 that desensitized the PPARγ-regulated anti-inflammatory responses in macrophages (117). In addition to promoting M2-like phenotypes, miR223 has also been shown to suppress M1-like pro-inflammatory responses through suppressing NFκB/JNK via inhibiting PBX/Knotted 1 Homeobox 1 (Pknox1) expression (118). This M1-suppressive activity of miR223 has been illustrated within murine intestinal macrophages, where miR223 suppresses C/EBPβ expression, thereby blunting the differentiation of THP-1 cells and peripheral human blood monocytes into inflammatory macrophages (119) (120).
2) miR155
In contrast to miR223, miR155 has been shown to facilitate the development of the pro-inflammatory M1-like phenotype. First identified as gene commonly induced in B-cell lymphomas, miR155 expression has since been shown to increase upon TLR activation (LPS, hypomethylated DNA or Pam3CSK4) or pro-inflammatory cytokine (TNF-α, IFNβ or IFNγ) stimulation within cultured murine macrophages (121). Expression was dependent on JNK signaling and increased levels of miR155 positively correlated with pro-inflammatory responses (O’Connell et al, 2007). Similarly, in a study by Tili el. (122) miR155 promoted TNF-α production and targeted several members of TLR4 signaling. During alcoholic liver disease, NF-κB activation induced miR155 expression in liver macrophages, which enhanced TNF-α synthesis by stabilizing its mRNA (123). In addition to promoting pro-inflammatory factors, miR155 facilitated inflammatory responses through direct suppression of anti-inflammatory regulators such as suppressor of cytokine signaling 1 (SOCS1) and phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1 (SHIP1) (124) (125). In addition, miR155 can promote M1 polarization through inhibition of IL13RA1, thus blocking STAT6 activation (126). Interestingly, despite numerous studies supporting its pro-inflammatory role, there is also evidence suggesting that in mouse bone marrow-derived macrophages miR155 down-regulates pro-inflammatory cytokine production in response to LPS stimulation, possibly through targeting TGF-β Activated Kinase 1/MAP3K7 Binding Protein 2 (TAB2) (127).
3) miR125b and Let7c
Another mechanism that microRNAs utilize to regulate macrophage polarization is by repressing the expression of critical transcription factors. miR125b is one such miRNA that promotes M1-like pro-inflammatory responses of macrophages. For example, when murine macrophages were induced by IFNγ, increased expression of miR125b suppressed its target gene IRF4, which is an important transcription factor that promotes M2 activation. The resulting response was an M1-like phenotype (128); however, in mouse Raw 264.7 macrophages, miR125b negatively regulated TNF-α production and was down-regulated upon LPS stimulation (122). In contrast, microRNA Let-7c has been shown to play a crucial role in maintaining M2 activation through suppression of the pro-inflammatory transcription factor C/EBPσ. In M1 polarized macrophages, ectopic expression of Let-7c reduced IL-12 levels and major histocompatibility complex class II surface expression, which collectively suggests suppressed inflammatory activity. Knockdown of Let-7c, on the other hand, caused reduced M2-associated activity of macrophages, despite stimulation with Th2 cytokines (107).
4) Other miRNAs
In addition to the above mentioned species, several other miRNAs are also reported to be involved in macrophage polarization. Among them, miR9 and miR127 enhanced M1 polarization by suppressing expression of peroxisome proliferator-activated receptor δ (PPAR δ) and B-cell lymphoma 6 protein (Bcl6), respectively (129) (130). On the other hand, miR124 has been shown to promote M2 polarization through STAT3 and TNF-α converting enzyme targeting (131). Other miRNAs that have been implicated in inducing M2-like phenotypes are miR132 and miR146a which inhibit NF-κB signaling pathways (132) (133). Alternatively, miR21, has an unclear role in macrophage polarization, with evidence suggesting a role in promoting both M1 (134) and M2 (135) activation.
5) lncRNAs
Long noncoding RNAs (lncRNA) are non-coding transcripts over 200 nucleotides. Recently, lncRNAs have been proposed to have a regulatory role across a broad spectrum of biological processes, including macrophage polarization. A recent study found that M1 and M2 polarized macrophages presented distinct lncRNA profiles (136). One example is lncRNA E330013P06, which was induced in macrophages isolated from insulin-resistant type 2 diabetic (T2D) mice and monocytes of T2D humans. Overexpression of lncRNA E330013P06 in macrophages enhanced expression of pro-inflammatory genes and inflammatory responses (137). Similarly, lncRNA-Cox2 was induced by inflammatory stimuli in murine BMDM and its overexpression enhanced or suppressed expression of a multitude of immune response genes, including several key regulators of macrophage polarization (138). While this is a burgeoning area of research, lncRNAs have been found to target NFκB and TNF-α signaling pathways, both of which are critical regulators of inflammatory responses (139) (140) (141).
Macrophage Polarization and Metainflammation
a. Pathogenic alteration of macrophage polarization during metainflammation
Alterations in macrophage polarization could contribute to obesity-induced insulin resistance. The residing macrophages in adipose tissues of lean individuals generally have anti-inflammatory, M2-like phenotypes (112) (142). Under the stress of obesity, a population of pro-inflammatory M1-like macrophages are recruited into adipose tissues. Specifically, Patsouris et al. demonstrated a clear contribution of pro-inflammatory macrophages to obesity-associated inflammation and insulin resistance, which could then be resolved through depletion of these macrophages (143). Additionally, Shi et al. suggested that during obesity, M1-like ATM undermined insulin responses of adipocytes and enhanced inflammatory responses through production of pro-inflammatory cytokines (33). In addition, obesity stress not only promoted infiltration of pro-inflammatory macrophages into adipose tissue, but also caused the M2-like residing macrophages to undergo a phenotypic switch to M1 (80).
b. Mechanisms of altered macrophage polarization during obesity
Several mechanisms of altered macrophage polarization during obesity have been recently suggested. These hypotheses are centered on several signaling pathways and regulation by other adipose tissue-resident cells.
T cells have long been recognized as critical regulators of macrophage development and function. During diet-induced obesity in mice, recruitment of CD8+ T cells into adipose tissue precedes the increase of ATM, and blocking T cell recruitment reduces the number of M1-like macrophages in the tissue with no effect on M2-like macrophage recruitment (144). Conversely, T regulator cells (Tregs) may contribute to M2 polarization and improve insulin response (145). In addition to T cells, adipocytes, the largest cell population within adipose tissue, have also been demonstrated as an orchestrator of ATM polarization. During the progression of obesity, the adipocyte secretome shifts to a pro-inflammatory profile that induces M1-like polarization (146). Furthermore, in vitro co-culture of BMDM with adipocytes enhanced surface expression of CD11c, a pro-inflammatory macrophage marker (147).
In recent years, several studies have implicated key signaling pathways as major players in the process of altered macrophage polarization during obesity. Arkan et al. described a role for IKKβ regulation of M1 polarization, since depletion of IKKβ in myeloid cells reduced tissue inflammation and improved insulin sensitivity in mice fed a high fat diet (148). Similarly, depletion of Mitogen-Activated Protein Kinase 8 (MAPK8) in hematopoietic cell lineages decreased adipose tissue inflammation and reduced insulin resistance (149). TLR4 signaling is another potent regulator of macrophage polarization. TLR4 is activated during obesity by several types of molecules, including saturated fatty acids and oxidized low-density lipoprotein (Ox-LDL) (150) (142) (151). Obesity induced an increase in TLR4 expression in ATM (33), and activation of TLR4 signaling in macrophages increased pro-inflammatory cytokines production, which blocked insulin signaling in adipocytes in co-culture studies (152).
Interleukin 6 (IL-6) has both pro-inflammatory and anti-inflammatory roles in regulating immune responses. In models of acute inflammation and sepsis, IL-6 promotes inflammation and mediates monocyte recruitment (153) (154). In contrast, the anti-inflammatory function of IL-6 was shown in a study by Mauer et al., in which disruption of IL-6 signaling in myeloid cells caused exaggerated systemic inflammation in high-fat diet fed obese mice (155). IL-6 was described to augment IL-4 signaling in mouse BMDMs through STAT3-mediated IL-4Rα expression, hence driving M2 polarization of mouse BMDMs and ATM. Braune et al. recently corroborated this duality of IL-6 mediated macrophage responses, showing that IL-6 promoted local proliferation of M2-like macrophages in adipose tissue during obesity, likely through sensitization to IL-4 through IL-4Rα upregulation (156).
In addition, epigenetic mechanisms are also involved in regulating macrophage polarization during obesity. DNA methyltransferase 1 (DNMT1)-mediated DNA methylation suppressed M2-like activation of ATM in obese mice, while inhibition of DNA methylation promoted ATM M2-like phenotype and down-regulated inflammatory markers in ATM. Interestingly, PPARγ1 expression, the previously described regulator of macrophage polarization, was controlled by DNA methylation in those cells and DNA-demethylation induced PPARγ1 expression and M2 macrophage polarization (157). Besides the above mentioned mechanisms, dietary factors associated with obesity are found to contribute to the altered macrophage functions and phenotypes. Links between systemic hyperlipidemia and altered macrophage phenotype have long ago been made, primarily in the context of impaired macrophage directed wound healing in diabetes (158, 159). While lipid accumulation within macrophages plays a critical role in the transition of macrophage to foam cell in the context of atherosclerosis, arthritis, and neurodegeneration, it is still unclear what role it might have in the context of obesity induced meta-inflammation(160). In addition, saturated fatty acids induced proinflammatory responses in mouse peritoneal macrophages (161) and macrophage cell line RAW 264.7 (162). In contrast, polyunsaturated fatty acids triggered anti-inflammatory effects in RAW 264.7 and in mouse intraperitoneal macrophages (163).
c. Secretome of polarized macrophages
Release of secretory products is a major way that macrophages interact with and regulate other cells to coordinate inflammatory or homeostatic responses. As one might predict, the secretome of M1 and M2 polarized macrophages are distinct (164). M1 macrophages generally produce pro-inflammatory cytokines such as TNF-α, IL-1β, IL-12, and IL-23 (165) (166), while M2 macrophages secrete anti-inflammatory cytokines and growth factors, such as IL-10 and TGFβ (167). For example, LPS, a classic inflammatory stimulus of M1 macrophages, induces M2 polarized human PBMC derived macrophages to produce significantly higher IL-10 and lower TNFα, IL-6 and IL-12p40 than their M1 counterparts (168). A recent study implicated inflammasome assembly as a mediator of these distinct secretory patterns (164). Among the macrophage-derived cytokines, IL-6 is a particularly interesting one as it is secreted by both M1 macrophages and subtypes of M2 macrophages (169). As mentioned above, IL-6 have both proinflammatory and anti-inflammatory functions, depending on the specific scenario. Obesity or exercise induced IL-6 elevation enhanced insulin secretion by inducing Glucagon-like peptide-1 (GLP-1) secretion from pancreas and intestine (170). In addition, IL-6 was needed for macrophage recruitment and myoblast proliferation, which promoted tissue repair of skeletal muscle (171).
In addition to cytokines, M1 macrophages are also characterized by production of nitric oxide (NO) and reactive oxygen species (ROS), which are mediated by SOCS3 (172) and Cytochrome B-245 β chain (CYBB alternatively named NADPH Oxidase 2 or NOX2) (173), respectively. NO and ROS have crucial regulatory roles in obesity-associated chronic inflammation (174) (175), (176) (177).
Recently, several studies have suggested that miRNAs are important components of the immune system’s secretome (178) (179). Studies on human cell lines revealed a critical role for Argonaute (AGO) proteins in mediating microRNA exportation out of cell membranes (180) (181). AGO proteins bind to miRNAs and protect them from nuclease degradation. AGO-associated miRNAs are released from cells either within vesicles or in vesicle-free forms and enter acceptor cells through membrane fusion, gap junction channels, or other unknown mechanisms (182).
Extracellular RNAs have now been accepted as a new communication venue between cells and tissues. However, our understanding of how they are produced and are packaged for secretion is still in its infancy, which much less known about their secretion by macrophages. Cultured human macrophages were able to transfer miR142 and miR223 to co-cultured hepato-carcinoma cells through gap junctions (183), and LPS stimulation could alter the level of miRNAs detected in the supernatant of human monocyte cell line THP-1-derived macrophages (178). (178). Recent data from our lab shows that the extracellular miRNA (exRNA) profile of BMDMs is different from their intracellular miRNAs (inRNA) profile, and varied with polarization status (Fig 1.). While the function of these secreted miRNAs are not yet fully understood, evidence from recent years’ studies suggest that extracellular RNAs could play critical roles in cell-cell communication. For example, miRNAs released by macrophages and other cell types have been shown to regulate cancer metastasis (184) (185), tissue remodeling (186) and immune response (187) (188). The role of extracellular miRNAs in mediating polarized macrophage function, especially in the scenario of obesity-induced metainflammation, however, is still unknown. Thus, this understudied area represents a new area of opportunity for developing novel strategies for therapeutic intervention.
Figure 1. Intracellular and Extracellular miRNA profile of BMDMs.
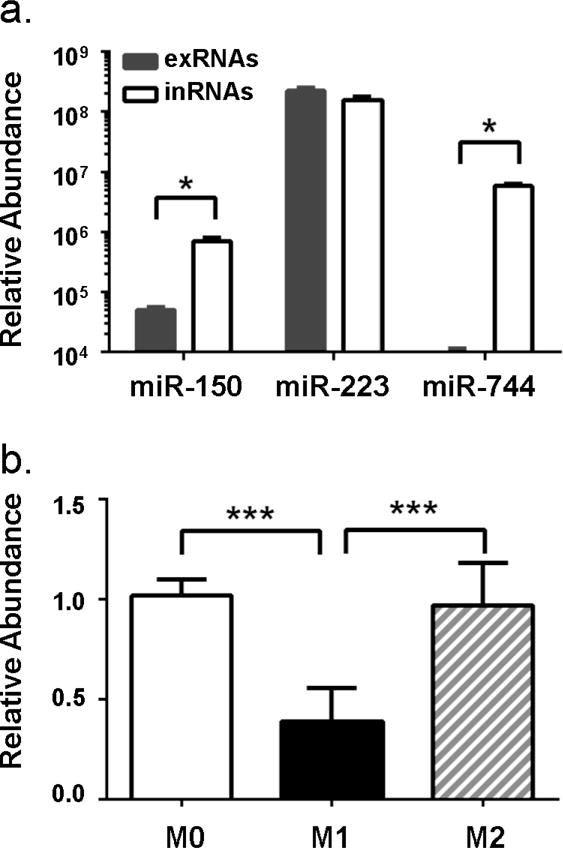
(a) Total extracellular RNAs (exRNAs) were extracted from conditioned medium of BMDMs 48 hours post-stimulation and intracellular RNAs (inRNAs) were extracted from the same batch of BMDMs. qRT-PCR analysis confirmed that miR-150 and miR-744 were highly expressed in the BMDMs, but secreted at low levels. Interestingly, miR-223, a potent regulator of macrophage polarization, was abundant in both the intra- and extra-cellular compartment. (b) BMDMs were either not activated (M0) or stimulated with 100 ng/mL LPS (M1) or 20 ng/mL IL-4 (M2). Total extracellular RNAs were extracted from cell culture supernatants 24 hours post-stimulation and miR-223 expression was quantified by qRT-PCR. exRNAs were normalized using Cel-miR-39 as spike-in controls. Data are presented as mean ± SEM; n=4, *, p<0.05 (student’s t test).
d. Meta-Inflammation moderates further disease pathogenesis
The skewing of macrophage polarization affects human physiology and unsurprisingly, pathology as well. Abnormally altered macrophage polarization greatly contributes to local tissue pathology. Atherosclerosis is well known as a chronic inflammatory disease (189), and is characterized by conglomeration of atheromatous immune cells and debris within the artery wall. Both M1-like and M2-like macrophages have been found in human atherosclerotic plaques (190) (94). Interestingly, shoulder regions of plaques, which are most prone to rupture and initiating sequelae of thrombosis and vessel occlusion, contained more M1-like pro-inflammatory macrophages (191), while regions that contained M2-like macrophages that were more stable and resistant to foam cell formation (192), which is critical for atherosclerosis progression.
Polarization of colonic macrophages is also involved in pathogenesis of inflammatory bowel disease (IBD). M1-like macrophages accumulated in the colon during IBD and became the predominant phenotype (193), which were likely to promote disease (194). On the other hand, IL-10-producing, M2-like macrophages also presented at sites of inflammation and were suggested to reduce inflammatory reactions and contribute to IBD resistance (195).
In addition to local effects, the skewing of macrophage activation induced by meta-inflammation has profound systemic impacts. For example, insulin resistance is closely associated with chronic inflammation (196), where pro-inflammatory cytokines released by macrophages are major contributing factors to the development of insulin resistance(26) (197). In adipose tissues, excess nutrients trigger inositol-requiring enzyme 1α (IRE1α) signaling, which impairs adaptive thermogenesis, disturbing the energetic homeostasis in favor of a M1 ATM phenotype (198). However, beyond the well-documented history of meta-inflammation and diabetes, there is emerging evidence implicating chronic macrophage activation in graft-vs-host diseases (GVHD) and the inflammatory conditions of osteoarthritis (199), systemic lupus erythematous, and gastrointestinal cancers (200). GVHD occurs when effector immune cells from a donor attack host tissue, and it has been suggested that host macrophages normally play a protective role through the engulfment of autoreactive immune cells (201). As acute GVHD has been epidemiologically linked to obese populations with insulin insensitivity, it is possible that a skewing of macrophage polarization plays a role in this epidemiologic finding (201) (202). Osteoarthritis has long been associated with metabolic syndrome, and it has since been suggested that a proinflammatory secretome of the predominant M1-skewed macrophage population inhibits the synthesis of protective extracellular matrix, accelerating cartilage degradation and bone resorption (203). Obesity has also been epidemiologically linked to increased incidence of gastrointestinal cancer, perhaps because M1-like macrophages possess a secretome that might promote carcinogenesis (204). In mouse models of lymphocyte-derived DNA induced systemic lupus erythematosus, transplantation of M2, but not M1 macrophages, relieved macrophage-depleted mice from lupus like pathology (205). While the precise mechanisms underlying these associations have yet to be uncovered, they suggest that they are significant contributors to the pathologic burden on human health.
Summary
In summary, the significance of macrophage infiltration and activation is beginning to be recognized as a pivotal instigator of meta-inflammation. In response to over-nutrition, peripheral tissues alter their metabolic phenotypes, release distinct secretome profiles, and shift the body’s homeostasis into one that promotes macrophage invasion, and supports various downstream inflammatory cascades.
Several important regulators are involved in regulation of macrophage polarization, including STAT, PPARα, CREB-C/EBP and IRFS. Several microRNAs modulate macrophage activation by suppressing expression of critical regulators that promote either M1 or M2 polarization. In addition, regulatory roles of lncRNAs have also been identified. Certainly the polarization of macrophages is affected under obesity stress, and T cell and adipocyte activity, signaling of regulatory proteins and changes in DNA methylation status contribute to those alternations. Polarized macrophages orchestrate tissue functions through secreting cytokines and/or reactive chemical species, which present distinct patterns between M1- and M2-like macrophages. Abnormally altered macrophage polarization not only contributes to local tissue pathology, but also has an extensive impact in promoting insulin resistance and its consequent symptoms. Despite the great achievements made in the past decades, a lot of questions on mechanisms of macrophage polarization and meta-inflammation are yet to be answered. The heterogeneous and plastic nature of macrophages make the interaction between macrophages and their microenvironment a complex and dynamic process. Our current knowledge of such interactions in vivo is limited. The signaling pathways underlying the impacts of polarized macrophages on physiological functions and pathological alterations are yet to be deciphered. New technologies including single cell analysis and computational biology approaches are being incorporated in this field and will hopefully help address those challenges. And transitions from scientific research to clinical application, such as discoveries of new molecular targets for therapies, will be one of the future directions. The metainflammation-induced alternations of macrophage polarization, and their impacts on tissue/organ functions are summarized in Figure 2.
Figure 2. Macrophage polarization modulated tissue/organ functions during obesity-induced metainflammation.
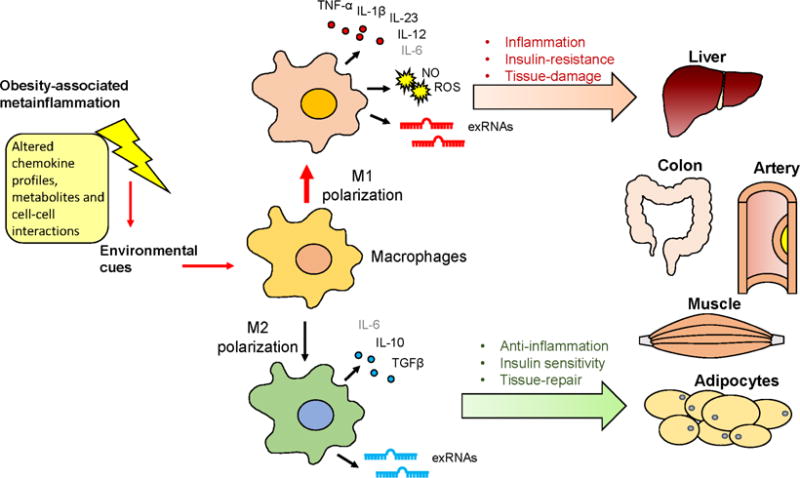
Obesity-associated metainflammation orchestrates macrophage polarization patterns through altering environmental cues, which generally favors an M1 activation state. M1- and M2-like macrophages differently modulate tissue/organ functions by secreting specific cytokines and/or reactive chemical species. Metainflammation-altered macrophage polarization profiles contribute to local tissue pathology at sites such as liver, colon and arterial walls, and further impose systemic effects by regulating insulin sensitivity in the liver, muscle and adipose tissues.
Acknowledgments
This work was supported by National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK 1R01DK098662 to B. Zhou) and the American Heart Association (Association Wide 17CPRE33660241 to M.M. Xu). All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors have read the journal’s policy on disclosure of potential conflicts of interest and the authors declare that they have no conflicts of interest with the contents of this article.
Disclose: The Authors have nothing to disclose.
References
- 1.Smith KB, Smith MS. Obesity Statistics. Prim Care. 2016;43(1):121–35. ix. doi: 10.1016/j.pop.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149(7):1514–20. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41(6):715–22. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268(35):26055–8. [PubMed] [Google Scholar]
- 9.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–75. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 12.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15(12):731–44. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 15.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44(3):450–62. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15(20):5647–58. [PMC free article] [PubMed] [Google Scholar]
- 17.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99(1):111–20. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 20.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–75. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37(6):1076–90. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue–understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92(2):386–95. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 25.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 26.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 27.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekimoto R, Fukuda S, Maeda N, Tsushima Y, Matsuda K, Mori T, et al. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc Natl Acad Sci U S A. 2015;112(16):E2058–66. doi: 10.1073/pnas.1409480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60(10):2474–83. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng C, Yang Q, Cao J, Xie N, Liu K, Shou P, et al. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 2016;7:e2167. doi: 10.1038/cddis.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32(7):307–14. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5(5):1196–203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 38.Chung KJ, Chatzigeorgiou A, Economopoulou M, Garcia-Martin R, Alexaki VI, Mitroulis I, et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol. 2017;18(6):654–64. doi: 10.1038/ni.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106(5):563–73. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, et al. Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell Metab. 2015;22(2):279–90. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23(5):623–30. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae J, Ricciardi CJ, Esposito D, Komarnytsky S, Hu P, Curry BJ, et al. Activation of pattern recognition receptors in brown adipocytes induces inflammation and suppresses uncoupling protein 1 expression and mitochondrial respiration. Am J Physiol Cell Physiol. 2014;306(10):C918–30. doi: 10.1152/ajpcell.00249.2013. [DOI] [PubMed] [Google Scholar]
- 45.Nohr MK, Bobba N, Richelsen B, Lund S, Pedersen SB. Inflammation Downregulates UCP1 Expression in Brown Adipocytes Potentially via SIRT1 and DBC1 Interaction. Int J Mol Sci. 2017;18(5) doi: 10.3390/ijms18051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumari M, Wang X, Lantier L, Lyubetskaya A, Eguchi J, Kang S, et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest. 2016;126(8):2839–54. doi: 10.1172/JCI86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding S, Lund PK. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr Opin Clin Nutr Metab Care. 2011;14(4):328–33. doi: 10.1097/MCO.0b013e3283478727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260(1):102–17. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawano Y, Nakae J, Watanabe N, Kikuchi T, Tateya S, Tamori Y, et al. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016;24(2):295–310. doi: 10.1016/j.cmet.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 53.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 54.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 55.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3(9):559–72. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 57.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 58.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17(5):306–21. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 59.Kumamoto Y, Camporez JP, Jurczak MJ, Shanabrough M, Horvath T, Shulman GI, et al. CD301b(+) Mononuclear Phagocytes Maintain Positive Energy Balance through Secretion of Resistin-like Molecule Alpha. Immunity. 2016;45(3):583–96. doi: 10.1016/j.immuni.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knudsen NH, Lee CH. Identity Crisis: CD301b(+) Mononuclear Phagocytes Blur the M1–M2 Macrophage Line. Immunity. 2016;45(3):461–3. doi: 10.1016/j.immuni.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Bjorntorp P, Sjostrom L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism. 1978;27(12 Suppl 2):1853–65. doi: 10.1016/s0026-0495(78)80004-3. [DOI] [PubMed] [Google Scholar]
- 62.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282(1):E46–51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 63.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–31. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Negrin KA, Roth Flach RJ, DiStefano MT, Matevossian A, Friedline RH, Jung D, et al. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One. 2014;9(9):e107265. doi: 10.1371/journal.pone.0107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59(2):347–57. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13(3):316–27. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9(4):e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kolios G, Valatas V, Manousou P, Xidakis C, Notas G, Kouroumalis E. Nitric oxide and MCP-1 regulation in LPS activated rat Kupffer cells. Mol Cell Biochem. 2008;319(1–2):91–8. doi: 10.1007/s11010-008-9881-7. [DOI] [PubMed] [Google Scholar]
- 69.Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58(4):1461–73. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127(1):43–54. doi: 10.1172/JCI88880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 2014;22(3):747–57. doi: 10.1002/oby.20615. [DOI] [PubMed] [Google Scholar]
- 72.Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol. 2014;232(3):344–55. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinto AR, Godwin JW, Rosenthal NA. Macrophages in cardiac homeostasis, injury responses and progenitor cell mobilisation. Stem Cell Res. 2014;13(3 Pt B):705–14. doi: 10.1016/j.scr.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell. 2017;169(3):510–22 e20. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray HW, Rubin BY, Rothermel CD. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983;72(4):1506–10. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brummer E, Morrison CJ, Stevens DA. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985;49(3):724–30. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pace JL, Russell SW, Torres BA, Johnson HM, Gray PW. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983;130(5):2011–3. [PubMed] [Google Scholar]
- 78.Napoli C, Paolisso G, Casamassimi A, Al-Omran M, Barbieri M, Sommese L, et al. Effects of nitric oxide on cell proliferation: novel insights. J Am Coll Cardiol. 2013;62(2):89–95. doi: 10.1016/j.jacc.2013.03.070. [DOI] [PubMed] [Google Scholar]
- 79.Engstrom A, Erlandsson A, Delbro D, Wijkander J. Conditioned media from macrophages of M1, but not M2 phenotype, inhibit the proliferation of the colon cancer cell lines HT-29 and CACO-2. Int J Oncol. 2014;44(2):385–92. doi: 10.3892/ijo.2013.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61(2):346–54. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dowal L, Parameswaran P, Phat S, Akella S, Majumdar ID, Ranjan J, et al. Intrinsic Properties of Brown and White Adipocytes Have Differential Effects on Macrophage Inflammatory Responses. Mediators Inflamm. 2017;2017:9067049. doi: 10.1155/2017/9067049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14(4):341–6. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 86.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84(3):431–42. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 87.Wienerroither S, Shukla P, Farlik M, Majoros A, Stych B, Vogl C, et al. Cooperative Transcriptional Activation of Antimicrobial Genes by STAT and NF-kappaB Pathways by Concerted Recruitment of the Mediator Complex. Cell Rep. 2015;12(2):300–12. doi: 10.1016/j.celrep.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 89.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20(5):623–35. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 90.Brombacher F, Arendse B, Peterson R, Holscher A, Holscher C. Analyzing classical and alternative macrophage activation in macrophage/neutrophil-specific IL-4 receptor-alpha-deficient mice. Methods Mol Biol. 2009;531:225–52. doi: 10.1007/978-1-59745-396-7_15. [DOI] [PubMed] [Google Scholar]
- 91.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380(6575):627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 92.Kapoor N, Niu J, Saad Y, Kumar S, Sirakova T, Becerra E, et al. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J Immunol. 2015;194(12):6011–23. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437(7059):759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 95.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400(6742):378–82. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 97.Sun W, Shen W, Yang S, Hu F, Li H, Zhu TH. miR-223 and miR-142 attenuate hematopoietic cell proliferation, and miR-223 positively regulates miR-142 through LMO2 isoforms and CEBP-beta. Cell Res. 2010;20(10):1158–69. doi: 10.1038/cr.2010.134. [DOI] [PubMed] [Google Scholar]
- 98.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451(7182):1125–9. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 99.Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33(5):699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7(1):161–71. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 101.N AG, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–58. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szanto A, Roszer T. Nuclear receptors in macrophages: a link between metabolism and inflammation. FEBS Lett. 2008;582(1):106–16. doi: 10.1016/j.febslet.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 103.Kim H. The transcription factor MafB promotes anti-inflammatory M2 polarization and cholesterol efflux in macrophages. Sci Rep. 2017;7(1):7591. doi: 10.1038/s41598-017-07381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106(41):17475–80. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim C, Wilcox-Adelman S, Sano Y, Tang WJ, Collier RJ, Park JM. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc Natl Acad Sci U S A. 2008;105(16):6150–5. doi: 10.1073/pnas.0800105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9(9):1028–36. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 107.Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, et al. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190(12):6542–9. doi: 10.4049/jimmunol.1202496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee B, Qiao L, Lu M, Yoo HS, Cheung W, Mak R, et al. C/EBPalpha regulates macrophage activation and systemic metabolism. Am J Physiol Endocrinol Metab. 2014;306(10):E1144–54. doi: 10.1152/ajpendo.00002.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59(4):489–510. doi: 10.1007/s00262-009-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J Immunol. 1999;163(3):1529–36. [PubMed] [Google Scholar]
- 111.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–8. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 112.Li C, Ying W, Huang Z, Brehm T, Morin A, Vella AT, et al. IRF6 regulates alternative activation by suppressing PPARgamma in male murine macrophages. Endocrinology. 2017 doi: 10.1210/en.2017-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tarassishin L, Suh HS, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. 2011;8:187. doi: 10.1186/1742-2094-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.El Chartouni C, Schwarzfischer L, Rehli M. Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology. 2010;215(9–10):821–5. doi: 10.1016/j.imbio.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 115.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11(10):936–44. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 116.Ying W, Kanameni S, Chang CA, Nair V, Safe S, Bazer FW, et al. Interferon tau alleviates obesity-induced adipose tissue inflammation and insulin resistance by regulating macrophage polarization. PLoS One. 2014;9(6):e98835. doi: 10.1371/journal.pone.0098835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ying W, Tseng A, Chang RC, Morin A, Brehm T, Triff K, et al. MicroRNA-223 is a crucial mediator of PPARgamma-regulated alternative macrophage activation. J Clin Invest. 2015;125(11):4149–59. doi: 10.1172/JCI81656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 119.Zhou H, Xiao J, Wu N, Liu C, Xu J, Liu F, et al. MicroRNA-223 Regulates the Differentiation and Function of Intestinal Dendritic Cells and Macrophages by Targeting C/EBPbeta. Cell Rep. 2015;13(6):1149–60. doi: 10.1016/j.celrep.2015.09.073. [DOI] [PubMed] [Google Scholar]
- 120.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121(6):984–95. doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A. 2005;102(10):3627–32. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179(8):5082–9. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 123.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286(2):1436–44. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31(2):220–31. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A. 2009;106(17):7113–8. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1) J Biol Chem. 2011;286(3):1786–94. doi: 10.1074/jbc.M110.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A. 2009;106(8):2735–40. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD, O’Connell RM, et al. MicroRNA-125b potentiates macrophage activation. J Immunol. 2011;187(10):5062–8. doi: 10.4049/jimmunol.1102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thulin P, Wei T, Werngren O, Cheung L, Fisher RM, Grander D, et al. MicroRNA-9 regulates the expression of peroxisome proliferator-activated receptor delta in human monocytes during the inflammatory response. Int J Mol Med. 2013;31(5):1003–10. doi: 10.3892/ijmm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu Z, et al. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194(3):1239–51. doi: 10.4049/jimmunol.1402088. [DOI] [PubMed] [Google Scholar]
- 131.Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF, et al. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res. 2013;23(11):1270–83. doi: 10.1038/cr.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu F, Li Y, Jiang R, Nie C, Zeng Z, Zhao N, et al. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp Lung Res. 2015;41(5):261–9. doi: 10.3109/01902148.2015.1004206. [DOI] [PubMed] [Google Scholar]
- 133.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caescu CI, Guo X, Tesfa L, Bhagat TD, Verma A, Zheng D, et al. Colony stimulating factor-1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA-21. Blood. 2015;125(8):e1–13. doi: 10.1182/blood-2014-10-608000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Z, Brandt S, Medeiros A, Wang S, Wu H, Dent A, et al. MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS One. 2015;10(2):e0115855. doi: 10.1371/journal.pone.0115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang Q, Zhang JJ, Zhang ZZ. The Protective Effect of Inhibition of PARP-1 on Inflammation Induced by PM2.5 in Human Bronchial Epithelial Cell Line. Sichuan Da Xue Xue Bao Yi Xue Ban. 2016;47(6):825–9. [PubMed] [Google Scholar]
- 137.Reddy MA, Chen Z, Park JT, Wang M, Lanting L, Zhang Q, et al. Regulation of inflammatory phenotype in macrophages by a diabetes-induced long noncoding RNA. Diabetes. 2014;63(12):4249–61. doi: 10.2337/db14-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341(6147):789–92. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krawczyk J, Kraj L, Ziarkiewicz M, Wiktor-Jedrzejczak W. Metabolic and nutritional aspects of cancer. Postepy Hig Med Dosw (Online) 2014;68:1008–14. doi: 10.5604/17322693.1118194. [DOI] [PubMed] [Google Scholar]
- 140.Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, et al. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27(3):370–81. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 141.Rapicavoli NA, Qu K, Zhang J, Mikhail M, Laberge RM, Chang HY. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762. doi: 10.7554/eLife.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 143.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 145.Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16(10):1484–92. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- 146.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. 2004;28(8):993–7. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 147.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18(6):816–30. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 149.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6(5):386–97. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 150.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100(11):1589–96. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 151.Wise LA, Radin RG, Kumanyika SK, Ruiz-Narvaez EA, Palmer JR, Rosenberg L. Prospective study of dietary fat and risk of uterine leiomyomata. Am J Clin Nutr. 2014;99(5):1105–16. doi: 10.3945/ajcn.113.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32(10):2010–9. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barkhausen T, Tschernig T, Rosenstiel P, van Griensven M, Vonberg RP, Dorsch M, et al. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit Care Med. 2011;39(6):1407–13. doi: 10.1097/CCM.0b013e318211ff56. [DOI] [PubMed] [Google Scholar]
- 154.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(6):705–14. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 155.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15(5):423–30. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Braune J, Weyer U, Hobusch C, Mauer J, Bruning JC, Bechmann I, et al. IL-6 Regulates M2 Polarization and Local Proliferation of Adipose Tissue Macrophages in Obesity. J Immunol. 2017;198(7):2927–34. doi: 10.4049/jimmunol.1600476. [DOI] [PubMed] [Google Scholar]
- 157.Wang X, Cao Q, Yu L, Shi H, Xue B, Shi H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight. 2016;1(19):e87748. doi: 10.1172/jci.insight.87748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chu X, Newman J, Park B, Nares S, Ordonez G, Iacopino AM. In vitro alteration of macrophage phenotype and function by serum lipids. Cell Tissue Res. 1999;296(2):331–7. doi: 10.1007/s004410051293. [DOI] [PubMed] [Google Scholar]
- 159.Iacopino AM. Diabetic periodontitis: possible lipid-induced defect in tissue repair through alteration of macrophage phenotype and function. Oral Dis. 1995;1(4):214–29. doi: 10.1111/j.1601-0825.1995.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 160.Vosper H, Patel L, Graham TL, Khoudoli GA, Hill A, Macphee CH, et al. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem. 2001;276(47):44258–65. doi: 10.1074/jbc.M108482200. [DOI] [PubMed] [Google Scholar]
- 161.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–70. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276(20):16683–9. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 163.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Torre-Minguela C, Barbera-Cremades M, Gomez AI, Martin-Sanchez F, Pelegrin P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci Rep. 2016;6:22586. doi: 10.1038/srep22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, et al. Alternative versus classical activation of macrophages. Pathobiology. 1999;67(5–6):222–6. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- 166.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bohlson SS, O’Conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front Immunol. 2014;5:402. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reales-Calderon JA, Aguilera-Montilla N, Corbi AL, Molero G, Gil C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics. 2014;14(12):1503–18. doi: 10.1002/pmic.201300508. [DOI] [PubMed] [Google Scholar]
- 169.Rodriguez-Menocal L, Faridi MH, Martinez L, Shehadeh LA, Duque JC, Wei Y, et al. Macrophage-derived IL-18 and increased fibrinogen deposition are age-related inflammatory signatures of vascular remodeling. Am J Physiol Heart Circ Physiol. 2014;306(5):H641–53. doi: 10.1152/ajpheart.00641.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–9. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2013;288(3):1489–99. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arnold CE, Whyte CS, Gordon P, Barker RN, Rees AJ, Wilson HM. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology. 2014;141(1):96–110. doi: 10.1111/imm.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, et al. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19(6):595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mattos RT, Medeiros NI, Menezes CA, Fares RC, Franco EP, Dutra WO, et al. Chronic Low-Grade Inflammation in Childhood Obesity Is Associated with Decreased IL-10 Expression by Monocyte Subsets. PLoS One. 2016;11(12):e0168610. doi: 10.1371/journal.pone.0168610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145–56. doi: 10.1159/000289203. [DOI] [PubMed] [Google Scholar]
- 176.Aroor AR, DeMarco VG. Oxidative stress and obesity: the chicken or the egg? Diabetes. 2014;63(7):2216–8. doi: 10.2337/db14-0424. [DOI] [PubMed] [Google Scholar]
- 177.Litvinova L, Atochin DN, Fattakhov N, Vasilenko M, Zatolokin P, Kirienkova E. Nitric oxide and mitochondria in metabolic syndrome. Front Physiol. 2015;6:20. doi: 10.3389/fphys.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ortega FJ, Moreno M, Mercader JM, Moreno-Navarrete JM, Fuentes-Batllevell N, Sabater M, et al. Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin Epigenetics. 2015;7:49. doi: 10.1186/s13148-015-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L110–L21. doi: 10.1152/ajplung.00423.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–33. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Turchinovich A, Tonevitsky AG, Burwinkel B. Extracellular miRNA: A Collision of Two Paradigms. Trends Biochem Sci. 2016;41(10):883–92. doi: 10.1016/j.tibs.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 183.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191(12):6250–60. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–4. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–46. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41(1):89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cochain C, Zernecke A. Macrophages and immune cells in atherosclerosis: recent advances and novel concepts. Basic Res Cardiol. 2015;110(4):34. doi: 10.1007/s00395-015-0491-8. [DOI] [PubMed] [Google Scholar]
- 191.Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225(2):461–8. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 192.Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res. 2011;108(8):985–95. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G59–73. doi: 10.1152/ajpgi.00123.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109(24):9517–22. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80(4):802–15. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 196.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43(11):1271–8. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 197.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582(1):97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18(5):519–29. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 199.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(1):22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 200.Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146(2):357–73. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hashimoto D, Chow A, Greter M, Saenger Y, Kwan WH, Leboeuf M, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208(5):1069–82. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fuji S, Takano K, Mori T, Eto T, Taniguchi S, Ohashi K, et al. Impact of pretransplant body mass index on the clinical outcome after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(12):1505–12. doi: 10.1038/bmt.2014.178. [DOI] [PubMed] [Google Scholar]
- 203.Hoff P, Buttgereit F, Burmester GR, Jakstadt M, Gaber T, Andreas K, et al. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2013;37(1):145–51. doi: 10.1007/s00264-012-1724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–23. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 205.Li F, Yang Y, Zhu X, Huang L, Xu J. Macrophage Polarization Modulates Development of Systemic Lupus Erythematosus. Cell Physiol Biochem. 2015;37(4):1279–88. doi: 10.1159/000430251. [DOI] [PubMed] [Google Scholar]


