SUMMARY
Background
Serum pepsinogen (SPG) and anti-Helicobacter pylori serology have been used for gastric risk stratification in Asia.
Aim
To assess utility of these markers in a Western population.
Methods
SPGI measurements were available for 21,895 Finnish male smokers in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. We used Cox proportional hazards models adjusted for potential confounders to estimate gastric cancer hazard ratios (HR) and 95% confidence intervals (95% CI) for low SPGI (<25μg/l). In a subset (n=3,555) with anti-H. pylori serology, these markers jointly defined the following: Group A (H. pylori[−], SPGI[normal]; reference group), Group B (H. pylori[+], SPGI[normal]), Group C (H. pylori[+], SPGI[low]) and Group D (H. pylori[−], SPGI[low]). Odds ratios (ORs) and 95% CI were calculated using multivariate logistic regression.
Results
There were 329 gastric cancers diagnosed an average of 13.9 years after baseline. Prediagnostic low SPGI was significantly associated with increased gastric cancer risk (HR 2.68, 95% CI 1.99–3.61). Among subjects with both SPGI and H. pylori serology, groups B, C and D had increased gastric cancer ORs (95% CI) of 1.79 (1.21–2.64), 3.85 (2.36–6.28) and 6.35 (2.20–18.34) respectively. CagA seropositives had significantly higher ORs than CagA seronegatives within group B (Pheterogeneity=0.01). For groups B and C, repeat SPGI level at 3 years did not further stratify gastric cancer risk.
Conclusions
Low SPGI was associated with increased gastric cancer risk in our large Finnish cohort. A single measurement of SPGI along with H. pylori whole cell and CagA serology provides potentially useful prediction of gastric cancer risk.
Keywords: GASTRIC CANCER, EPIDEMIOLOGY, HELICOBACTER PYLORI, SCREENING
INTRODUCTION
Chronic infection with Helicobacter pylori (H. pylori) is well-known to be a strong risk factor for gastric cancer 1–3. H. pylori colonization of the gastric mucosa induces inflammation that causes chronic gastritis and mucosal atrophy that may eventually lead to gastric cancer 4, 5. Serologic response to infection by H. pylori can be assessed by measuring anti-H. pylori immunoglobulin G (IgG) antibodies using assays based on whole cell sonicate or one or more individual bacterial antigens 6.
Altered levels of serum pepsinogens (SPG), which are mainly produced by the chief cells of the fundic glands of the stomach, reflect the atrophic status (i.e., gland loss) of gastric mucosa 7, 8. SPG levels not only reveal the past infection status or current atrophy of the stomach, respectively, but have also been shown to be predictive of gastric cancer risk9, 10. However, previous studies in Western populations are limited, failing to adjust for potential confounders 11–13 and/or to quantitate risk 14–16, mostly because of small sample sizes. Even in Asian populations, there are only a few prospective studies investigating the main effect of SPGI with gastric cancer risk 17, 18.
Anti-H. pylori antibodies may undergo seroreversion with time and/or progression of disease, and may be undetectable later in the course of disease 19, 20. SPG levels are normal among H. pylori- infected individuals without atrophic gastritis 21 as well as in some cases of gastric cancer, particularly with diffuse-type histology 17, 22. Thus, the combination of the two markers has been suggested to overcome the limits of each, and this has been applied in Japan as a screening tool for gastric cancer, an approach known as the “ABC(D) method” 21, 23. A recent meta-analysis of East Asian studies reported a gastric cancer meta-HR as high as 13 times in the highest risk group 24. Previous studies in Western populations that examined the joint association of serum pepsinogen and anti-H. pylori seropositivity with gastric cancer risk have been limited in sample size (less than 100 cases) 11, 13, 25, did not provide overall risk estimates for the combined effects 26–28, and/or were not adjusted for possible confounders 12.
Gastric cancer is a heterogenous disease, with important epidemiologic differences in among subtypes. For instance, with regards to anatomical subsites, H. pylori is a major risk factor for noncardia but not for cardia gastric cancer 29. Divergent incidence trends have been reported for these subtypes in different populations 30, 31. While intestinal-type gastric cancer is often related to environmental factors such as H. pylori or diet, diffuse-type cancer is more closely associated with genetic predisposition 32. Furthermore, some studies report stronger associations with higher anti-H. pylori antibody titer and/or infection with cytotoxin-associated gene A (CagA) virulence factor-positive strains 33, 34.
The high mortality rate of gastric cancer is mostly a consequence of late detection, stemming from the lack of specific symptoms of the disease 35, 36. Gastric cancer when found early may be curable by endoscopic or minimally invasive surgery 37. In countries of high gastric incidence where general population screening by endoscopy is not routinely conducted, triaging high risk individuals for definitive evaluation by endoscopy would be useful. However, the utility of non-invasive risk stratification by blood tests has not been evaluated outside of a few high-income Asian countries. Therefore, the aims of this study are to evaluate the association of low serum pepsinogen I (SPGI) with gastric cancer risk overall and by subtypes and to assess the combination of H. pylori serology and SPGI as a joint predictor of gastric cancer risk, in a prospective cohort study conducted in a Western population.
METHODS
Study Population
The current analysis represents an extension of prior reports 14, 15, 38, with inclusion of additional cancer cases and consideration of repeated SPGI measurements. Subjects were from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study, a randomized, double-blinded, placebo-controlled, 2 × 2 factorial trial of daily supplementation of alpha-tocopherol (50 mg) and/or beta-carotene (20 mg) for the primary prevention of lung cancer 39, 40. A total of 29,133 Caucasian male smokers aged 50–69 years were originally recruited between 1985 and 1988 in southwestern Finland. At baseline, study participants completed questionnaires on demographic characteristics, self-reported medical history, life-style factors and dietary history. Fasting blood samples were collected at baseline and after 3 years’ intervention, stored in serum aliquots at −70 °C until testing. The study was approved by the Institutional Review Boards of both the National Cancer Institute, Bethesda, Maryland, USA and the National Public Health Institute, Helsinki, Finland. All participants provided written informed consent.
SPGI was measured at baseline and follow-up for ATBC participants who continued in the study for more than 3–5 years (n=21,895, 75% of the original cohort). Low SPGI in either blood sample triggered referral for upper gastrointestinal endoscopy, as previously reported 14, 15. In the current study, baseline measurements were used for determining the association between SPGI and incidence of gastric cancer.
Anti-H. pylori antibody status determined in prior nested-case control analyses of pancreas, biliary tract, esophagus, lung, colorectal, and gastric cancers 38, 41–48 was used to evaluate the joint effect of SPGI and anti-H. pylori antibodies on gastric cancer risk for a total of 3,555 subjects with both measures.
Identification and Classification of Cancer Cases
The intervention phase ended in April, 1993, but subjects have been passively followed-up using the Finnish Cancer Registry which has nearly 100% case coverage of the ATBC cohort 49. Diagnoses of gastric cancer were classified according to the International Classification of Diseases, Ninth Revision (ICD-9) as cardia (ICD-9 code 151.0) or noncardia (ICD-9 codes 151.1–151.9). Two histological subtypes according to Lauren classification, intestinal- and diffuse-types, were separately assessed 50. The median time to gastric cancer diagnosis was 13.9 (Standard Deviation, SD, 6.8) years.
Laboratory Analysis
SPGI analyses were performed by radioimmunoassay in two laboratories. Serum samples were assayed at the laboratory of I. M. Samloff, Sepulveda, California, USA during 1989–1991 51. After that facility was damaged by an earthquake, the remaining samples were assayed at the laboratory of M. Härkönen, Helsinki, Finland from 1992–199314, 15. The SPGI measurements from the two laboratories were standardized and transformed for compatibility, with low SPGI defined as 25 μg/L or less, as previously reported 14, 15, 38.
Anti-H. pylori IgG antibodies were measured by whole cell enzyme-linked immunosorbent assays (ELISA) 52 or multiplex bead-based assays 53, 54, as previously described 38, 41–48. The two methods were standardized to create an indicator variable for seropositivity and the continuous values dichotomized into low vs. high titer among seropositives, as previously reported 55. Antibodies to the H. pylori virulence factor CagA antigen were also measured by ELISA or bead-based assays and classified for statistical analysis as present vs. absent, as previously reported 55. Furthermore, positivity for anti-CagA antibodies was considered indicative of anti-H. pylori seropositivity regardless of other test results, since anti-CagA antibodies can remain positive relatively longer than anti-H. pylori antibodies 56. Antibody titer and anti-CagA seropositivity were evaluated for further refinement of anti-H. pylori associations with gastric cancer risk.
Statistical Analyses
Gastric cancer hazard ratios (HRs) and 95% confidence intervals (95% CI) associated with low SPGI were estimated using Cox proportional hazards models. For each participant, follow-up time was calculated from the date of randomization until the diagnosis of cancer, death or December 31, 2014. In the subset of subjects (n=3,555) with both baseline SPGI and H. pylori serology information available, odds ratios (ORs) and 95% CI were estimated using logistic regression models for the following 4 categories: Group A (H. pylori[−], SPGI[normal]; reference group), Group B (H. pylori[+], SPGI[normal]), Group C (H. pylori[+], SPGI[low]) and Group D (H. pylori[−], SPGI[low]). Subgroup analyses were performed to evaluate variation by anti-H. pylori antibody titer and anti-CagA seropositivity within anti-H. pylori seropositive groups. We calculated the ratio of the ORs and p-heterogeneity for subtypes of gastric cancer in case-case comparisons 57. The associations with histologic subtypes were assessed in both the Cox proportional hazards model and in the logistic models.
The effects of changes between baseline and 3-year follow-up SPGI within each ABCD group were analyzed using logistic regression. The 3-year follow-up measurements were available for 3,462 subjects, approximately 97.4% of the participants with both baseline SPGI and H. pylori serology information. Lag analyses were conducted to estimate marker associations with incident gastric cancer occurring less than 5 years, 5–10 years, and more than 10 years after enrollment. Sensitivity analyses were performed by: 1) excluding overlapping and unspecified subsites for noncardia cancer, 2) limiting to only cancer-free controls and 3) using a previously defined gastric cancer nested case-control set 45. To address the concern for potential bias in case ascertainment due to pepsinogen measurement, two sensitivity analyses were performed. First, a log-rank test assessed whether gastric cancer incidence overall differed between the participants with vs. without SPGI. Second, a chi-square test assessed difference in early (IA, IB) vs. late (II–IV) stage distributions of these incident cancers.
For all analyses, except for the log-rank model, minimally adjusted models included age at randomization and type of assigned intervention in order to account for the experimental study design. Based on known or suspected associations with gastric cancer risk, additional covariates for the full models included Body Mass Index (BMI; kg/m2, continuous), pack years of smoking (continuous), alcohol drinking (g/day, continuous), highest level of education (categorical), fruit intake (g/day), vegetable intake (g/day). The fully adjusted models excluded approximately 5% of subjects who did not have information on dietary factors.
Statistical analyses were conducted using SAS 9.3 (SAS Institute Inc, Cary, NC). All P values were two-sided, and were considered significant for P <0.05.
RESULTS
The baseline characteristics of the total cohort and gastric cancer cases, and subjects with both H. pylori serology and pepsinogen measurements in subset analysis are presented in Table 1. Within the cohort analysis, cancer cases had more years of smoking and lower consumption of fruit and vegetables. Low SPGI was more common among gastric cancer cases than controls. In the subset analysis, gastric cancer cases consumed less vegetables and showed a higher prevalence of low SPGI. All other variables showed no difference between the cases and controls.
Table 1.
Baseline characteristics for ATBC study participants who did or did not develop gastric cancer in the overall study cohort and in the subgroup with both H. pylori serology and serum pepsinogen I measurements
| Cohort analysis (n=21,895) | Subset analysis (n=3,555) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| entire cohort | gastric cancer | controls | gastric cancer | |||||
| N | 21,895 | 329 | 3,291 | 264 | ||||
| Age, y (SD) | 57.0 | (5.0) | 57.3 | (4.9) | 58.2 | (4.9) | 58.0 | (4.9) |
| Body mass index, kg/m2 (SD) | 26.3 | (3.7) | 26.5 | (3.7) | 26.2 | (3.7) | 26.5 | (3.7) |
| Pack years of smoking (SD) | 36.3 | (18.0) | 37.6 | (19.0) | 36.8 | (17.9) | 38.7 | (19.9) |
| Alcohol intake, g (SD) | 17.3 | (20.3) | 17.3 | (20.5) | 17.3 | (21.1) | 18.1 | (21.7) |
| Daily fruit intake, g (SD) | 221.4 | (196.5) | 205.0 | (161.8) | 217.9 | (185.9) | 201.3 | (157.6) |
| Daily vegetable intake, g (SD) | 297.7 | (113.8) | 289.2 | (106.3) | 291.5 | (111.2) | 284.9 | (103.8) |
| Some high school, college, or technical school (%) | 14,772 | 67.5 | 223 | 67.8 | 2,222 | 67.5 | 177 | 67.1 |
| Low SPGI (< 25μg/l), n (%) | 1,791 | 8.2 | 58 | 17.6 | 298 | 9.1 | 51 | 19.3 |
| H. pylori seropositivity, n (%) | 2,536 | 77.1 | 224 | 84.9 | ||||
Abbreviations: ATBC - Alpha-Tocopherol, Beta-Carotene Cancer Prevention, SD - Standard deviation, SPGI – serum pepsinogen I
Table 2 shows the association of low baseline SPGI with subsequent gastric cancer risk. In the fully adjusted Cox model (model 2), low SGPI at baseline conferred 2.7-fold higher risk for gastric cancer (95% CI 1.99–3.61) as compared to normal SPGI. Analysis by anatomical subsite showed HRs of 2.95 (95% CI 2.11–4.12) for noncardia and 2.01 (95% CI 1.05–3.83) for cardia gastric cancers. HRs were significantly elevated for intestinal-type gastric cancer (HR 2.57, 95% CI 1.57–4.21), but not for diffuse-type gastric cancer (HR 0.92, 95% CI 0.33–2.55).
Table 2.
Association of prediagnostic low serum pepsinogen I and subsequent gastric cancer risk in the ATBC Study
| number of cases | person years | model 1 | model 2 | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Gastric cancer total | 329 | 5,724 | 2.53 | 1.89–3.37 | 2.68 | 1.99–3.61 |
| Anatomical subsite | ||||||
| Noncardia | 245 | 4,274 | 2.76 | 1.99–3.83 | 2.95 | 2.11–4.12 |
| Cardia | 84 | 1,449 | 1.93 | 1.04–3.59 | 2.01 | 1.05–3.83 |
| Lauren classification | ||||||
| Intestinal | 115 | 1,759 | 2.33 | 1.43–3.81 | 2.57 | 1.57–4.21 |
| Diffuse | 63 | 1,101 | 1.04 | 0.41–2.61 | 0.92 | 0.33–2.55 |
| Others | 151 | 2,864 | 3.49 | 2.35–5.17 | 3.74 | 2.49–5.61 |
Abbreviations: ATBC - Alpha-Tocopherol, Beta-Carotene Cancer Prevention, HR – hazard ratio, CI – confidence interval
model 1: adjusted for age at randomization and type of intervention
model 2: model 1 + body mass index, pack years of smoking, alcohol drinking, education, fruit intake, and vegetable intake
In the subset with both SPGI and H. pylori serology information available, Groups B, C, D had significantly elevated risks for total gastric cancer as compared to group A, with ORs (95% CI) of 1.79 (1.21–2.64), 3.85 (2.36–6.28), and 6.35 (2.20–18.34), respectively, Ptrend <0.0001 (Table 3). ORs (95% CI) restricted to noncardia gastric cancer were higher for groups B, C and D (Ptrend <0.0001), 3.59 (2.02–6.39), 7.49 (3.84–14.61), and 16.55 (5.26–52.04) respectively. Associations with cardia gastric cancer were not statistically significant for any group, nor were Ptrends statistically significant.
Table 3.
Combination of anti-H. pylori seropositivity and pepsinogen I on risk of gastric cancer in ATBC Study
| control (n=3,291) | case (n=264) | model 1 | model 2 | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| Total Gastric Cancer (n=264) | ||||||||
| Group A | 736 | 22.4 | 35 | 13.3 | ref | ref | ||
| Group B | 2,257 | 68.6 | 178 | 67.4 | 1.70 | 1.17–2.48 | 1.79 | 1.21–2.64 |
| Group C | 279 | 8.5 | 46 | 17.4 | 3.71 | 2.32–5.93 | 3.85 | 2.36–6.28 |
| Group D | 19 | 0.6 | 5 | 1.9 | 5.94 | 2.09–16.92 | 6.35 | 2.20–18.34 |
| p-trend | <0.0001 | <0.0001 | ||||||
|
| ||||||||
| Anatomical subsite | ||||||||
|
| ||||||||
| Noncardia (n=197) | ||||||||
| Group A | 736 | 22.4 | 14 | 7.1 | ref | ref | ||
| Group B | 2,257 | 68.6 | 143 | 72.6 | 3.49 | 2.00–6.09 | 3.59 | 2.02–6.39 |
| Group C | 279 | 8.5 | 35 | 17.8 | 7.40 | 3.89–14.07 | 7.49 | 3.84–14.61 |
| Group D | 19 | 0.6 | 5 | 2.5 | 15.62 | 5.07–48.12 | 16.55 | 5.26–52.04 |
| p-trend | <0.0001 | <0.0001 | ||||||
| Cardia (n=67) | ||||||||
| Group A | 736 | 22.4 | 21 | 31.3 | ref | ref | ||
| Group B | 2,257 | 68.6 | 35 | 52.2 | 0.53 | 0.31–0.92 | 0.58 | 0.33–1.03 |
| Group C | 279 | 8.5 | 11 | 16.4 | 1.30 | 0.61–2.77 | 1.40 | 0.63–3.11 |
| Group D | 19 | 0.6 | 0 | 0.0 | - | - | ||
| p-trend | 0.65 | 0.80 | ||||||
Abbreviations: ATBC - Alpha-Tocopherol, Beta-Carotene Cancer Prevention, OR – odds ratio, CI – confidence interval, SPGI- Serum pepsinogen I,
Group A: H. pylori (−) and normal SPGI, Group B: H. pylori (+) and normal SPGI, Group C: H. pylori (+) and low SPGI, Group D: H. pylori (−) and low SPGI
model 1: adjusted for age at randomization and type of intervention
model 2: model 1 + body mass index, pack years of smoking, alcohol drinking, education, fruit intake, and vegetable intake
H. pylori titer was not associated with risk of noncardia gastric cancer either within group B, the normal SPGI and H. pylori seropositive group (Pheterogeneity=0.23), or within group C, the low SPGI and anti-H. pylori seropositive group (Pheterogeneity=0.52) (Table 4). On the other hand, the CagA seropositive group showed significantly higher OR (4.34, 95% CI 2.41–7.79) than the CagA seronegative group OR (2.45, 95% CI 1.23–4.88) within group B (Pheterogeneity =0.01). There was no significant difference by CagA status in group C (Pheterogeneity =0.06).
Table 4.
Combination of anti-H. pylori titer and CagA seropositivity with pepsinogen I on risk of noncardia gastric cancer in ATBC Study
| control (n=3,291) | case (n=197) | model 1 | model 2 | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| Anti-H. pylori antibody titer in all noncardia cancer (n=197) | ||||||||
| Group A | 736 | 22.4 | 14 | 7.1 | ref | ref | ||
| Group B + low titer H. pylorib | 1,571 | 47.7 | 92 | 46.7 | 3.21 | 1.82–5.68 | 3.34 | 1.85–6.03 |
| Group B + high titer H. pylori | 686 | 20.8 | 51 | 25.9 | 4.15 | 2.27–7.58 | 4.17 | 2.23–7.79 |
| Group C + low titer H. pylorib | 186 | 5.7 | 25 | 12.7 | 7.96 | 4.03–15.73 | 8.09 | 4.00–16.37 |
| Group C + high titer H. pylori | 93 | 2.8 | 10 | 5.1 | 6.34 | 2.72–14.78 | 6.31 | 2.59–15.35 |
| Group D | 19 | 0.6 | 5 | 1.9 | 15.66 | 5.08–48.25 | 16.57 | 5.27–52.13 |
| p-trend | <0.0001 | <0.0001 | ||||||
| Anti-CagA seropositivity in all noncardia cancer (n=186)a | ||||||||
| Group A | 736 | 24.1 | 14 | 7.5 | ref | ref | ||
| Group B + CagA (−)c | 590 | 19.4 | 25 | 13.4 | 2.37 | 1.22–4.60 | 2.45 | 1.23–4.88 |
| Group B + CagA (+) | 1,451 | 47.6 | 110 | 59.1 | 4.18 | 2.38–7.36 | 4.34 | 2.41–7.79 |
| Group C + CagA (−)c | 46 | 1.5 | 11 | 5.9 | 14.14 | 6.04–33.11 | 13.33 | 5.47–32.47 |
| Group C + CagA (+) | 207 | 6.8 | 21 | 11.3 | 6.03 | 2.99–12.15 | 6.28 | 3.04–12.98 |
| Group D | 19 | 0.6 | 5 | 2.7 | 15.71 | 5.09–48.42 | 16.73 | 5.31–52.68 |
| p-trend | <0.0001 | <0.0001 | ||||||
Abbreviations: ATBC - Alpha-Tocopherol, Beta-Carotene Cancer Prevention, OR – odds ratio, CI – confidence interval, SPGI- Serum pepsinogen I,
Group A: H. pylori (−) and normal SPGI, Group B: H. pylori (+) and normal SPGI, Group C: H. pylori (+) and low SPGI, Group D: H. pylori (−) and low SPGI
model 1: adjusted for age at randomization and type of intervention
model 2: model 1 + body mass index, pack years of smoking, alcohol drinking, education, fruit intake, and vegetable intake
excluded with missing CagA status (11 cases & 242 controls) or H. pylori negative/CagA positive cases (157 controls)
Within group B high titer vs. low titer H. pylori (Pheterogeneity = 0.23), group C high titer vs. low titer H. pylori (Pheterogeneity =0.52) in model 2
Within group B CagA (+) vs. CagA (−) (Pheterogeneity = 0.01), group C CagA (+) vs. CagA (−) (Pheterogeneity =0.06) in model 2
The trend across groups was statistically significant in intestinal-type gastric cancer (Ptrend <0.0001) but not in diffuse-type gastric cancer in multivariate analysis (Ptrend =0.25) (Table 5).
Table 5.
Combination of anti-H. pylori seropositivity and pepsinogen I on risk of noncardia gastric cancer by Lauren classification in ATBC Study
| control (n=3,291) | case (n=197) | model 1 | model 2 | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| Intestinal Type Gastric Cancer (n=69) | ||||||||
| Group A | 736 | 22.4 | 5 | 7.3 | ref | ref | ||
| Group B | 2,257 | 68.6 | 51 | 73.9 | 3.47 | 1.37–8.73 | 4.23 | 1.52–11.80 |
| Group C | 279 | 8.5 | 12 | 17.4 | 7.02 | 2.42–20.39 | 8.65 | 2.72–27.50 |
| Group D | 19 | 0.6 | 1 | 1.5 | 8.86 | 0.98–80.45 | 11.95 | 1.25–114.23 |
| p-trend | <0.0001 | <0.0001 | ||||||
| Diffuse Type Gastric Cancer (n=49) | ||||||||
| Group A | 736 | 22.4 | 5 | 10.2 | ref | ref | ||
| Group B | 2,257 | 68.6 | 40 | 81.6 | 2.69 | 1.05–6.86 | 2.57 | 1.00–6.59 |
| Group C | 279 | 8.5 | 4 | 8.2 | 2.27 | 0.60–8.64 | 1.74 | 0.41–7.46 |
| Group D | 19 | 0.6 | 0 | 0.0 | - | - | ||
| p-trend | 0.15 | 0.25 | ||||||
| Other Type Gastric Cancer (n=79) | ||||||||
| Group A | 736 | 22.4 | 4 | 5.1 | ref | ref | ||
| Group B | 2,257 | 68.6 | 52 | 65.8 | 4.53 | 1.63–12.58 | 4.25 | 1.52–11.84 |
| Group C | 279 | 8.5 | 19 | 24.1 | 14.69 | 4.90–44.01 | 13.92 | 4.61–42.09 |
| Group D | 19 | 0.6 | 4 | 5.1 | 46.21 | 10.60–201.47 | 43.81 | 9.89–194.11 |
| p-trend | <0.0001 | <0.0001 | ||||||
Abbreviations: ATBC - Alpha-Tocopherol, Beta-Carotene Cancer Prevention, OR – odds ratio, CI – confidence interval, SPGI- Serum pepsinogen I
Group A: H. pylori (−) and normal SPGI, Group B: H. pylori (+) and normal SPGI, Group C: H. pylori (+) and low SPGI, Group D: H. pylori (−) and low SPGI
model 1: adjusted for age at randomization and type of intervention
model 2: model 1 + body mass index, pack years of smoking, alcohol drinking, education, fruit intake, vegetable intake
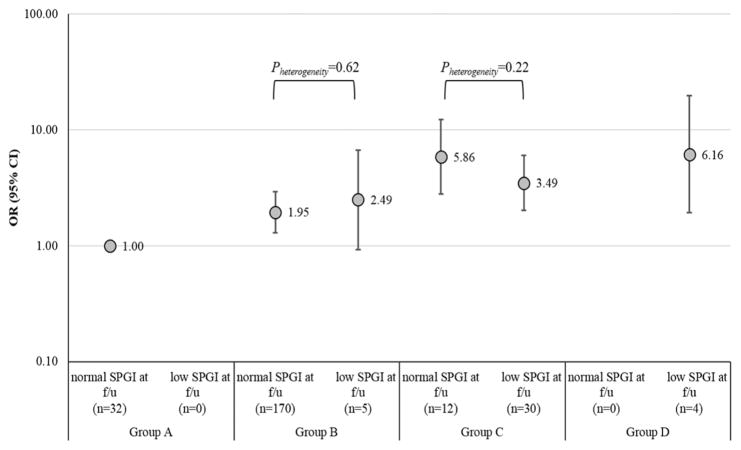
Gastric cancer risks associated with 3-year change of SPGI are shown in Figure 1. Within groups B and C, gastric cancer ORs did not significantly differ between individuals with normal vs. low SPGI at follow-up.
Figure 1.
Association of prediagnostic and 3-year follow-up measurement of serum pepsinogen I and subsequent gastric cancer risk in the ATBC Study
Abbreviations: ATBC – Alpha-Tocopherol, Beta-Carotene Cancer Prevention, f/u – follow-up, OR – odds ratio, CI – confidence interval, SPGI - Serum pepsinogen I,
Group A: H. pylori (−) and normal SPGI, Group B: H. pylori (+) and normal SPGI, Group C: H. pylori (+) and low SPGI, Group D: H. pylori (−) and low SPGI
Model adjusted for age at randomization, type of intervention, pack years of smoking, alcohol drinking, education, fruit intake, vegetable intake
* Numbers in parentheses indicate gastric cancer cases in each group at follow-up
Cases diagnosed before follow-up measurements (≤3 years) were excluded from the analysis (n=5)
In a lag analysis of prediagnostic SPGI and subsequent gastric cancer risk, HR was highest within 5 years after baseline (4.73, 95% CI 1.88–11.88). The estimate is accentuated in noncardia and intestinal-type gastric cancers with HR (95% CI) of 5.97 (2.15–16.56) and 5.63 (1.30–24.32), respectively (Supporting Information Table 1).
Similarly, in the combined analysis of SPGI and anti-H. pylori seropositivity, group D, which is mainly the association of low SPGI (anti-H. pylori seronegative group), had the highest OR when restricted to cancers diagnosed < 5 years after enrollment (OR 16.93, 95% CI 2.85–100.43). The OR decreased to 5.98 (95% CI 1.24–28.93) in lag 5–10 years and 3.16 (95% CI 0.39–25.67) in lag > 10 years (Supporting Information Figure 1). On the other hand, when SPGI was normal the association of anti-H. pylori seropositivity was lowest in lag < 5 years with OR 0.64 (95% CI 0.22–1.91) and increased to OR 1.58 (95% CI 0.86–2.90) in lag 5–10 years and OR 2.43 (95supp% CI 1.38–4.30) in lag > 10 years.
Three separate sensitivity analyses restricting the noncardia cases excluding overlapping and unspecified site (Supporting Information Table 2), using only non-cancer controls from subset of the cohort with both SPGI and H. pylori serology information available from previous nested case-control sets (Supporting Information Figure 2), and testing within one nested case-control set for gastric cancer risk (Supporting Information Figure 3) did not change the risk estimates or the trend of risk.
Based on the log-rank test, there was no significant difference in gastric cancer incidence between those with SPGI testing and those without (p=0.38). Furthermore, the fraction of early gastric cancer did not differ between these two groups (p=0.19).
DISCUSSION
We report the largest study of the association of SPGI and H. pylori serology with gastric cancer risk in a Western population. Our analyses were based on combined marker categories similar to the Japanese “ABC(D)” method which defines low pepsinogen as PGI ≤ 70ng/ml and PGI/PG II ≤ 3.
Despite the difference in definition of low pepsinogen, the risk estimates for noncardia gastric cancer derived from our study are similar to estimates for overall gastric cancer from previous studies in Asia, where cardia cancer is relatively uncommon 58–62 (Supporting Information Table 3).
There are only limited studies that further stratify H. pylori seropositivity within ABCD groups in relation to gastric cancer risk. In our study, titer of anti-H. pylori antibodies was not associated with level of risk among participants with normal SPGI status (i.e., group B). Among individuals with low SPGI status (i.e., group C), the non-significantly higher OR we found for low anti-H. pylori antibody titer may reflect longer exposure to H. pylori infection and more severe disease progression leading to diminished antibody production 63. Previous reports in Asian populations have been inconsistent, associating increased gastric cancer risk with high antibody titer in some studies 59, 64 and with low titer in others 33, 65. To the best of our knowledge, this study is the first to investigate H. pylori antibody titer in relation to SPGI status in a Western population.
Mucosal atrophy represents the intermediate outcome in the causal pathway from H. pylori infection to gastric cancer. Antibodies to CagA have been associated with higher risk of gastric cancer among H. pylori seropositive populations 34, presumably because CagA positive H. pylori strains cause more severe mucosal damage than negative strains. We found anti-CagA seropositivity was associated with gastric cancer risk among individuals with normal pepsinogen (group B), but not with low pepsinogen (group C). Thus, our data implies that CagA-positive infection confers no additional risk once mucosal damage has occurred.
Another point to note is the assessment of the usefulness of repeated measurement of SPGI in predicting gastric cancer risk (Figure 1). Our analysis shows that a 3-year follow-up of SPGI does not differentiate risk within baseline categories of B and C. The current analysis cannot provide evidence for other follow-up intervals but, in our lag analysis for prediagnostic SPGI, the association of SPGI with gastric cancer was highest in the first five years prior to diagnosis (Supporting Information Table 1). Therefore, we cautiously conclude that repeated measurement may not be necessary for SPGI and H. pylori serology assessed at baseline. For individuals with normal levels of SPGI, measurement of anti-CagA antibodies may provide better discrimination of gastric cancer risk.
The current Cox regression analysis associated low SPGI with gastric cancer risk, in line with our previous nested case-control analysis of cases diagnosed through April 2006 38. We found a stronger association within shorter time intervals with the highest HR observed for cancers occurring within 5 years after baseline (Supporting Information Table 1). Our findings replicate previous studies (Supporting Information Table 4), not only regarding the significant association of low SPGI with gastric cancer overall, but also higher risk estimates for noncardia subsites and intestinal-type histology.
The major strength of our study is the use of prediagnostic samples analyzed for SPGI and anti-H. pylori seropositivity at baseline, with a long follow-up period. Another strength is that we have the largest number of cases (n=329) ever studied in a Western population, allowing us for a more comprehensive analysis across group categories and anatomical and histological subtypes, as well as accounting for potential confounder effects. While this study has a large number of gastric cancer cases, sub-group analyses are likely to be underpowered.
Although both men (as compared to women) and smokers (as compared to non-smokers) have higher risks of gastric cancer, our findings based on male smokers may have limited generalizability. Nonetheless, the risk estimates were similar to the previous studies. Another possible limitation of our study is that the SPGI measurement selected high-risk individuals for further screening with endoscopy, but we did not find evidence of excess cancer diagnoses among the screened fraction of the cohort compared to those who were unscreened. Moreover, the analysis incorporating H. pylori serology information was based on availability of data from prior nested case-control datasets for various cancers, but the results were unchanged by restriction to the matched gastric cancer case-control set. We also did not gather information about possible H. pylori eradication therapy post-enrollment.
In conclusion, we found that the joint consideration of SPGI and anti-H. pylori whole cell and CagA seropositivity is a potentially useful predictor for the development of gastric cancer, especially noncardia gastric cancer, in a large population study of a Western population. This noninvasive and relatively inexpensive method could be used to identify high risk individuals for definitive evaluation by endoscopy and/or to advise on H. pylori eradication. Risk stratification could potentially be improved by incorporating other factors, such as an individual’s genetic and epigenetic information 66. Further studies of cost-effectiveness and of risk-specific screening intervals are warranted for development of personalized screening guidelines.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, US National Cancer Institute, National Institutes of Health, Department of Health and Human Services. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was supported by funding provided by the Intramural Research Program of the National Cancer Institute and US Public Health Service contracts HHSN261201500005C.
Footnotes
AUTHORSHIP
Guarantor of the article: Minkyo Song
Author contributions: MS, MCC and CSR designed the study. SJW, GM, NDF, JK, RZSS, CCA, SM and DA collected the data. MS performed the statistical analysis. MS drafted the manuscript. MS, MCC, SJW, GM, NDF, JK, RZSS, CCA, SM, DA and CSR interpreted the results. MS, MCC and CSR edited and revised the manuscript. All authors approved the final version of the manuscript.
References
- 1.Schistosomes liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; Lyon. 7–14 June 1994; 1994. pp. 1–241. [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13(7):851–6. doi: 10.1046/j.1365-2036.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers EJ, Uyterlinde AM, Pena AS, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345(8964):1525–8. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Piazuelo MB. Helicobacter pylori Infection and Gastric Adenocarcinoma. US Gastroenterol Hepatol Rev. 2011;7(1):59–64. [PMC free article] [PubMed] [Google Scholar]
- 6.Kosunen TU, Seppala K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet. 1992;339(8798):893–5. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 7.Samloff IM. Immunologic studies of human group I pepsinogens. J Immunol. 1971;106(4):962–8. [PubMed] [Google Scholar]
- 8.Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83(1 Pt 2):204–9. [PubMed] [Google Scholar]
- 9.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–79. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 10.Huang YK, Yu JC, Kang WM, et al. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(11):e0142080. doi: 10.1371/journal.pone.0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aromaa A, Kosunen TU, Knekt P, et al. Circulating anti-Helicobacter pylori immunoglobulin A antibodies and low serum pepsinogen I level are associated with increased risk of gastric cancer. Am J Epidemiol. 1996;144(2):142–9. doi: 10.1093/oxfordjournals.aje.a008901. [DOI] [PubMed] [Google Scholar]
- 12.Knekt P, Teppo L, Aromaa A, Rissanen H, Kosunen TU. Helicobacter pylori IgA and IgG antibodies, serum pepsinogen I and the risk of gastric cancer: changes in the risk with extended follow-up period. Int J Cancer. 2006;119(3):702–5. doi: 10.1002/ijc.21884. [DOI] [PubMed] [Google Scholar]
- 13.Vohlonen I, Pukkala E, Malila N, et al. Risk of gastric cancer in Helicobacter pylori infection in a 15-year follow-up. Scand J Gastroenterol. 2016;51(10):1159–64. doi: 10.1080/00365521.2016.1183225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varis K, Sipponen P, Laxen F, et al. Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Helsinki Gastritis Study Group. Scand J Gastroenterol. 2000;35(9):950–6. doi: 10.1080/003655200750023011. [DOI] [PubMed] [Google Scholar]
- 15.Varis K, Taylor PR, Sipponen P, et al. Gastric cancer and premalignant lesions in atrophic gastritis: a controlled trial on the effect of supplementation with alpha-tocopherol and beta-carotene. The Helsinki Gastritis Study Group. Scand J Gastroenterol. 1998;33(3):294–300. doi: 10.1080/00365529850170892. [DOI] [PubMed] [Google Scholar]
- 16.Lomba-Viana R, Dinis-Ribeiro M, Fonseca F, Vieira AS, Bento MJ, Lomba-Viana H. Serum pepsinogen test for early detection of gastric cancer in a European country. Eur J Gastroenterol Hepatol. 2012;24(1):37–41. doi: 10.1097/MEG.0b013e32834d0a0a. [DOI] [PubMed] [Google Scholar]
- 17.Oishi Y, Kiyohara Y, Kubo M, et al. The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study. Am J Epidemiol. 2006;163(7):629–37. doi: 10.1093/aje/kwj088. [DOI] [PubMed] [Google Scholar]
- 18.Ren JS, Kamangar F, Qiao YL, et al. Serum pepsinogens and risk of gastric and oesophageal cancers in the General Population Nutrition Intervention Trial cohort. Gut. 2009;58(5):636–42. doi: 10.1136/gut.2008.168641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuipers EJ, Pena AS, van Kamp G, et al. Seroconversion for Helicobacter pylori. Lancet. 1993;342(8867):328–31. doi: 10.1016/0140-6736(93)91473-y. [DOI] [PubMed] [Google Scholar]
- 20.Weck MN, Gao L, Brenner H. Helicobacter pylori infection and chronic atrophic gastritis: associations according to severity of disease. Epidemiology. 2009;20(4):569–74. doi: 10.1097/EDE.0b013e3181a3d5f4. [DOI] [PubMed] [Google Scholar]
- 21.Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - “ABC method”. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(7):405–14. doi: 10.2183/pjab.87.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correa P. Serum pepsinogens in gastric cancer screening. Dig Dis Sci. 2010;55(8):2123–5. doi: 10.1007/s10620-010-1248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasazuki S. The ABC Method and Gastric Cancer: Evidence From Prospective Studies. J Epidemiol. 2016;26(12):611–612. doi: 10.2188/jea.JE20160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terasawa T, Nishida H, Kato K, et al. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PLoS One. 2014;9(10):e109783. doi: 10.1371/journal.pone.0109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen XZ, Schottker B, Castro FA, et al. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7(13):17182–93. doi: 10.18632/oncotarget.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen S, Vollset SE, Derakhshan MH, et al. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56(7):918–25. doi: 10.1136/gut.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurilovich S, Belkovets A, Reshetnikov O, et al. Stomach-specific Biomarkers (GastroPanel) Can Predict the Development of Gastric Cancer in a Caucasian Population: A Longitudinal Nested Case-Control Study in Siberia. Anticancer Res. 2016;36(1):247–53. [PubMed] [Google Scholar]
- 28.Tu H, Sun L, Dong X, et al. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Am J Gastroenterol. 2017;112(5):704–715. doi: 10.1038/ajg.2017.55. [DOI] [PubMed] [Google Scholar]
- 29.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22(3):375–87. doi: 10.1007/s10552-010-9707-2. [DOI] [PubMed] [Google Scholar]
- 30.Camargo MC, Anderson WF, King JB, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60(12):1644–9. doi: 10.1136/gut.2010.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song M, Kang D, Yang JJ, et al. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric Cancer. 2015;18(3):580–9. doi: 10.1007/s10120-014-0411-x. [DOI] [PubMed] [Google Scholar]
- 32.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–61. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatemichi M, Sasazuki S, Inoue M, Tsugane S, Group JS. Clinical significance of IgG antibody titer against Helicobacter pylori. Helicobacter. 2009;14(3):231–6. doi: 10.1111/j.1523-5378.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125(6):1636–44. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–49. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosati G, Ferrara D, Manzione L. New perspectives in the treatment of advanced or metastatic gastric cancer. World J Gastroenterol. 2009;15(22):2689–92. doi: 10.3748/wjg.15.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min YW, Min BH, Lee JH, Kim JJ. Endoscopic treatment for early gastric cancer. World J Gastroenterol. 2014;20(16):4566–73. doi: 10.3748/wjg.v20.i16.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy G, Kamangar F, Dawsey SM, et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst. 2011;103(14):1123–9. doi: 10.1093/jnci/djr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 40.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 41.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93(12):937–41. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 42.Limburg PJ, Stolzenberg-Solomon RZ, Colbert LH, et al. Helicobacter pylori seropositivity and colorectal cancer risk: a prospective study of male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1095–9. [PubMed] [Google Scholar]
- 43.Abnet CC, Kamangar F, Dawsey SM, et al. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40(6):681–7. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]
- 44.Cook MB, Dawsey SM, Diaw L, et al. Serum pepsinogens and Helicobacter pylori in relation to the risk of esophageal squamous cell carcinoma in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1966–75. doi: 10.1158/1055-9965.EPI-10-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook MB, Kamangar F, Weinstein SJ, et al. Iron in relation to gastric cancer in the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2033–42. doi: 10.1158/1055-9965.EPI-12-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koshiol J, Flores R, Lam TK, et al. Helicobacter pylori seropositivity and risk of lung cancer. PLoS One. 2012;7(2):e32106. doi: 10.1371/journal.pone.0032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu G, Murphy G, Michel A, et al. Seropositivity to Helicobacter pylori and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2416–9. doi: 10.1158/1055-9965.EPI-13-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy G, Michel A, Taylor PR, et al. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology. 2014;60(6):1963–71. doi: 10.1002/hep.27193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trial cohort--accuracy and delay. Acta Oncol. 2002;41(4):381–8. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 50.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 51.Samloff IM. Pepsinogens I and II: purification from gastric mucosa and radioimmunoassay in serum. Gastroenterology. 1982;82(1):26–33. [PubMed] [Google Scholar]
- 52.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55(10):2111–5. [PubMed] [Google Scholar]
- 53.Michel A, Waterboer T, Kist M, Pawlita M. Helicobacter pylori multiplex serology. Helicobacter. 2009;14(6):525–35. doi: 10.1111/j.1523-5378.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 54.Gao L, Weck MN, Michel A, Pawlita M, Brenner H. Association between chronic atrophic gastritis and serum antibodies to 15 Helicobacter pylori proteins measured by multiplex serology. Cancer Res. 2009;69(7):2973–80. doi: 10.1158/0008-5472.CAN-08-3477. [DOI] [PubMed] [Google Scholar]
- 55.Sung H, Camargo MC, Yu K, et al. Association of 4p14 TLR locus with antibodies to Helicobacter pylori. Genes Immun. 2015;16(8):567–70. doi: 10.1038/gene.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiota S, Matsunari O, Watada M, Yamaoka Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010;5(12):1885–93. doi: 10.2217/fmb.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35(5):782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charvat H, Sasazuki S, Inoue M, et al. Prediction of the 10-year probability of gastric cancer occurrence in the Japanese population: the JPHC study cohort II. Int J Cancer. 2016;138(2):320–31. doi: 10.1002/ijc.29705. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134(6):1445–57. doi: 10.1002/ijc.28470. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda F, Shikata K, Hata J, et al. Combination of Helicobacter pylori Antibody and Serum Pepsinogen as a Good Predictive Tool of Gastric Cancer Incidence: 20-Year Prospective Data From the Hisayama Study. J Epidemiol. 2016;26(12):629–636. doi: 10.2188/jea.JE20150258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuno S, Miki I, Ishida T, et al. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci. 2010;55(11):3132–7. doi: 10.1007/s10620-010-1154-0. [DOI] [PubMed] [Google Scholar]
- 62.Sasazuki S, Inoue M, Iwasaki M, et al. Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1341–7. doi: 10.1158/1055-9965.EPI-05-0901. [DOI] [PubMed] [Google Scholar]
- 63.Kishikawa H, Kimura K, Takarabe S, Kaida S, Nishida J. Helicobacter pylori Antibody Titer and Gastric Cancer Screening. Dis Markers. 2015;2015:156719. doi: 10.1155/2015/156719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe M, Kato J, Inoue I, et al. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131(11):2632–42. doi: 10.1002/ijc.27514. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki G, Cullings H, Fujiwara S, et al. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1224–8. doi: 10.1158/1055-9965.EPI-06-1048. [DOI] [PubMed] [Google Scholar]
- 66.Shah S, Bonder MJ, Marioni RE, et al. Improving Phenotypic Prediction by Combining Genetic and Epigenetic Associations. Am J Hum Genet. 2015;97(1):75–85. doi: 10.1016/j.ajhg.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.