Abstract
Purpose of review
Reception and transmission of signals across the plasma membrane has been a function generally attributed to transmembrane proteins. In the last three years, however, a growing number of reports have further acknowledged important contributions played by membrane lipids in the process of signal transduction.
Recent findings
In particular, the constituency of membrane lipids can regulate how proteins with SH2 domains and molecules like K-Ras expose their catalytic domains to the cytosol and interact with effectors and second messengers. Recent reports have also shown that the degree of saturation of phospholipids can reduce the activation of certain G-protein coupled receptors, as well as signaling downstream to Toll-like receptor 4 with consequences to NFkB activation and inflammation. Levels of specific gangliosides in the membrane were reported to activate integrins in a cell-autonomous manner affecting tumor cell migration. Furthermore, high resolution of the association of cholesterol with the Smoothened receptor has clarified its participation in sonic hedgehog signaling. These are some of the key advancements that have further propelled our understanding of the broad versatile contributions of membrane lipids in signal transduction.
Summary
As we gain definitive detail regarding the impact of lipid-protein interactions and their consequences to cell function, the options for therapeutic targeting expand with the possibility of greater specificity.
Keywords: cell signaling, protein-lipid interactions, polyunsaturated fatty acids
Introduction
Cellular membranes are highly dynamic structures assembled by amphipathic lipids and proteins. Because these associations are affinity-based, membranes are considered fluid, allowing rotational (movement around their individual axes perpendicular to the membrane), translational (movement across and parallel to the membrane), and transbilayer lipid movement (lipids flipping between opposite external planes) [1–3].
Lipid composition confers important properties on the plasma membrane (PM). Asymmetry, for instance, is largely a product of enriched varieties of lipids within distinct membrane leaflets. In mammalian cells, the PM cytoplasmic leaflet usually contains more phosphatidylethanolamine (PE) and phosphatidylserine (PS) when compared with the outer leaflet rich in sphingolipids [4]. In addition, lipids can exhibit different overall geometric shapes based on inherent properties, such as head group size and the length and/or saturation of their acyl chain tails [5,6]. Maintenance of membrane fluidity, despite the presence of saturated lipids, is sustained by integrated sterols that interfere with acyl chain packing [1,7]. Conversely, because sterols themselves are inflexible they can increase membrane rigidity if associated with flexible unsaturated lipid bilayer [7]. It is worth noting that cholesterol deposition can similarly be asymmetrical and differs greatly among organelles [8,9]. Furthermore, both saturation states and the presence of cholesterol are sufficient to prevent lipid miscibility and lead to specific lipid domains formed by associations of liquid ordered and disordered phases [1,7]. These physical properties can act as the underlying template for membrane curvature, thickness, and tension that, as will be discussed, play important roles in cell signaling.
Furthermore, membrane lipids are known to act as secondary messengers during cell signaling. Many G-protein coupled receptors activate the phosphatidylinositol signaling pathway, whereby phospholipase C hydrolyzes phosphatidylinositol 4,5-biphosphate (PIP2) into two secondary messengers: inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) [10]. In turn, DAG activates protein kinase C and IP3 increases intracellular calcium levels promoting a plethora of cellular responses, including transcription, cell growth and immune responses. Interestingly, another membrane lipid, sphingosine, has been shown to modulate protein kinase C [11].
More recently, membrane lipids have been shown to alter integral membrane receptor signaling either through direct or indirect stoichiometric interactions. Investigations within the last five years have identified important roles of lipids in the regulation of membrane protein receptors during cell signaling. These functions have been uncovered due to recent developments in crystal structure resolution and identification of lipid binding sites in the context of 3D structures. These technical advances have paved the way to a better understanding as to how the composition of the PM offers both a tremendous level of versatility and plasticity in cell signaling. This short review highlights work done, chiefly within the last two years, that have significantly expanded our view of the contribution of membrane lipids in cell signaling.
Membrane lipid composition and cell signaling
It is well accepted that the composition of distinct phospholipids in eukaryotic membranes is essential for maintaining cellular homeostasis. Furthermore, the macrodomain heterogeneity caused by lipid phases can promote segregation and crowding that, in turn, limits lateral diffusion in a manner that affects cell signaling by either facilitating or hindering protein-protein interactions [1]. In addition, the local constituency of phospholipids has been recently shown to impact signaling by regulating the exposure of specific protein domains to the cytosol, as discussed below.
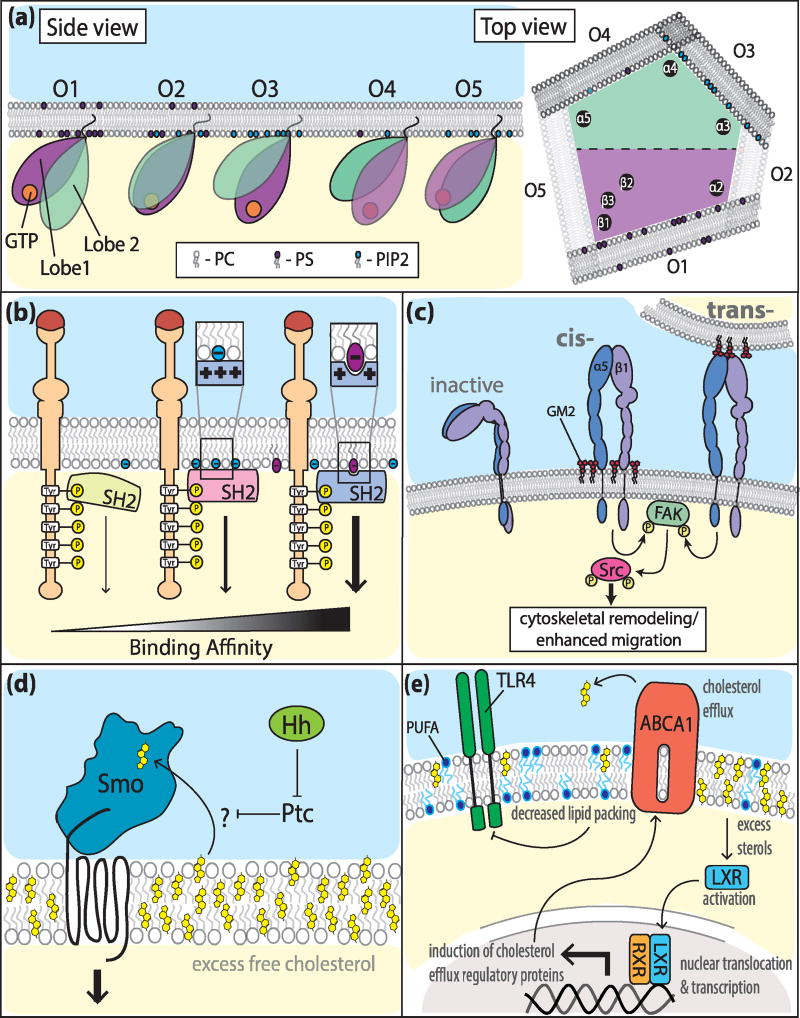
Lipid composition affects how signaling proteins associate with the PM. A recent study has uncovered that the function of K-Ras can be greatly influenced by the composition of phospholipids in the PM as they offer distinct affinity to the protein [12**]. Ras, a small GTPase that regulates multiple signaling pathways and is known to be linked to several cancers, is only fully functional once bound to the PM. Importantly, the orientation of the K-Ras catalytic domain can alter its ability to interact with regulator and effector proteins (Figure 1a). Consequently, how the protein is anchored in the PM has profound effects in its ability to signal. Using all-atom molecular dynamics simulations, Li and Buck found that the association of K-Ras with distinct phospholipids modulates its orientation and therefore its function. The authors showed five possible cytosolic topologies of Ras depending on the type of anionic lipids present at the membrane, resulting in alterations in the exposure of the catalytic domain and therefore its ability to signal [12**]. Interestingly, it also appears that the affinity of K-Ras to membrane lipids is nucleotide-dependent [13]. Nuclear magnetic resonance showed that GDP-bound Ras, unlike its GTP-bound counterpart, has the switch region exposed to the cytosol in a manner that facilitates its interaction with exchange factor proteins, GEFs [13]. These interactions allow Ras reversion to its active form. One could also predict that this association might enable a more prolonged K-Ras activity as GTPase-activating proteins (GAPs) are hindered together with the hydrolysis that renders K-Ras inactive. The type of binding specificity found for K-Ras is predicted to be broad and affect multiple proteins known to interact with lipids in the PM.
Figure 1. Impact of lipid membrane composition on signaling responses.
(a) Side view shows relative orientations (O1-5) of K-RAS4A influenced by anionic membrane composition. Top view illustrates a view of K-RAS4A from its rotational axis, showing which GTPase residues are in closest proximity to the membrane at the various orientations. Darkness of the bilayer indicates more prevalent orientations. (b) SH2 domain modulation of receptor tyrosine kinase signal transduction based membrane lipid binding affinity. In the absence of such affinity, SH2 domains have transient signaling in comparison to those with ACPs. Specific binding pockets in addition to ACPs confer higher affinity and sustain binding/prolong signal transduction. (c) Signaling through α5β1 integrin promotes cytoskeletal remodeling and migration through interactions with GM2. The mechanism of interaction involves direct binding of GM2 with the integrin receptor in either cis or trans configurations. (d) Sterol-dependent signal transduction of Hh mediated de-repression of Smo. Subcellular location of sterol incorporation has yet to be established, though molecular bobbing induced by excess free membrane cholesterol could provide the impetus for association. (e) Reduction of TLR4 mediated inflammatory signaling through decreased membrane lipid packing facilitated by membrane enrichment of PUFAs and LXR/ABCA1 mediated cholesterol efflux. Abbreviations (not found in text): focal adhesion kinase (FAK), Sarcoma proto-oncogene tyrosine-protein kinase (Src), Patched (Ptc), Toll-like receptor 4 (TLR4), retinoid X receptor (RXR).
Moreover, lipid subtypes can provide versatile anchoring platforms that modulate protein-protein interactions and downstream signaling. Along these lines, one of the most well-known modules for protein-protein interactions in cell signaling are the Src homology (SH) domains. Notably, SH2 domains are true binding modules essential to downstream signaling in multiple phosphotyrosine (pY) receptor pathways [14]. Two decades ago, it was reported that SH2 domains bind to lipids in a manner that either inhibit [15] or promote [10] their activity. Nonetheless, these findings have remained controversial as they lacked mechanistic details. In a recent study, Park and colleagues took on the challenge and systematically evaluated the role of lipids in regulating SH2 domain-mediated protein-protein interactions and downstream signaling events [16**]. They provided sound evidence that most SH2 domains bind with high affinity to PM lipids through their so-called “alternate cationic patches” (ACPs), which are domains adjacent to hydrophobic and/or aromatic residues reminiscent of membrane-binding protein lipid-binding sites (Figure 1b). The study demonstrated that SH2 modules have in fact dual specificity with lipid and protein-binding domains. In this manner, the relative locations of the ACPs and pY pocket dictate SH2 domain protein orientation and favor flexible lipid-mediated regulatory mechanisms for pY-signaling outcomes based on lipid binding affinity (Figure 1b). The authors further found that the morphology of the lipid binding ACPs allows for SH2 containing proteins to interact differently with the PM; groove-forming ACPs bind to lipid headgroups, while flat ACPs interact non-specifically with anionic lipids (Figure 1b). In summation, these different associations are predicted to influence the motility of the SH2-containing proteins and their interaction with distinct downstream effectors.
Recent work also revealed direct lipid-dependent receptor signaling. Enrichment of ganglioside GM2 in the PM of cancer cells has been previously associated with tumor progression and epithelial-mesenchymal transition [17*]. Kundu and colleagues provided mechanistic evidence for a novel role of ganglioside GM2 in promoting tumor cell migration in vitro [18**]. Using a variety of in vitro analysis, the group showed that GM2 directly interacts and promotes the activation of beta1 integrins both in cis and in trans (Figure 1c). They demonstrated that activation of beta1 integrins by GM2 promotes downstream signaling with consequent phosphorylation of Erk-MAPK resulting in cytoskeletal changes and increased migration. The findings are paradigm shifting because they provide the first evidence that lipid subtypes can activate integrins in a cell-autonomous manner. It also offers a potential therapeutic strategy not previously considered.
Increases in membrane cholesterol impact on receptor signaling
Structural studies have revealed that cholesterol is a positive regulator for G-protein coupled receptor (GPCR) signaling. The recently resolved Smoothened (Smo) extracellular cholesterol binding cysteine-rich domain is necessary for proper hedgehog (Hh) signal transduction [19**]. As such, this site has been likened to an allosteric agonist region. Mutations or antagonists that conformationally obscure this site prevent Hh signaling [19**]. Furthermore, sterol-induced conformational changes are sufficient to activate Smo [20*] (Figure 1d). The context of this extracellular binding site and the specific source of these sterols require further investigation, though accumulation of cellular cholesterol has been tied to increased activation [21].
As stated previously, membrane lipid composition is dynamic and highly titrated. Activation of liver X receptor (LXR) by increased oxysterol concentrations induces ATP-binding cassette transporter (ABCA1) expression and cholesterol efflux [22]. If efflux is hindered, cholesterol enrichment can be cytotoxic and has been correlated with Toll-like receptor (TLR) activation [23]. Interestingly, LXR activity has been shown to antagonize TLR induced inflammatory gene expression, though whether these signaling pathways are directly tethered has been contentious [23]. However, recent work by Ito and colleagues in macrophages has shown that LXR/ABCA1 induced decreases in raft cholesterol content disrupt TLR4 recruitment of adaptor molecules myeloid differentiation primary response gene 88 (MyD88) and tumor necrosis factor receptor associated factor 6 (TRAF6), preventing downstream inflammatory gene expression [24*] (Figure 1e).
Clearly membrane composition has a significant impact on cellular homeostasis and pathology. It is thus understandable that membrane lipid targeted therapy is a rapidly growing field. However, because protein-lipids interfaces are complex and highly dynamic, a more concrete understanding of how exactly lipids interface with cell surface receptors will be critical in designing targeted therapies.
Extent of phospholipid saturation and cell signaling
Cell signaling events can also be influenced by the degree of saturation present in the phospholipid chains. Alterations in fatty acid saturation is a key property that controls stiffness and elasticity of PMs, and is a well-known mechanism for adaptation to distinct temperatures. It has been previously shown that polyunsaturated fatty acids (PUFAs) can impair lipopolysaccharide (LPS)-mediated activation of TLR4 which eventually promotes the activation of the nuclear factor kappa B (NFkB) [25]. Nonetheless, the mechanisms associated with this process have remained elusive until recently. In an elegant paper by Schoeniger and colleagues, the authors demonstrated that, unlike the predicted mechanism at the level of gene expression, PUFA enrichment affects TLR4 at the membrane [26**]. In fact, the findings provide evidence that PUFAs can disrupt PM microdomains and significantly impair stimulation of TLR4 [27]. The presence of lipid rafts is indeed critical for TLR4 activation. They serve as dynamic platforms for the assembly of specific proteins that enable the organization of protein-ligand interactions [27]. Through this work, Schoeniger and colleagues revealed that slight changes in membrane lipid composition, resulting in minor modulation of the physical-chemical properties of membrane microdomains (rafts), are sufficient to alter cell signal transduction with impressive consequences to inflammation.
Importantly, diet can affect the saturation of phospholipids in the PM. By manipulating the diet of fruit flies, Randall and colleagues could reduce the proportion of polyunsaturated membrane phospholipids by 7-fold [28**]. Interestingly, this was associated with significant effects on the visual performance of the flies. Specifically, photoreceptor responses were reduced 2- to 3- fold. The authors further showed that specific GPCR activation in Drosophila photoreceptors cells relies on by mechanical forces in the membrane induced by PIP hydrolysis by phospholipase C.
Diet was also shown to alter the levels of cell surface Notch1 in endothelial cells with significant consequences to Notch signaling, inflammation and atherosclerosis. We found that high cholesterol gavage of mice for three consecutive days significantly reduced Notch1 protein level in the endothelium of the aorta, a decrease that was not associated with changes in transcript levels [29]. The specific mechanism associated with these changes in Notch1 are currently unclear. However, the associated decrease in Notch signaling resulted in binding of inflammatory cells to the tunica intima and a robust induction of atherosclerotic plaque formation similar to heterozygous Notch1 animals subjected to a normal diet [29].
Plasma membrane proteins contribute to the formation of lipid microdomains
Oligomerized integral proteins and those anchored with cytoskeletal elements can also limit lipid lateral diffusion of PM lipids, retaining certain lipids in the inner leaflet. For example, cortical actin assemblies (asters), known to bind and therefore immobilize PS in the inner leaflet, have also been purported to induce clustering of glycophosphatidylinositol (GPI)-anchored proteins on the outer leaflet with important consequences to cell signaling [30]. Proteins tethered by lipid associations, such as GPI, palmitoyl, myristoyl, or cholesterol, are already preferentially segregated to higher ordered lipid raft domains [31]. Through cholesterol mediated interactions, associations between aster immobilized inner membrane fatty PS and fatty outer membrane lipid anchors are also thought to drive raft formation [32]. Like lipid raft domains, vesicular invaginations known as caveolae are formed by scaffolds of caveolin bound with membrane cholesterol. Importantly, through mediating cell signaling and endocytosis, caveolae also work indirectly to mediate cholesterol transport and homeostasis [33].
Interplay between lipids and proteins in vesicular formation, trafficking and signaling
Cell signaling is highly dependent on the delivery and removal of receptors to the cell surface and clearly the dynamics of exo- and endocytosis rely on specific protein-lipid interactions. Recent work has highlighted the regulatory role of both lipids and proteins during vesicular trafficking and intracellular vesicular transport that contributes to cell signaling. For example, glycerolphospholipids have been shown to regulate vesicular transport. The sorting of glycosylphosphatidylinositol lipid anchors in the endoplasmic reticulum and sphingolipids can change the ability of SNAREs (soluble N-ethylmaleimide sensitive fusion [NSF] attachment protein receptors) to promote membrane fusion [34, 35].
In addition, the process of vesicle formation is another important way by which lipid-protein interactions are known to affect cell signaling. Briefly, membrane curvature necessary for vesicular formation is facilitated by hydrophobic mismatch as results of protein crowding, as well as scaffolding mechanisms with the aid of coat proteins [36*]. Hydrophobic mismatch causes proteins, which have large extramembrane components diffusing in the membrane, to induce molecular crowding in order to lower the accessible membrane surface area and reduced lipids [37].
Conclusion
In addition to their multiple roles in providing unique and critical physical and chemical properties to plasma membranes, lipids have more recently emerged as important regulators of cell signaling. Their new roles are mediated either by their association with receptors and second messengers, as direct ligands to receptors, or by regulating the assembly of complex signaling networks. These advances were made possible by the improvement in structural technology and increased ability to identify specific lipid moieties within specific subdomains. It is likely that this trend will continue and broaden opportunities for potential therapeutic exploitation.
Key points.
Lipid composition contributes to cell signaling and homeostasis.
Membrane lipids can interact directly by associating with receptors or to second messenger molecules.
Lipid saturation states and lipid enrichment can also modulate assembly of signaling networks.
Acknowledgments
Funding sources: This work was supported by National Institutes of Health 2P01HL030568-31 (to MLIA) and by a Ruth L. Kirschstein National Research Service Award GM007185 (to HS).
Footnotes
Disclosures: The authors have no conflicts to disclose
References and recommended reading
Papers of particular interest, published within the period of the review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Goni FM. The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim Biophys Acta. 2014;1838(6):1467–76. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Razi Naqvi K, Gonzalez-Rodriguez J, Cherry RJ, Chapman D. Spectroscopic technique for studying protein rotation in membranes. Nat New Biol. 1973;245(147):249–51. doi: 10.1038/newbio245249a0. [DOI] [PubMed] [Google Scholar]
- 3.Rothman JE, Kennedy EP. Rapid transmembrane movement of newly synthesized phospholipids during membrane assembly. Proc Natl Acad Sci U S A. 1977;74(5):1821–5. doi: 10.1073/pnas.74.5.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Kroon AI, Rijken PJ, De Smet CH. Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Prog Lipid Res. 2013;52(4):374–94. doi: 10.1016/j.plipres.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Zwaal RF, Comfurius P, van Deenen LL. Membrane asymmetry and blood coagulation. Nature. 1977;268(5618):358–60. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- 6.Daleke DL. Phospholipid flippases. J Biol Chem. 2007;282(2):821–5. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- 7.Mondal M, Mesmin B, Mukherjee S, Maxfield FR. Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol Biol Cell. 2009;20(2):581–8. doi: 10.1091/mbc.E08-07-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membr Biol. 2004;21(3):193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18(6):361–74. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae YS, Cantley LG, Chen CS, et al. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(8):4465–9. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 11.Hannun YA, Bell RM. Regulation of protein kinase C by sphingosine and lysosphingolipids. Clin Chim Acta. 1989;185(3):333–45. doi: 10.1016/0009-8981(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 12**.Li ZL, Buck M. Computational Modeling Reveals that Signaling Lipids Modulate the Orientation of K-Ras4A at the Membrane Reflecting Protein Topology. Structure. 2017;25(4):679–89. e2. doi: 10.1016/j.str.2017.02.007. Using all-atom molecular simulations and other experimental strategies, the authors showed that distinct anionic lipids modulate binding and cytosolic orientation of K-Ras4A. Consequently, the availability / exposure of its catalytic domain and ability to interact with effector molecules relies on its interaction with membrane lipids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazhab-Jafari MT, Marshall CB, Smith MJ, et al. Oncogenic and RASopathy-associated K-RAS mutations relieve membrane-dependent occlusion of the effector-binding site. Proc Natl Acad Sci U S A. 2015;112(21):6625–30. doi: 10.1073/pnas.1419895112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim WA, Pawson T. Phosphotyrosine signaling: evolving a new cellular communication system. Cell. 2010;142(5):661–7. doi: 10.1016/j.cell.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rameh LE, Chen CS, Cantley LC. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83(5):821–30. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 16**.Park MJ, Sheng R, Silkov A, et al. SH2 Domains Serve as Lipid-Binding Modules for pTyr-Signaling Proteins. Mol Cell. 2016;62(1):7–20. doi: 10.1016/j.molcel.2016.01.027. Using a combination of structural, biochemical and biophysical data, the authors showed that SH2 domains can anchor themselves in the plasma membrane through specific interactions with anionic lipids. These interactions both facilitate and enhance the affinity for the phosphor-tyrosine domains of activated transmembrane receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Mahata B, Banerjee A, Kundu M, et al. TALEN mediated targeted editing of GM2/GD2-synthase gene modulates anchorage independent growth by reducing anoikis resistance in mouse tumor cells. Sci Rep. 2015;5:9048. doi: 10.1038/srep09048. In this study, the authors showed that Inactivation of GM2 synthase reduced tumor progression and anchorage independent growth of tumor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Kundu M, Mahata B, Banerjee A, et al. Ganglioside GM2 mediates migration of tumor cells by interacting with integrin and modulating the downstream signaling pathway. Biochim Biophys Acta. 2016;1863(7 Pt A):1472–89. doi: 10.1016/j.bbamcr.2016.04.004. The authors showed that GM2 gangliosides are able to interact in cis and in trans with beta1 integrins and promote their activation with important downstream signaling consequences including changes in the cytoskeleton and increase in cell migration. [DOI] [PubMed] [Google Scholar]
- 19**.Byrne EF, Sircar R, Miller PS, et al. Structural basis of Smoothened regulation by its extracellular domains. Nature. 2016;535(7613):517–22. doi: 10.1038/nature18934. Crystal structures of Smoothened generated from this study provided evidence cholesterol’s ability to directly regulate Frizzled class GPCR transmembrane domain conformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Huang P, Nedelcu D, Watanabe M, et al. Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell. 2016;166(5):1176–87. e14. doi: 10.1016/j.cell.2016.08.003. In this study, authors identify cholesterol as endogenous activator of Smoothened and used crystal structures to demonstrate conformational changes of Smo induced by cholesterol binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luchetti G, Sircar R, Kong JH, et al. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. In: Pan D, editor. eLife. Vol. 5. 2016. p. e20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bovenga F, Sabba C, Moschetta A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015;21(4):517–26. doi: 10.1016/j.cmet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Joseph SB, Castrillo A, Laffitte BA, et al. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–9. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 24*.Ito A, Hong C, Rong X, et al. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife. 2015;4:e08009. doi: 10.7554/eLife.08009. Authors provide mechanistic evidence of how LXR/Abca1 mediated cholesterol efflux reduces inflammatory signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIsaac SM, Stadnyk AW, Lin TJ. Toll-like receptors in the host defense against Pseudomonas aeruginosa respiratory infection and cystic fibrosis. J Leukoc Biol. 2012;92(5):977–85. doi: 10.1189/jlb.0811410. [DOI] [PubMed] [Google Scholar]
- 26**.Schoeniger A, Fuhrmann H, Schumann J. LPS- or Pseudomonas aeruginosa-mediated activation of the macrophage TLR4 signaling cascade depends on membrane lipid composition. PeerJ. 2016;4:e1663. doi: 10.7717/peerj.1663. Using a combination of confocal analysis and signaling experimentation, the authors demonstrate that small alterations in polyunsaturated fatty acids are sufficient to alter lipid rafts and impair activation of TLR4 with downstream consequences to NFkB signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumann J, Leichtle A, Thiery J, Fuhrmann H. Fatty acid and peptide profiles in plasma membrane and membrane rafts of PUFA supplemented RAW264.7 macrophages. PLoS One. 2011;6(8):e24066. doi: 10.1371/journal.pone.0024066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Randall AS, Liu CH, Chu B, et al. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J Neurosci. 2015;35(6):2731–46. doi: 10.1523/JNEUROSCI.1150-14.2015. Changing the diet of fruit flies, these authors effectively changed the saturation of fatty acids in the plasma membrane affecting the responses of light sensitive channels (TRP and TRPL) in photoreceptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briot A, Civelek M, Seki A, et al. Endothelial NOTCH1 is suppressed by circulating lipids and antagonizes inflammation during atherosclerosis. J Exp Med. 2015;212(12):2147–63. doi: 10.1084/jem.20150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritzsche M, Li D, Colin-York H, et al. Self-organizing actin patterns shape membrane architecture but not cell mechanics. Nat Commun. 2017;8:14347. doi: 10.1038/ncomms14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomishige M, Sako Y, Kusumi A. Regulation mechanism of the lateral diffusion of band 3 in erythrocyte membranes by the membrane skeleton. J Cell Biol. 1998;142(4):989–1000. doi: 10.1083/jcb.142.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18(6):361–74. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membr Biol. 2004;21(3):193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabeling C, Yu H, Wang L, et al. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci U S A. 2015;112(13):E1614–23. doi: 10.1073/pnas.1421190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogiso H, Taniguchi M, Okazaki T. Analysis of lipid-composition changes in plasma membrane microdomains. J Lipid Res. 2015;56(8):1594–605. doi: 10.1194/jlr.M059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Milovanovic D, Honigmann A, Koike S, Gottfert F, Pahler G, Junius M, et al. Hydrophobic mismatch sorts SNARE proteins into distinct membrane domains. Nat Commun. 2015;6:5984. doi: 10.1038/ncomms6984. Here the authors showed that hydrophobic mismatch due to cholesterol-mediated thickening of the membrane can promote clustering of proteins and formations of specific membrane domains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busch DJ, Houser JR, Hayden CC, Sherman MB, Lafer EM, Stachowiak JC. Intrinsically disordered proteins drive membrane curvature. Nat Commun. 2015;6:7875. doi: 10.1038/ncomms8875. [DOI] [PMC free article] [PubMed] [Google Scholar]