Abstract
Chymase is the most efficient angiotensin II-forming enzyme in the human body and has been implicated in a wide variety of human diseases that also implicate its many other protease actions. Largely thought to be the product of mast cells, the identification of other cellular sources including cardiac fibroblasts and vascular endothelial cells demonstrates a more widely dispersed production and distribution system in various tissues. Furthermore, newly emerging evidence for its intracellular presence in cardiomyocytes and smooth muscle cells opens an entirely new compartment of chymase-mediated actions that were previously thought to be limited to the extracellular space. This review illustrates how these multiple chymase-mediated mechanisms of action can explain the “residual risk” in clinical trials of cardiovascular disease using conventional renin-angiotensin system blockade.
Keywords: myocardial infarction, diabetes, atherosclerosis, ischemia reperfusion injury, heart failure
Introduction
The destructive and protective functions of mast cell proteases have led to the labeling of the mast cell as a contextual chameleon.1 The major protease contents of the mast cell include tryptase, chymase, carboxypeptidase A, and dipeptidyl peptidase I (DPP I). The widely diverse actions of mast cells are determined by the dynamic changes in mast cell contents that is dependent on the type of stress and resulting local environment.1–5 Of these mast cell products, the serine protease chymase (E.C. 3.4.21.39) has received a great deal of attention over the past 30 years. This ~30 kD protein is the predominant angiotensin II (Ang II)-forming enzyme in the human heart and has a catalytic efficiency 20-fold greater than that of angiotensin converting enzyme (ACE).6,7 Although this function is central to the role of renin angiotensin system (RAS) blockade in the treatment of cardiovascular disease, the translation of chymase inhibition to the bedside has been lacking. One reason is the misleading notion that chymase inhibition may be unnecessary because Ang II type I (AT1) receptor blockers will negate any excess Ang II formation that escapes ACE inhibition. The second reason stems from a reluctance to interpret the limited efficacy of RAS inhibitors in major clinical trials as a failure of these therapies to directly access the intracellular sites at which Ang II is formed.8,9
Recent reviews have addressed the question of “residual risk” of ACE inhibitors, AT1 receptor blockade, or their combinational use in the many stroke, hypertension, diabetes, and heart failure trials reported over the past 20 years.8–12 The discussion of these analyses has largely focused on the complete suppression of Ang II formation or the production of other functionally active Ang peptide derivatives.8,9 The need for new therapeutic targets is now fueled by the failure of dual RAS blockade with the addition of the direct renin inhibitor aliskiren in patients with chronic heart failure13,14 or with type II diabetes.15 Likewise, the success of neprilysin blockade added to AT1 receptor blockade in patients with heart failure demonstrates only a 20% benefit over a ACE inhibitor-based treatment approach.16,17
This review will highlight the extensive evidence implicating chymase in pathobiology, with an emphasis on: 1) human and animal studies demonstrating the multifunctional roles of chymase in tissue injury and remodeling, 2) clinical applications of chymase inhibition that complement ACE inhibitor and/or AT1 receptor blockade, and 3) intracellular location and alternative cellular sources of chymase in addition to mast cells.
Multifunctional Mechanisms of Chymase in Tissue Remodeling
Besides being the most efficient Ang II-forming enzyme, chymase has an amazing number of roles in tissue remodeling that are mediated by its direct protease actions, such as:
cleavage of matricellular proteins and peptides, including laminin and fibronectin important in cell survival;18–20 gap junction proteins essential to regulation of intestinal permeability;21 and insulin-derived growth factor 1 (IGF-1), which negates the beneficial effects of IGF-1 in ischemia/reperfusion injury;22 and
activation of peptide and enzyme precursors, including matrix metalloproteinases (MMPs) such as MMP-9;23–26 transforming growth factor-β (TGF-β);27–29 stem cell factor (SCF);30 kallikrein, which produces bradykinin (BK) and causes further mast cell degranulation and chemotaxis;31,32 Interleukin-6 (IL-6) and IL-1β;33 pre-proendothelin I (pre-proET1);34 and IL-18, which is involved the pathophysiology of atopic dermatitis.35
Chymase in Human Pathobiology
The ubiquitous nature and multiple functions of chymase explain why the literature is replete with the importance of mast cells and chymase in various human organ pathologies. Table 1 provides a list of the many disease pathologies that represent a partial list of reports of chymase in human disease.1–5 Many underscore the mechanistic role of chymase in MMP activation in atherosclerosis,36–47 vulnerability of the atherosclerotic plaque and aneurysm formation,48–50 and angiogenesis in tumor progression. 51–53 In concert with its major role as an Ang II forming enzyme in the human heart,6,7,54 chymase has been associated with TGF-β activation and ET-1 formation from pre-proET-1 in pulmonary fibrosis55,56 and chronic obstructive pulmonary disease,57 chronic kidney disease,58,59 polycystic kidney disease,60 diabetic nephropathy61–63 and retinopathy,64 kidney transplant rejection,65 and keloid formation in the skin.66,67 With regard to its direct protease actions, chymase has been implicated in the direct breakdown of placental matricellular proteins and vascular permeability in preeclampsia,68–70 Crohn’s disease,71 and atopic dermatitis.72 Finally, a number of studies have demonstrated chymase-mediated vasoconstriction in isolated human internal mammary arteries, coronary arteries, and saphenous veins (Figure 2).73–77
Table 1.
Chymase Upregulation in Human Disease.
| Pathological Condition | Reference |
|---|---|
| Infarct coronary arteries | 36–39 |
| Atherosclerotic and aneurysmal aortas | 40–49 |
| Pathogenesis of angiogenesis in small cell lung cancer and tumor progression | 51,52 |
| Gastric cancer | 53 |
| Atrial myocardium of patients with ischemic and valvular heart disease | 54 |
| Idiopathic pulmonary fibrosis | 55,56 |
| Chronic obstructive pulmonary disease | 57 |
| Veins of arteriovenous fistula of patients and kidney with end stage renal disease | 58,59 |
| Hypertensive nephropathy | 59 |
| Kidney parenchyma of polycystic kidney disease | 60 |
| Coronary and renal arteries and kidney parenchyma of patients with diabetes | 61–63 |
| Rejected kidney allograft | 65 |
| Skin keloid | 66,67 |
| Preeclampsia | 68–70 |
| Submucosa and muscularis intestinal layers of patients with Crohn’s disease | 71 |
| Atopic dermatitis | 72 |
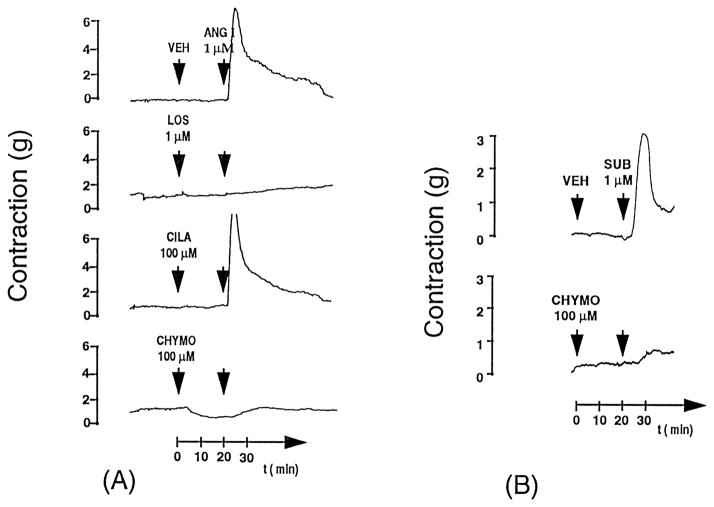
Figure 2. Original tracings of human coronary artery ring preparations from patients with heart failure.
Panel A demonstrates the pressor response to 1 μmol/L Ang I or chymase-specific substrate [Pro11DAla12] Ang I (SUB) with cilazaprilat (CILA, 100 μmol/L), chymostatin (CHYMO, 100 μmol/L), chymostatin and cilazaprilat together, and losartan (LOS, 1 μmol/L). Arrowheads indicate the time of application. The inhibitors were added 20 minutes before the substrates. VEH indicates vehicle. Losartan and chymostatin completely blocked the pressor response to Ang I, whereas cilazaprilat was ineffective. As further confirmation of the chymase function, the chymase specific substrated (SUB) pro-Dala-Ang I contraction is completely by chymostatin.75 Reproduced with permission of the American Heart Association.
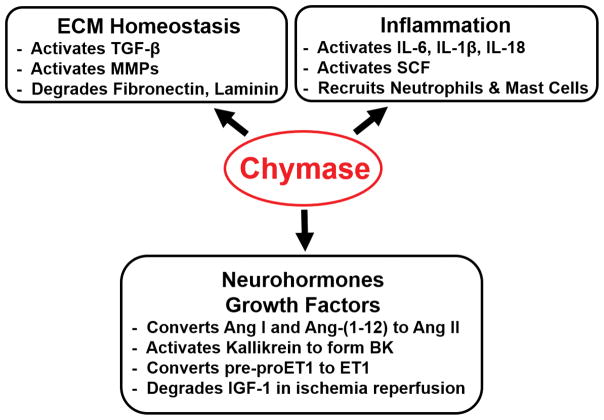
Altogether, chymase has a pervasive presence in human organ systems, with direct and indirect protease actions that mediate acute tissue injury and chronic remodeling. However, the net effect of chymase in tissue remodeling is best exemplified by the widely divergent effects of its presence in mediating extracellular matrix (ECM) breakdown in atopic dermatitis and excessive ECM formation in skin keloids. As will be emphasized in this review, the ultimate phenotype of chymase activation depends on the nature and timing of the stress and the prevailing milieu. In ECM homeostasis, cellular and tissue remodeling, chymase is at the epicenter of a critical balance of: (1) pro-fibrotic ET-1 and Ang II formation, myofibroblast formation, and TGF-β activation, (2) the anti-fibrotic protease activation of MMPs, direct digestion of matricellular proteins, and formation of BK, and (3) inflammatory cytokines and SCF activation and chemotaxis of inflammatory cells (Figure 1).
Figure 1.
Multifunctional Actions of Chymase in Acute and Chronic Tissue Injury and Remodeling.
Chymase in Preclinical Animal Models
Transgenic Mice
The problem with addressing the role of chymase in preclinical animal studies is that the mouse and rat have multiple isoforms of chymase that forms and degrades Ang II (β-chymase). Because of these diverse catalytic specificities, the clinical translatability of pharmacologic blockade cannot be guaranteed, as humans have only the α-chymase isoform.78,79 Nevertheless, the mouse mast cell protease-4 (MCP-4) is the isoform most comparable to human α-chymase because it has similar substrate specificity, tissue distribution, serglycin proteoglycan-binding properties, and the ability to convert Ang I to Ang II.78 It is of interest that the MCP-4-knockout mouse at baseline defines the importance of a functional chymase in mediating MMP activation and ECM homeostasis.80,81 In these mice, histological analysis reveals an increase in collagen in lung and ear tissue and a marked increase in fibronectin. Thus, at baseline there appears to be a net increase of ECM production that results from the reduction of chymase activation of MMP-2 and -9 and a decrease in chymase-mediated degradation of fibronectin.
Accordingly, acute tissue injury that is largely mediated by MMP activation, matricellular breakdown, and inflammatory cell infiltration is ameliorated in MCP-4-knockout mice. Knockout of MCP-4 also improves left ventricular (LV) infarct size and LV dilatation and function after cardiac ischemia/reperfusion injury.22 Moreover, inflammatory cell infiltration and MMP and TGF-β activation are significantly decreased resulting in reduced fibrosis from bleomycin-induced lung injury.82 MCP-4 knockout also leads to decreased blister formation in a transgenic model of bullous pemphigoid,83 protection against ulceration and scarring after a scald dermal burn injury,84 and attenuation of aortic aneurysm formation in a model of elastase perfusion of the aorta.85 Other studies in MCP-4-knockout mice demonstrate decreased conversion of pre-proET1 to ET-1 and the reduction of pulmonary fibrosis in response to bleomycin injury86 and atherosclerosis.87 Ulceration and subsequent scarring from burn injury have also been linked to MCP-4 digestion of the tight junction protein claudin,88 which is prevented in MCP-4-knockout mice but restored by addition of chymase to injured skin. Knockout of MCP-4 also affords protection in mouse models of immune complex-induced encephalomyelitis,89 arthritis,90 and glomerulonephritis.91
However, there are equally important protease actions of MCP-4 that provide protection from inflammation through degradation of inflammatory cytokines in models of sepsis,92 acute spinal cord93 and post-traumatic brain94 injury, and allergic airway disease,95,96 or in the breakdown of snake venom.97 It is of interest that knockout of MCP-4 prevents the degradation of fibronectin and decreases the homing of inflammatory cells and fibrosis in a 7-day model of ureteral obstruction.98
Conversely, transgenic mouse models expressing human chymase are reported to have a three-fold increase in LV Ang II levels,99 in addition to the presence of LV hypertrophy, mild hypertension,100 and increased MMP-9 activity and IL-6 levels. It is of interest that human chymase transgenic mice have leukocytosis and emaciation, with a reduction in skin and visceral fat, and oligotrichia as well.100 Moreover, streptozotocin-induced diabetes is associated with higher plasma glucose levels and a reduced survival rate in these transgenic mice.101 A unique rat smooth muscle chymase, rat vascular chymase (RVCH), has been isolated from the spontaneously hypertensive rat (SHR).102 Transgenic mice with conditional expression of RVCH targeted to smooth muscle cells have hypertension, with a hypertensive arteriopathy manifested by medial thickening in the mesenteric and pulmonary arteries and an increased vasoconstrictor response to phenylephrine.103 Although RVCH has 80% homology to rat mast cell proteases I and II, it has a substrate specificity and Km and Kcat values similar to those of the human chymase (α-chymase).
Thus, it is abundantly clear that human chymase and murine MCP-4, apart from Ang II forming capacity, mediate acute and subacute inflammatory insults that involve the multiple mechanisms of action outlined in Figure 1. In considering the translational implications of studies in mice, MCP-4-knockout mice have a baseline status of ECM production, while the transgenic mice overexpressing human chymase have a generalized emaciated appearance with evidence of systemic inflammation. Taken together, the in vivo phenotypes must be reviewed in the context of the nature of the acute stress, target organ, and timing after the acute stress. Many more pharmacologic studies in the next sections are conducted over a longer period of time and further demonstrate the many functions of chymase in tissue remodeling.
Pharmacologic Studies in Rodents
Although rats and mice have a collection of β-chymases that degrade Ang II,78,79 a number of studies in these animals document the beneficial effects of chymase inhibitors in a wide variety of conditions that again highlight the many destructive protease actions of chymase (Table 2).104–110 There are many more pharmacologic studies that evaluate chymase inhibitors on organ function and remodeling in the more clinically relevant hamster, which has an α-chymase with substrate specificity similar to that of the human chymase and lacks the β-chymases.78,79,112–115 These studies demonstrate a number of beneficial effects of prolonged chymase inhibition in models of more chronic tissue remodeling and function (Table 2).116–128 Chymase-mediated activation of SCF has an important role in the accumulation of mast cells, and these studies also demonstrated a decrease in the number of mast cells and other inflammatory cells with chymase inhibition130 and, importantly, functional stabilization of the mast cell membrane.131 The transgenic studies of MCP-4-knockout mice and the pharmacological studies in mice, rats, and particularly hamsters clearly show the multifunctional role of chymase in the pathophysiology of acute and chronic tissue injury and remodeling.
Table 2.
Preclinical studies in mice, rats and hamsters
| Condition | Model | Chymase Inhibition Effects | Reference |
|---|---|---|---|
| Intermittent Hypoxia | mouse | Decreased perivascular fibrosis and cardiomyocyte hypertrophy, inflammatory cytokines, oxidative stress, Ang II, and superoxide production in LV myocardium | 104 |
| Dextran sodium sulfate-induced colitis | mouse | Decreased neutrophil infiltration and MMP-9 activity resulting in improved disease activity index and histological scores | 105 |
| Ang II induced aneurysm | ApoE- deficient mouse | Decreased MMP-9 activity and macrophage infiltration. Prevented Ang II-induced aortic aneurysm formation in ApoE-deficient mice | 106 |
| Increased salt diet | mouse | Suppressed hypertension and decreased plasma Ang II and aldosterone | 107 |
| Myosin-immunized myocarditis | rat | Decreased MMP-9 activation, TGF-β expression, and macrophage infiltration; Improved survival and LV function 4 weeks after immunization | 108 |
| Stroke-Prone SHR Rat | rat | Decreased aortic MMP-9 activity, TNF-α, MCP-1 levels and macrophage infiltration; Improved vascular function in vitro and survival | 111 |
| Indomethacin- induced colitis | rat | Decreased intestinal wall MMP-9 activation and myeloperoxidase activity; Decreased intestinal lesions and damage | 110 |
| Lipopolysaccharide induced liver injury | hamster | Improved liver function and reduced liver necrosis and fibrosis; Decreased liver MMP-9 activation and myeloperoxidase and TNF-α levels | 116 |
| Carbon tetrachloride- induced chronic liver failure | hamster | Decreased liver myofibroblasts and fibrosis and decreased liver Ang II levels | 117 |
| Elastase-induced aneurysm formation | hamster | Decreased abdominal aortic aneurysm size and mast cell infiltration | 118 |
| Streptozotocin- induced diabetes | hamster | Decreased LV NOX4-induced oxidative stress, malonaldehyde levels, and interstitial fibrosis; Attenuated kidney oxidative stress, decreased renal fibrosis and TGF-β, and improved renal function | 119–121 |
| Cigarette smoking and bleomycin- induced lung injury | hamster | Decreased lung TGF-β signaling, ET-1 levels, and pulmonary hypertension and fibrosis | 122,123 |
| Coronary artery ligation | hamster | Improved LV systolic function and hemodynamics, hypertrophy and fibrosis; Improved survival | 124–126 |
| Obstructed kidney | hamster | Attenuated tubulointerstitial fibrosis and TGF-β and α-smooth muscle actin expression | 127 |
| Prolonged high-fat diet | hamster | Prevented lipid deposition in the aortas | 128 |
Pharmacologic Studies in Dogs
The dog provides one of the better animal models of human chymase biochemistry and physiology, in addition to the baboon132 and macaque.129 These species have only α-chymase with Ang II-forming capacity and MMP-activating potential similar to that of human chymase, without the potential of Ang II degradation of β-chymases.78,79 In the dog, chymase inhibition attenuates neointimal hyperplasia 2 weeks after carotid balloon injury133 and 4 weeks after grafted veins.134 Prolonged chymase inhibition in dogs prevents progression of aortic abdominal aneurysm 8 weeks after elastase treatment,135 development of fibrosis in the pacing tachycardia model of heart failure,136 and development of ventricular arrhythmias after myocardial infarction.137 The next section delves further into the multiple protease actions of chymase in the stretch stimulus of mitral regurgitation (MR) and ischemia/reperfusion injury in the dog.
Potential Cardiovascular Clinical Applications for Chymase Inhibition
Volume Overload
Chymase has a critical role in the myocardial wall thinning and LV chamber dilation in a pure volume overload (VO) in humans with isolated MR,138–140 in dogs with experimentally produced MR,141–145 and in mice146 and rats with aortocaval fistula (Figure 3).147–152 Chymase activity is increased in the left atrium of MR patients,54,153 in the LV of the MR dog,141–143 and in LV of the aortocaval fistula mouse146 and rat.149–152 Although LV ACE activity and Ang II levels are upregulated in the VO heart, AT1 receptor blockade or ACE inhibition exacerbates ECM loss and LV dilatation due to the anti-fibrotic effects of these drugs.144,145 The myocardial response to a pure VO is distinct from the response to pressure overload in that, as opposed to ECM accumulation, there is extensive loss of interstitial collagen and breakdown of matricellular stabilizing proteins, resulting in disruption of the focal adhesion complex and cardiomyocyte elongation and thinning.154–156 In addition to forming Ang II, chymase activates kallikrein and in part may be responsible for increased LV BK in a pure VO in the rat149,150 and dog.156 BK not only perpetuates collagen loss due to its anti-fibrotic effects but also promotes mast cell recruitment and degranulation, resulting in increased chymase activity that is further accentuated by the increase in BK with ACE inhibition.150,157 Chronic chymase inhibition in the dog with isolated MR decreases MMP activation and elevated LV BK levels, prevents fibronectin degradation, and restores the focal adhesion complex, resulting in improved cardiomyocyte shortening and LV systolic function.156
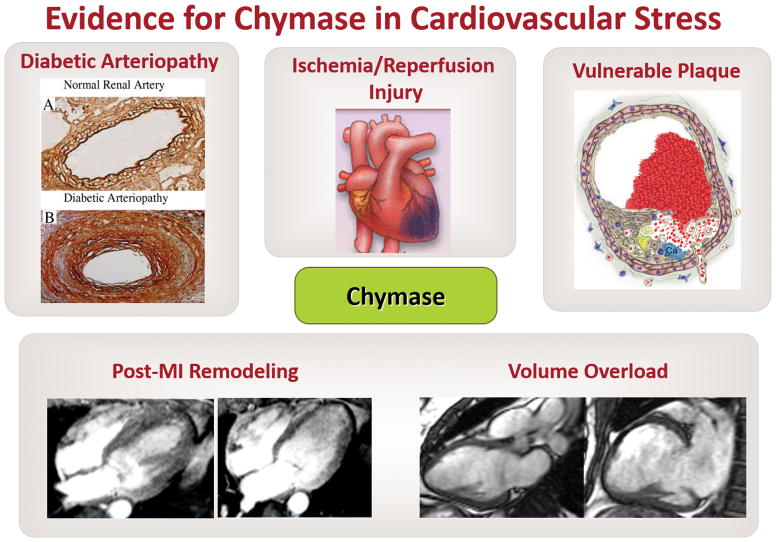
Figure 3. Evidence from human pathology and preclinical animal models supports the important role for chymase in cardiovascular stress.
Upper left panel: demonstrates a cross-section of a human renal artery with marked increase in chymase (brown) extending from the endothelium to adventitia in a patient with diabetes (reproduced with permission of the American Heart Association).252 Center and upper right panels: cartoons demonstrate the potential role of chymase in attenuating ischemia/reperfusion injury and promoting stablization of the vulnerable athersclerotic plaque (reproduced with permission of the American Heart Association). Bottom right panel: demonstrate the marked increase in LV volume and LV wall thinning in a patient with chronic MR (right image) compared with a normal subject (left image) (reproduced with permission of the American Heart Association).253 Bottom left panel: demonstrates the increase in LV volume and apical wall thinning in a patient 6 months after an antero-apical myocardial infarction (right image) compared with three days after myocardial infarction (MI; left image) (reproduced with permission of the American Heart Journal).254 Targeting the multifunctional roles of chymase (Figure 1) in each of these conditions by chymase inhibition will complement conventional RAS blockade in promoting better outcomes and tissue protection.
More recently, we have reported fibroblast production of chymase in the LV of hearts with chronic VO in vivo and in isolated adult rat cardiac fibroblasts subjected to cyclical stretch in vitro.152 In addition, chymase mediates autophagic digestion of pro-collagen and fibronectin within cardiac fibroblasts after cyclical stretch in vitro and VO in vivo.152 These results indicate that the more plentiful fibroblasts, not just the sparsely populated mast cells, account for the abundance of chymase in the heart. Moreover, this finding offers a new chymase-mediated mechanism of ECM loss—through stimulation of fibroblast autophagic digestion of matricellular proteins in the context of a pure VO.
Myocardial Infarction
Understanding the underlying pathophysiology and timing of chymase activation in the context of its many potential direct protease actions may be an important factor in attenuation of acute/subacute tissue injury that translates into improvement in LV remodeling (Figure 3) and a better long-term outcome. More recently, chymase has been identified as an important pharmacologic target in limiting cardiac ischemia/reperfusion injury in the dog158 and pig.159 Again, these models demonstrate a significant acute decrease in MMP-9 activation, a decrease in neutrophil and mast cell infiltration, preservation of fibronectin and laminin, protection of the focal adhesion complex, and a decrease in apoptosis. Furthermore, studies in the MCP-4 knockout mouse identifies IGF-1 as a proteolytic target for chymase.22 All of these chymase-mediated actions result in decreased infarct size and improved LV function.
In the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II), the ACE inhibitor enalapril started within 24 hours after myocardial infarction does not improve survival over 180 days;160 whereas, treatment with captopril at 48 hours and 14 days after myocardial infarction significantly decreased LV dilation over 3 months to 1 year.161,162 In patients dying of myocardial infarction, chymase activity is increased nearly three-fold in both the infarcted and the non-infarcted portions of the LV and represents 90% of Ang II-forming capacity compared to ACE.163 In addition, the left atrium shows the greatest increase in chymase activity, which also suggests that hemodynamic factors such as increased wall stress may be an important stimulus for chymase production/activation.163 In the dog during ischemia/reperfusion injury, in vivo cardiac microdialysis shows an increase in interstitial fluid chymase activity in the infarct and non-infarct regions of the heart, thereby supporting an early activation of chymase with acute myocardial infarction.158
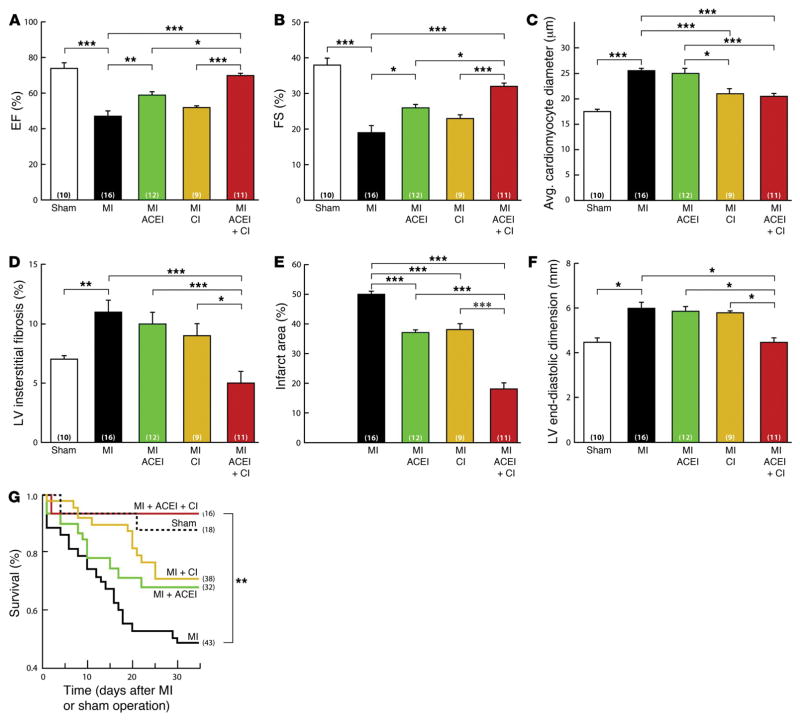
Thus, early chymase inhibition coupled with later ACE inhibition may provide the best outcome in the first month after myocardial infarction. In the hamster model of total left anterior descending artery occlusion, chronic ACE inhibition combined with chymase inhibition (as opposed to chymase or ACE inhibition alone) significantly improves LV remodeling and function, cardiomyocyte hypertrophy, and cardiac fibrosis, as well as survival 1 month after coronary occlusion (Figure 4).157 This study also demonstrates a BK-mast cell interaction that triggers mast cell degranulation and an increase in interstitial fluid chymase activity. This effect is abrogated in the mast cell-deficient mouse or with BK type 2 receptor antagonism in wild-type mice.157 This finding suggests a novel mechanism of “ACE escape” with regard to failure of ACE inhibitor therapy. More importantly, combined therapy with ACE and chymase inhibition attenuates the potential pro-inflammatory effects of BK attendant with ACE inhibition, along with the multiple chymase-mediated protease actions (Figure 1).157 The results of this study raise the question of whether the benefits of chronic ACE inhibition in myocardial infarction may be limited by the ACE inhibitor–dependent chymase release into the cardiac interstitial fluid space.
Figure 4. LV function and mortality after left anterior descending (LAD) artery occlusion in hamster.
The combination of ACE inhibition (ACEi) and chymase inhibition (CI) produces the greatest improvement in LV ejection fraction (EF) (A), fractional shortening (FS) (B), cardiomyocyte diameter (C), LV fibrosis (D), infarct area (E), LV end-diastolic dimension (F), and survival (G) 1 month after LAD occlusion. Drug administration was started within 24 hours after LAD occlusion.157 Reproduced with permission of the American Society of Clinical Investigation.
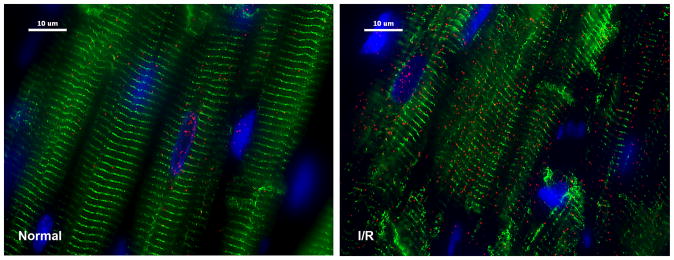
One of the newest discoveries regarding chymase is its presence within cardiomyocytes after ischemia/reperfusion in the dog.158 Figure 5 demonstrates a marked influx of chymase (red) and breakdown of desmin (green) in the cardiomyocyte after 1 hour of occlusion and 2 hours of reperfusion. This now raises many questions regarding the possible role of chymase in cardiomyocyte ischemia/reperfusion injury that may involve activation of intracellular MMPs that have been connected with cardiomyocyte myofibrillar degradation and injury. 164–166 Currently, a new chymase inhibitor, BAY1142524, is being tested for treating patients with LV dysfunction after myocardial infarction in a phase 2 clinical trial (NCT02452515 — clinicaltrials.gov).
Figure 5. Chymase inside dog cardiomyocytes during ischemia/reperfusion.
Adult dogs subjected to 60 minutes of LAD occlusion and 100 minutes of reperfusion (right panel) or normal controls (left panel). Ischemia/reperfusion LV led to a marked increase in chymase (red) in cardiomyocytes with breakdown of desmin (green, right). I/R: ischemia/reperfusion; blue: DAPI.158 Reproduced with permission of Plos One.
Vulnerable Atherosclerotic Plaque
In the setting of non-ST elevation myocardial infarction, which is the predominant presentation in the U.S. today, plaque stability is the key focus and the therapeutic target in the follow-up of these patients (Figure 3). Much of the subsequent risk for myocardial infarction resides in coronary arteries without significant angiographic stenosis, highlighting the importance of the vulnerable plaque. In the Providing Regional Observations to Study Predictors of Events in the Coronary Tree study (PROSPECT), the mean angiographic stenosis of lesions that subsequently produced ischemic events was 32%.167 Over the past 30 years, much attention has been devoted to the identification of the prevalence and detection of the thin-cap fibroatheroma with consensus of opinion regarding its propensity to rupture.168–171
Increases in the number of mast cells and the levels of chymase36–39 and MMPs172 have been identified in the atherosclerotic shoulder region of the infarct-related artery in the human, implicating chymase in plaque rupture by activation of MMPs.36–39,46 In addition, chymase has been implicated in plaque erosion by inducing apoptosis in endothelial cells through direct protease actions that degrade collagen.173 Other studies have demonstrated that chymase-mediated degradation of vitronectin and fibronectin, necessary for maintenance of focal adhesions, results in subsequent inactivation of focal adhesion kinase and Akt and activation of caspase-8 and -9.174,175 Several excellent reviews of these aspects of mast cell proteases and chymase provide a strong argument for chymase inhibition in patients presenting with acute coronary syndrome, expecially those who are at high risk for plaque rupture in the ensuing 6 months.176–178 Finally, chymase may also be directly or indirectly involved in intimal lipid deposition mediated through degradation of ECM177 and in cholesterol efflux by proteolysis of high density lipoprotein 3.179
The identification of increased chymase in the infarct-related artery36–39 and the multiple studies demonstrating a predominant vasoconstrictor response in the coronary artery73–75 further support a role for chymase in the pathogenesis of acute myocardial infarction. Taken together, there is a strong case made by these studies in humans as well as preclinical animal models that chymase may be an important target in acute and chronic atherosclerotic vascular disease.
Diabetes
A connection between diabetes and mast cell chymase in the heart, kidney, and vasculature has been documented in humans and preclinical animal models.180 Advanced glycation end products (AGEs) have been shown to induce chymase expression and a predominance of chymase-dependent Ang II generation (70%) over ACE-dependent generation in human vascular smooth muscle cells via receptor for advanced glycation endproduct (RAGE)-ERK1/2 MAP kinase–dependent mechanism.61 Furthermore, a three-fold increase in chymase immunostaining is demonstrated in renal and coronary arteries in diabetic versus nondiabetic patients.61 A marked increase in chymase, AGEs, RAGE, and phosphorylated ERK1/2 has also been detected in the diabetic coronary arterial wall, spanning the endothelium, media, and adventitia.61 It is of interest that in addition to mast cells, vascular smooth muscle cells are also identified as a major site of increased chymase expression in the human diabetic kidney. Finally, expression of chymase in the glomerulus and tubulointerstitial areas correlates with the increase in blood pressure and the severity of proteinuria.62
The suggestion of a non-mast cell source of chymase is consistent with in vitro studies in human vascular smooth muscle cells demonstrating that high glucose increases both chymase and ACE mRNA.63 In the rat vascular smooth muscle cell, high glucose causes an increase in chymase expression and a switch from ACE-dependent to chymase-dependent Ang II formation.181 In addition to Ang II formation, studies in hamsters119–121 and rats182,183 have connected the beneficial effects of chymase inhibition to the attenuation of oxidative stress, TGF-β activation, and fibrosis in the kidney and heart in the streptozotocin-induced diabetes model. It is well accepted that diabetic nephropathy is a microvascular complication of type II diabetes mellitus. Notably, leptin receptor-deficient db/db mice exhibit a switch from ACE-dependent to chymase-dependent afferent arteriole vasoconstriction and Ang II formation.184,185
In addition to the effects in vascular smooth muscle cells, exogenous high glucose upregulates chymase production in isolated cardiomyocytes and fibroblasts.186–188 This intracrine function further underscores the potential of intracellular chymase-mediated Ang II signaling that is minimally affected by ACE inhibitors and AT1 receptor blockers acting predominantly on the cell surface. A caution for combined therapy of conventional RAS inhibition has been signaled by the results of the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), in which combined AT1 receptor blockade and ACE inhibition associated with more adverse events, without an increase in benefit compared with either monotherapy in patients with cardiovascular disease or diabetes mellitus.189
The next section will discuss a de novo, noncanonical intracellular Ang II-forming process that proceeds from angiotensinogen to Ang-(1-12) to Ang II via chymase. The intracellular Ang II-forming capacity of chymase, in addition to its other protease actions (Figure 1), provides a rationale for chymase inhibition in narrowing the residual risk in clinical trials of RAS blockade in diabetes without a deleterious decrease in blood pressure.
Intracellular location and multiple cellular sources of chymase other than mast cells
At this point, it must be stressed that studies of chymase-mediated Ang II formation and/or cleavage of proteins and peptides have been largely focused on the chymase actions in the extracellular space of organs and tissue. As alluded to above, using cardiac microdialysis in vivo in the dog has revealed a compartmentalization of Ang II formation, with Ang II formation mediated by chymase in the cardiac interstitium and by ACE in the intravascular space.190 In the ACE-knockout mouse, tissue chymase activity is upregulated such that tissue Ang II levels are preserved in the heart, lung, and kidney while circulating Ang II levels are undetectable.191 As alluded to in the previous section, this tissue compartmentalization of chymase has now been expanded to include its role in the intracrine RAS.192–194
Chymase-mediated Ang II formation in neonatal cardiomyocytes and fibroblasts in response to glucose stress,186–188 production of chymase by fibroblasts in response to VO in the rat,152 and chymase production in smooth muscle cells in the human with diabetes62 also generate questions regarding the compartmentalization of chymase’s direct protease actions and Ang II formation within the cell. Results from a growing number of studies suggest that angiotensin-(1-12) [Ang-(1-12)] serves as an alternate intracellular precursor for the generation of Ang II.195–197,195–204 In particular, this concept is supprted by the presence of Ang-(1-12) along with chymase in left atrial myocytes of patients undergoing a Cox Maze procedure for atrial fibrillation ablation.54,204 In support of this contention, conversion of Ang-(1-12) to Ang II by chymase within the adult rat cardiomyocyte results in an increase in the duration of the action potential followed by the generation of early afterdepolarizations.205 In these experiments, a chymase inhibitor dialyzed intracellularly with Ang-(1-12) prevented the effect of intracellular Ang-(1-12) delivery on the potassium currents.205 Importantly, this study directly demonstrates for the first time that chymase has an intracellular function that is affected when the AT1 receptor blocker is delivered with Ang-(1-12) intracellularly, suggesting that intracellular AT1 receptors are required for the signaling.205 It is important to note that in the human heart failure heart, endothelial cells and other interstitial cells are identified as a major source of chymase in addition to mast cells.206 Taken together, these results suggest that mast cells, endothelial cells, and fibroblasts are a major source of chymase in the human heart and raise the provocative question of whether chymase is also produced within cardiomyocytes or is largely taken up via endocytosis.
Earlier studies have demonstrated increased Ang-(1-12) uptake in neonatal myocytes cultured from the spontaneously hypertensive rat.198 We have recently demonstrated that chymase is taken up by mouse cardiac myocytes (HL-1 cells)158 and adult rat cardiac fibroblasts152 by a dynamin-mediated process. It is of interest that other serine proteases, including neutrophil elastase and cathepsin G, have been shown to enter cancer cells by clathrin-mediated endocytosis.207 However, the actual uptake mechanism in vivo remains elusive and the in vitro addition of the active protease may not reliably recapitulate the in vivo condition. Two recent studies have reported the delivery of exosomes containing Ang II208 and AT1 receptors209 into cells, thereby providing another potential mechanism of chymase trafficking directly into the cardiomyocyte cytoplasm.
With regard to cellular uptake of RAS components, it has been 46 years since radiolabeled Ang II injected into the circulation of the rat in vivo was found in the nucleus.210 Subsequently, AT1 and AT2 receptors have been located in the nuclear membrane211,212 and shown to produce angiotensinogen/renin mRNA and nitric oxide (NO),213 respectively, in response to Ang II. Ang II has been shown to bind to chromatin214 with a subsequent increase in RNA synthesis and gene transcription of angiotensinogen and renin.215 Ang II and its AT1 receptor in endo-lysosomes produce increased cytosolic Ca2+.216,217 AT1 and AT2 receptors have also been identified on the inner mitochondrial membrane, and when activated, regulate NO production and respiration in isolated mitochondria (Figure 6).218,219 Intracellular microinjection of Ang II directly in single kidney proximal tubular cells increases [Ca2+] levels in the presence of extracellular losartan, suggesting activation of intracellular AT1 and AT2 receptors.220 Loading of renal mesangial cell nuclei with fluorescent NO prior to incubation with Ang II (1 nM) produces NO that is blocked by the non-specific AT2 receptor antagonist PD-123319 but not the AT1 receptor blocker losartan.220
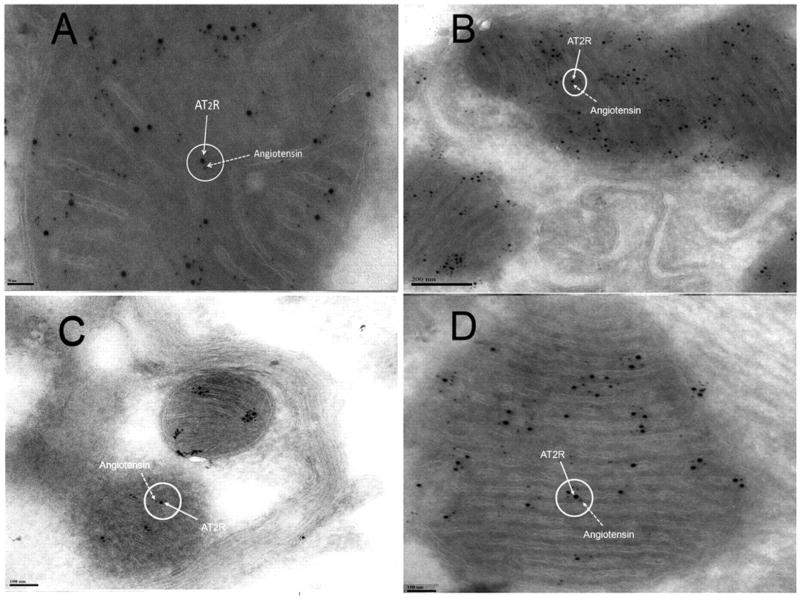
Figure 6.
Immunogold transmission electron microscopy using gold-labeled anti-AT2R antibody (12 nm gold) and a gold-labeled anti-Ang antibody (6 nm gold) demonstrates the localization of the AT2 receptor and binding thereof to Ang in the mitochondria of mouse hepatocytes (A), kidney tubular cells (B), neurons (C), and cardiac myocytes (D).218 Reproduced with permission of PNAS.
Overall, these studies reveal the presence of AT1 and AT2 receptors on various cellular organelles with documented secondary physiological and/or second messenger effects based on Ang II microinjection into intact cells.220 Future work must unravel the intracrine from extracellular Ang II biology. The challenge will be to separate the similar intracellular and extracellular actions of Ang II, and will require studies that incorporate intracellular Ang II trafficking.221–223
Serine Protease Activation and Inhibition in vivo
Chymase is one of a large family of serine proteases in mast cells that have essential roles in blood coagulation, apoptosis, inflammation, and host immunity, among other processes.1–5 Indeed, immune cells express a wide variety of serine proteases, such as granzymes in cytotoxic lymphocytes; elastase, proteinase 3, and cathepsin G in neutrophils; and chymase and tryptase in mast cells. All of these serine proteases have somewhat similar actions in tissue remodeling, as outlined in Figure 1. Although beyond the scope of this chymase review, additional therapeutic options in the innovative area of protease inhibition deserves some mention.
For example, the prominent role of neutrophils and Cathepsin G in ischemia reperfusion injury of the heart has led to the development of dual chymase-cathepsin G inhibitors.224,225 Administration of Cathepsin G-chymase inhibitor immediately after reperfusion of the ischemic myocardium demonstrates the acute (24 hour) and longer term effects (7 days) after 30 minutes of occlusion in mice treated daily after reperfusion.226 At 24 hours dual inhibition significantly reduce the increase in pro-inflammatory signaling pathways, Signal Transducer And Activator Of Transcription 3ST and Nuclear Factor-κB, myeloperoxidase, and accumulation of pro-inflammatory cytokines TNF-α- and IL-1β. At 7 days, dual inhibition reduces myofibroblast density and accumulation of profibrotic connective tissue growth factor, and TGF-β gene expression that is associated with a decrease in interstitial collagen. Altogether, this results in a decrease in infarct area and significant attenuation of LV dilatation and improvement in LV function at 7 days. Collectively, these data underscore the importance of that dual inhibition of Cathepsin G and chymase in the early inflammatory influx and later extracellular matrix acculation in the ischemia reperfusion injury.
In addition to chymase, mast cell tryptase is present in the shoulder region of the vulnerable atherosclerotic plaque36–38 and in abdominal aortic aneurysm.227 A recent review on the status of currently available tryptase inhibitors reports newly available tryptase inhibitors (APC-366 and RWJ-58643) showing promise in clinical trials of psoriasis and ulcerative colitis.228 Neutrophil elastase and mast cell tryptase are key proteases in mediating lung injury and remodeling in chronic obstructive lung disease, cystic fibrosis, and α1-antitrypsin deficiency. Neutrophil elastase is one of the main targets of α1-antitrypsin, an acute-phase reactant protein that functions primarily as a serine protease inhibitor.229 The recent Randomized, placebo-controlled trial of augmentation therapy in Alpha 1-Proteinase Inhibitor Deficiency (RAPID) and the RAPID Extension trials confirmed the benefits of α1-antitrypsin therapy in slowing disease progression in patients with α1-antitrypsin deficiency.230
In the absence of a pathophysiologic stimulus, chymase and other serine proteases are held in check by members of the serpin superfamily, including α1-antitrypsin, α1-antichymotrypsin, and α2-macroglobulin, maintaining the balance of protease actions in tissue and in the circulation.231,232 Providing support for this contention, we have used cardiac microdialysis to measure Ang II-forming capacity from the chymase-specific substrate [Pro11-D-Ala12] Ang I infused through a microdialysis probe into the dog heart in vivo.158 At baseline, chymase-mediated Ang II formation is negligible, whereas Ang II production increases significantly after occlusion and reperfusion of the left anterior descending artery. Other studies have shown that chymase-mediated conversion of Ang I to Ang II is almost completely inhibited in human heart tissue extracts when incubated with skin interstitial fluid, which interestingly has high concentrations of α1-antitrypsin.233 Numerous studies of mast cells in skin blister formation have emphasized the important in vivo interplay between serpins and mast cell chymase/tryptase in addition to the physiologic effects of heparin binding, which also inhibits the protease activities.234 The identification of chymase production by cells other than mast cells raises questions regarding whether the same inhibitory packaging that is specific to mast cells is also in place in endothelial cells and fibroblasts when they are stimulated to produce chymase.
The importance of serpins in vivo has been demonstrated in transgenic mouse models of SERPINA3, the murine homologue of the human α1-antichymotrypsin. SERPINA3 overexpression promotes greater sarcolemma membrane integrity and stability in dystrophic mouse models and also promotes increased membrane residence of integrins.235 SERPINA3N inhibits decorin degradation by granzyme B and leads to enhanced collagen remodeling and reinforcement of the adventitia that augments the rate of rupture and death in a mouse model of abdominal aortic aneurysm.236 In vitro studies of smooth muscle cells plated on fibronectin demonstrated that in the absence of the proteinase inhibitors (α1-antitrypsin, α1-antichymotrypsin, and α2-macroglobulin), fibronectin is degraded and that promotes apoptosis and caspase activation that is prevented in the presence of inhibitors.237 This interaction is further complicated in vivo by the fact that serpins may be degraded by proteinases such as MMPs238–241 and also by chymase, which has a four-fold greater catalytic efficiency than that of MMPs in serpin degradation.242 Thus, a chymase inhibitor may also preserve serpins indirectly by inhibiting MMPs and by directly preventing degradation of serpins.
On the other hand, cathepsin C, also known as DPP1, is a cysteine exopeptidase that is expressed in many inflammatory cells, but is especially abundant in mast cells.1–3,243 DPP1 is clinically attractive because it is the upstream activator of tryptases, chymases, elastase, and cathepsin G, removing N-terminal pro-dipeptides from the zymogen forms of these proteases. The cathepsin C-knockout mouse lacks chymase activity in mast cells.1–3,243 Although a substantial number of cathepsin C inhibitors have been developed, especially for their ability to decrease neutrophil-driven elastase activity in lung disease,244 further clinical development has stalled due to challenges with drug design. In addition, there remains a question of whether cathepsin C protease activation in humans is as important as it appears to be in mice.243
Since mast cells contain large amounts of chymase and other proteases involved in tissue injury and adverse remodeling, there has been some interest in mast cell stabilizers in cardiovascular disease.176,177,180 However, the bidirectional function of mast cells in mediating injury as well as host immunity is problematic for chronic implementation of mast cell stabilizers. Another potential concern is that chronic mast cell stabilization in chronic MR in the dog results in a decrease in LV systolic function that is further verified in isolated adult dog cardiomyocytes, where mast cell stabilization causes a marked depression of the cardiomyocyte calcium transient and fractional shortening.245 As a result, inhibiting chymase or a combination of proteases is a more appropriate therapeutic approach. Such an approach is now especially enticing given that cells other than mast cells produce excessive chymase under pathologic stimuli such as hypoxia,246,247 hemodynamic stretch,152 and excessive glucose.186–188 This finding, coupled with the known benefits of mast cell contents in host immunity and attenuation of inflammation,1–3 in addition to the alternative cellular sources of chymase, may dampen the enthusiasm for mast cell stabilizers in humans with LV dysfunction.
Synopsis
As documented by this review, there is a plethora of evidence for chymase upregulation in cardiovascular disease in humans and for the beneficial effects of its blockade in preclinical animal models of myocardial infarction, ischemia reperfusion injury, cardiac VO, aortic aneurysm formation, atherosclerosis, and diabetic kidney and cardiovascular disease (Figure 3). In addition, increased chymase expression has been reported in African Americans,248 in aging rats,249 in females versus males subjected to pressure overload,250 and in oophorectomized rats.251 Race, age, and gender add to the potential for chymase in explaining the “residual risk” associated with ACE inhibition and AT1 receptor blockade in the treatment of cardiovascular disease. This concept, coupled with multifunctional protease actions of chymase and the multiple cellular sources of chymase other than mast cells, provide a strong rationale that chymase blockade will provide complementary or synergistic actions with current RAS and/or adrenergic blockade. This viewpoint is further fueled by emerging evidence for its intracellular presence and actions that are observed even in the presenceof ACE inhibition or AT1 receptor blockade. The challenge for the future is to 1) further understand mechanisms of intracellular and extracellular chymase activation and how this may affect therapeutic efficacy in acute or chronic cardiovascular disease, 2) document synergism with conventional therapy in preclinical animal models, and finally, 3) translate this functional information into targeted clinical trials.
Supplementary Material
Acknowledgments
Source of Funding: This work was supported by grants from Department of Veteran Affairs for Merit Review (Grant 1CX000993-01 and 1BX003664-01 to LJD) and NIH (Grant P01 HL051952 to CMF, JFC and LJD).
Non-standard Abbreviations and Acronyms
- AGEs
advanced glycation end products
- Ang II
angiotensin II
- ACE
angiotensin converting enzyme
- AT1
angiotensin II type I
- DPP I
dipeptidyl peptidase I
- BK
bradykinin
- ECM
extracellular matrix
- IGF-1
insulin-derived growth factor 1
- IL
Interleukin
- LV
left ventricular
- MCP-4
mast cell protease-4
- MMPs
matrix metalloproteinases
- MR
mitral regurgitation
- NO
nitric oxide
- pre-proET1
pre-proendothelin I
- RVCH
rat vascular chymase
- RAGE
receptor for advanced glycation endproduct
- RAS
renin angiotensin system
- SHR
spontaneously hypertensive rat
- SCF
stem cell factor
- TGF-β
transforming growth factor-β
- VO
volume overload
Footnotes
Conflicts of Interest: None.
References
- 1.Trivedi NN, Caughey GH. Mast cell peptidases: chameleons of innate immunity and host defense. Am J Respir Cell Mol Biol. 2010;42:257–267. doi: 10.1165/rcmb.2009-0324RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol. 2011;716:212–234. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caughey GH. Mast cell proteases as pharmacological targets. Eur J Pharmacol. 2016;778:44–55. doi: 10.1016/j.ejphar.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He A, Shi GP. Mast cell chymase and tryptase as targets for cardiovascular and metabolic diseases. Curr Pharm Des. 2013;19:1114–1125. doi: 10.2174/1381612811319060012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heutinck K, ten Berge I, Hack C, Hamann J, Rowshani A. Serine proteases of the human immune system in health and disease. Mol Immunol. 2010;47:1943–1955. doi: 10.1016/j.molimm.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Balcells E, Meng QC, Johnson WH, Jr, Oparil S, Dell’Italia LJ. Ang II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273:H1769–H1774. doi: 10.1152/ajpheart.1997.273.4.H1769. [DOI] [PubMed] [Google Scholar]
- 7.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:2234–22357. [PubMed] [Google Scholar]
- 8.Reyes S, Varagic J, Ahmad S, VonCannon J, Kon ND, Wang H, Groban L, Cheng CP, Dell’Italia LJ, Ferrario CM. Novel cardiac intracrine mechanisms based on Ang-(1–12)/Chymase axis require a revision of therapeutic approaches in human heart disease. Curr Hypertens Rep. 2017;19:16. doi: 10.1007/s11906-017-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario CM, Mullick AE. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol Res. 2017;125(Pt A):57–71. doi: 10.1016/j.phrs.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugts JJ, van Vark L, Akkerhuis M, Bertrand M, Fox K, Mourad JJ, Boersma E. Impact of renin–angiotensin system inhibitors on mortality and major cardiovascular endpoints in hypertension: A number-needed-to-treat analysis. Int J Cardiol. 2015;181:425–429. doi: 10.1016/j.ijcard.2014.11.179. [DOI] [PubMed] [Google Scholar]
- 11.Baker WL, Coleman CI, Kluger J, Reinhart KM, Talati R, Quercia R, Phung OJ, White CM. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors or angiotensin II-receptor blockers for ischemic heart disease. Ann Intern Med. 2009;151:495, 861–871. doi: 10.7326/0003-4819-151-12-200912150-00162. [DOI] [PubMed] [Google Scholar]
- 12.Dusing R. Mega clinical trials which have shaped the RAS intervention clinical practice. Ther Adv Cardiovasc Dis. 2016;10:133–150. doi: 10.1177/1753944716644131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gheorghiade EM, Böhm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJ, Krum H, Abraham WT, Dickstein K, Køber LV, Desai AS, Solomon SD, Greenlaw N, Ali MA, Chiang Y, Shao Q, Tarnesby G, Massie BM ATMOSPHERE Committees Investigators. Aliskiren, Enalapril, or Aliskiren and Enalapril in Heart Failure. N Engl J Med. 2016;374:1521–1532. doi: 10.1056/NEJMoa1514859. [DOI] [PubMed] [Google Scholar]
- 15.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA ALTITUDE Investigators. Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes. N Engl J Med. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 16.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 17.Hubers SA, Brown NJ. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation. 2016;133:1115–11124. doi: 10.1161/CIRCULATIONAHA.115.018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vartio T, Seppa H, Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981;256:471–477. [PubMed] [Google Scholar]
- 19.Hara M, Matsumori A, Ono K, Kido H, Hwang MW, Miyamoto T, et al. Mast cells cause apoptosis of cardiomyocytes and proliferation of other intramyocardial cells in vitro. Circulation. 1999;100:1443–1449. doi: 10.1161/01.cir.100.13.1443. [DOI] [PubMed] [Google Scholar]
- 20.Leskinen MJ, Lindstedt KA, Wang Y, Kovanen PT. Mast cell chymase induces smooth muscle cell apoptosis by a mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler Thromb Vasc Biol. 2003;23:238–243. doi: 10.1161/01.atv.0000051405.68811.4d. [DOI] [PubMed] [Google Scholar]
- 21.Fu Z, Thorpe M, Hellman L. rMCP-2, the major rat mucosal mast cell protease, an analysis of its extended cleavage specificity and its potential role in regulating intestinal permeability by the cleavage of cell adhesion and junction proteins. PLoS One. 2015;10:e0131720. doi: 10.1371/journal.pone.0131720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tejada T, Tan L, Torres RA, Calvert JW, Lambert JP, Zaidi M, Husain M, Berce MD, Naib H, Pejler G, Abrink M, Graham RM, Lefer DJ, Naqvi N, Husain A. IGF-1 degradation by mouse mast cell protease 4 promotes cell death and adverse cardiac remodeling days after a myocardial infarction. Proc Natl Acad Sci U S A. 2016;113:6949–6954. doi: 10.1073/pnas.1603127113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urata H. Chymase and matrix metalloproteinase. Hypertens Res. 2007;30:3–4. doi: 10.1291/hypres.30.3. [DOI] [PubMed] [Google Scholar]
- 24.Fang KC, Raymond WW, Blount JL, Caughey GH. Dog mast cell alpha-chymase activates progelatinase B by cleaving Phe88-Gln89 and Phe91-Glu92 bonds of catalytic domain. J Biol Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 25.Fang KC, Raymond WW, Lazarus SC, Caughey GH. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J Clin Invest. 1996;97:1589–1596. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saarinen J, Kalkkinen N, Welgus HG, Kovanen PT. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994;269:18134–18140. [PubMed] [Google Scholar]
- 27.Wang Y, Shiota N, Leskinen MJ, Lindstedt KA, Kovanen PT. Mast cell chymase inhibits smooth muscle cell growth and collagen expression in vitro: TGFβ1-dependent and -independent effects. Arterioscler Thromb Vasc Biol. 2001;21:1928–1933. doi: 10.1161/hq1201.100227. [DOI] [PubMed] [Google Scholar]
- 28.Zhao XY, Zhao LY, Zheng QS, Su JL, Guan H, Shang FJ, Niu XL, He YP, Lu XL. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem. 2008;310:159–166. doi: 10.1007/s11010-007-9676-2. [DOI] [PubMed] [Google Scholar]
- 29.Lindstedt KA, Wang Y, Shiota N, et al. Activation of paracrine TGF-β1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- 30.Longley BJ, Tyrrell L, Ma Y, Williams DA, Halaban R, Langley K, Lu HS, Schechter NM. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci USA. 1997;94:9017–9021. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forteza R, Lauredo I, Abraham WM, Conner GE. Bronchial tissue kallikrein activity is regulated by hyaluronic acid binding. Am J Respir Cell Mol Biol. 1999;21:666–674. doi: 10.1165/ajrcmb.21.6.3651. [DOI] [PubMed] [Google Scholar]
- 32.He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other infammatory cells in vivo. Brit J Pharmacol. 1998;125:1491–1500. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizutani H, Schechter N, Lazarua G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1β to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174:821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kido H, Nakano A, Okishama N, Wakabayashi H, Kishi F, Nakaya Y, Yoshizumi M, Tamaki T. Human chymase, an enzyme forming novel bioactive 31-amino acid length endothelins. Biol Chem. 1998;379:885–891. doi: 10.1515/bchm.1998.379.7.885. [DOI] [PubMed] [Google Scholar]
- 35.Omoto Y, Tokime K, Yamanaka K, Habe K, Morioka T, Kurokawa I, Tsutsui H, Yamanishi K, Nakanishi K, Mizutani H. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J Immunol. 2006;177:8315–8319. doi: 10.4049/jimmunol.177.12.8315. [DOI] [PubMed] [Google Scholar]
- 36.Laine P, Kaartinen M, Penttilä A, Panula P, Paavonen T, Kovanen PT. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99:361–369. doi: 10.1161/01.cir.99.3.361. [DOI] [PubMed] [Google Scholar]
- 37.Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1084–1088. doi: 10.1161/01.cir.92.5.1084. [DOI] [PubMed] [Google Scholar]
- 38.Kaartinen M, Penttilä A, Kovanen PT. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90:1669–1678. doi: 10.1161/01.cir.90.4.1669. [DOI] [PubMed] [Google Scholar]
- 39.Borland JA, Kelsall C, Yacoub MH, Chester AH. Expression, localization and function of ACE and chymase in normal and atherosclerotic human coronary arteries. Vascul Pharmacol. 2005;42:99–108. doi: 10.1016/j.vph.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Kaartinen M, Penttilä A, Kovanen PT. Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arterioscler Thromb. 1994;14:966–972. doi: 10.1161/01.atv.14.6.966. [DOI] [PubMed] [Google Scholar]
- 41.Cicha I, Wörner A, Urschel K, Beronov K, Goppelt-Struebe M, Verhoeven E, Daniel WG, Garlichs CD. Carotid plaque vulnerability: a positive feedback between hemodynamic and biochemical mechanisms. Stroke. 2011;42:3502–3510. doi: 10.1161/STROKEAHA.111.627265. [DOI] [PubMed] [Google Scholar]
- 42.Borland JA, Kelsall C, Yacoub MH, Chester AH. Expression, localization and function of ACE and chymase in normal and atherosclerotic human coronary arteries. Vascul Pharmacol. 2005;42:99–108. doi: 10.1016/j.vph.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Kovanen PT. Chymase-containing mast cells in human arterial intima: implications for atherosclerotic disease. Heart Vessels. 1997;12S:125–127. [PubMed] [Google Scholar]
- 44.Uehara Y, Urata H, Sasaguri M, Ideishi M, Sakata N, Tashiro T, Kimura M, Arakawa K. Increased chymase activity in internal thoracic artery of patients with hypercholesterolemia. Hypertension. 2000;35:55–60. doi: 10.1161/01.hyp.35.1.55. [DOI] [PubMed] [Google Scholar]
- 45.Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M, Ideishi M, Arakawa K. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399–1405. doi: 10.1161/01.hyp.33.6.1399. [DOI] [PubMed] [Google Scholar]
- 46.Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18:1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- 47.Bot I, Mäyränpää SMI, Heikkilä HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis. 2006;17:611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- 48.Nishimoto M, Takai S, Fukumoto H, Tsunemi K, Yuda A, Sawada Y, Yamada M, Jin D, Sakaguchi M, Nishimoto Y, Sasaki S, Miyazaki M. Increased local angiotensin II formation in aneurysmal aorta. Life Sci. 2002;71:2195–2205. doi: 10.1016/s0024-3205(02)01998-7. [DOI] [PubMed] [Google Scholar]
- 49.Furubayashi K, Takai S, Jin D, Miyazaki M, Katsumata T, Inagaki S, Kimura M, Tanaka K, Nishimoto M, Fukumoto H. Chymase activates promatrix metalloproteinase-9 in human abdominal aortic aneurysm. Clin Chim Acta. 2008;388:214–216. doi: 10.1016/j.cca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Tsunemi K, Takai S, Nishimoto M, Yuda A, Hasegawa S, Sawada Y, Fukumoto H, Sasaki S, Miyazaki M. Possible roles of angiotensin II-forming enzymes, angiotensin converting enzyme and chymase-like enzyme, in the human aneurysmal aorta. Hypertens Res. 2002;25:817–822. doi: 10.1291/hypres.25.817. [DOI] [PubMed] [Google Scholar]
- 51.Ibaraki T, Muramatsu M, Takai S, Jin D, Maruyama H, Orino T, Katsumata T, Miyazaki M. The relationship of tryptase- and chymase-positive mast cells to angiogenesis in stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2005;28:617–621. doi: 10.1016/j.ejcts.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 52.de Souza DA, Junior, Santana AC, da Silva EZ, Oliver C, Jamur MC. The role of mast cell specific chymases and tryptases in tumor angiogenesis. Biomed Res Int. 2015:142359. doi: 10.1155/2015/142359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guidolin D, Ruggieri S, Annese T, Tortorella C, Marzullo A, Ribatti D. Spatial distribution of mast cells around vessels and glands in human gastric carcinoma. Clin Exp Med. 2017 Jan 19; doi: 10.1007/s10238-017-0452-7. doi:0.1007/s10238–017–0452–7. [DOI] [PubMed] [Google Scholar]
- 54.Nagata S, Varagic J, Kon ND, Wang H, Groban L, Simington SW, Ahmad S, Dell’Italia LJ, VonCannon JL, Deal D, Ferrario CM. Differential expression of the angiotensin-(1–12)/chymase axis in human atrial tissue. Ther Adv Cardiovasc Dis. 2015;9:168–180. doi: 10.1177/1753944715589717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Chang CS, Kim EK, Lee JW, Matthay MA, Golden JA, Elicker BM, Jones K, Collard HR, Wolters PJ. Lung mast cell density defines a subpopulation of patients with idiopathic pulmonary fibrosis. Histopathology. 2012;61:98–106. doi: 10.1111/j.1365-2559.2012.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kosanovic D, Dahal BK, Wygrecka M, Reiss I, Günther A, Ghofrani HA, Weissmann N, Grimminger F, Seeger W, Schermuly RT, Banat GA. Mast cell chymase: an indispensable instrument in the pathological symphony of idiopathic pulmonary fibrosis? Histol Histopathol. 2013;28:691–699. doi: 10.14670/HH-28.691. [DOI] [PubMed] [Google Scholar]
- 57.Kosanovic D, Dahal BK, Peters DM, Seimetz M, Wygrecka M, Hoffmann K, Antel J, Reiss I, Ghofrani HA, Weissmann N, Grimminger F, Seeger W, Schermuly RT. Histological characterization of mast cell chymase in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Pulm Circ. 2014;4:128–136. doi: 10.1086/675642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wasse H, Rivera AA, Huang R, Martinson DE, Long Q, McKinnon W, Naqvi N, Husain A. Increased plasma chymase concentration and mast cell chymase expression in venous neointimal lesions of patients with CKD and ESRD. Semin Dial. 2011;24:688–693. doi: 10.1111/j.1525-139X.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welker P, Krämer S, Groneberg DA, Neumayer HH, Bachmann S, Amann K, Peters H. Increased mast cell number in human hypertensive nephropathy. Am J Physiol Renal Physiol. 2008;295:F1103–F1109. doi: 10.1152/ajprenal.00374.2007. [DOI] [PubMed] [Google Scholar]
- 60.McPherson EA, Luo Z, Brown RA, LeBard LS, Corless CC, Speth RC, Bagby SP. Chymase-like angiotensin II-generating activity in end-stage human autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2004;15:493–500. doi: 10.1097/01.asn.0000109782.28991.26. [DOI] [PubMed] [Google Scholar]
- 61.Koka V, Wang W, Huang XR, Kim-Mitsuyama S, Truong LD, Lan HY. Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation. 2006;113:1353–1360. doi: 10.1161/CIRCULATIONAHA.105.575589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang XR, Chen WY, Truong LD, Lan HY. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol. 2003;14:1738–1747. doi: 10.1097/01.asn.0000071512.93927.4e. [DOI] [PubMed] [Google Scholar]
- 63.Cristovam PC, Arnoni CP, de Andrade MC, Casarini DE, Pereira LG, Schor N, Boim MA. ACE-dependent and chymase-dependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med (Maywood) 2008;233:1035–1043. doi: 10.3181/0708-RM-229. [DOI] [PubMed] [Google Scholar]
- 64.Maruichi M, Oku H, Takai S, Muramatsu M, Sugiyama T, Imamura Y, Minami M, Ueki M, Satoh B, Sakaguchi M, Miyazaki M, Ikeda T. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: a possible involvement of chymase in the pathogenesis of macular hole patients. Curr Eye Res. 2004;29:321–325. doi: 10.1080/02713680490516161. [DOI] [PubMed] [Google Scholar]
- 65.Yamada M, Ueda M, Naruko T, Tanabe S, Han YS, Ikura Y, Ogami M, Takai S, Miyazaki M. Mast cell chymase expression and mast cell phenotypes in human rejected kidneys. Kidney Int. 2001;59:1374–1381. doi: 10.1046/j.1523-1755.2001.0590041374.x. [DOI] [PubMed] [Google Scholar]
- 66.Dong X, Zhang C, Ma S, Wen H. Mast cell chymase in keloid induces profibrotic response via transforming growth factor-β1/Smad activation in keloid fibroblasts. Int J Clin Exp Pathol. 2014;15(7):3596–3607. [PMC free article] [PubMed] [Google Scholar]
- 67.Dong X, Zhang C, Ma S, Wen H. High concentrations of mast cell chymase facilitate the transduction of the transforming growth factor-β1/Smads signaling pathway in skin fibroblasts. Exp Ther Med. 2015;9:955–960. doi: 10.3892/etm.2015.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Gu Y, Lewis DF, Alexander JS, Granger DN. Elevated plasma chymotrypsin-like protease (chymase) activity in women with preeclampsia. Hypertens Pregnancy. 2010;29:253–261. doi: 10.3109/10641950802001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Alexander JS. Role of chymase in preeclampsia. Curr Vasc Pharmacol. 2013;11:606–615. doi: 10.2174/1570161111311050005. [DOI] [PubMed] [Google Scholar]
- 70.Gu Y, Lewis DF, Alexander JS, Wang Y. Placenta-derived chymotrypsin-like protease (CLP) disturbs endothelial junctional structure in preeclampsia. Reprod Sci. 2009;16:479–488. doi: 10.1177/1933719108329818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andoh A, Deguchi Y, Inatomi O, Yagi Y, Bamba S, Tsujikawa T, Fujiyama Y. Immunohistochemical study of chymase-positive mast cells in inflammatory bowel disease. Oncol Rep. 2006;16:103–107. [PubMed] [Google Scholar]
- 72.Badertscher K, Brönnimann M, Karlen S, Braathen LR, Yawalkar N. Mast cell chymase is increased in chronic atopic dermatitis but not in psoriasis. Arch Dermatol Res. 2005;296:503–506. doi: 10.1007/s00403-005-0542-3. [DOI] [PubMed] [Google Scholar]
- 73.Richard V, Hurel-Merle S, Scalbert E, Ferry G, Lallemand F, Bessou JP, Thuillez C. Functional evidence for a role of vascular chymase in the production of angiotensin II in isolated human arteries. Circulation. 2001;104:750–752. doi: 10.1161/hc3201.094971. [DOI] [PubMed] [Google Scholar]
- 74.Tom B, Garrelds IM, Scalbert E, Stegmann AP, Boomsma F, Saxena PR, Danser AH. ACE-versus chymase-dependent angiotensin II generation in human coronary arteries: a matter of efficiency? Arterioscler Thromb Vasc Biol. 2003;23:251–256. doi: 10.1161/01.atv.0000051875.41849.25. [DOI] [PubMed] [Google Scholar]
- 75.Wolny A, Clozel JP, Rein J, Mory P, Vogt P, Turino M, Kiowski W, Fischli W. Functional and biochemical analysis of angiotensin II-forming pathways in the human heart. Cir Res. 1997;80:219–227. doi: 10.1161/01.res.80.2.219. [DOI] [PubMed] [Google Scholar]
- 76.McDonald JE, Padmanabhan N, Petrie MC, Hillier C, Connell JM, McMurray JJ. Vasoconstrictor effect of the angiotensin-converting enzyme-resistant, chymase-specific substrate [Pro(11)(D)-Ala(12)] angiotensin I in human dorsal hand veins: in vivo demonstration of non-ace production of angiotensin II in humans. Circulation. 2001;104:1805–1808. doi: 10.1161/hc4001.097220. [DOI] [PubMed] [Google Scholar]
- 77.Nishimoto M, Takai S, Sawada Y, Yuda A, Kondo K, Yamada M, Jin D, Sakaguchi M, Asada K, Sasaki S, Miyazaki M. Chymase-dependent angiotensin II formation in the saphenous vein versus the internal thoracic artery. J Thorac Cardiovasc Surg. 2001;121:729–734. doi: 10.1067/mtc.2001.112467. [DOI] [PubMed] [Google Scholar]
- 78.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 79.Chandrasekharan UM, Sanker S, Glynias MJ, Karnik SS, Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996;271:502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- 80.Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 81.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reber LL, Daubeuf F, Pejler G, Abrink M, Frossard N. Mast cells contribute to bleomycin-induced lung inflammation and injury in mice through a chymase/mast cell protease 4-dependent mechanism. J Immunol. 2014;192:1847–1854. doi: 10.4049/jimmunol.1300875. [DOI] [PubMed] [Google Scholar]
- 83.Lin L, Bankaitis E, Heimbach L, Li N, Abrink M, Pejler G, An L, Diaz LA, Werb Z, Liu Z. Dual targets for mouse mast cell protease-4 in mediating tissue damage in experimental bullous pemphigoid. J Biol Chem. 2011;286:37358–37367. doi: 10.1074/jbc.M111.272401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Younan G, Suber F, Xing W, Shi T, Kunori Y, Abrink M, Pejler G, Schlenner SM, Rodewald HR, Moore FD, Jr, Stevens RL, Adachi R, Austen KF, Gurish MF. The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. J Immunol. 2010;185:7681–7690. doi: 10.4049/jimmunol.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, Ennis TL, Gurish MF, Libby P, Shi GP. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Houde M, Jamain MD, Labonté J, Desbiens L, Pejler G, Gurish M, Takai S, D’Orléans-Juste P. Pivotal role of mouse mast cell protease 4 in the conversion and pressor properties of Big-endothelin-1. J Pharmacol Exp Ther. 2013;346:31–37. doi: 10.1124/jpet.112.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Houde M, Desbiens L, Schwertani A, Pejler G, Iglarz M, D’Orléans-Juste P. Endothelin receptor antagonist macitentan or deletion of mouse mast cell protease 4 delays lesion development in atherosclerotic mice. Life Sci. 2016;159:71–75. doi: 10.1016/j.lfs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 88.Bankova LG, Lezcano C, Pejler G, Stevens RL, Murphy GF, Austen KF, Gurish MF. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J Immunol. 2014;192:2812–2820. doi: 10.4049/jimmunol.1301794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desbiens L, Lapointe C, Gharagozloo M, Mahmoud S, Pejler G, Gris D, D’Orléans-Juste P. Significant contribution of mouse mast cell protease 4 in early phases of experimental autoimmune encephalomyelitis. Mediators Inflamm. 2016;2016:9797021. doi: 10.1155/2016/9797021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magnusson SE, Pejler G, Kleinau S, Abrink M. Mast cell chymase contributes to the antibody response and the severity of autoimmune arthritis. FASEB J. 2009;23:875–882. doi: 10.1096/fj.08-120394. [DOI] [PubMed] [Google Scholar]
- 91.Scandiuzzi L, Beghdadi W, Daugas E, Abrink M, Tiwari N, Brochetta C, Claver J, Arouche N, Zang X, Pretolani M, Monteiro RC, Pejler G, Blank U. Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol. 2010;185:624–633. doi: 10.4049/jimmunol.0902129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piliponsky AM, Chen CC, Rios EJ, Treuting PM, Lahiri A, Abrink M, Pejler G, Tsai M, Galli SJ. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am J Pathol. 2012;181:875–886. doi: 10.1016/j.ajpath.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nelissen S, Vangansewinkel T, Geurts N, Geboes L, Lemmens E, Vidal PM, Lemmens S, Willems L, Boato F, Dooley D, Pehl D, Pejler G, Maurer M, Metz M, Hendrix S. Mast cells protect from post-traumatic spinal cord damage in mice by degrading inflammation-associated cytokines via mouse mast cell protease 4. Neurobiol Dis. 2014;62:260–972. doi: 10.1016/j.nbd.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 94.Hendrix S, Kramer P, Pehl D, Warnke K, Boato F, Nelissen S, Lemmens E, Pejler G, Metz M, Siebenhaar F, Maurer M. Mast cells protect from post-traumatic brain inflammation by the mast cell-specific chymase mouse mast cell protease-4. FASEB J. 2013;27:920–929. doi: 10.1096/fj.12-204800. [DOI] [PubMed] [Google Scholar]
- 95.Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, Wernersson S. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 96.Waern I, Lundequist A, Pejler G, Wernersson S. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal Immunol. 2013;6:911–920. doi: 10.1038/mi.2012.129. [DOI] [PubMed] [Google Scholar]
- 97.Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, Schlenner SM, Feyerabend TB, Rodewald HR, Pejler G, Tsai M, Galli SJ. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121:4180–4191. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beghdadi W, Madjene LC, Claver J, Pejler G, Beaudoin L, Lehuen A, Daugas E, Blank U. Mast cell chymase protects against renal fibrosis in murine unilateral ureteral obstruction. Kidney Int. 2013;84:317–326. doi: 10.1038/ki.2013.98. [DOI] [PubMed] [Google Scholar]
- 99.Chen LY, Li P, He Q, Jiang LQ, Cui CJ, Xu L, Liu LS. Transgenic study of the function of chymase in heart remodeling. J Hypertens. 2002;20:2047–2055. doi: 10.1097/00004872-200210000-00025. [DOI] [PubMed] [Google Scholar]
- 100.Koga T, Urata H, Inoue Y, Hoshino T, Okamoto T, Matsunaga A, Suzuki M, Miyazaki J, Ideishi M, Arakawa K, Saku K. Human chymase expression in a mice induces mild hypertension with left ventricular hypertrophy. Hypertens Res. 2003;26:759–768. doi: 10.1291/hypres.26.759. [DOI] [PubMed] [Google Scholar]
- 101.Rafiq K, Sherajee SJ, Fan YY, Fujisawa Y, Takahashi Y, Matsuura J, Hase N, Urata H, Nakano D, Hitomi H, Nishiyama A. Blood glucose level and survival in streptozotocin-treated human chymase transgenic mice. Chin J Physiol. 2011;54:30–35. doi: 10.4077/cjp.2011.amm008. [DOI] [PubMed] [Google Scholar]
- 102.Guo C, Ju H, Leung D, Massaeli H, Shi M, Rabinovitch M. A novel vascular smooth muscle chymase is upregulated in hypertensive rats. J Clin Invest. 2001;107:703–715. doi: 10.1172/JCI9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ju H, Gros R, You X, Tsang S, Husain M, Rabinovitch M. Conditional and targeted overexpression of vascular chymase causes hypertension in transgenic mice. Proc Natl Acad Sci U S A. 2001;98:7469–7474. doi: 10.1073/pnas.131147598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsumoto C, Hayashi T, Kitada K, Yamashita C, Miyamura M, Mori T, Ukimura A, Ohkita M, Jin D, Takai S, Miyazaki M, Okada Y, Kitaura Y, Matsumura Y. Chymase plays an important role in left ventricular remodeling induced by intermittent hypoxia in mice. Hypertension. 2009;54:164–171. doi: 10.1161/HYPERTENSIONAHA.109.131391. [DOI] [PubMed] [Google Scholar]
- 105.Ishida K, Takai S, Murano M, Nishikawa T, Inoue T, Murano N, Inoue N, Jin D, Umegaki E, Higuchi K, Miyazaki M. Role of chymase dependent matrix metalloproteinase-9 activation in mice with dextran sodium sulfate induced colitis. J Pharmacol Exp Ther. 2008;324:422–426. doi: 10.1124/jpet.107.131946. [DOI] [PubMed] [Google Scholar]
- 106.Inoue N, Muramatsu M, Jin D, Takai S, Hayashi T, Katayama H, Kitaura Y, Tamai H, Miyazaki M. Effects of chymase inhibitor on angiotensin II-induced abdominal aortic aneurysm development in apolipoprotein E-deficient mice. Atherosclerosis. 2009;204:359–364. doi: 10.1016/j.atherosclerosis.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 107.Devarajan S, Yahiro E, Uehara Y, Habe S, Nishiyama A, Miura S, Saku K, Urata H. Depressor effect of chymase inhibitor in mice with high salt-induced moderate hypertension. Am J Physiol Heart Circ Physiol. 2015;309:H1987–H1996. doi: 10.1152/ajpheart.00721.2014. [DOI] [PubMed] [Google Scholar]
- 108.Palaniyandi SS, Nagai Y, Watanabe K, Ma M, Veeraveedu PT, Prakash P, Kamal FA, Abe Y, Yamaguchi K, Tachikawa H, Kodama M, Aizawa Y. Chymase inhibition reduces the progression to heart failure after autoimmune myocarditis in rats. Exp Biol Med. 2007;232:1213–1221. doi: 10.3181/0703-RM-85. [DOI] [PubMed] [Google Scholar]
- 109.Kanemitsu H, Takai S, Tsuneyoshi H, Nishina T, Yoshikawa K, Miyazaki M, Ikeda T, Komeda M. Chymase inhibition prevents cardiac fibrosis and dysfunction after myocardial infarction in rats. Hypertens Res. 2006;29:57–64. doi: 10.1291/hypres.29.57. [DOI] [PubMed] [Google Scholar]
- 110.Kakimoto K, Takai S, Murano M, Ishida K, Yoda Y, Inoue T, Jin D, Umegaki E, Higuchi K. Significance of chymase-dependent matrix metalloproteinase-9 activation on indomethacin-induced small intestinal damages in rats. J Pharmacol Exp Ther. 2010;332:684–689. doi: 10.1124/jpet.109.162933. [DOI] [PubMed] [Google Scholar]
- 111.Takai S, Jin D, Chen H, Li W, Yamamoto H, Yamanishi K, Miyazaki M, Higashino H, Yamanishi H, Okamura H. Chymase inhibition improves vascular dysfunction and survival in stroke-prone spontaneously hypertensive rats. J Hypertens. 2014;32:1637–1648. doi: 10.1097/HJH.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 112.Kunori Y, Muroga Y, Iidaka M, Mitsuhashi H, Kamimura T, Fukamizu A. Species differences in angiotensin II generation and degradation by mast cell chymases. J Recept Signal Transduct Res. 2005;25:35–44. doi: 10.1081/rrs-200054355. [DOI] [PubMed] [Google Scholar]
- 113.Miyazaki M, Takai S. Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci. 2006;100:391–397. doi: 10.1254/jphs.cpj06008x. [DOI] [PubMed] [Google Scholar]
- 114.Akasu M, Urata H, Kinoshita A, Sasaguri M, Ideishi M, Arakawa K. Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension. 1998;32:514–520. doi: 10.1161/01.hyp.32.3.514. [DOI] [PubMed] [Google Scholar]
- 115.Takai S, Shiota N, Yamamoto D, Okunishi H, Miyazaki M. Purification and characterization of angiotensin II-generating chymase from hamster cheek pouch. Life Sci. 1996;58:591–597. doi: 10.1016/0024-3205(95)02328-3. [DOI] [PubMed] [Google Scholar]
- 116.Imai Y, Takai S, Jin D, Komeda K, Tashiro K, Li ZL, Otsuki Y, Okamura H, Hayashi M, Uchiyama K. Chymase inhibition attenuates lipopolysaccharide/ d-galactosamine-induced acute liver failure in hamsters. Pharmacology. 2014;93:47–56. doi: 10.1159/000357684. [DOI] [PubMed] [Google Scholar]
- 117.Komeda K, Takai S, Jin D, Tashiro K, Hayashi M, Tanigawa N, Miyazaki M. Chymase inhibition attenuates tetrachloride-induced liver fibrosis in hamsters. Hepatol Res. 2010;40:832–840. doi: 10.1111/j.1872-034X.2010.00672.x. [DOI] [PubMed] [Google Scholar]
- 118.Tsunemi K, Takai S, Nishimoto M, Jin D, Sakaguchi M, Muramatsu M, Yuda A, Sasaki S, Miyazaki M. A specific chymase inhibitor, 2-(5-formylamino-6-oxo-2-phenyl-1,6-dihydropyrimidine-1-yl)-N-[[3,4-dioxo-1-phenyl-7-(2-pyridyloxy)]-2-heptyl]acetamide (NK3201), suppresses development of abdominal aortic aneurysm in hamsters. J Pharmacol Exp Ther. 2004;309:879–883. doi: 10.1124/jpet.103.063974. [DOI] [PubMed] [Google Scholar]
- 119.Takai S, Jin D, Ohzu M, Tanaka K, Miyazaki M. Chymase inhibition provides pancreatic islet protection in hamsters with streptozotocin-induced diabetes. J Pharmacol Sci. 2009;110:459–465. doi: 10.1254/jphs.09115fp. [DOI] [PubMed] [Google Scholar]
- 120.Maeda Y, Inoguchi T, Takei R, Sawada F, Sasaki S, Fujii M, Kobayashi K, Urata H, Nishiyama A, Takayanagi R. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am J Physiol Renal Physiol. 2010;299:F1328–F1338. doi: 10.1152/ajprenal.00337.2010. [DOI] [PubMed] [Google Scholar]
- 121.Maeda Y, Inoguchi T, Takei R, Hendarto H, Ide M, Inoue T, Kobayashi K, Urata H, Nishiyama A, Takayanagi R. Chymase inhibition prevents myocardial fibrosis through the attenuation of NOX4-associated oxidative stress in diabetic hamsters. J Diabetes Investig. 2012;3:354–361. doi: 10.1111/j.2040-1124.2012.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang T, Han SX, Zhang SF, Ning YY, Chen L, Chen YJ, He GM, Xu D, An J, Yang T, Zhang XH, Wen FQ. Role of chymase in cigarette smoke-induced pulmonary artery remodeling and pulmonary hypertension in hamsters. Respir Res. 2010;11:36. doi: 10.1186/1465-9921-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kosanovic D, Luitel H, Dahal BK, Cornitescu T, Janssen W, Danser AH, Garrelds IM, De Mey JG, Fazzi G, Schiffers P, Iglarz M, Fischli W, Ghofrani HA, Weissmann N, Grimminger F, Seeger W, Reiss I, Schermuly RT. Chymase: a multifunctional player in pulmonary hypertension associated with lung fibrosis. Eur Respir J. 2015;46:1084–1094. doi: 10.1183/09031936.00018215. [DOI] [PubMed] [Google Scholar]
- 124.Hoshino F, Urata H, Inoue Y, Saito Y, Yahiro E, Ideishi M, Arakawa K, Saku K. Chymase inhibitor improves survival in hamsters with myocardial infarction. J Cardiovasc Pharmacol. 2003;41(Suppl 1):S11. [PubMed] [Google Scholar]
- 125.Jin D, Takai S, Yamada M, Sakaguchi M, Kamoshita K, Ishida K, Sukenaga Y, Miyazaki M. Impact of chymase inhibitor on cardiac function and survival after myocardial infarction. Cardiovasc Res. 2003;60:413–420. doi: 10.1016/s0008-6363(03)00535-2. [DOI] [PubMed] [Google Scholar]
- 126.Jin D, Takai S, Yamada M, Sakaguchi M, Miyazaki M. Beneficial effects of cardiac chymase inhibition during the acute phase of myocardial infarction. Life Sci. 2002;71:437–446. doi: 10.1016/s0024-3205(02)01689-2. [DOI] [PubMed] [Google Scholar]
- 127.Fan YY, Nishiyama A, Fujisawa Y, Kobori H, Nakano D, Matsuura J, Hase N, Hitomi H, Kiyomoto H, Urata H, Kohno M. Contribution of chymase-dependent angiotensin II formation to the progression of tubulointerstitial fibrosis in obstructed kidneys in hamsters. J Pharmacol Sci. 2009;111:82–90. doi: 10.1254/jphs.09152fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uehara Y, Urata H, Ideishi M, Arakawa K, Saku K. Chymase inhibition suppresses high-cholesterol diet-induced lipid accumulation in the hamster aorta. Cardiovasc Res. 2002;55:870–876. doi: 10.1016/s0008-6363(02)00458-3. [DOI] [PubMed] [Google Scholar]
- 129.Kervinen J, Crysler C, Bayoumy S, Abad MC, Spurlino J, Deckman I, Greco MN, Maryanoff BE, de Garavilla L. Potency variation of small-molecule chymase inhibitors across species. Biochem Pharmacol. 2010;80:1033–1041. doi: 10.1016/j.bcp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 130.Zhang S, Anderson DF, Bradding P, Coward WR, Baddeley SM, MacLeod JD, McGill JI, Church MK, Holgate ST, Roche WR. Human mast cells express stem cell factor. J Pathol. 1998;186:59–66. doi: 10.1002/(SICI)1096-9896(199809)186:1<59::AID-PATH140>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 131.He S, Gaca MD, McEuen AR, Walls AF. Inhibitors of chymase as mast cell-stabilizing agents: contribution of chymase in the activation of human mast cells. J Pharmacol Exp Ther. 1999;291:517–523. [PubMed] [Google Scholar]
- 132.Hoit BD, Shao Y, Kinoshita A, Gabel M, Husain A, Walsh RA. Effects of angiotensin II generated by an angiotensin converting enzyme-independent pathway on left ventricular performance in the conscious baboon. J Clin Invest. 1995;95:1519–1527. doi: 10.1172/JCI117824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kishi K, Muramatsu M, Jin D, Furubayashi K, Takai S, Tamai H, Miyazaki M. The effects of chymase on matrix metalloproteinase-2 activation in neointimal hyperplasia after balloon injury in dogs. Hypertens Res. 2007;30:77–83. doi: 10.1291/hypres.30.77. [DOI] [PubMed] [Google Scholar]
- 134.Takai S, Jin D, Sakaguchi M, Miyazaki M. A single treatment with a specific chymase inhibitor, TY-51184, prevents vascular proliferation in canine grafted veins. J Pharmacol Sci. 2004;94:443–448. doi: 10.1254/jphs.94.443. [DOI] [PubMed] [Google Scholar]
- 135.Furubayashi K, Takai S, Jin D, Muramatsu M, Ibaraki T, Nishimoto M, Fukumoto H, Katsumata T, Miyazaki M. The significance of chymase in the progression of abdominal aortic aneurysms in dogs. Hypertens Res. 2007;30:349–357. doi: 10.1291/hypres.30.349. [DOI] [PubMed] [Google Scholar]
- 136.Matsumoto T, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in progression of heart failure. Circulation. 2003;107:2555–2558. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- 137.Jin D, Takai S, Sakaguchi M, Okamoto Y, Muramatsu M, Miyazaki M. An antiarrhythmic effect of a chymase inhibitor after myocardial infarction. J Pharmacol Exp Ther. 2004;309:490–497. doi: 10.1124/jpet.103.061465. [DOI] [PubMed] [Google Scholar]
- 138.Ahmed M, Gladden JD, Litovsky S, McGiffin D, Gupta H, Lloyd S, Denney T, Dell’Italia LJ. Myofibrillar degeneration, oxidative stress and post-surgical systolic dysfunction in patients with isolated mitral regurgitation and pre surgical left ventricular ejection fraction > 60% J Am Coll Cardiol. 2011;55:671–679. doi: 10.1016/j.jacc.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schiros CG, Dell’Italia LJ, Gladden JD, Clark D, 3rd, Aban I, Gupta H, Lloyd SG, McGiffin DC, Perry G, Denney TS, Jr, Ahmed MI. Magnetic resonance imaging with 3-dimensional analysis of left ventricular remodeling in isolated mitral regurgitation: implications beyond dimensions. Circulation. 2012;125:2334–2342. doi: 10.1161/CIRCULATIONAHA.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmed MI, Guichard JL, Rajasekaran NS, Ahmad S, Mariappan N, Litovsky S, Gupta H, Lloyd SG, Denney TS, Powell PC, Aban I, Collawn JF, Davies JE, McGiffin DC, Dell’Italia LJ. Disruption of desmin-mitochondrial architecture in patients with regurgitant mitral valves and preserved ventricular function. J Thorac Cardiovasc Surg. 2016;152:1059–1070. doi: 10.1016/j.jtcvs.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dell’Italia LJ, Meng QC, Balcells E, Straeter-Knowlen IM, Hankes GH, Dillon R, Cartee RE, Orr R, Bishop SP, Oparil S, Elton TS. Increased ACE and chymase-like activity in cardiac tissue of dogs with chronic mitral regurgitation. Am J Physiol. 1995;269:H2065–H2073. doi: 10.1152/ajpheart.1995.269.6.H2065. [DOI] [PubMed] [Google Scholar]
- 142.Stewart JA, Wei C-C, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi P, Janicki JS, Dell’Italia LJ. Cardiac mast cell and chymase mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2013;35:311–319. doi: 10.1016/s0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 143.Zheng J, Chen Y, MD, Pat B, Dell’Italia LA, Tillson M, Dillon AR, Powell P, Shi K, Shah N, Denney T, Husain A, Dell’Italia LJ. Mircroarray identifies extensive downregulation of noncollagen extracellular matrix and profibrotic growth factor genes in chronic isolated mitral regurgitation in the dog. Circulation. 2009;119:2086–2095. doi: 10.1161/CIRCULATIONAHA.108.826230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dell’Italia LJ, Balcells E, Meng QC, Su X, Schultz D, Bishop SP, Machida N, Straeter-Knowlen IM, Hankes GH, Dillon R, Cartee RE, Oparil S. Volume overload cardiac hypertrophy is unaffected by ACE inhibitor treatment in the dog. Am J Physiol. 1997;273:H961–H970. doi: 10.1152/ajpheart.1997.273.2.H961. [DOI] [PubMed] [Google Scholar]
- 145.Perry GJ, Wei CC, Su X, Bishop SP, Hankes GH, Dillon GH, Mukherjee R, Spinale FG, Dell’Italia LJ. Angiotensin II receptor blockade does not improve LV function and remodeling in subacute mitral regurgitation in the dog. J Am Coll Cardiol. 2002;39:1374–1379. doi: 10.1016/s0735-1097(02)01763-1. [DOI] [PubMed] [Google Scholar]
- 146.Perry GJ, Mori T, Wei CC, Xu XY, Chen YF, Oparil S, Lucchesi P, Dell’Italia LJ. Genetic variation in angiotensin-converting enzyme does not prevent development of cardiac hypertrophy or upregulation of angiotensin II in response to aortocaval fistula. Circulation. 2001;103:1012–1016. doi: 10.1161/01.cir.103.7.1012. [DOI] [PubMed] [Google Scholar]
- 147.Ryan TD, Rothstein E, Aban I, Tallaj J, Husain A, Lucchesi PA, Dell’Italia LJ. Left ventricular eccentric remodeling is mediated by bradykinin and precedes myocyte elongation in rats with volume overload. J Am Coll Cardiol. 2007;49:811–821. doi: 10.1016/j.jacc.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 148.Yancey D, Guichard J, Zhou L, Murphy MP, Johnson MS, Benavides GS, Collawn JF, Darley-Usmar V, Dell’Italia LJ. Cardiomyocyte Mitochondrial Oxidative Stress and Cytoskeletal Breakdown in the Volume Overloaded Heart. Am J Physiol. 2015;308:H651–663. doi: 10.1152/ajpheart.00638.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wei CC, Lucchesi PA, Tallaj J, Bradley WE, Powell PC, Dell’Italia LJ. Cardiac interstitial bradykinin and mast cells modulate pattern of LV remodeling in volume overload in rats. Am J Physiol. 2003;285:H784–H792. doi: 10.1152/ajpheart.00793.2001. [DOI] [PubMed] [Google Scholar]
- 150.Wei CC, Chen Y-W, Shi Ke, Powell L, Powell P, Bradley WE, Dell’Italia LJ. Cardiac Kallikrein Kinin system is upregulated in chronic volume overload and mediates inflammatory induced collagen loss. PLOS One. 2012;7:e40110. doi: 10.1371/journal.pone.0040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fu L, Wei CC, Powell PC, Bradley WE, Collawn JF, Dell’Italia LJ. Volume overload induces autophagic degradation of procollagen in cardiac fibroblasts. J Mol Cell Cardiol. 2015;89:241–250. doi: 10.1016/j.yjmcc.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fu L, Wei CC, Powell PC, Bradley WE, Ahmad S, Ferrario CM, Collawn JF, Dell’Italia LJ. Increased fibroblast chymase production mediates procollagen autophagic digestion in volume overload. J Mol Cell Cardiol. 92:1–9. doi: 10.1016/j.yjmcc.2016.01.019. 3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One. 2011;6:e28501-1–9. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sabri A, Rafiq K, Seqqat R, Kolpakov MA, Dillon R, Dell’italia LJ. Sympathetic activation causes focal adhesion signaling alteration in early compensated volume overload attributable to isolated mitral regurgitation in the dog. Circ Res. 2008;102:1127–1136. doi: 10.1161/CIRCRESAHA.107.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seqqat R, Guo X, Rafiq K, Kolpakov MA, Guo J, Koch WJ, Houser SR, Dell’Italia LJ, Sabri A. Beta1-adrenergic receptors promote focal adhesion signaling downregulation and myocyte apoptosis in acute volume overload. J Mol Cell Cardiol. 2012;53:240–249. doi: 10.1016/j.yjmcc.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pat B, Killingsworth C, Shi K, Zheng J, Powell P, Chen Y, Walcott G, Ahmed M, Desai R, Gladden JD, Wei CC, Hase N, Kobayashi T, Granzier H, Denney T, Tilson M, Dillon AR, Husain A, Dell’Italia LJ. Chymase inhibition prevents fibronectin and myofibrillar loss and improves cardiomyocyte function and LV torsion angle in dogs with isolated mitral regurgitation. Circulation. 2010;122:1488–1595. doi: 10.1161/CIRCULATIONAHA.109.921619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wei CC, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, Naqvi N, Powell PC, Shi K, Takahashi Y, Saku K, Urata H, Dell’Italia LJ, Husain A. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest. 2010;120:1229–1239. doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng J, Wei CC, Hase N, Shi K, Killingsworth CR, Litovsky SH, Powell PC, Kobayashi T, Ferrario CM, Rab A, Aban I, Collawn JF, Dell’Italia LJ. Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog. PLoS One. 2014;9:e94732. doi: 10.1371/journal.pone.0094732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther. 2011;339:143–151. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Swedberg K, Held P, Kjekshus J, Rasmussen K, Ryden L, Wedel H. Effects of the early administration of Enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II) N Engl J Med. 1992;327:678–684. doi: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
- 161.Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–86. doi: 10.1056/NEJM198807143190204. [DOI] [PubMed] [Google Scholar]
- 162.Sharpe N, Smith H, Murphy J, Greaves S, Hart H, Gamble G. Early prevention of left ventricular dysfunction after myocardial infarction with angiotensin-converting-enzyme inhibition. Lancet. 1991;337:872–876. doi: 10.1016/0140-6736(91)90202-z. [DOI] [PubMed] [Google Scholar]
- 163.Ihara M, Urata H, Shirai K, Ideishi M, Hoshino F, Suzumiya J, Kikuchi M, Arakawa K. High cardiac angiotensin-II-forming activity in infarcted and non-infarcted human myocardium. Cardiology. 2000;94:247–253. doi: 10.1159/000047325. [DOI] [PubMed] [Google Scholar]
- 164.Baghirova S, Hughes BG, Poirier M, Kondo MY, Schulz R. Nuclear matrix metalloproteinase-2 in the cardiomyocyte and the ischemic-reperfused heart. J Mol Cell Cardiol. 2016;94:153–161. doi: 10.1016/j.yjmcc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 165.Gao L, Zheng YJ, Gu SS, Tan JL, Paul C, Wang YG, Yang HT. Degradation of cardiac myosin light chain kinase by matrix metalloproteinase-2 contributes to myocardial contractile dysfunction during ischemia/reperfusion. J Mol Cell Cardiol. 2014;77:102–112. doi: 10.1016/j.yjmcc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 166.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85:413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 167.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 168.Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, Santamore WP. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 169.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 170.Ahmadi A, Leipsic J, Blankstein R, Taylor C, Hecht H, Stone GW, Narula J. Do plaques rapidly progress prior to myocardial infarction? The interplay between plaque vulnerability and progression. Circ Res. 2015;117:99–104. doi: 10.1161/CIRCRESAHA.117.305637. [DOI] [PubMed] [Google Scholar]
- 171.Niccoli G, Stefanini GG, Capodanno D, Crea F, Ambrose JA, Berg R. Are the culprit lesions severely stenotic? J Am Coll Cardiol Img. 2013;6:1108–1114. doi: 10.1016/j.jcmg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 172.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997;272:7127–7131. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- 174.Heikkilä HM, Lätti S, Leskinen MJ, Hakala JK, Kovanen PT, Lindstedt KA. Activated mast cells induce endothelial cell apoptosis by a combined action of chymase and tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2008;28:309–314. doi: 10.1161/ATVBAHA.107.151340. [DOI] [PubMed] [Google Scholar]
- 175.Lindstedt KA, Leskinen MJ, Kovanen PT. Proteolysis of the pericellular matrix: a novel element determining cell survival and death in the pathogenesis of plaque erosion and rupture. Arterioscler Thromb Vasc Biol. 2004;24:1350–1358. doi: 10.1161/01.ATV.0000135322.78008.55. [DOI] [PubMed] [Google Scholar]
- 176.Shi GP, Kovanen PT. Mast cells as effectors in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:265–271. doi: 10.1161/ATVBAHA.114.303570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Libby P, Shi GP. Mast cells as mediators and modulators of atherogenesis. Circulation. 2007;115:2471–2473. doi: 10.1161/CIRCULATIONAHA.107.698480. [DOI] [PubMed] [Google Scholar]
- 178.Arakawa K, Urata H. Hypothesis regarding the pathophysiological role of alternative pathways of angiotensin II formation in atherosclerosis. Hypertension. 2000;36:638–641. doi: 10.1161/01.hyp.36.4.638. [DOI] [PubMed] [Google Scholar]
- 179.Lindstedt L, Lee M, Castro GR, Fruchart JC, Kovanen PT. Chymase in exocytosed rat mast cell granules effectively proteolyzes apolipoprotein Al-containing lipoproteins, so reducing the cholesterol efflux-inducing ability of serum and aortic intimal fluid. J Clin Invest. 1996;97:2174–2182. doi: 10.1172/JCI118658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shi GP, Bot I, Kovanen PT. Mast cells in human and experimental cardiometabolic diseases. Nat Rev Cardiol. 2015;12:643–658. doi: 10.1038/nrcardio.2015.117. [DOI] [PubMed] [Google Scholar]
- 181.Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res. 2007;101:455–464. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- 182.Zheng M, Huang W, Bai J, Nie X, Wang W. Chymase inhibition protects diabetic rats from renal lesions. Mol Med Rep. 2016;14:121–128. doi: 10.3892/mmr.2016.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cristovam PC, Carmona AK, Arnoni CP, Maquigussa E, Pereira LG, Boim MA. Role of chymase in diabetic nephropathy. Exp Biol Med (Maywood) 2012;237:985–992. doi: 10.1258/ebm.2012.011356. [DOI] [PubMed] [Google Scholar]
- 184.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, Harrison-Bernard LM. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298:F37–F48. doi: 10.1152/ajprenal.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park S, Bivona BJ, Ford SM, Jr, Xu S, Kobori H, de Garavilla L, Harrison-Bernard LM. Direct evidence for intrarenal chymase-dependent angiotensin II formation on the diabetic renal microvasculature. Hypertension. 2013;61:465–471. doi: 10.1161/HYPERTENSIONAHA.111.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kumar R, Yong QC, Thomas CM, Baker KM. Intracardiac intracellular angiotensin system in diabetes. Am J Physiol Regul Integr Comp Physiol. 2012;302:R510–R17. doi: 10.1152/ajpregu.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol. 2007;293:H939–H948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 188.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol. 2008;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 189.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 190.Dell’Italia LJ, Meng QC, Balcells E, Wei C-C, Palmer R, Hageman GR, Durand J, Hankes GH, Oparil S. Compartmentalization of angiotensin II generation in the dog heart: Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wei CC, Tian B, Perry G, Meng QC, Chen YF, Oparil S, Dell’Italia LJ. Differential ANG II generation in plasma and tissue of mice with decreased expression of the ACE gene. Am J Physiol. 2002;282:H2254–H2258. doi: 10.1152/ajpheart.00191.2001. [DOI] [PubMed] [Google Scholar]
- 192.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab. 2007;18:208–214. doi: 10.1016/j.tem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 193.Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32:1551–1565. doi: 10.1016/j.peptides.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF, Dell Italia LJ. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol. 2016;311:H404–H414. doi: 10.1152/ajpheart.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, Dell’Italia LJ. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond) 2014;126:461–469. doi: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 197.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol. 2008;294:H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS ONE. 2011:e15759. doi: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol. 2009;296:H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nagata S, Kato J, Kuwasako K, Kitamura K. Plasma and tissue levels of proangiotensin-12 and components of the renin-angiotensin system (RAS) following low- or high-salt feeding in rats. Peptides. 2010;31:889–892. doi: 10.1016/j.peptides.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 201.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 202.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol. 2008;294:H2242–H224. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nagata S, Kato J, Kuwasako K, Asami M, Kitamura K. Plasma and tissue concentrations of proangiotensin-12 in rats treated with inhibitors of the renin-angiotensin system. Hypertension Res. 2012;35:234–238. doi: 10.1038/hr.2011.165. [DOI] [PubMed] [Google Scholar]
- 204.Ahmad S, Simmons S, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of Angiotensin II from Angiotensin-(1–12) in human atrial tissue. PLoS ONE. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.De Mello WC, Dell’Itallia LJ, Varagic J, Ferrario CM. Intracellular angiotensin-(1–12) changes the electrical properties of intact cardiac muscle. Mol Cell Biochem. 2016;422:31–40. doi: 10.1007/s11010-016-2801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, Husain A. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J Clin Invest. 1993;91:1269–1281. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gregory AD, Hale P, Perlmutter DH, Houghton AM. Clathrin pit-mediated endocytosis of neutrophil elastase and cathepsin G by cancer cells. J Biol Chem. 2012;287:35341–35350. doi: 10.1074/jbc.M112.385617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol. 2015;89:268–279. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II Type 1 Receptors. Circulation. 2015;131:2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Robertson AL, Jr, Khairallah PA. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science. 1971;172:1138–1139. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- 211.Booz GW, Conrad KM, Hess AL, Singer HA, Baker KM. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology. 1992;130:3641–3649. doi: 10.1210/endo.130.6.1597161. [DOI] [PubMed] [Google Scholar]
- 212.Tang SS, Rogg H, Schumacher R, Dzau V. Characterization of nuclear angiotensin-II-binding sites in rat liver and comparison with plasma membrane receptors. Endocrinology. 1992;131:374–380. doi: 10.1210/endo.131.1.1612017. [DOI] [PubMed] [Google Scholar]
- 213.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol. 2009;296:F1484–1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Re RN, Vizard DL, Brown J. Angiotensin II receptors in chromatin fragments generated by micrococcal nuclease. Biochem Biophys Res Commun. 1984;119:220–227. doi: 10.1016/0006-291x(84)91641-3. [DOI] [PubMed] [Google Scholar]
- 215.Eggena P, Zhu JH, Clegg K, Barrett JD. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension. 1993;22:496–501. doi: 10.1161/01.hyp.22.4.496. [DOI] [PubMed] [Google Scholar]
- 216.Deliu E, Tica AA, Motoc DG, Cristina Brailoiu GC, Brailoiu E. Intracellular angiotensin II activates rat myometrium. Am J Physiol. 2011;301:C559–C665. doi: 10.1152/ajpcell.00123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Re RN. Lysosomal action of intracrine angiotensin II. Focus on “Intracellular angiotensin II activates rat myometrium. ” Am J Physiol. 2011;301:C553–C554. doi: 10.1152/ajpcell.00232.2011. [DOI] [PubMed] [Google Scholar]
- 218.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Valenzuela R, Costa-Besada MA, Iglesias-Gonzalez J, Perez-Costas E, Villar-Cheda B, Garrido-Gil P, Melendez-Ferro M, Soto-Otero R, Lanciego JL, Henrion D, Franco R, Labandeira-Garcia JL. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 2016;7:e2427. doi: 10.1038/cddis.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating AT1 receptors in proximal tubule cell. Am J Physiol. 2006;290(6):F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr Opinion Neph Hyper. 2008;17:168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 222.Cook JL, Re RN. Intracrine Renin-Angiotensin System: A New Paradigm in Cardiovascular and Renal Control. Lessons from in vitro studies and a related intracellular angiotensin II transgenic mouse model. Am J Physiol. 2012;302:R482–R493. doi: 10.1152/ajpregu.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Re RN. A possible mechanism for the progression of chronic renal disease and congestive heart failure. J Am Soc Hypertens. 2015;9:54–63. doi: 10.1016/j.jash.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 224.de Garavilla L, Greco MN, Sukumar N, Chen ZW, Pineda AO, Mathews FS, Di Cera E, Giardino EC, Wells GI, Haertlein BJ, Kauffman JA, Corcoran TW, Derian CK, Eckardt AJ, Damiano BP, Andrade-Gordon P, Maryanoff BE. A Novel, Potent Dual Inhibitor of the Leukocyte Proteases Cathepsin G and Chymase: Molecular mechanisms and anti-inflammatory activity in vivo. J Biol Chem. 2005;280:18001–18007. doi: 10.1074/jbc.M501302200. [DOI] [PubMed] [Google Scholar]
- 225.Maryanoff BE, de Garavilla L, Greco MN, Haertlein BJ, Wells GI, Andrade-Gordon P, Abraham WM. Dual inhibition of cathepsin G and chymase is effective in animal models of pulmonary inflammation. Am J Respir Crit Care Med. 2010;181:247–253. doi: 10.1164/rccm.200904-0627OC. [DOI] [PubMed] [Google Scholar]
- 226.Hooshdaran B, Kolpakov MA, Guo X, Miller SA, Wang T, Tilley DG, Rafiq K, Sabri A. Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury. Basic Res Cardiol. 2017;112:62. doi: 10.1007/s00395-017-0652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang Y, Shi GP. Mast cell chymase and tryptase in abdominal aortic aneurysm formation. Trends Cardiovasc Med. 2012;22:150–150. doi: 10.1016/j.tcm.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ni WW, Cao MD, Huang W, Meng L, Wei JF. Tryptase inhibitors: a patent review. Expert Opin Ther Pat. 2017;27:919–928. doi: 10.1080/13543776.2017.1322064. [DOI] [PubMed] [Google Scholar]
- 229.Parr DG, Lara B. Clinical utility of alpha-1 proteinase inhibitor in the management of adult patients with severe alpha-1 antitrypsin deficiency: a review of the current literature. Drug Des Devel Ther. 2017;11:2149–2162. doi: 10.2147/DDDT.S105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rahaghi FF, Miravitlles M. Long-term clinical outcomes following treatment with alpha 1-proteinase inhibitor for COPD associated with alpha-1 antitrypsin deficiency: a look at the evidence. Respir Res. 2017;18:105. doi: 10.1186/s12931-017-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Raymond WW, Su S, Makarova A, Wilson TM, Carter MC, Metcalfe DD, Caughey GH. Alpha 2-macroglobulin capture allows detection of mast cell chymase in serum and creates a reservoir of angiotensin II-generating activity. J Immunol. 2009;182:5770–5777. doi: 10.4049/jimmunol.0900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Walter M, Sutton RM, Schechter NM. Highly efficient inhibition of human chymase by a(2)-macroglobulin. Arch Biochem Biophys. 1999;368:276–284. doi: 10.1006/abbi.1999.1309. [DOI] [PubMed] [Google Scholar]
- 233.Kokkonen JO, Saarinen J, Kovanen PT. Regulation of local angiotensin II formation in the human heart in the presence of interstitial fluid. Inhibition of chymase by protease inhibitors of interstitial fluid and of angiotensin-converting enzyme by Ang-(1–9) formed by heart carboxypeptidase A-like activity. Circulation. 1997;95:1455–1463. doi: 10.1161/01.cir.95.6.1455. [DOI] [PubMed] [Google Scholar]
- 234.Schechter NM, Sprows JL, Schoenberger OL, Lazarus GS, Cooperman BS, Rubin H. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989;264:21308–21315. [PubMed] [Google Scholar]
- 235.Schips T, Kanisicak O, Sargent MA, Molkentin JD. Genetic overexpression of Serpina3n attenuates muscular dystrophy in mice. Tjondrokoesoemo A. Hum Mol Genet. 2016;25:1192–1202. doi: 10.1093/hmg/ddw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ang LS, Boivin WA, Williams SJ, Zhao H, Abraham T, Carmine-Simmen K, McManus BM, Bleackley RC, Granville DJ. Serpina3n attenuates granzyme B-mediated decorin cleavage and rupture in a murine model of aortic aneurysm. Cell Death Dis. 2011;8(2):e209. doi: 10.1038/cddis.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ikari Y, Mulvihill E, Schwartz SM. alpha 1-Proteinase inhibitor, alpha 1-antichymotrypsin, and alpha 2-macroglobulin are the antiapoptotic factors of vascular smooth muscle cells. J Biol Chem. 2001;276:11798–11803. doi: 10.1074/jbc.M008503200. [DOI] [PubMed] [Google Scholar]
- 238.Mast AE, Enghild JJ, Nagase H, Suzuki K, Pizzo SV, Salvesen G. Kinetics and physiologic relevance of the inactivation of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, and antithrombin III by matrix metalloproteinases-1 (tissue collagenase), -2 (72-kDa gelatinase/type IV collagenase), and -3 (stromelysin) J Biol Chem. 1991;266:15810–15816. [PubMed] [Google Scholar]
- 239.Desrochers PE, Jeffrey JJ, Weiss SJ. Interstitial collagenase (matrix metalloproteinase-1) expresses serpinase activity. J Clin Invest. 1991;87(6):2258–2265. doi: 10.1172/JCI115262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Desrochers PE, Weiss SJ. Proteolytic inactivation of alpha-1-proteinase inhibitor by a neutrophil metalloproteinase. J Clin Invest. 1988;81:1646–1650. doi: 10.1172/JCI113500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schechter NM, Sprows JL, Schoenberger OL, Lazarus GS, Cooperman BS, Rubin H. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989;264:21308–21315. [PubMed] [Google Scholar]
- 242.Wolters PJ, Laig-Webster M, Caughey GH. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am J Respir Cell Mol Biol. 2000;22:183–190. doi: 10.1165/ajrcmb.22.2.3767. [DOI] [PubMed] [Google Scholar]
- 243.Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- 244.Laine DI, Busch-Petersen J. Inhibitors of cathepsin C (dipeptidyl peptidase I) Expert Opin Ther Pat. 2010;20:497–506. doi: 10.1517/13543771003657172. [DOI] [PubMed] [Google Scholar]
- 245.Pat B, Killingsworth C, Chen Y, Gladden JD, Walcott G, Powell PC, Denney T, Gupta H, Desai R, Tillson M, Dillon AR, Dell’Italia LJ. Mast cell stabilization decreases cardiomyocyte and LV function in dogs with isolated mitral regurgitation. J Card Fail. 2010;16:769–776. doi: 10.1016/j.cardfail.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Veerappan A, Thompson M, Savage AR, Silverman ML, Chan WS, Sung B, Summers B, Montelione KC, Benedict P, Groh B, Vicencio AG, Peinado H, Worgall S, Silver RB. Mast cells and exosomes in hyperoxia-induced neonatal lung disease. Am J Physiol. 2016;310:L1218–L232. doi: 10.1152/ajplung.00299.2015. [DOI] [PubMed] [Google Scholar]
- 247.Xie G, Liu Y, Yao Q, Zheng R, Zhang L, Lin J, Guo Z, Du S, Ren C, Yuan Q, Yuan Y. Hypoxia-induced angiotensin II by the lactate-chymase-dependent mechanism mediates radioresistance of hypoxic tumor cells. Sci Rep. 2017;7:42396. doi: 10.1038/srep42396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Forman JP, Price DA, Stevanovic R, Fisher NDL. Racial differences in renal vascular response to angiotensin blockade with captopril or candesartan. J Hypertens. 2007;25:877–882. doi: 10.1097/HJH.0b013e32803cae1a. [DOI] [PubMed] [Google Scholar]
- 249.Froogh G, Pinto JT, Le Y, Kandhi S, Aleligne Y, Huang A, Sun D. Chymase-dependent production of angiotensin II: an old enzyme in old hearts. Am J Physiol. 2017;312:H223–H231. doi: 10.1152/ajpheart.00534.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li J, Jubair S, Janicki JS. Estrogen inhibits mast cell chymase release to prevent pressure overload-induced adverse cardiac remodeling. Hypertension. 2015;65:328–334. doi: 10.1161/HYPERTENSIONAHA.114.04238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang H, Jessup JA, Zhao Z, Da Silva J, Lin M, MacNamara LM, Ahmad S, Chappell MC, Ferrario CM, Groban L. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2. Lewis rats. PLoS One. 2013;8:e76992. doi: 10.1371/journal.pone.0076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 253.Zheng J, Yancey DM, Ahmed MI, Wei CC, Powell PC, Shanmugam M, Gupta H, Lloyd SG, McGiffin DC, Schiros CG, Denney TS, Jr, Babu GJ, Dell’Italia LJ. Increased sarcolipin expression and adrenergic drive in humans with preserved left ventricular ejection fraction and chronic isolated mitral regurgitation. Circ Heart Fail. 2014;7:194–202. doi: 10.1161/CIRCHEARTFAILURE.113.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Foster RE, Johnson DB, Barilla F, Blackwell GG, Orr R, Roney M, Stanley AW, Jr, Pohost GM, Dell’Italia LJ. Changes in left ventricular mass and volumes in patients receiving angiotensin-converting enzyme inhibitor therapy for left ventricular dysfunction after Q-wave myocardial infarction. Am Heart J. 1998;136:269–275. doi: 10.1053/hj.1998.v136.89405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.