Introduction
Radiofrequency ablation is an effective treatment strategy for ischemic and non-ischemic cardiomyopathy-related ventricular tachycardia (VT). The role of substrate-guided ablation, performed using electrogram characteristics (low amplitude, fractionated or isolated potentials) as scar surrogates, is expanding due to frequent hemodynamic instability during entrainment mapping of scar-related VT. Late gadolinium-enhancement on cardiac magnetic resonance imaging (LGE-MRI) can accurately characterize the trans-mural extent, location, and configuration of ventricular scar1. Integration of LGE-MRI into electroanatomical mapping (EAM) during VT ablation was shown, in preliminary studies, to be feasible and to provide accurate localization of VT substrate and re-entry circuits2–4. However, studies to date examining the impact of MRI-scar integration on procedural outcomes have lacked control groups, precluding any comparisons with standard practice. We performed a study to: 1) demonstrate the feasibility in clinical practice of integrating MRI-derived scar for guidance of VT ablation; 2) report on the peri-procedural performance of LGE-MRI in identifying the arrhythmogenic substrate; and 3) examine the impact of MRI-guided ablation on procedural length and acute and long-term outcomes.
Study Design
In this prospective multi-center study, we enrolled 24 consecutive patients with ischemic (n=9) and/or non-ischemic cardiomyopathy (n=15), referred for catheter ablation of scar-related monomorphic VT. Patients were assigned, at the discretion of the treating physician (not randomized), to undergo either MRI-derived scar guided ablation or traditional ablation. Clinical characteristics of patients in both groups were statistically comparable with a higher tendency to use scar-integration for patients with prior failed ablation, lower LVEF and epicardial circuits (Supplemental Table 1).
MRI acquisition and analysis, as well as standard ablation strategy, are fully described in the supplement. Briefly, an EP study, using programmed stimulation, was performed at the procedure onset to induce the clinical VT(s), and to enable the identification and ablation of VT critical sites by pace- or entrainment-mapping. Additional ablation targets included areas with fractionated, isolated and low-voltage potentials. In MRI-scar guided ablation, registration of LGE-map and EAM was accomplished, and regions exhibiting LGE were carefully interrogated to identify low-voltage or abnormal electrograms. Entrainment or pace-mapping maneuvers were then performed in regions with LGE to ascertain their involvement in clinical VT. LGE areas were ablated if found to be implicated in VT and areas with LGE and abnormal electrograms were ablated in a substrate modification strategy if VT could not be induced/mapped. Figure 1 displays examples of VT ablation performed with and without MRI-scar integration.
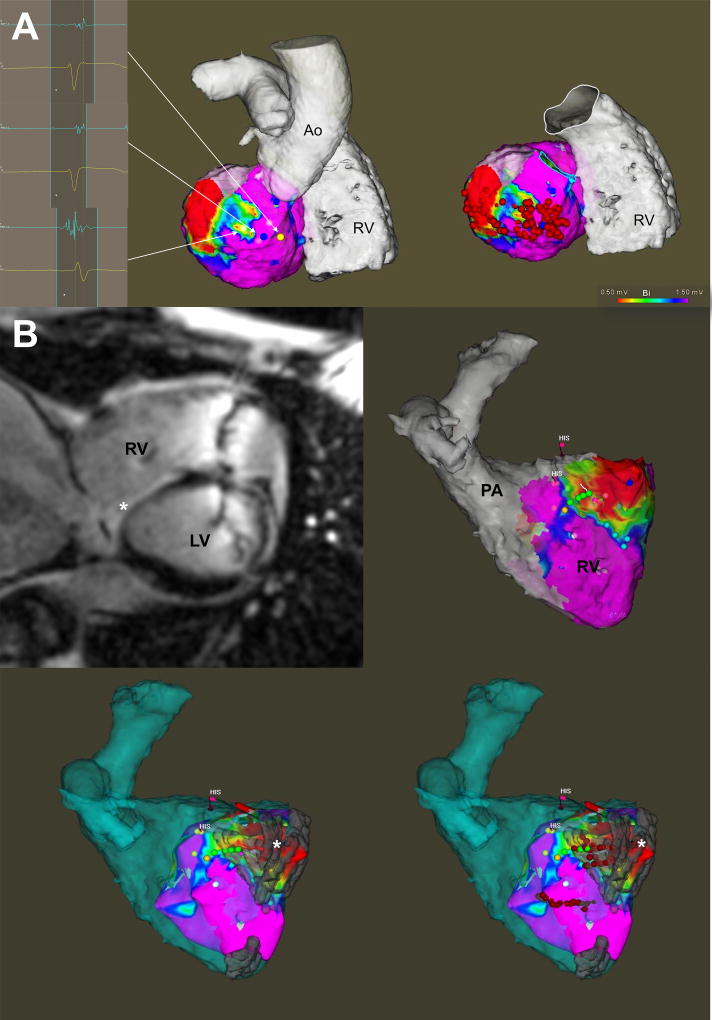
Figure.
Panel A displays a representative example of VT ablation performed without MRI-derived scar integration. Endocardial EAM identified scar at the basal infero-lateral region of the left ventricle, the ablation of which terminated clinical VT. Panel B displays a representative example of VT ablation performed with MRI-derived scar integration. Voltage map of the RV was constructed and merged with the previously acquired MRI (both segmented anatomical shell in blue as well as scar in grey). A basal septal and inferior RV scar noted on LGE-MRI (marked by an asterisk) closely matched the areas of low voltage, fractionated and isolated potentials found on EAM. Substrate modification was performed within the scar. No sustained VT was inducible subsequently.
Patients were followed post-procedure with regular ICD interrogations, Holter-monitoring, or ECG-recordings. The primary endpoint was long-term freedom from target-VT recurrence.
Results
Supplemental Table 1 and Supplemental Figure 2 exhibit procedural and MRI-scar data, respectively. In the scar-integration group, all patients with inducible-VTs had evidence of LGE on MRI, and ablation within or at the border of LGE sites terminated the arrhythmia in all these patients (100% match between clinical VT critical sites and LGE). In patients without LGE on MRI, sustained VT could not be induced. In one patient with non-ischemic cardiomyopathy, MRI revealed LGE in the mid-inferior wall, correlating with low-voltage and abnormal potentials on EAM. In this patient, VT could not be elicited on various maneuvers and substrate modification was done using radiofrequency energy in that area. Data on interrogation of LGE areas for abnormal electrograms was available in 9/13 patients in scar-merge group. Abnormal electrograms were co-localized with LGE in all patients and were targeted by ablation in patients in whom a substrate modification approach was adopted. Procedural, fluoroscopy, and ablation times, as well as ablation burden were similar in both study arms (p>0.05). Acute procedural success (clinical VT termination and/or non-inducibility) was achieved in all patients.
During a median follow-up of 38.5 IQR[26–53] months, 16(73%) patients had VT recurrence (7 in the study group vs. 9 controls, Supplemental Table 2). After adjusting for age, gender, cardiac substrate, epicardial ablation, and LVEF, MRI-guided ablation was associated with reduced VT recurrence during follow-up (HR 0.12, 95% CI 0.02–0.75, p=0.023). Follow-up echocardiographic studies were performed in a subset of patients and described in the Supplement.
Discussion
The current study is the first to evaluate the impact of integration of LGE-MRI scar into EAM during VT ablation on long-term procedural outcome using a control group for comparison. The main findings of this study are: 1) integration of MRI-derived scar during VT-ablation is feasible; 2) MRI-defined scar accurately identifies VT-substrate without differences upon procedural or fluoroscopy time; 3) acute ablation success rates were similar in procedures performed with and without scar-merging; and 4) after adjusting for clinical parameters, MRI-guided ablation was associated with decreased risk of long-term VT recurrence.
Incorporation of the LGE-derived scar facilitates VT ablation in the setting of hemodynamic instability that precludes conventional electrophysiological mapping and dense point-by-point voltage mapping, and may shorten procedure time devoted to substrate identification. LGE-MRI can also complement VT-substrate mapping by focusing substrate-mapping on scar regions. Indeed, regional necrosis/fibrosis is accompanied by hypertrophy of adjacent viable tissue (as a result of remodeling)5, exhibit normal voltage and be glossed over after identification of sparse neighboring ‘healthy’ points. Mid-wall scar may also contain critical parts of VT-reentry circuits and, while missed upon bipolar-voltage mapping, can be displayed upon integration of MRI-scar. We believe that scar-integration enhances the operator’s ability to target substrate that would otherwise have been missed, potentially improving patient outcomes. In addition, MRI-scar integration may impact safety by avoiding unnecessary ablation of healthy myocardium in procedures guided solely by voltage-mapping. In fact, false-positive low-voltage may be due to poor contact, thinner walls, or epicardial fat, leading to over-estimation of arrhythmogenic substrate. Study limitations are listed in the supplement.
Supplementary Material
Acknowledgments
Sources of Funding: The study was funded by the National Institutes of Health (grant nos. K23HL089333 and R01HL116280) as well as by a Biosense Webster grant to Dr. Nazarian and Dr. Henrikson; the Roz and Marvin H. Weiner and Family Foundation; the Dr. Francis P. Chiaramonte Foundation; Marilyn and Christian Poindexter; and the Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund.
Footnotes
Disclosures: Dr. Nazarian is a scientific advisor to Medtronic, CardioSolv, and Biosense Webster. All others authors have no financial disclosures that are relevent to the topic of the manuscript.
References
- 1.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 2.Bogun FM, Desjardins B, Good E, Gupta S, Crawford T, Oral H, Ebinger M, Pelosi F, Chugh A, Jongnarangsin K, Morady F. Delayed-Enhanced Magnetic Resonance Imaging in Nonischemic Cardiomyopathy. J Am Coll Cardiol. 2009;53:1138–1145. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijnmaalen AP, van der Geest RJ, van Huls van Taxis CFB, Siebelink H-MJ, Kroft LJM, Bax JJ, Reiber JHC, Schalij MJ, Zeppenfeld K. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011;32:104–114. doi: 10.1093/eurheartj/ehq345. [DOI] [PubMed] [Google Scholar]
- 4.Andreu D, Berruezo A, Ortiz-Perez JT, Silva E, Mont L, Borras R, de Caralt TM, Perea RJ, Fernandez-Armenta J, Zeljko H, Brugada J. Integration of 3D Electroanatomic Maps and Magnetic Resonance Scar Characterization Into the Navigation System to Guide Ventricular Tachycardia Ablation. Circ Arrhythmia Electrophysiol. 2011;4:674–683. doi: 10.1161/CIRCEP.111.961946. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki T, Miller CF, Hansford R, Yang J, Caffo BS, Zviman MM, Henrikson CA, Marine JE, Spragg D, Cheng A, Tandri H, Sinha S, Kolandaivelu A, Zimmerman SL, Bluemke DA, Tomaselli GF, Berger RD, Calkins H, Halperin HR, Nazarian S. Myocardial Structural Associations With Local ElectrogramsClinical Perspective. Circ Arrhythmia Electrophysiol. 2012;5 doi: 10.1161/CIRCEP.112.970699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.