Significance
Memories for emotional events tend to persist, raising a fundamental question about how the brain prioritizes significant memories. Past studies have pointed to a central role for the amygdala in mediating this endogenous memory enhancement. However, the premise that the amygdala can causally enhance declarative memory has not been directly tested in humans. Here we show that brief electrical stimulation to the human amygdala can enhance declarative memory for specific images of neutral objects without eliciting a subjective emotional response, likely by engaging other memory-related brain regions. The results show the human amygdala has a general capacity to initiate enhancement of specific declarative memories rather than a narrower role limited to indirectly mediating emotional effects on memory.
Keywords: amygdala, hippocampus, memory enhancement, brain stimulation, memory consolidation
Abstract
Emotional events are often remembered better than neutral events, a benefit that many studies have hypothesized to depend on the amygdala’s interactions with memory systems. These studies have indicated that the amygdala can modulate memory-consolidation processes in other brain regions such as the hippocampus and perirhinal cortex. Indeed, rodent studies have demonstrated that direct activation of the amygdala can enhance memory consolidation even during nonemotional events. However, the premise that the amygdala causally enhances declarative memory has not been directly tested in humans. Here we tested whether brief electrical stimulation to the amygdala could enhance declarative memory for specific images of neutral objects without eliciting a subjective emotional response. Fourteen epilepsy patients undergoing monitoring of seizures via intracranial depth electrodes viewed a series of neutral object images, half of which were immediately followed by brief, low-amplitude electrical stimulation to the amygdala. Amygdala stimulation elicited no subjective emotional response but led to reliably improved memory compared with control images when patients were given a recognition-memory test the next day. Neuronal oscillations in the amygdala, hippocampus, and perirhinal cortex during this next-day memory test indicated that a neural correlate of the memory enhancement was increased theta and gamma oscillatory interactions between these regions, consistent with the idea that the amygdala prioritizes consolidation by engaging other memory regions. These results show that the amygdala can initiate endogenous memory prioritization processes in the absence of emotional input, addressing a fundamental question and opening a path to future therapies.
Emotional events often stand out in one’s recollections, suggesting that the brain prioritizes memories by affective salience (1–6). Many studies have pointed to a key role for the amygdala in prioritizing emotional memories (1–13). In particular, patients with bilateral damage to the amygdala displayed good memory in general but, relative to healthy participants, typically showed no additional improvement in declarative memory for emotional words, pictures, or stories (14). Evidence from functional neuroimaging studies of emotional memory in healthy participants has also supported the role of the amygdala in enhancing memory for emotional material (8–13, 15). For example, during emotional memory encoding, both activity in the amygdala and measures of functional connectivity with other medial temporal lobe (MTL) structures correlated with the extent of subsequent emotional memory enhancement (8, 9, 11–13, 15). Further, increases in stress hormones during or after an emotional experience can facilitate memory consolidation and often correlate with fMRI activation of the amygdala (5, 6, 16–21). These results from studies in humans have underscored the importance of the amygdala but have left questions about its causal role in the prioritization of emotional memories.
A large body of work in experimental animals has sought to address these questions and has led to the hypothesis that the amygdala directly modulates memory consolidation in other brain regions (5, 6, 16–18, 21–25). A key set of findings was that direct activation of the basolateral complex of the amygdala (BLA) via electrical stimulation or local norepinephrine microinfusions even during nonemotional events improved memory when tested on subsequent days (5, 6, 16, 18, 22–25). Further, direct activation of the BLA modulated neuronal activity and markers of synaptic plasticity in the hippocampus and perirhinal cortex (16–18, 21, 24), two structures important for declarative memory that are directly innervated by the BLA (26). For example, memory-enhancing BLA stimulation during the study phase of an object-recognition memory task elicited intrahippocampal gamma synchrony that was suggested to be related to cellular memory consolidation by influencing spike-timing–dependent plasticity in the hippocampus (24). In another study, hippocampal inactivation eliminated the amygdala-mediated enhancement of object-recognition memory (23). These and other studies (6, 16, 18) have led to the view that an emotional experience engages the amygdala, which in turn enhances memory for that experience through modulation of synaptic plasticity-related processes underlying memory consolidation in other brain regions (6, 16, 18). This model predicts that direct stimulation of the human amygdala could enhance memory in a manner analogous to emotion’s enhancing effects on long-term memory.
Although data from studies examining the role of the human amygdala in memory and emotion have broadly agreed with results from experimental animals, several recent reports have questioned the extent to which the human amygdala plays a direct role in modulating memory processes. For example, in addition to its role in emotional memory enhancement, the amygdala is also thought to contribute to emotional arousal and the subjective experience of emotion (27, 28). Thus, it is possible that activation of the amygdala may influence a memory by associating it with an emotional context (29) or indirectly by triggering emotional arousal (30) rather than, or in combination with, directly modulating memory processes (31). For instance, if stimulation of the amygdala led to an emotional experience, it could induce further rumination or reappraisal of the material even after the stimulus was removed from view, thereby potentially strengthening memory encoding for that material. Moreover, studies in humans that have manipulated postencoding memory processes via noxious arousal (e.g., nociceptive shocks) or cognitive factors (e.g., selective attention or distinctiveness) have shown that each manipulation can potentially contribute to emotional memory enhancement, although the strength of the evidence linking the memory effects of each of these manipulations specifically to the amygdala varies considerably (6, 30, 32, 33). Further, most of these postencoding memory-enhancement effects have been demonstrated for stimuli that already have an emotional meaning (30, 32, 33), making it difficult to dissociate the possible roles of the amygdala in emotion and memory. Finally, none of these studies of emotional memory in humans has involved directly stimulating the amygdala, and those that have stimulated the amygdala apart from memory testing have found that subjective emotional responses and autonomic responses are elicited infrequently and only at relatively high levels of stimulation (34–36). Thus, it remains unknown whether direct electrical stimulation of the human amygdala would be sufficient to enhance memory and whether any such modulation would depend upon eliciting a subjective or physiological emotional response.
The present study therefore sought to test in humans whether directly engaging the amygdala can prioritize memory processes. We specifically asked whether brief electrical stimulation applied directly to the human amygdala could enhance recognition memory for specific images of neutral objects without eliciting an emotional experience. Fourteen patients with depth electrodes placed in the amygdala for clinical purposes performed a yes/no recognition memory task for neutral images of objects. During encoding, half of the images were followed by a 1-s low-amplitude (0.5 mA) electrical stimulation to the amygdala. In addition, patients were subsequently presented with 10 trials of amygdala stimulation and 10 trials of sham stimulation in a random order and were asked to judge which trials included the amygdala stimulation. Amygdala stimulation reliably improved later object-recognition memory with no elicited emotional response, and this memory enhancement was reflected by specific neuronal oscillations between the amygdala and memory-related regions. The results agree with past studies in experimental animals and show the human amygdala has a general capacity to initiate enhancement of specific declarative memories rather than a narrower role limited to indirectly mediating emotional effects on memory.
Results
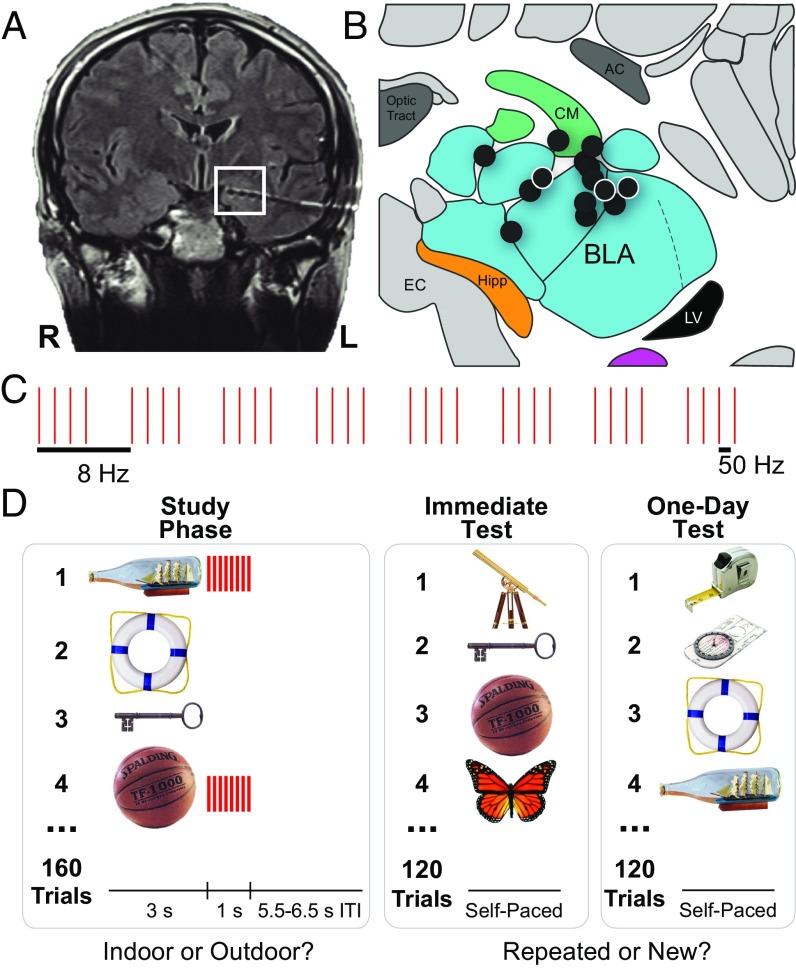
The influence of brief amygdala stimulation on recognition-memory performance for neutral object images was assessed in 14 epileptic patients (see Figs. S1 and S2 and Tables S1–S3 for information on patients and locations of electrodes). Fig. 1 summarizes the object-recognition memory procedure and depicts that 1 s of low-amplitude electrical stimulation (eight trains of 50 Hz, 0.5 mA biphasic pulses) was delivered at the offset of image presentation for a randomly selected half of the objects during the study phase of the task (Materials and Methods). Memory performance was tested immediately after the study phase and one day later.
Fig. 1.
The procedure used to stimulate the human amygdala and to test recognition memory. (A) A representative postoperative coronal MRI showing electrode contacts in the amygdala (white square). (B) Illustration of left amygdala (coronal slice) with black circles indicating estimated centroids of bipolar stimulation in or near the BLA in all 14 patients. (See Fig. S1 for more precise localizations.) White borders denote right-sided stimulation. All patients had at least one bipolar stimulation contact in the BLA. AC, anterior commissure; CM, centromedial complex of the amygdala; EC, entorhinal cortex; Hipp, head of the hippocampus; LV, lateral ventricle (temporal horn). Adapted with permission from ref. 54, copyright Elsevier 2007. (C) Schematic of the 1-s stimulation pulse sequence (each pulse = 500 μs biphasic square wave; pulse frequency = 50 Hz; train frequency = 8 Hz). (D) Schematic of the recognition-memory task in which the amygdala was stimulated after the presentation of half of the objects in the study phase and recognition memory was tested on unique subsets of images immediately and one day after the study phase.
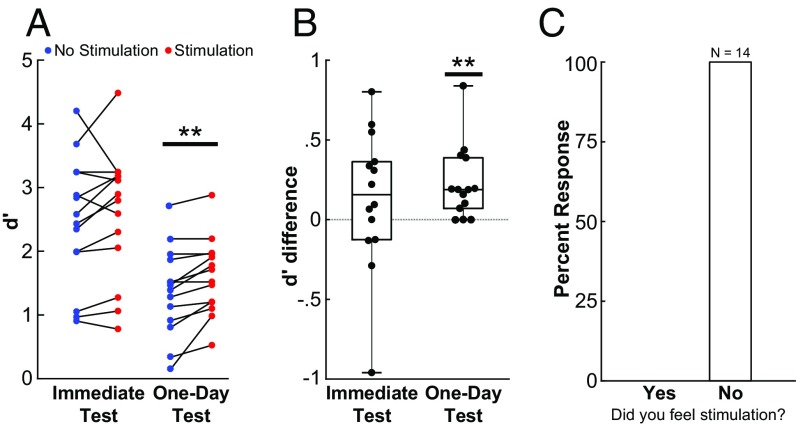
Fig. 2 shows the memory performance as a standard discriminability index (d′) and indicates that brief stimulation of the amygdala following some images during the study phase enhanced recognition memory relative to control (no-stimulation) images on the following day [stimulation vs. no stimulation d′: mean(SEM) = 1.60(0.16) vs. 1.37(0.19), t (13) = 3.69, P = 0.003, Cohen’s d = 0.99]. By comparison, there was no reliable effect of stimulation on the immediate test [stimulation vs. no stimulation d′: mean(SEM) = 2.68(0.24) vs. 2.55(0.24), t (13) = 1.07, P = 0.30, Cohen’s d = 0.29]. A repeated-measures ANOVA revealed an overall effect of test day [F(1,13) = 94.60, P < 0.001] and an overall effect of stimulation [F(1,13) = 6.11, P = 0.03]. The better overall performance on the immediate test raises the possibility that any benefit of amygdala stimulation might not be distinguishable at this time point in this particular task (i.e., a ceiling effect). Nevertheless, the reliability of the observed memory benefit on the one-day test is consistent with the presumed role for the amygdala in modulating long-term memory-consolidation processes (6, 16, 18, 21, 37, 38). No differences in yes/no reaction times during the immediate or one-day test were observed between stimulation and unstimulated control conditions (Fig. S3). No carryover effects of stimulation on memory were observed for control images that followed stimulation trials during the study phase (Fig. S4). Thus, although these results do not distinguish which putative psychological processes of recognition memory were influenced by amygdala stimulation (e.g., episodic-like recollection and/or item-specific familiarity), the lack of carryover indicates that the memory enhancement was temporally specific. That is, objects in the stimulation condition were remembered better than control objects initially viewed only seconds (3–5 s) apart.
Fig. 2.
Brief electrical stimulation to the amygdala in humans enhanced subsequent declarative memory without eliciting an emotional response. (A) Recognition-memory performance for each patient plotted as discriminability index (d′). All data were normally distributed without statistical outliers (Materials and Methods). The one-day memory-enhancement effect is significant even with the largest individual positive effect removed, and the memory-enhancement effect in the immediate test remained nonsignificant with the largest negative individual effect removed. (B) Recognition-memory test performance plotted for each patient as the difference in d′ in the stimulation and no-stimulation conditions (scatter plots). Overlaid box-and-whisker plots show the median, range, and interquartile range for each condition. (C) Reported responses of patients when they were asked in subsequent testing whether they felt any sensation of stimulation. All patients responded “No” to every trial regardless of whether actual or sham stimulation had been delivered. Additionally, no patient indicated subjective awareness of stimulation during the study. **P < 0.005 (see text).
Fig. 2 also shows the results of a subsequent awareness test in which subjects were asked to decide if amygdala stimulation had been administered across 10 amygdala-stimulation and 10 sham-stimulation trials that were presented in random order. All 14 patients denied subjective awareness of the amygdala stimulation on every trial. In addition, no patient reported emotional responses associated with amygdala stimulation during the stimulation awareness test or during recognition-memory testing. Moreover, similar amygdala-stimulation parameters caused no detectable autonomic changes in patients (n = 7) undergoing stimulation parameter screening (Fig. S5). Thus, the amygdala stimulation used in the present study was capable of reliably enhancing one-day object-recognition memory performance without eliciting a subjective or objective emotional response. Indeed, the patients were unable to discriminate if or when any stimulation was being delivered.
Patients with epilepsy often exhibit cognitive deficits as a consequence of chronic seizures, antiepileptic medications, and associated neuronal dysfunction (39–41). Thus, an important question was whether the benefit of amygdala stimulation was attenuated in patients with poorer baseline memory function. The patients with weakest baseline memory performance actually tended to show the greatest stimulation-mediated improvements in recognition memory on the one-day test, suggesting that the benefit was possibly attenuated in patients with higher baseline memory scores (Fig. S6). In particular, the magnitude of the one-day memory enhancement was negatively correlated with all three baseline neuropsychological performance measures [Rey Auditory Verbal Learning Test discrimination index, r (12) = −0.61, P = 0.02; Rey–Osterrieth Complex Figure Delayed Recall, r (12) = −0.56, P = 0.04; and Wechsler Adult Intelligence Scale (WAIS) IV Full-Scale IQ, r (12) = −0.37, P = 0.18] (Fig. S4). As an example, the patient with the greatest baseline memory deficits routinely failed to recognize researchers and physicians she had met on multiple prior occasions, but she exhibited the greatest stimulation-induced memory-enhancement effect. If this patient's memory-enhancement effect was treated as an outlier and excluded from analysis (although statistically it was not considered an outlier; see SI Materials and Methods), the group memory-enhancement effect remained significant [t (11) = 4.24 P = 0.001, Cohen's d = 1.17]. In addition, individual memory-enhancement effects persisted for several patients despite the occurrence of seizures between the encoding phase and the one-day recognition test (Table S2). These observations highlight the robustness of the amygdala-mediated memory-enhancement effect and suggest that amygdala stimulation could provide substantial memory benefits even for patients with significant baseline cognitive deficits.
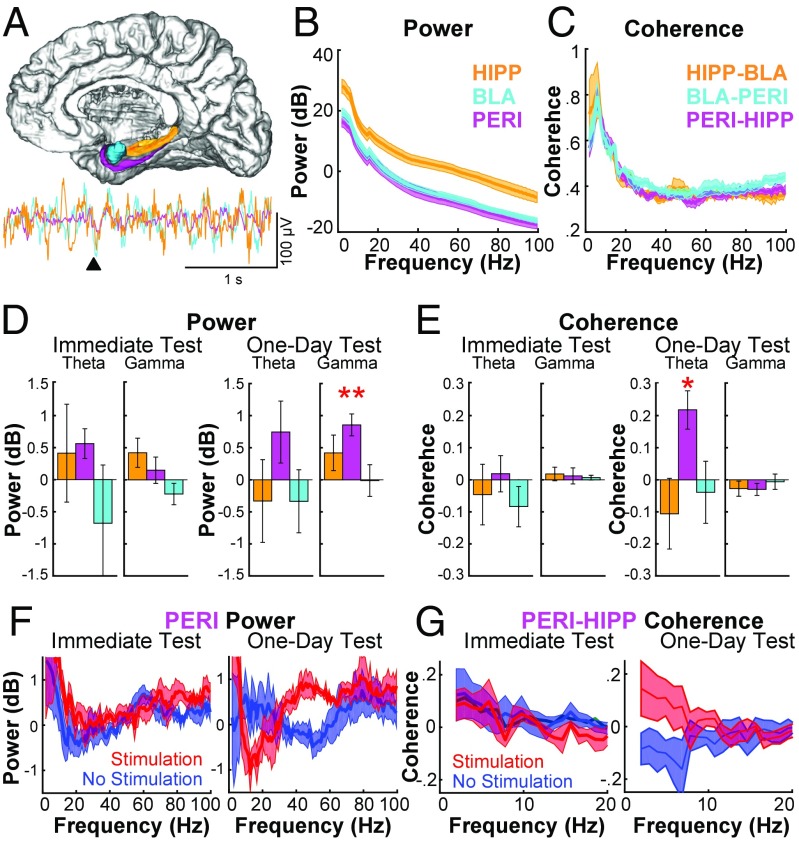
We next sought to identify neural correlates of the amygdala-mediated memory enhancement within MTL structures. The hippocampus and perirhinal cortex are both important for normal recognition memory (42–48). Each receives direct innervation from the BLA (26), and amygdala-mediated memory enhancement in rats requires intact hippocampal function (23). Thus, for the entire subset of patients with electrodes localized concurrently in the amygdala, hippocampus, and perirhinal cortex (n = 5 patients), local field potentials (LFPs) from each region were recorded simultaneously during the immediate and one-day recognition-memory tests (Fig. S2); stimulation artifacts precluded analysis of LFPs from the study session itself. The enhancement effect on the one-day test across these five patients (mean stimulation − control d′ difference = 0.22) was similar to the mean (0.23) across all 14 patients (for patients S3, S6, S8, S10, and S14: stimulation-control d′ differences on the one-day test were 0.40, 0.00, 0.07, 0.44, and 0.19, respectively, and on the immediate test were 0.06, 0.36, 0.31, 0.55, and 0.60, respectively). An example of these recordings (Fig. 3A) illustrates that LFP oscillations were apparent in the theta (here 5–7 Hz) and gamma (30–55 Hz) ranges, although the intermittent nature of the gamma oscillations obscured peaks in the average spectrograms. Analyses of neuronal oscillations during the first 0.5 s of recognition-memory trials were used to investigate how prior amygdala stimulation during the study phase affected subsequent oscillatory interactions during retrieval within the MTL. Spectral power within each region and coherence between each region-to-region pair were baseline-normalized, and main results were calculated as the difference between the oscillatory activity for remembered object images in the stimulation condition vs. the no-stimulation condition (Materials and Methods and Fig. 3 B–G). Recognition during the one-day test but not during the immediate test exhibited increased power in perirhinal cortex in the gamma frequency range for remembered objects previously followed by stimulation compared with remembered objects without stimulation [t(4) = 5.01, P = 0.007, Cohen’s d = 2.24]. Furthermore, LFPs during the one-day test, but not during the immediate test, revealed increased coherence of hippocampal–perirhinal oscillations in the theta frequency range for remembered objects previously followed by stimulation compared with remembered objects without stimulation [t(4) = 3.62, P = 0.02, Cohen’s d = 1.67] (Fig. 3 D–G). These results suggest that amygdala stimulation benefitted memory by influencing neural activity in brain regions normally important for recognition memory.
Fig. 3.
Recognition of specific object images one day following amygdala stimulation evokes increases in perirhinal gamma power and perirhinal–hippocampal theta coherence. (A) Illustration of the BLA (cyan), hippocampus (HIPP, orange), and perirhinal cortex (PERI, magenta) and a representative LFP from each region during a recognition test trial (black triangle indicates image onset). The 3D brain model was adapted with permission from the Albany Medical College Virtual Brain Model (www.amc.edu/academic/software). (B and C) Average overall power (B) and coherence (C) for the hippocampus, BLA, and perirhinal cortex electrodes during the one-day test (n = 5 subjects). The artifact at 18 Hz in the power spectra resulted from different multitaper parameters used for low-frequency and higher frequency ranges (Materials and Methods). (D and E) Difference in power (D) and coherence (stimulation − no-stimulation conditions) (E) for the theta (5–7 Hz) and gamma (30–55 Hz) bands. All error bars represent the SEM. *P < 0.05; **P < 0.01 after correcting for multiple comparisons; see Materials and Methods. (F and G) Spectral plot of differences in perirhinal gamma power (F) and perirhinal–hippocampal theta coherence (G) in the stimulation and no-stimulation conditions.
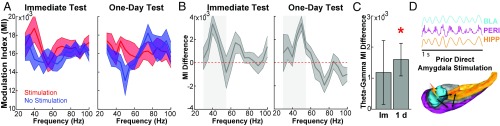
To examine the interactions between theta and gamma oscillations, we calculated a modulation index of cross-frequency (theta–gamma) phase-amplitude coupling (see Materials and Methods for details and refs. 49–51). Specifically, we calculated the extent to which the phase of the BLA theta oscillations modulated the amplitude of the perirhinal gamma oscillations during accurate retrieval of images previously encoded with and without stimulation. During both the immediate and one-day tests, the phase of the amygdala theta oscillations indeed modulated the amplitude of perirhinal gamma oscillations, particularly around 50 Hz, and did so to a significantly greater extent on the one-day test for remembered objects in the stimulated condition than for remembered objects in the no-stimulation control condition [t(4) = 3.04, P = 0.04, Cohen’s d = 1.36] (Fig. 4). Thus, accurately recognizing objects from the stimulation condition during the one-day test was accompanied by oscillatory patterns suggesting that network interactions between the amygdala and other memory regions underlie the memory enhancement.
Fig. 4.
Increased modulation of perirhinal gamma power by amygdala theta phase during accurate recognition of objects from the stimulation condition. (A) A phase–amplitude modulation index (MI) is plotted for interactions between BLA theta phase (5–7 Hz) and perirhinal cortex gamma amplitude for remembered objects in the stimulation and no-stimulation conditions at the immediate and one-day recognition tests. (B) MI differences in the stimulation and no-stimulation conditions by spectral frequency in the perirhinal cortex. The shaded region denotes the gamma band between 30–55 Hz. (C) Cumulative MI difference between stimulation and no-stimulation conditions for the gamma range in the perirhinal cortex. During the one-day test, the MI was increased for remembered images in the stimulation condition relative to remembered images in the no-stimulation condition, *P < 0.05 (D) Schematic representation of oscillatory activity during the one-day recognition test in the BLA, hippocampus (HIPP), and perirhinal cortex (PERI) for objects in the stimulation condition. The oscillations depict increased theta interactions between the three regions and gamma power in perirhinal cortex modulated by those theta oscillations. Im, immediate test. All error bars and bands represent the SEM.
Discussion
The present study demonstrates that brief electrical stimulation to the human amygdala reliably improved long-term recognition memory for images of neutral objects without eliciting an emotional response. This memory enhancement was accompanied by neuronal oscillations during retrieval that reflected increased interactions between the amygdala, hippocampus, and perirhinal cortex. The experiment was motivated by prior research in experimental animals in which activation of the amygdala by direct electrical stimulation or local microinfusions of norepinephrine improved subsequent memory and modulated neuronal activity or synaptic plasticity in the hippocampus and perirhinal cortex (5, 6, 16, 18, 22–25). Indeed, our approach was directly modeled on recent studies in rats in which brief electrical amygdala stimulation enhanced recognition memory of novel neutral objects but in which direct assessment of subjective experience was not possible (Fig. S7) (22–24). In rats, amygdala stimulation elicited intrahippocampal gamma synchrony that was reflected as coordinated timing of action potentials of hippocampal CA3 pyramidal neurons with membrane potentials in downstream neurons in hippocampal region CA1 (24), and hippocampal inactivation eliminated the amygdala-mediated memory enhancement (23). The current results suggest fundamentally similar mechanisms of memory enhancement across rats and humans and indicate a causal role for the amygdala in memory that, in humans, is dissociable from subjective emotional experience. Although the amygdala’s role in emotional memory has been well established, the current findings suggest that the amygdala can play a discrete role in modulating memory consolidation apart from emotional experience.
These results also suggest an important role for the hippocampus and perirhinal cortex in amygdala-mediated enhancement of long-term memory, which is consistent with prior evidence of the importance of these MTL regions for recognition memory more generally (42–48). Moreover, the enhancement-related patterns of oscillations observed during the one-day memory test resembled the theta-modulated gamma burst pattern of the amygdala stimulation delivered during the study phase. This reappearance of theta-modulated gamma oscillations in the MTL during retrieval could possibly indicate a reactivation of an amygdala-induced network state or could reflect the overall importance of theta and gamma oscillations for appropriate medial temporal lobe function. In either case, it is known that many of the cellular consolidation processes related to long-term synaptic plasticity in memory networks unfold over the course of hours (6, 16, 18, 21, 37, 38). Thus, it is possible that amygdala stimulation initiated specific synaptic plasticity-related molecular cascades, resulting in a cumulative benefit to memory that emerged only over a protracted period as cellular processes progressed. One notion is that increased glutamatergic release from amygdala terminals in regions such as the hippocampus up-regulates the induction of immediate but small molecular changes at active synapses, which in turn increase the likelihood that those synapses will undergo slower but more robust molecular and structural changes over subsequent hours to days (17, 31). In this view, plasticity in memory structures initiated by viewing of the object images would be magnified by amygdala stimulation.
Our study raises several technical considerations. First, as in any study examining the effects of direct electrical stimulation in patients undergoing intracranial electrode monitoring, our study necessarily consisted of patients with intractable epilepsy rather than healthy individuals. Intracranial monitoring typically samples broad territories of brain representing putatively normal and pathological tissue to accurately localize and define the boundaries of the patient’s specific seizure focus or network (52). Although specific foci of each patient’s brain were presumed to be functioning abnormally, we accounted for the clinical heterogeneity across patients by using a within-subject design, comparing each patient’s performance and electrophysiological responses in the stimulated vs. unstimulated conditions. Also, memory-enhancement effects were observed regardless of anatomical proximity to seizure onsets, and this specifically included several subjects in whom the hippocampus ipsilateral to amygdala stimulation was independently implicated in seizure onsets (Table S2). Additionally, analogous memory-enhancement results resembled those from three prior studies in rats without seizures, suggesting that our findings are unrelated to epilepsy. Second, although we examined whether brief, low-amplitude amygdala stimulation elicited an explicit subjective emotional response, further studies of the potential effects of low-amplitude amygdala stimulation on subtler implicit evaluations of emotional valence and arousal are needed. Third, this study did not examine the neurophysiological effects of amygdala stimulation during the encoding phase due to recorded signals being obscured by stimulation artifacts. Future studies using recording techniques that eliminate or minimize such stimulation artifacts are needed to determine the effects of amygdala stimulation on hippocampal and perirhinal oscillatory activity during encoding. Finally, although the memory-enhancement effect from amygdala stimulation was reliable and significant across the majority of tested patients, it is possible that different stimulation parameters (i.e., location, frequency, duration, and timing of stimulation) might induce more robust amygdala-mediated memory enhancement. Future studies will calibrate amygdala stimulation parameters to amplify memory-enhancement effects in patients suffering from memory impairments.
Human brain stimulation not only reveals potential causal mechanisms of normal brain function but also has therapeutic implications. Although chronically implanted stimulation devices are used to treat a variety of neurological and psychiatric disorders safely and effectively, effective means for improving cognitive disorders and specifically enhancing memory remain elusive (39–41, 49, 53). Our description of amygdala-mediated memory enhancement provides unique results with respect to target and effects relative to prior efforts in humans (39–41, 49, 53). For instance, alternative strategies directly targeting the hippocampus or entorhinal cortex have yielded inconsistent effects on memory among individuals and across studies (39–41). Further, some recent reports suggest that real-time classification of the brain’s encoding state may be needed to produce proper timing of stimulation and memory enhancement during poor encoding states (39, 41). Although we found robust and reliable memory enhancement without such closed-loop classification of the memory-encoding state, knowledge of the memory-encoding state with stimulation only for poorly encoded items could possibly strengthen the enhancement of stimulated relative to unstimulated memories. This potential closed-loop approach to memory enhancement is aided by our demonstration that amygdala stimulation can produce reliable, temporally specific memory-enhancement effects even when delivered following an experience by allowing determination of the memory-encoding state during an experience.
The consistent memory enhancement we observed may be related to the proposed role for the amygdala as an overarching modulator of downstream memory structures. Capitalizing upon this endogenous capacity to engage memory networks may offer therapeutic advantages. Indeed, to date no other invasive or noninvasive human brain-stimulation studies have shown temporally specific memory enhancement across periods longer than a few minutes after initial stimulation and encoding (39–41, 49, 50, 53). Indeed, with amygdala-mediated memory enhancement, we observe temporally specific learning that is robust after a single trial and persists to the following day. Notably, memory enhancement occurred broadly among the majority of subjects tested, even in subjects with baseline memory deficits, and likewise did not impair any individual subject’s memory the next day. Finally, accurate memory retrieval related to clear electrophysiological signatures of prior stimulation-enhanced consolidation, providing evidence of amygdala stimulation’s impact upon downstream memory structures.
In conclusion, our study constitutes direct evidence for a causal role of the human amygdala in the initiation of temporally specific memory enhancement and consequent functional changes in the hippocampus and perirhinal cortex without eliciting a subjective emotional response. By directly adapting an analogous paradigm from object-recognition memory-enhancement experiments in experimental animals, we extend previous knowledge while making observations relevant to humans. Parallel efforts across species will foster future studies of the network, cellular, and molecular mechanisms of amygdala-mediated memory enhancement with relevance to normal and pathological memory. Such work will reveal the basic mechanisms of endogenous memory prioritization while also advancing translational interventions to treat human memory impairment.
Materials and Methods
Participants.
Fourteen participants (five females) with drug-resistant epilepsy volunteered and provided written informed consent (Tables S1–S3). The study protocol was approved by the Emory University Institutional Review Board. Standard stereotactic depth electrode arrays were implanted into the brain parenchyma by a neurosurgeon (J.T.W. or R.E.G.) for the sole purpose of clinical seizure investigation. The number of patients was predetermined by a formal power (target = 0.8) analysis.
Brain Stimulation and Recording.
Electrode contacts were localized manually following coregistration of pre- and postoperation brain scans (Figs. S1 and S2). Stimulation parameters were chosen to replicate those used in three prior rat studies that demonstrated amygdala-mediated memory enhancement (22–24). Specifically, stimulation was delivered to the BLA in current-regulated, charge-balanced, biphasic rectangular pulses at 0.5 mA for 1 s in eight trains of four pulses at 50 Hz. LFPs were recorded at a sampling rate of either 500 or 1,000 Hz. No seizure activity or afterdischarges to stimulation were detected during testing or in a thorough posttest review of all recorded LFP channels by a clinical epileptologist (R.E.F.).
Behavioral Tasks.
Recognition-memory task procedure.
During the study phase, each participant viewed 160 images of neutral object images (51), one trial at a time, and was asked to make an “indoor” vs. “outdoor” judgment with respect to the image on each trial. A fixation screen was presented for a jittered duration of 0.5–1.5 s immediately before each image onset. Each image was then presented for 3 s, and in a randomly selected half of the trials (stimulation condition), offset of the image coincided with the onset of 1 s of stimulation to the BLA. The intertrial interval was 5 s. Memory was tested via a self-paced yes/no (i.e., repeated/new) recognition-memory test for half the images (40 from the stimulation condition and 40 from the no-stimulation condition) immediately after the end of the study session; memory for the remaining images (40 from the stimulation condition and 40 from the no-stimulation condition) was tested approximately one day later (M = 22 h, range = 20–25 h delay). Forty new images served as foils, and different foils were used for each test. After making yes/no judgments, participants also made sure/not sure certainty judgments. Analysis of certainty judgments was not performed because some subjects did not seem to understand the certainty judgment instructions (e.g., some participants paired sure with all “yes” responses and not sure with all “no” responses). No electrical stimulation was delivered during either recognition-memory test.
Stimulation awareness test procedure.
After the one-day recognition test, participants were administered 20 trials in which a button was pressed to deliver either stimulation or sham stimulation (no current) and were asked “Did you feel any stimulation?” Stimulation to the BLA using the same parameters as during the memory task was delivered in only a randomly selected half of the trials, and participants were not told which trials omitted the stimulation.
Electrophysiological Data Analysis.
Analyses of electrophysiological data focused on oscillations in LFPs recorded simultaneously from the BLA, hippocampus, and perirhinal cortex in five patients with electrodes simultaneously present in all three regions (Fig. S2). Data from the first 0.5 s of each correctly answered trial (before yes/no judgments) in both the immediate and one-day memory tests were analyzed. Analyses focused on the test sessions because electrical stimulation was delivered during the study session only after object images were presented and because the electrical stimulation produced substantial electrical artifacts. In addition, for several of the five patients in the LFP analyses, there were relatively few incorrect images in the stimulation condition on either test. Thus, comparing LFPs between correct and incorrect trials was not feasible. Please see Supporting Information for a detailed description of LFP analyses.
Supplementary Material
Acknowledgments
We thank the patients, physicians, and staff of the Emory University Hospital Epilepsy Monitoring Unit for their time and trust, and Crystal Chu, Anna Rogers, and David L. Brody for their support and input on earlier versions of the manuscript. Effort and resources for this work were supported in part by NIH Grants KL2TR000455 (to K.R.B.), R01NS088748 (to D.L.D.), and R01MH100318 (to J.R.M.) and National Research Service Award F30 MH095491 (to D.I.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The datasets generated and code used to analyze the current study’s data are available from the corresponding author on reasonable request.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714058114/-/DCSupplemental.
References
- 1.Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- 2.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 3.Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL. Memory–A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL. Making lasting memories: Remembering the significant. Proc Natl Acad Sci USA. 2013;110:10402–10407. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng J, et al. Amygdala-hippocampal dynamics during salient information processing. Nat Commun. 2017;8:14413. doi: 10.1038/ncomms14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proc Natl Acad Sci USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill L, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 12.Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- 15.Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 16.Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci. 2002;22:9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McReynolds JR, Anderson KM, Donowho KM, McIntyre CK. Noradrenergic actions in the basolateral complex of the amygdala modulate Arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiol Learn Mem. 2014;115:49–57. doi: 10.1016/j.nlm.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahill L, Prins B, Weber M, McGaugh JL. β-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 20.Segal SK, Stark SM, Kattan D, Stark CE, Yassa MA. Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiol Learn Mem. 2012;97:465–469. doi: 10.1016/j.nlm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paré D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol. 2003;70:409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 22.Bass DI, Partain KN, Manns JR. Event-specific enhancement of memory via brief electrical stimulation to the basolateral complex of the amygdala in rats. Behav Neurosci. 2012;126:204–208. doi: 10.1037/a0026462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bass DI, Nizam ZG, Partain KN, Wang A, Manns JR. Amygdala-mediated enhancement of memory for specific events depends on the hippocampus. Neurobiol Learn Mem. 2014;107:37–41. doi: 10.1016/j.nlm.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bass DI, Manns JR. Memory-enhancing amygdala stimulation elicits gamma synchrony in the hippocampus. Behav Neurosci. 2015;129:244–256. doi: 10.1037/bne0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manns JR, Bass DI. The amygdala and prioritization of declarative memories. Curr Dir Psychol Sci. 2016;25:261–265. doi: 10.1177/0963721416654456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 1996;375:552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Hamann S. Mapping discrete and dimensional emotions onto the brain: Controversies and consensus. Trends Cogn Sci. 2012;16:458–466. doi: 10.1016/j.tics.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: Activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- 29.Yonelinas AP, Ritchey M. The slow forgetting of emotional episodic memories: An emotional binding account. Trends Cogn Sci. 2015;19:259–267. doi: 10.1016/j.tics.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talmi D. Enhanced emotional memory: Cognitive and neural mechanisms. Curr Dir Psychol Sci. 2013;22:430–436. [Google Scholar]
- 31.Richter-Levin G, Akirav I. Emotional tagging of memory formation–In the search for neural mechanisms. Brain Res Brain Res Rev. 2003;43:247–256. doi: 10.1016/j.brainresrev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Schwarze U, Bingel U, Sommer T. Event-related nociceptive arousal enhances memory consolidation for neutral scenes. J Neurosci. 2012;32:1481–1487. doi: 10.1523/JNEUROSCI.4497-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunsmoor JE, Murty VP, Davachi L, Phelps EA. Emotional learning selectively and retroactively strengthens memories for related events. Nature. 2015;520:345–348. doi: 10.1038/nature14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meletti S, et al. Emotions induced by intracerebral electrical stimulation of the temporal lobe. Epilepsia. 2006;47:47–51. doi: 10.1111/j.1528-1167.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 35.Lanteaume L, et al. Emotion induction after direct intracerebral stimulations of human amygdala. Cereb Cortex. 2007;17:1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- 36.Bijanki KR, et al. Case report: Stimulation of the right amygdala induces transient changes in affective bias. Brain Stimul. 2014;7:690–693. doi: 10.1016/j.brs.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne JD, Kensinger EA. Sleep’s role in the consolidation of emotional episodic memories. Curr Dir Psychol Sci. 2010;19:290–295. [Google Scholar]
- 38.Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Nuerosci. 2012;6:108. doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs J, et al. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron. 2016;92:983–990. doi: 10.1016/j.neuron.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 40.Suthana N, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ezzyat Y, et al. Direct brain stimulation modulates encoding states and memory performance in humans. Curr Biol. 2017;27:1251–1258. doi: 10.1016/j.cub.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 43.Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 44.Gabrieli JDE. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- 45.Yassa MA, Stark CEL. Multiple signals of recognition memory in the medial temporal lobe. Hippocampus. 2008;18:945–954. doi: 10.1002/hipo.20452. [DOI] [PubMed] [Google Scholar]
- 46.Fernández G, Tendolkar I. The rhinal cortex: ‘gatekeeper’ of the declarative memory system. Trends Cogn Sci. 2006;10:358–362. doi: 10.1016/j.tics.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Brown MW. Hippocampal and perirhinal functions in recognition memory. Nat Rev Neurosci. 2008;9:405, author reply 405. doi: 10.1038/nrn2154-c1. [DOI] [PubMed] [Google Scholar]
- 48.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamani C, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 50.Wang JX, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guillory SA, Bujarski KA. Exploring emotions using invasive methods: Review of 60 years of human intracranial electrophysiology. Soc Cogn Affect Neurosci. 2014;9:1880–1889. doi: 10.1093/scan/nsu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laxton AW, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 54.Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3rd Ed Academic; Cambridge, MA: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.