Significance
How climate change and biological invasions interact to affect biodiversity is of major concern to conservation. Quantitative evidence for the nature of climate change–invasion interactions is, however, limited. For the soil ecosystem fauna, such evidence is nonexistent. Yet across the globe, soil-dwelling animals regulate belowground functioning and have pronounced influences on aboveground dynamics. Using springtails as an exemplar taxon, widely known to have species-specific effects on below- and aboveground dynamics, we show that across a wide latitudinal span (16–54°S), alien species have greater ability to tolerate climate change-associated warming than do their indigenous counterparts. The consequences of such consistent differences are profound given globally significant invasions of soil systems by springtails.
Keywords: climate change, introduced species, soil fauna, thermal limits, laboratory evolution
Abstract
Soil systems are being increasingly exposed to the interactive effects of biological invasions and climate change, with rising temperatures expected to benefit alien over indigenous species. We assessed this expectation for an important soil-dwelling group, the springtails, by determining whether alien species show broader thermal tolerance limits and greater tolerance to climate warming than their indigenous counterparts. We found that, from the tropics to the sub-Antarctic, alien species have the broadest thermal tolerances and greatest tolerance to environmental warming. Both groups of species show little phenotypic plasticity or potential for evolutionary change in tolerance to high temperature. These trait differences between alien and indigenous species suggest that biological invasions will exacerbate the impacts of climate change on soil systems, with profound implications for terrestrial ecosystem functioning.
The impacts of climate change and biological invasions continue to increase in magnitude (1, 2), yet investigation of their interactions remains fragmented and incomplete (3). The general expectation is that such interactions will benefit alien over indigenous species, and thus have negative consequences for native biodiversity (4). Soil systems are globally important reservoirs of diversity, influence aboveground ecological dynamics, and are critical for food security (5). They are also highly susceptible to climate change and to biological invasions, which both have major effects on soil ecosystem functioning and are expected to interact to the detriment of these systems (6, 7). Understanding how climate change–invasion interactions will play out in soil systems is, therefore, pressing (8). For the soil biota, increasing temperature is one of the most significant components of climate change (9). Differential fitness effects of rising temperatures on indigenous compared with alien species must necessarily be mediated by consistent thermal trait differences between these two groups of species (10). Moreover, if such effects are general, these trait differences between alien and indigenous species should be maintained across a broad latitudinal—and hence climatic—range. No study has yet evaluated for any group of soil macrobiota whether such latitudinally extensive differences in thermal traits exist that advantage alien species under warming conditions, and thus whether forecast impacts are likely to be realized.
To assess this expectation, we investigated the thermal limits to activity in 16 alien and 14 indigenous species of springtails (Collembola) spanning a climatic gradient from the Australian tropics (16°S) to south of the Antarctic Polar Front (54°S) (Fig. 1). Springtails are a globally distributed, functionally significant component of the soil biota (5, 11). The group includes many alien species that have invaded soil systems from the polar regions to the tropics (6, 12). Critical thermal limits to activity form a useful proxy for adult fitness because organisms are incapable of movement outside of these limits, thus reducing fitness (13, 14), with demographic effects reflected in associations between critical thermal limits and geographic ranges in several taxa (15, 16). Upper critical thermal limits are also now widely used to assess tolerance to climate change-associated warming in a wide range of organisms (17, 18). Using a single consistent method [in contrast with multiple methods represented in and which complicate interpretations of meta-analyses (19)], the F2 generation of populations of each species to overcome parental effects and the potential for laboratory adaptation (20), and taking account of the influence of phylogenetic relatedness (21), we investigated both basal thermal tolerance and the extent of phenotypic plasticity in the upper critical thermal limits.
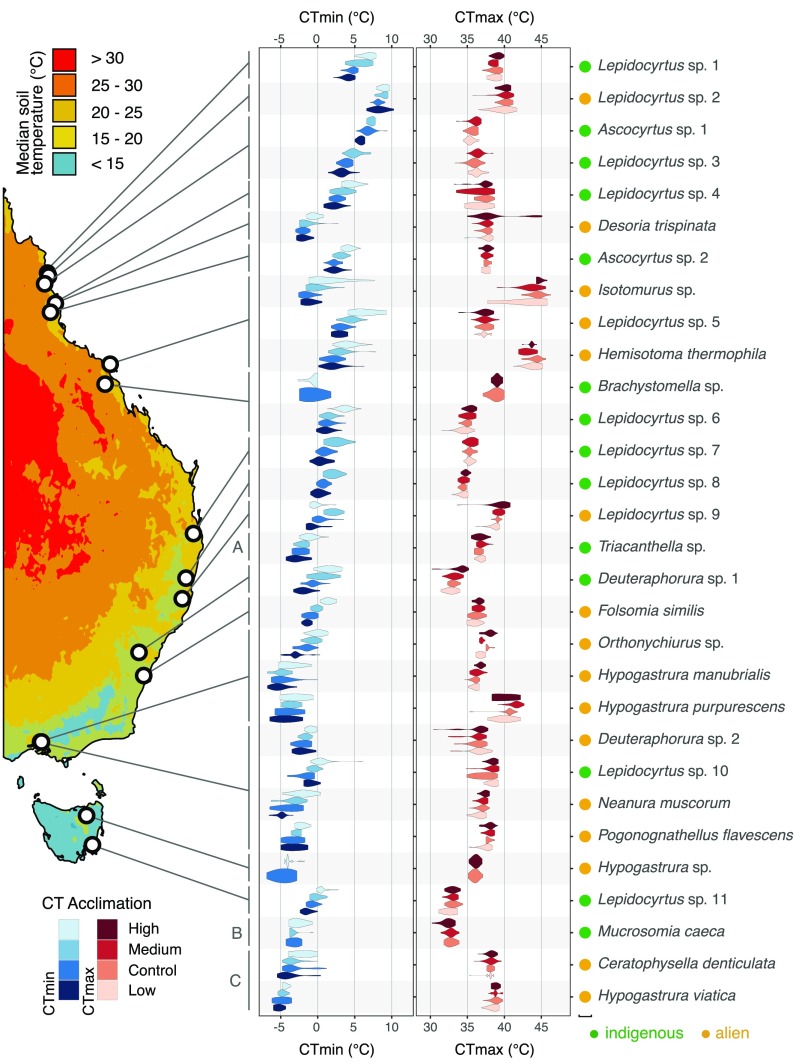
Fig. 1.
Geographic variation in basal critical thermal limits and their phenotypic plasticity across Australia. Violin plots display the frequency density of individual critical thermal limits data (smoothed with a Gaussian kernel) for control and acclimation populations of each species. In each case the control value is the basal tolerance of the species, whereas the other values refer to experimental acclimations (low, medium, high; 5 °C below, 5 °C above, 10 °C above control values, respectively) (SI Appendix, Table S8). Soil temperature contours represent the median daytime temperature 2.5-cm below the soil surface, estimated using methods detailed in SI Appendix. Lord Howe Island (A: 31.5°S, 159.0°E) and the sub-Antarctic sites of South Georgia (B: 54.2°S, 36.6°W, south of the Antarctic Polar Front) and Macquarie Island (C: 54.5°S, 158.9°E) are not shown. Indigenous and alien species are indicated by green circles and orange circles, respectively, adjacent to species names.
Although phenotypic plasticity of thermal tolerance traits may only play a limited role in reducing exposure to climate change in some ectotherms (22), it has been identified as a potentially significant mediator of responses to thermal challenge (23), including in springtails (9, 24). Phenotypic plasticity is also thought to be a major contributor to the success of invasive species (25, 26). In addition, total thermal tolerance range and lower critical thermal limits play a role in determining range shifts in response to environmental change (16). Thus, we not only assessed basal tolerance, but also phenotypic plasticity for both upper and lower critical thermal limits. We evaluated whether climate-related variation in upper and lower critical thermal limits, thermal tolerance range, and warming tolerance—the difference between species’ upper critical thermal limits and maximum environmental temperatures (17)—differ systematically between the indigenous and alien springtail species. Tests were conducted using phylogenetic generalized least squares (PGLS) (21), and a phylogenetic tree constructed from molecular markers to take into account phylogenetic signal in the traits measured.
Evolutionary responses have the potential to reduce substantially the impacts of climate change on populations (27, 28). Therefore, we also examined the extent to which thermal traits might evolve in springtails and whether this potential differs between indigenous and alien species. We used laboratory natural selection (29) to assess the extent of evolutionary potential in the critical thermal limits of an indigenous and alien species from the tropics and another such pair of species from the temperate zone, held at elevated temperatures just below those that substantially depress fitness in these species, so reflecting extreme environmental conditions.
Results
Critical Limits and Warming Tolerance.
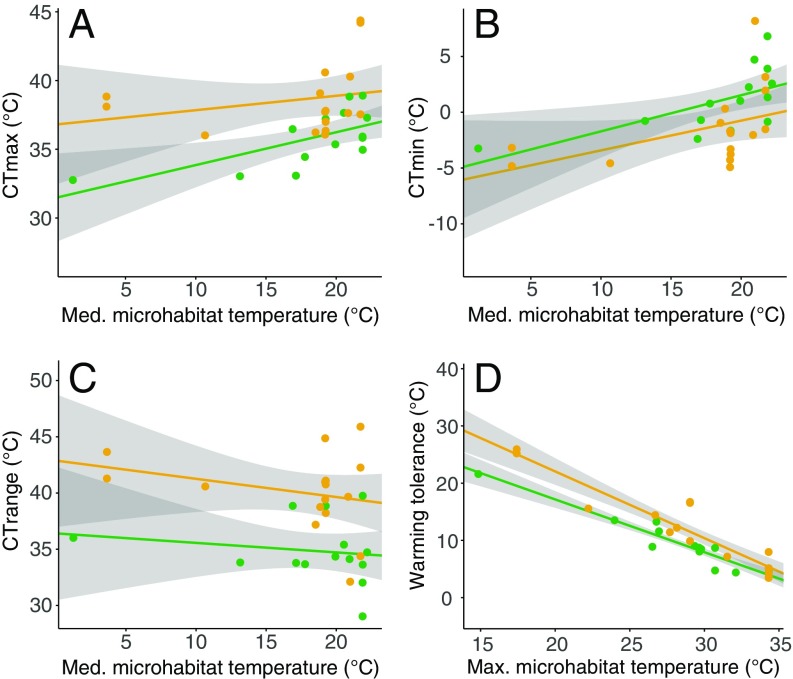
Although critical thermal maximum (CTmax) increased by 0.16 ± 0.07 °C (mean ± SE) on average for each 1 °C increase in soil microhabitat temperature [obtained from MODIS Land surface temperatures (Materials and Methods)] across latitude, the indigenous species had, on average, a 2.9 ± 0.8 °C lower CTmax than their alien counterparts (Fig. 2A), with little evidence of phylogenetic signal in the trait (Table 1). For critical thermal minimum (CTmin), the magnitude of change with microhabitat temperature variation was similar (Fig. 2B) (0.18 ± 0.08 °C increase per 1 °C change in microhabitat temperature). The difference between the two groups was, however, smaller (0.5 ± 0.9 °C), with an important contribution of phylogenetic relatedness to trait variation, which rendered the difference between the indigenous and alien species nonsignificant (Table 1). Although CTmax and CTmin were not significantly related (SI Appendix, Fig. S1 and Table S1), similar covariation of each of these variables with microhabitat temperature meant that tolerance range showed no association with microhabitat temperature. Nonetheless, a large difference in tolerance range, of on average 3.4 ± 1.1 °C (Fig. 2C), was found between the indigenous and alien species, with strong phylogenetic signal in the trait (Table 1). Thus, overall, upper and lower critical temperatures varied in similar ways with microhabitat temperature across latitude, resulting in no microhabitat-associated variation in thermal tolerance range. Consistently large differences among the indigenous and alien species were, however, found in CTmax and tolerance range. Warming tolerance declined by 1.1 ± 0.08 °C with every 1 °C increase in soil microhabitat temperature across latitude for both groups, but the alien species had, on average, a 2.8 ± 0.8 °C larger warming tolerance than the indigenous species (Fig. 2D), with no trait variation accounted for by phylogenetic relatedness (Table 1). Alien species were, therefore, on average more tolerant of warming across latitude than their indigenous counterparts.
Fig. 2.
Variation between indigenous and alien springtails in thermal tolerance from tropical to polar habitats. For each trait, the x axis shows median or maximum soil microhabitat temperature for the sites at which species’ populations were collected. Lines represent the fits of ordinary least-squares regression for indigenous (green symbols) and alien (orange symbols) species separately with 95% confidence bands (gray shading). No interaction terms were significant, indicating no slope differences between indigenous and alien species in any of the traits (analytical outcomes, excluding interaction terms, in Table 1). (A) Critical thermal maximum, (B) critical thermal minimum, (C) critical thermal range, (D) warming tolerance calculated as the difference between the highest microhabitat temperature of the warmest month and the critical thermal maximum. For comparative purposes, plots against minimum and maximum environmental temperatures are provided as SI Appendix, Fig. S3.
Table 1.
Outcome of PGLS analyses
| CT values and warming and tolerance ranges | Estimate ± SE | T | P |
| CTmax | |||
| Intercept | 35.79 ± 1.37 | 26.13 | <0.001 |
| Median soil temperature | 0.16 ± 0.07 | 2.25 | 0.033 |
| Status (indigenous) | −2.93 ± 0.81 | −3.62 | 0.001 |
| F(2,27) = 8.46, P = 0.001, R2 = 0.34, MLλ = 0.00 | |||
| CTmin | |||
| Intercept | −4.10 ± 1.90 | −2.15 | 0.040 |
| Median soil temperature | 0.18 ± 0.08 | 2.32 | 0.028 |
| Status (indigenous) | 0.54 ± 0.94 | 0.57 | 0.571 |
| F(2,27) = 2.76, P = 0.081, R2 = 0.11, MLλ = 0.44 | |||
| Tolerance range | |||
| Intercept | 39.57 ± 2.38 | 16.66 | <0.001 |
| Median soil temperature | −0.006 ± 0.096 | −0.07 | 0.947 |
| Status (indigenous) | −3.35 ± 1.14 | −2.95 | 0.007 |
| F(2,27) = 4.39, P = 0.022, R2 = 0.19, MLλ = 0.49 | |||
| Warming tolerance | |||
| Intercept | 42.83 ± 2.30 | 18.62 | <0.001 |
| Maximum soil temperature | −1.08 ± 0.08 | −13.98 | <0.001 |
| Status (indigenous) | −2.82 ± 0.79 | −3.57 | 0.001 |
| F(2,27) = 101, P < 0.001, R2 = 0.87, MLλ = 0.00 | |||
Outcome of PGLS analyses showing change in thermal tolerance (°C) with median (or maximum for warming tolerance) daytime soil surface temperature (°C) and the difference among indigenous and alien species. MLλ is the maximum-likelihood estimate of Pagel’s λ (21), a measure of phylogenetic effect where 0 indicates no effect and 1 indicates a strong effect equivalent to that expected under a BM model of evolutionary change.
Evolutionary Potential and Phenotypic Plasticity.
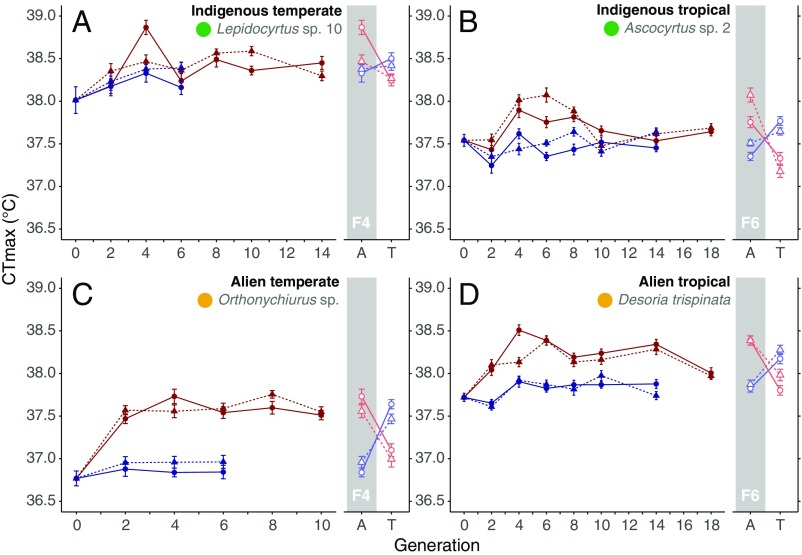
Across the four species examined for evolutionary responses to elevated temperatures, CTmax showed little evolutionary response over 10–18 generations. Although the treatments were significant in three of the four species (SI Appendix, Table S2), observed responses to selection per generation across all species were small, ranging from 0 to 0.89 °C (Fig. 3 and SI Appendix, Table S3), with a maximum overall response of 0.6 °C in the selected lines of the alien temperate Orthonychiurus sp. Moreover, the responses in CTmax remained within the scope of developmental phenotypic plasticity typical for all four species (Fig. 3 and SI Appendix, Table S4), accounting for the smaller than expected observed responses of CTmax to selection (SI Appendix, Table S3). These changes in CTmax were much more limited than those of CTmin, which showed larger intergenerational changes, and typically larger than those achievable through developmental plasticity (SI Appendix, Fig. S2 and Tables S5 and S6).
Fig. 3.
The response of critical thermal maximum to laboratory natural selection in four species of springtails. Mean CTmax (±SE) for the temperate indigenous Lepidocyrtus sp. 10 (A), tropical indigenous species Ascocyrtus sp. 2 (B), temperate alien Orthonychiurus sp. (C), and tropical alien Desoria trispinata (D) for each of two lines (filled circles/triangles and solid/dashed lines) evolved under an elevated temperature (red: tropical = 27 °C, temperate = 25 °C) and control lines (blue: tropical = 20 °C, temperate = 15 °C). Open symbols to the right indicate the outcomes of a reciprocal transplant experiment at generations four or six, determining the contribution of developmental plasticity to CTmax. Here, springtails from selection and control groups were reared under either their standard acclimation temperature (A) or transplanted to the thermal environment of the opposing group (T) and reared for one generation. Intergenerational change in CTmax is typically within the scope of developmental plasticity. Effect sizes are typically less than 1 °C (see SI Appendix, Tables S2 and S4 for statistics).
Although acclimation responses varied substantially among the traits and species (Fig. 1), acclimation response ratio (ARR), a measure of phenotypic plasticity (22), showed no systematic difference between the indigenous and alien groups either for CTmax or CTmin (SI Appendix, Table S7), resulting in no influence on warming tolerances either. Overall, CTmin typically showed greater phenotypic plasticity (ARR mean: 0.137, range: 0.037–0.238) than CTmax (ARR mean: 0.047, range: 0.008–0.109) [ANOVA F(1,58) = 64.05, P < 0.0001], but no variation in ARR was found with microhabitat temperature or with basal tolerance in either trait (SI Appendix, Table S7). Theory suggests that broader performance curves in temperate species relative to tropical ones might differentially influence responses to acclimation exposures of similar magnitudes (17), but such differences were not detected.
Discussion
In plants and in some animal groups, indigenous and alien species frequently differ systematically either in basal trait values or, over the short-term, in phenotypic plasticity, which may account, at least in part, for the success of alien species (25, 26, 30, 31). Only a few studies have also sought to interpret these differences in a climate change context and typically just for specific locations (10, 32, 33). Hence, evidence that climate change may favor alien species over their indigenous counterparts via differences in trait values is limited (3).
Drawing on extensive latitudinal coverage, we show that, on average, alien soil-dwelling springtails have higher CTmax than their indigenous counterparts across a broad latitudinal range, despite quite limited variation with climate across latitude [as has been found previously for insects (34)]. For these differences in CTmax between the indigenous and alien species to translate into differential susceptibility to habitat temperature change, forecast survival in the face of such change should show consistent differences between the two groups of species. In the springtail species investigated here, warming tolerance—a widely used measure of thermal safety margins—or capability to cope with elevated temperatures (17, 18, 35, 36), and calculated as the difference between maximum microhabitat temperature and CTmax, shows just such variation. On average, the difference between the groups is 3 °C across the climate zones investigated. A difference in warming tolerance of 3 °C on average between the groups is substantial, especially given forecasts of future change to global mean temperatures of this magnitude or larger by 2100 (37). Such differences in warming tolerance suggest that, as soils warm, alien species will on average have greater time available for activity [given that they will likely seek thermal refuges before critical values are reached (36, 38)], providing them with fitness benefits over their indigenous counterparts.
For this difference to have a longer-term effect on differences between indigenous and invasive species, CTmax should show limited evolutionary potential as climates change (18, 27, 28). The laboratory selection experiment indicated not only that the potential for evolutionary change in CTmax is limited, especially by comparison with the situation for CTmin, but also that this potential does not differ between either the indigenous or alien species, or the tropical and temperate climatic areas from which the species were sourced. Phenotypic plasticity similarly shows no consistent variation between the indigenous and alien springtail groups, despite significant interspecific variation in the traits and their plasticity (Fig. 1). Such limited effects of phenotypic plasticity and low potential for evolutionary change in CTmax in the springtail species assessed (and in contrast to substantial phenotypic plasticity and potential for evolutionary change in CTmin) are in keeping with what has been found for other animal groups (22, 34). They indicate that thermal tolerance differences between the indigenous and alien groups are likely to persist through time. Nonetheless, thermal conditions different to those adopted in the laboratory selection experiment, such as occasional extreme events (39), might be more effective in driving evolutionary change in CTmax (28). Indeed, past evolutionary responses to divergent thermal conditions, in conjunction with the ecological conditions that promote the success of alien species (7), may explain the observed trait differences between the two groups of species in the first place. Such a mechanistic macrophysiology of biological invasion remains to be developed.
Given that trait differences between the alien and indigenous groups will likely endure, springtail assemblages should, with ongoing climate change, be more prone to dominance by alien species, irrespective of whether these assemblages are tropical, temperate, or more polar. Indeed, evidence from some areas suggests that such a process is already underway (6, 12, 32). Clearly, interspecific variation exists in thermal traits and warming tolerance among the springtail species investigated here (Fig. 1), and other life-history traits might also play a role (15), suggesting that specific predictions may be complicated. Nonetheless, the general trends we demonstrate support growing concerns that soil systems will be significantly impacted by interactions among climate change and biological invasion (7, 8).
The broader influence of such climate change–invasion interactions is likely to be realized through changes to soil-system dynamics. Springtails are widely known to have considerable effects on soil system functioning, which can result in aboveground changes to system properties and functioning, influencing higher trophic levels (40, 41). For example, soil-dwelling springtails alter aboveground plant biomass and either lower or increase herbivore reproduction depending on the specific host plant (42). Moreover, these effects differ substantially between different springtail species, and influence belowground faunal interactions, such as between earthworms and springtails, again affecting the aboveground component of systems (11, 42). These findings, widespread invasion of soil systems by springtails (6), warming-related dominance of springtail assemblages (12), and different functional effects between alien and indigenous springtail species (43), indicate that interactions between warming and soil invasions will result in substantial changes to system functioning. Thus, differential success of alien over indigenous species as climates change is likely not only to have an effect on soil biodiversity and belowground ecological functioning, but also on aboveground ecosystems. In consequence, our work suggests that biological invasions stand to increase substantially the already pronounced impacts of climate change on terrestrial ecosystems across the planet.
Materials and Methods
Extended protocol descriptions are provided in the SI Appendix.
Collection, Identification, and Alien Species Assignment.
The 30 springtail species were collected typically from across Australia (SI Appendix, Table S8) between 2013 and 2016. The focus was on hemiedaphic (litter-dwelling) species. Individuals were initially assigned to species in the field and at least 200 individuals collected per species. Collections were returned to the laboratory typically within 1 wk of collection. Species were identified to genus, and where species had been described, to species level using available keys (e.g., refs. 44 and 45). DNA barcoding was used to confirm species identifications. Mitochondrial DNA extraction and sequencing of the cytochrome c oxidase subunit I gene was undertaken by the Biodiversity Institute of Ontario, University of Guelph, Canada, following standard protocols developed for springtails (46). Sequences of 74 specimens from 23 species were compared with the >75,000 springtail sequences available through the Barcode of Life Data Systems (BOLD; www.barcodinglife.org) (SI Appendix, Table S9). Individuals that could not be identified using available keys and which were not represented in BOLD were examined by one of the authors (C.J.-S.), in discussion with other systematic experts, and assigned to uniquely identifiable species based on morphological characteristics or a barcoding gap of at least 2.5% (47). Sequences are available on BOLD (www.boldsystems.org) as part of Project COLMU (Collembola of Monash University). Species that were clearly identified as indigenous to Australia or to the sub-Antarctic islands in faunal treatments and those similarly identified as alien to either of these regions were retained as such in the classification of species as alien or indigenous. Undescribed species not represented in BOLD previously, or represented only from individuals already collected across Australia, New Zealand, or south of the Wallace line were considered indigenous. Following previous authors (6), undescribed species that had sequences present in BOLD from other distant tropical regions (such as the Neotropics) or from the Holarctic (typically Europe) were considered alien species (SI Appendix, Tables S8 and S9). Given the extensive nature of the BOLD information on springtails (>75,000 sequences, representing several thousand species), and the systematic expertise we consulted, we have high confidence in the species assignments to alien or indigenous species.
Site Microclimate Characteristics.
The soil microclimate characteristics of each site were calculated using remote-sensed daytime land-surface temperature data from the MODIS satellite network (MOD11C2 v006; 30 arcseconds spatial resolution; 8-d temporal resolution from January 2001 to December 2015; doi:10.5067/MODIS/MOD11C2.006), which were linearly transformed to account for the diffusion of heat from the land surface to 2.5-cm below the soil surface. The slope of this linear transformation was derived from the microclim dataset (48), which contains validated estimates of soil temperature for each hour in a 24-h cycle of an average day in each month of an average year under varying shade conditions. The median (MODIS soil median), 99% quantile (MODIS soil99), and maximum soil temperature (MODIS soil max; i.e., the warmest 8-d mean) of each site were calculated from our linearly transformed MODIS time series.
Colony Maintenance.
Species were reared at constant rearing temperatures that typically reflect the average soil temperatures of the sites at which they were collected (SI Appendix, Table S8) in controlled-temperature incubators (MIR-154; SANYO Electric) on a 12-h light:12-h dark light cycle. Incubator temperatures were monitored using Hygrochron iButtons (DS 1923-F5; Maxim Integrated) (SI Appendix, Table S8). The F2 generation was the focus of this work (SI Appendix, Fig. S4) to minimize any carryover effects from the environment of origin, including parental effects, and to reduce the possibility of adaptation to laboratory conditions (20). Between 50 and 200 adults from the collected (F0) individuals were randomly assigned to two to four 60-mL pots lined with moistened Plaster-of-Paris:Charcoal powder (9:1) substrates. De-ionized water was added once to twice a week to maintain high humidity and, depending on the species, individuals were fed two to three times a week with algae from the bark of Platanus sp. or on slime mold ad libitum (49), enabling individuals to select nutrients optimally. Adults from the F2 generation [average egg to adult development time among the species is 74.16 d ± 40.56 (SD)] were used for most experiments (SI Appendix, Fig. S4).
Acclimation to Assess Phenotypic Plasticity.
Before the experimental trials, all species were subject to temperature treatments (referred to “acclimation” hereafter), undertaken in controlled-temperature incubators (MIR-154; SANYO), with temperatures verified using Hygrochron iButtons and under 12-h light:12-h dark conditions (SI Appendix, Table S8). Acclimation treatments lasted 7 d, given that complete responses usually occur within less time in terrestrial arthropods (50). Low, medium, and high acclimation temperatures were set 5 °C below, and 5 and 10 °C above standard rearing temperatures, respectively (SI Appendix, Fig. S4). For control temperatures, individuals were subject to the same manipulation as those in the acclimation treatments.
Critical Thermal Limit and Warming Tolerance Determinations.
Critical thermal limits (CTmax and CTmin) were determined following standard methods (24, 51). Assays began at the control (rearing) temperature for each species to ensure that comparisons between the control and acclimated groups could be made. Heating (for CTmax) and cooling (for CTmin) rates were set at 0.05 °C/min, rates that are within the range and close to the mean (for increasing temperatures) of those recorded for tropical to temperate microhabitats, and which empirical data and modeling show provide comparable acclimation and warming tolerance estimates across species and environments (24). Temperature of the substrate was recorded with a type K thermocouple, using a digital thermometer (Model RDXL 12SD; Omega Engineering). CTmin and CTmax were defined as temperature at which a loss of righting response occurred (24, 51). Because body mass may contribute to variation in critical thermal limits (e.g., refs. 9 and 52), species’ mean body mass (mg) was determined from a randomly selected, separate sample of 40 adult individuals for each species using a high-resolution (0.1 μg) microbalance (Mettler-Toledo XP2U) (SI Appendix, Table S8). For each species, basal thermal tolerance was calculated as the mean CTmin for the lower critical thermal limit and mean CTmax for the upper critical thermal limit, obtained from individuals reared under control conditions and subjected to the same temperature for acclimation. Basal thermal tolerance range was calculated as the difference between these two mean values. To determine the extent of phenotypic plasticity, the ARR (22) was calculated for each CTmin and CTmax for each species. Here, the ARR was calculated as the largest difference between mean CTmin (or CTmax) across any of the acclimation treatments, divided by the maximum temperature range represented by those treatments. Warming tolerance was calculated as the difference between mean basal CTmax (individuals reared under control conditions) of each species and the MODIS 99% quantile (MODIS soil99).
Selection Experiment.
Laboratory natural selection (29) was used to investigate the ways in which critical thermal limits respond to elevated rearing temperatures. Four species were selected for this experiment, representing a tropical alien (Desoria trispinata), a tropical indigenous (Ascocyrtus sp. 2), a temperate alien (Orthonychiurus sp.), and a temperate indigenous species (Lepidocyrtus sp. 10). Laboratory natural selection rather than artificial selection was chosen to minimize the risk of sterilization and other cellular damage that can be associated with exposure to extreme temperatures (53–55). Selection and control groups were initiated for each species from the F2 generation of field-caught individuals. F2 individuals were used to minimize any carryover effects from the environment of origin, including parental effects, and to reduce the possibility of adaptation to laboratory conditions (20, 53). Each group contained two independent replicate lines starting with 150 individuals divided into two separate vials per replicate line. Control lines were maintained under the original rearing temperatures (temperate = 15 °C, tropical = 20 °C), while selection lines were maintained under warmer temperatures (temperate = 25 °C, tropical = 27 °C). Temperatures for the selection treatment were based on results from a pilot study, which indicated that 25 °C and 27 °C were the highest temperatures at which the temperate and tropical species could still reproduce, respectively (SI Appendix, Table S10). Throughout the experiment, generations remained discrete, and eggs from replicate vials within each replicate selected and control line were randomly combined within generations to maintain genetic diversity. Population size in each line (control and selection lines) was on average 775 individuals. Critical thermal limits were assessed, as for the interspecific comparisons, for adults of each species before selection commencing (at generation 0), then every second generation for individuals in the selection and control groups up to generation 10, and every fourth generation thereafter. Approximately 45 individuals were assessed per replicate line per treatment at each sampling period. The degree of plasticity associated with any phenotypic changes observed during the selection experiment was assessed using a reciprocal transplant experiment, investigating developmental plasticity. This involved switching individuals from the selection conditions to the control conditions and vice versa. Individuals were switched within 1 d of hatching at generation four for the temperate species and generation six for the tropical species. These different generations were used because of the slower development time of the temperate species. Thermal limits were assessed when the switched individuals had reached adulthood.
Statistical Analyses.
All analyses were conducted in R v3.3.1 (56). PGLS (57), as implemented in the caper v0.5.2 (58) and APE (59) packages, was used to investigate relationships between species mean critical thermal limits (either CTmin, CTmax, or thermal tolerance range), environmental characteristics (MODIS soil median), springtail species mean mass, and species status (alien or indigenous).
A phylogeny for the species was constructed based on joint considerations of two recent molecular phylogenies for the group (60, 61), with species relative positions based on the cytochrome c oxidase subunit I gene phylogeny, or in a few cases on morphological similarity adjudicated by one of us (C.J.-S.). The barcoding placements were obtained from a neighbor-joining tree using standard methods. For the final tree, branch lengths were assigned using Grafen’s method (62), and the tree (SI Appendix, Fig. S5) is available as a Newick file. Initially, two covariance matrices were constructed following either Brownian motion (BM) or Ornstein–Uhlenbeck (OU) models of evolution (63). Akaike Information Criterion values of BM and OU models were compared to identify which model of evolution provided the best fit to observed data using the APE (59) and nlme v3.1-131 (64) packages. Phylogenetically corrected models based on BM covariance matrices were a consistently better fit than those based on other evolutionary assumptions (SI Appendix, Table S11), thus the outcomes of these models are reported primarily, although for comparative purposes we provide the OU outcomes as well (SI Appendix, Table S12). For the BM models, a maximum-likelihood approach provided Pagel’s λ (21) to indicate the degree of phylogenetic correlation in the data. The PGLS approach was used to investigate relationships between warming tolerance, maximum soil temperature (MODIS soil maximum), species mass, and species status, and to investigate relationships between CTmin and CTmax. Analyses were repeated using ordinary least-squares approaches as implemented in the linear model function of R v3.3.1, and coefficients were typically similar to those found in the PGLS models (SI Appendix, Table S13). Throughout, mass did not appear as a significant term in the models, and in no cases did slopes of the relationships between critical thermal limit traits and environmental features differ between the alien and indigenous species groups. For investigations of the ARR and its relationship with mean trait values, ordinary least-squares methods indicated no significant relationships and PGLS bore out these conclusions.
For the selection experiment, to analyze differences in critical thermal limits between selection and control lines, nested mixed-effect model analyses were conducted using the lmer function in the lme4 package (v1.1-13) (65) in R (v3.3.1). “Treatment” (control or selection) and “generation number” were treated as fixed effects, and “replicate line” was nested within treatment as a random effect (66). Nested mixed-effect analyses were also undertaken to analyze data from the reciprocal transplant experiment examining developmental plasticity. This involved comparing the critical thermal limits of the selection, control, and reciprocally transplanted lines at the respective generation of the reciprocal transplant experiment, with “replicate line” nested within treatment (control, selected, reciprocally transplanted) as a random effect. Separate analyses were performed for each of the four species.
Supplementary Material
Acknowledgments
We thank Ian Aitkenhead, Jess Hoskins, Ben Wegener, Oliver Stuart, and several volunteers for assisting with colony maintenance; Louis Deharveng, Wanda M. Weiner, Sun Xin, and Mikhail Potapov for assisting with species verification; and Ary Hoffmann, Melodie McGeoch, Philippe Vernon, and Craig White for commenting on a previous version of the work. This research was funded by Australian Research Council Grant DP140102815 and Australian Antarctic Science Grant 4307. The International Barcoding of Life Project (iBOL) partly supported the sequencing.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data used for this work are deposited in Monash Figshare (10.4225/03/59fa71b5291c8).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715598115/-/DCSupplemental.
References
- 1.Hawkins E, et al. Estimating changes in global temperature since the pre-industrial period. Bull Am Meteorol Soc. 2017;98:1841–1856. [Google Scholar]
- 2.Seebens H, et al. No saturation in the accumulation of alien species worldwide. Nat Commun. 2017;8:14435. doi: 10.1038/ncomms14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulme PE. Climate change and biological invasions: Evidence, expectations, and response options. Biol Rev Camb Philos Soc. 2017;92:1297–1313. doi: 10.1111/brv.12282. [DOI] [PubMed] [Google Scholar]
- 4.Walther G-R, et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol Evol. 2009;24:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- 6.Cicconardi F, et al. MtDNA metagenomics reveals large-scale invasion of belowground arthropod communities by introduced species. Mol Ecol. 2017;26:3104–3115. doi: 10.1111/mec.14037. [DOI] [PubMed] [Google Scholar]
- 7.Coyle DR, et al. Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biol Biochem. 2017;110:116–133. [Google Scholar]
- 8.Ricciardi A, et al. Invasion science: A horizon scan of emerging challenges and opportunities. Trends Ecol Evol. 2017;32:464–474. doi: 10.1016/j.tree.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 9.van Dooremalen C, Berg MP, Ellers J. Acclimation responses to temperature vary with vertical stratification: Implications for vulnerability of soil-dwelling species to extreme temperature events. Glob Change Biol. 2013;19:975–984. doi: 10.1111/gcb.12081. [DOI] [PubMed] [Google Scholar]
- 10.Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW. Linking climate change and biological invasions: Ocean warming facilitates nonindigenous species invasions. Proc Natl Acad Sci USA. 2002;99:15497–15500. doi: 10.1073/pnas.242437499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cragg R, Bardgett RD. How changes in soil faunal diversity and composition within a trophic group influence decomposition processes. Soil Biol Biochem. 2001;33:2073–2081. [Google Scholar]
- 12.Terauds A, Chown SL, Bergstrom DM. Spatial scale and species identity influence the indigenous-alien diversity relationship in springtails. Ecology. 2011;92:1436–1447. doi: 10.1890/10-2216.1. [DOI] [PubMed] [Google Scholar]
- 13.Chown SL, Nicolson SW. Insect Physiological Ecology. Mechanisms and Patterns. Oxford Univ Press; Oxford: 2004. [Google Scholar]
- 14.Sinclair BJ, et al. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol Lett. 2016;19:1372–1385. doi: 10.1111/ele.12686. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard J, Kearney MR, Hoffmann AA. Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Glob Change Biol. 2014;20:1738–1750. doi: 10.1111/gcb.12521. [DOI] [PubMed] [Google Scholar]
- 16.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nat Clim Chang. 2012;2:686–690. [Google Scholar]
- 17.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araújo MB, et al. Heat freezes niche evolution. Ecol Lett. 2013;16:1206–1219. doi: 10.1111/ele.12155. [DOI] [PubMed] [Google Scholar]
- 19.Chown SL, Gaston KJ. Macrophysiology—Progress and prospects. Funct Ecol. 2016;30:330–344. [Google Scholar]
- 20.Sgrò CM, Partridge L. Evolutionary responses of the life history of wild-caught Drosophila melanogaster to two standard methods of laboratory culture. Am Nat. 2000;156:341–353. [Google Scholar]
- 21.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 22.Gunderson AR, Stillman JH. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc Biol Sci. 2015;282:20150401. doi: 10.1098/rspb.2015.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevin LM, Hoffmann AA. Evolution of phenotypic plasticity in extreme environments. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160138. doi: 10.1098/rstb.2016.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen JL, Chown SL, Janion-Scheepers C, Clusella-Trullas S. Interactions between rates of temperature change and acclimation affect latitudinal patterns of warming tolerance. Conserv Physiol. 2016;4:cow053. doi: 10.1093/conphys/cow053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao H, D’Antonio CM, Chen B, Huang Q, Peng S. How much do phenotypic plasticity and local genetic variation contribute to phenotypic divergences along environmental gradients in widespread invasive plants? A meta-analysis. Oikos. 2016;125:905–917. [Google Scholar]
- 26.Forsman A. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity (Edinb) 2015;115:276–284. doi: 10.1038/hdy.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- 28.Diamond SE. Evolutionary potential of upper thermal tolerance: Biogeographic patterns and expectations under climate change. Ann N Y Acad Sci. 2017;1389:5–19. doi: 10.1111/nyas.13223. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs AG. Laboratory selection for the comparative physiologist. J Exp Biol. 1999;202:2709–2718. doi: 10.1242/jeb.202.20.2709. [DOI] [PubMed] [Google Scholar]
- 30.van Kleunen M, Weber E, Fischer M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett. 2010;13:235–245. doi: 10.1111/j.1461-0248.2009.01418.x. [DOI] [PubMed] [Google Scholar]
- 31.McKnight E, García-Berthou E, Srean P, Rius M. Global meta-analysis of native and nonindigenous trophic traits in aquatic ecosystems. Glob Change Biol. 2017;23:1861–1870. doi: 10.1111/gcb.13524. [DOI] [PubMed] [Google Scholar]
- 32.Chown SL, Slabber S, McGeoch M, Janion C, Leinaas HP. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc Biol Sci. 2007;274:2531–2537. doi: 10.1098/rspb.2007.0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolkovich EM, Cleland EE. Phenological niches and the future of invaded ecosystems with climate change. AoB Plants. 2014;6:plu013. doi: 10.1093/aobpla/plu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann AA, Chown SL, Clusella-Trullas S. Upper thermal limits in terrestrial ectotherms: How constrained are they? Funct Ecol. 2013;27:934–949. [Google Scholar]
- 35.Diamond SE, et al. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob Change Biol. 2012;18:448–456. [Google Scholar]
- 36.Sunday JM, et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc Natl Acad Sci USA. 2014;111:5610–5615. doi: 10.1073/pnas.1316145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.England MH, Katjar JB, Maher N. Robust warming projections despite recent hiatus. Nat Clim Chang. 2015;5:394–396. [Google Scholar]
- 38.Boiteau G, MacKinley P. Role of avoidance behavior in the response of Folsomia candida to above-freezing cooling temperatures. Entomol Exp Appl. 2013;147:50–60. [Google Scholar]
- 39.Kingsolver JG, Buckley LB. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160147. doi: 10.1098/rstb.2016.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ngosong C, Gabriel E, Ruess L. Collembola grazing on arbuscular mycorrhiza fungi modulates nutrient allocation in plants. Pedobiologia (Jena) 2014;57:171–179. [Google Scholar]
- 41.Forey E, Coulibaly SFM, Chauvat M. Flowering phenology of a herbaceous species (Poa annua) is regulated by soil Collembola. Soil Biol Biochem. 2015;90:30–33. [Google Scholar]
- 42.Scheu S, Theenhaus A, Jones TH. Links between the detritivore and the herbivore system: Effects of earthworms and Collembola on plant growth and aphid development. Oecologia. 1999;119:541–551. doi: 10.1007/s004420050817. [DOI] [PubMed] [Google Scholar]
- 43.Leinaas HP, Bengtsson J, Janion-Scheepers C, Chown SL. Indirect effects of habitat disturbance on invasion: Nutritious litter from a grazing resistant plant favors alien over native Collembola. Ecol Evol. 2015;5:3462–3471. doi: 10.1002/ece3.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenslade P, Ireson J, Skarzynski D. Biology and key to the Australian species of Hypogastrura and Ceratophysella (Collembola: Hypogastruridae) Austral Entomol. 2014;53:53–74. [Google Scholar]
- 45.Fjellberg A. Fauna Entomologica Scandinavica. Vol 42 Brill; Leiden, The Netherlands: 1998. The Collembola of Fennoscandia and Denmark. Part I: Poduromorpha. [Google Scholar]
- 46.Hogg ID, Hebert PDN. Biological identification of springtails (Hexapoda: Collembola) from the Canadian Arctic, using mitochondrial DNA barcodes. Can J Zool. 2004;82:749–754. [Google Scholar]
- 47.Meyer CP, Paulay G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005;3:e422. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kearney MR, Isaac AP, Porter WP. Microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci Data. 2014;1:140006. doi: 10.1038/sdata.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoskins JL, Janion-Scheepers C, Chown SL, Duffy GA. Growth and reproduction of laboratory-reared neanurid Collembola using a novel slime mould diet. Sci Rep. 2015;5:11957. doi: 10.1038/srep11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weldon CW, Terblanche JS, Chown SL. Time-course for attainment and reversal of acclimation to constant temperature in two Ceratitis species. J Therm Biol. 2011;36:479–485. [Google Scholar]
- 51.Everatt MJ, Bale JS, Convey P, Worland MR, Hayward SAL. The effect of acclimation temperature on thermal activity thresholds in polar terrestrial invertebrates. J Insect Physiol. 2013;59:1057–1064. doi: 10.1016/j.jinsphys.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro PL, Camacho A, Navas CA. Considerations for assessing maximum critical temperatures in small ectothermic animals: Insights from leaf-cutting ants. PLoS One. 2012;7:e32083. doi: 10.1371/journal.pone.0032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vollmer JH, Sarup P, Kaersgaard CW, Dahlgaard J, Loeschcke V. Heat and cold-induced male sterility in Drosophila buzzatii: Genetic variation among populations for the duration of sterility. Heredity (Edinb) 2004;92:257–262. doi: 10.1038/sj.hdy.6800405. [DOI] [PubMed] [Google Scholar]
- 54.Jørgensen KT, Sørensen JG, Bundgaarda J. Heat tolerance and the effect of mild heat stress on reproductive characters in Drosophila buzzatii. J Therm Biol. 2006;31:280–286. [Google Scholar]
- 55.Zizzari ZV, Ellers J. Effects of exposure to short-term heat stress on male reproductive fitness in a soil arthropod. J Insect Physiol. 2011;57:421–426. doi: 10.1016/j.jinsphys.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 56.R Core Team 2016. R: A Language and Environment for Statistical Computing, version 3.3.1 (R Foundation for Statistical Computing, Vienna)
- 57.Garland T, Jr, Ives AR. Using the past to predict the present: Confidence intervals for regression equations in phylogenetic comparative methods. Am Nat. 2000;155:346–364. doi: 10.1086/303327. [DOI] [PubMed] [Google Scholar]
- 58.Orme CDL, et al. 2013 The caper package: Comparative analysis of phylogenetics and evolution in R. Available at https://cran.r-project.org/web/packages/caper/vignettes/caper.pdf. Accessed April 20, 2017.
- 59.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 60.D’Haese CA. Were the first springtails semi-aquatic? A phylogenetic approach by means of 28S rDNA and optimization alignment. Proc Biol Sci. 2002;269:1143–1151. doi: 10.1098/rspb.2002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malcicka M, Berg MP, Ellers J. Ecomorphological adaptations in Collembola in relation to feeding strategies and microhabitat. Eur J Soil Biol. 2017;78:82–91. [Google Scholar]
- 62.Grafen A. The phylogenetic regression. Philos Trans R Soc Lond B Biol Sci. 1989;326:119–157. doi: 10.1098/rstb.1989.0106. [DOI] [PubMed] [Google Scholar]
- 63.Cooper N, Thomas GH, Venditti C, Meade A, Freckleton RP. A cautionary note on the use of Ornstein Uhlenbeck models in macroevolutionary studies. Biol J Linn Soc Lond. 2016;118:64–77. doi: 10.1111/bij.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinheiro J, et al. 2017 Package ‘nlme’. Available at https://cran.r-project.org/web/packages/nlme/nlme.pdf. Accessed February 6, 2017.
- 65.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 66.Zuur A, Leno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. Springer; New York: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.