Significance
In vertebrates, steroid hormones regulate developmental transition from juveniles to adults. Insect steroid hormone, 20-hydroxyecdysone (20E), coordinates with juvenile hormone (JH) to regulate metamorphosis; however, the precise cross-talk mechanism is not well understood. Here, we report that JH and 20E antagonize each other’s biosynthesis in a major endocrine organ of Drosophila larvae: JH suppresses ecdysone biosynthesis and inhibits metamorphosis, whereas 20E suppresses JH biosynthesis and promotes metamorphosis. These data answer a long-standing question on how the mutual antagonism between the two major insect hormones regulates metamorphosis and may help to understand the hormonal regulation of developmental transition in mammals.
Keywords: juvenile hormone, 20-hydroxyecdysone, ring gland, hormone biosynthesis, antagonistic action
Abstract
In both vertebrates and insects, developmental transition from the juvenile stage to adulthood is regulated by steroid hormones. In insects, the steroid hormone, 20-hydroxyecdysone (20E), elicits metamorphosis, thus promoting this transition, while the sesquiterpenoid juvenile hormone (JH) antagonizes 20E signaling to prevent precocious metamorphosis during the larval stages. However, not much is known about the mechanisms involved in cross-talk between these two hormones. In this study, we discovered that in the ring gland (RG) of Drosophila larvae, JH and 20E control each other’s biosynthesis. JH induces expression of a Krüppel-like transcription factor gene Kr-h1 in the prothoracic gland (PG), a portion of the RG that produces the 20E precursor ecdysone. By reducing both steroidogenesis autoregulation and PG size, high levels of Kr-h1 in the PG inhibit ecdysteriod biosynthesis, thus maintaining juvenile status. JH biosynthesis is prevented by 20E in the corpus allatum, the other portion of the RG that produces JH, to ensure the occurrence of metamorphosis. Hence, antagonistic actions of JH and 20E within the RG determine developmental transitions in Drosophila. Our study proposes a mechanism of cross-talk between the two major hormones in the regulation of insect metamorphosis.
The transition from the juvenile stage to adulthood is a key developmental event for reaching reproductive maturation. In animals, this process is regulated by steroid hormones and their corresponding nuclear receptors. In mammals, such regulatory steroids are two main classes of sex hormones: androgens in males and estrogens in females (1). In insects, major developmental transitions, including larval–larval molting and larval–pupal–adult metamorphosis, are elicited by pulses of 20-hydroxyecdysone (20E; the main active form of insect steroid hormones) (2). However, each major developmental transition in insects is coordinated by the sesquiterpenoid juvenile hormone (JH). The balance of the two hormones defines the outcome of each developmental transition: during the middle larval instars, a high level of JH ensures that 20E pulse only triggers larval–larval molting, while during the last larval instar, JH titer declines sharply and 20E pulse initiates metamorphosis (3–6). Thus, JH prevents 20E-induced metamorphosis and is therefore referred to as the “status quo” hormone (7, 8). A number of studies have shown either the effect of JH on 20E titer (9–12) or the effect of 20E on JH biosynthesis (13, 14), but the actual model of this mutual regulation has not been determined in any insect species (15, 16).
20E, in conjunction with its nuclear receptor complex composed of the ecdysone receptor (EcR) and ultraspiracle (USP) proteins, triggers a transcriptional cascade consisting of 20E primary-response genes and a subsequent array of 20E secondary-response genes, and thus induces each molt (2). During larval–pupal metamorphosis, 20E induces programmed cell death to eliminate larval tissues and promotes adult tissue formation from imaginal discs, mainly through two 20E primary-response genes, Br-C and E93 (16, 17). Methoprene-tolerant (Met), a bHLH-PAS transcription factor, is a JH receptor that mediates the “status quo” actions of JH (18–20). Krüppel homolog 1 (Kr-h1) is a JH primary-response gene that encodes a zinc-finger transcription factor (21–24). Kr-h1 transduces JH signals to prevent 20E-induced gene expression during larval stages to ensure that metamorphosis occurs only in the absence of JH and Kr-h1 (22, 25, 26). Therefore, Kr-h1 is regarded as an antimetamorphic factor (7, 8). The repression of Br-C and E93 by Kr-h1 in peripheral target tissues partly accounts for the cross-talk between these two hormones (21–24, 27–31). Nevertheless, detailed studies are required to clarify the precise molecular mechanisms whereby Kr-h1 mediates the JH-20E cross-talk to achieve the antimetamorphic action at the level of hormone biosynthesis.
In the fruit fly, Drosophila melanogaster, a paralog of Met named Germ-cell expressed (Gce) functions as an alternate JH receptor (32–36). In the presence of JH, Met/Gce binds to a JH response region (JHRR; which contains three E-box–like motifs) in the Kr-h1 promoter and activates Kr-h1 expression (24). It is well documented that JH signaling is not essential during the early larval stages but is required for preventing metamorphosis (3, 4). The “status quo” action of JH in this dipteran insect is comparatively subtle but crucial: JH prevents 20E-triggered programmed cell death of the larval fat body and differentiation of the optic lobe in the adult brain (3, 4, 33). Interestingly, the JH-deficient animals, Met gce double mutants, and Kr-h1 mutants die around pupation with delayed developmental timing (the period from egg laying to pupariation) (3, 4, 11, 21, 33, 35, 36). These results are in contrast with those in the beetle, Tribolium castaneum, in which knockdown of Met or Kr-h1 by systemic RNAi results in precocious metamorphosis (19, 22). It should be of great value to understand where and how JH signaling prevents 20E-induced metamorphosis by using the classic genetic and developmental model insect Drosophila.
Interestingly, we detected high levels of Kr-h1 in the prothoracic gland (PG), a portion of the ring gland (RG), which produces the 20E precursor ecdysone. The RG is the major endocrine organ in Drosophila larvae, which consists of the PG producing ecdysone, the corpus allatum (CA) producing JH, and the corpus cardiacum producing a number of peptides including the adipokinetic hormone. We further determined that a major target organ of JH signaling is the PG, in which Kr-h1 transduces JH signals to inhibit ecdysone biosynthesis. Moreover, we discovered that JH and 20E, produced in the two different portions of the RG, inhibit each other’s biosynthesis. This study answers a long-standing question on how the mutual antagonism between JH and 20E regulates insect metamorphosis.
Results and Discussion
Drosophila PG Shows High Levels of Kr-h1 Expression When JH Titers Are High.
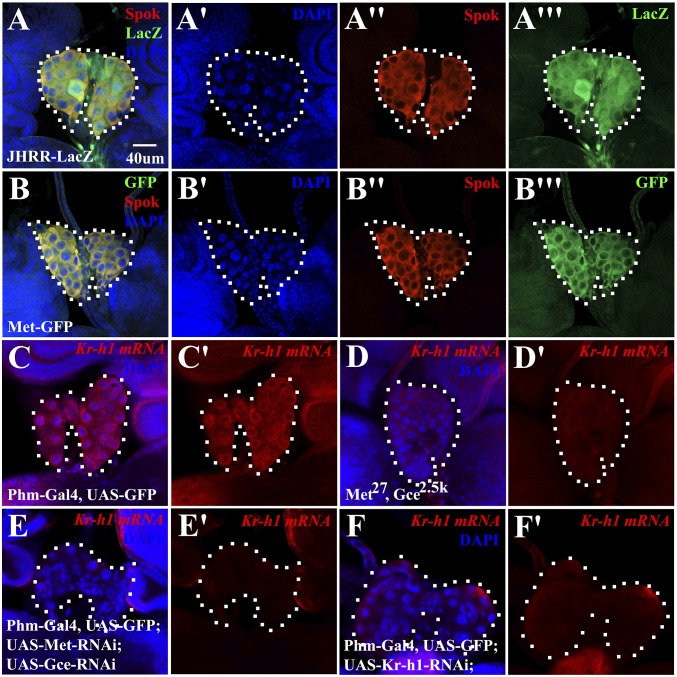
We have previously generated an LacZ reporter JHRR-LacZ based on the JHRR of Drosophila Kr-h1 promoter, which recapitulates the responsiveness of Kr-h1 to JH and Met/Gce (24). This reporter was employed to estimate the expression pattern of Kr-h1 during the early wandering stage when the JH titers are high (ref. 24 and references therein). JHRR-LacZ was detected in various larval tissues, including the fat body, salivary glands, PG, and the adult midgut progenitor cells (AMP), but not in the larval midgut cells (Fig. S1, Left). PBac{Met-GFP.FPTB} is a transgenic line that carries a genomic BAC construct expressing the Drosophila Met protein that is C-terminally tagged with EGFP (Met-GFP), and this transgene is able to rescue the Met gce double mutants (Met27 gce2.5K) to adults. Consistently, Met-GFP was also detected in the larval fat body, salivary glands, PG, and AMP, but not in the larval midgut cells (Fig. S1, Right). As revealed by a recent study using Bac recombineering and transgenic knock-in techniques, both Met and gce showed expression patterns similar to those shown in Fig. S1 (37). These results are consistent with the well-recognized role of JH signaling in targeting larval tissues to suppress the 20E-induced programmed cell death and in regulating the formation of adult organs during the larval–pupal metamorphosis (7, 8). Interestingly, we found that the PG shows abundant expression of JHRR-LacZ (Figs. S1, Left and S2 A and A′), but JHRR-LacZ was barely detected in the fat body and PG of the Met gce double mutants (24) (Fig. S2 B and B′). The expression of JHRR-LacZ in the PG was further confirmed by the colocalization of JHRR-LacZ with Spookier (Spok), a PG-specific enzyme catalyzing an essential step of ecdysone biosynthesis (38) (Fig. 1 A and A′′′). Similarly, Met-GFP is highly expressed and colocalized with Spok in the PG (Fig. 1 B and B′′′). Moreover, in situ hybridization revealed high Kr-h1 expression in the PG of the wild-type animals but no detectable Kr-h1 expression in the PG of the Met gce double mutants (Fig. 1 C and D′ and Fig. S2 C and C′). Given that JH has been shown to regulate 20E titer in both Drosophila and the silkworm Bombyx mori (9–12), our observations imply that JH may control 20E titer by regulating ecdysone biosynthesis in the PG. These data suggest that JH exerts its action by modulating gene expression in multiple tissues, including the PG of Drosophila.
Fig. 1.
Expression of JHRR-LacZ, Met-GFP, and Kr-h1. (A–B′′′) Spok colocalizes with JHRR-LacZ and Met-GFP in the PG. (Scale bar, 40 μm.) (A–A′′′) Spok (red), JHRR-LacZ (green), DAPI (blue). (B–B′′′) Spok (red), Met-GFP (green), DAPI (blue). (C–F′) Kr-h1 expression in the PG is significant in the wild-type larvae (C and C′) but decreased in the Met gce double mutant (D and D′), it is also decreased when Met and Gce or Kr-h1 was knocked down by RNAi (E–F′). In situ hybridization was performed using antisense probes of Kr-h1. Kr-h1 antisense (red), DAPI (blue).
Knockdown of Met and gce or Kr-h1 in the PG Triggers Initiation of Metamorphosis.
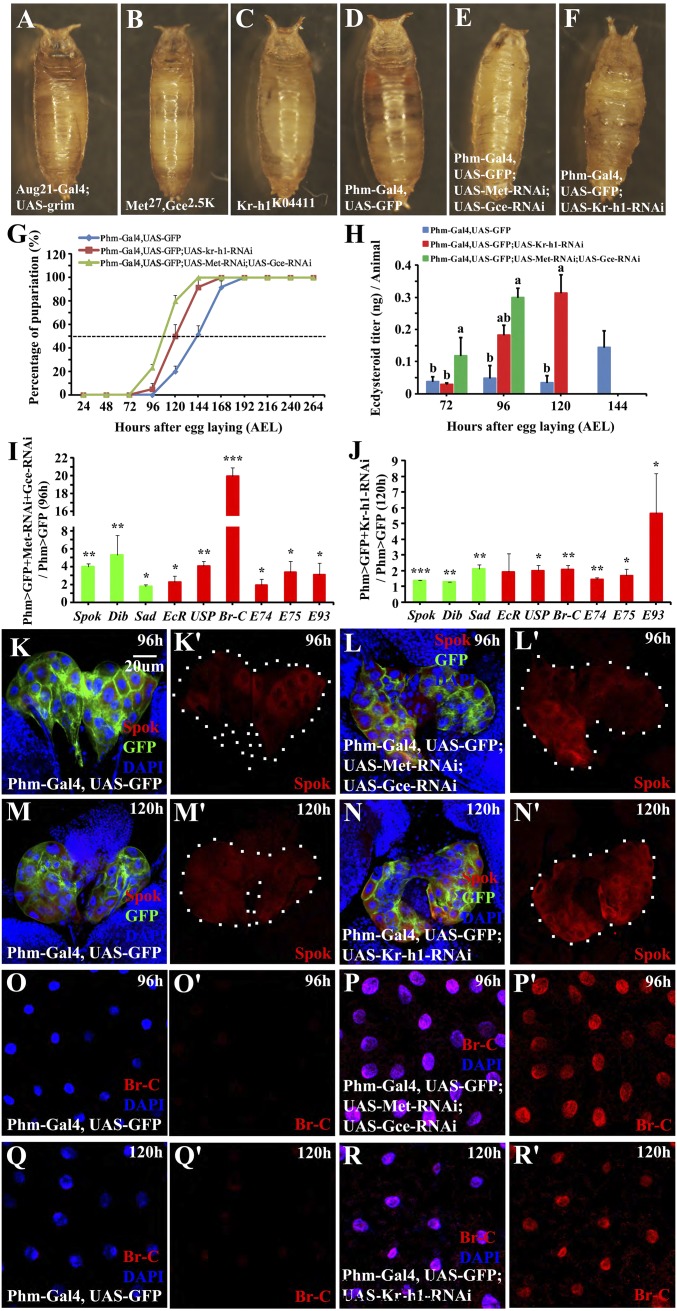
We then sought to investigate the possible roles of different target tissues in mediating JH actions. As reported previously (3, 4, 11, 21, 33, 35, 36), the JH-deficient animals Aug21-Gal4;UAS-grim, the double mutant Met27 gce2.5K animals, and the Kr-h1K04411 mutants die around pupation with delayed rather than precocious developmental timing. Meanwhile, all of the three genotypes failed to undergo normal head eversion (Fig. 2 A–C). To investigate the function of JH signaling in different target tissues, we depleted expression of Met and gce or Kr-h1 tissue-specifically using RNAi. Several Gal4 lines, including the PTTH-producing neuron-specific PTTH-Gal4, the fat body-specific Lsp2-Gal4 and Ppl-Gal4, the AMP-specific Esg-Gal4, the salivary gland-specific FKH-Gal4, and the PG-specific Phm-Gal4, were individually crossed with UAS-Met-RNAi, UAS-gce-RNAi, or UAS-Kr-h1-RNAi. Knockdown of Met and gce or Kr-h1 in PTTH-producing neurons, fat body, AMP, or salivary glands neither caused lethality nor significantly affected developmental timing (Fig. S3). In contrast, knockdown of Met and gce or of Kr-h1 in the PG (Fig. 1 E and F′) resulted in complete lethality and failure of head eversion during the pupal stage, showing lethal phenotypes similar to Aug21-Gal4;UAS-grim, Met27 gce2.5K, or Kr-h1K04411 animals (Fig. 2 D–F). Consistent with our observations, a recent PG-specific RNAi screen study also showed that knockdown of Kr-h1 in the PG resulted in pupal lethality (39). Moreover, knockdown of Met and gce or Kr-h1 in the PG resulted in smaller body sizes and pupariation ∼36 h and 24 h earlier, respectively, compared with the pupariation time in wild-type animals (Fig. 2 D–G). Therefore, attenuation of JH signaling specifically in the PG accelerates larval development resulting in precocious metamorphosis, unlike in the JH-deficient animals, Met gce double mutants, and Kr-h1 mutants. The tissue-specific RNAi results suggest that Drosophila PG is a key target organ mediating JH action, which is consistent with the high expression level of Kr-h1 detected in the PG (Fig. S1).
Fig. 2.
Down-regulation of Met and Gce or Kr-h1 in the PG results in an increase in ecdysone biosynthesis and precocious metamorphosis. (A–F) Lethal phenotypes; (G) developmental timing and percentage of pupariation; and (H) ecdysteroid titers of the indicated genotypes. (I and J) qRT-PCR measurements of gene expression. Fold changes shown are relative to control. Green bars indicate Halloween genes and red bars show key genes in the 20E-triggered transcriptional cascade. (K–N′) Spok protein level in the PG. Spok (red), GFP (green), DAPI (blue). (O–R′) Br-C protein level in the fat body. Br-C (red), DAPI (blue). For the t test: *P < 0.05; **P < 0.01; ***P < 0.001. ANOVA: bars labeled with different lowercase letters are significantly different (P < 0.05).
JH Represses Ecdysone Biosynthesis in the PG.
It is well documented that the timing of metamorphosis is coordinated by the rise in 20E titers (16). Therefore, we tested whether JH inhibits ecdysone biosynthesis in the PG and thus prevents premature pupariation (9–12). Indeed, knockdown of Met and gce or Kr-h1 in the PG dramatically induced a premature increase in ecdysteroid (mainly 20E and ecdysone) titers (Fig. 2H). This premature increase in ecdysteroid titers is most likely due to an increase in ecdysone biosynthesis in the PG, as we detected an increase in mRNA levels of three Halloween genes (Spok, Dib, and Sad) (ref. 38 and references therein) as well as the protein level of Spok in response to the PG-specific knockdown of Met and gce or Kr-h1 (Fig. 2 I–N′). Consistent with the increase in ecdysteroid titers, expression of EcR, USP, and several 20E primary-response genes (Br-C, E74, E75, and E93) in the whole body as well as the Br-C protein levels in the fat body were elevated in Met gce or Kr-h1 RNAi animals (Fig. 2 I, J, and O–R′). These data show that JH normally represses ecdysone biosynthesis in the PG to prevent premature pupariation.
Kr-h1 Overexpression in the PG Inhibits Ecdysone Biosynthesis and Blocks Metamorphosis.
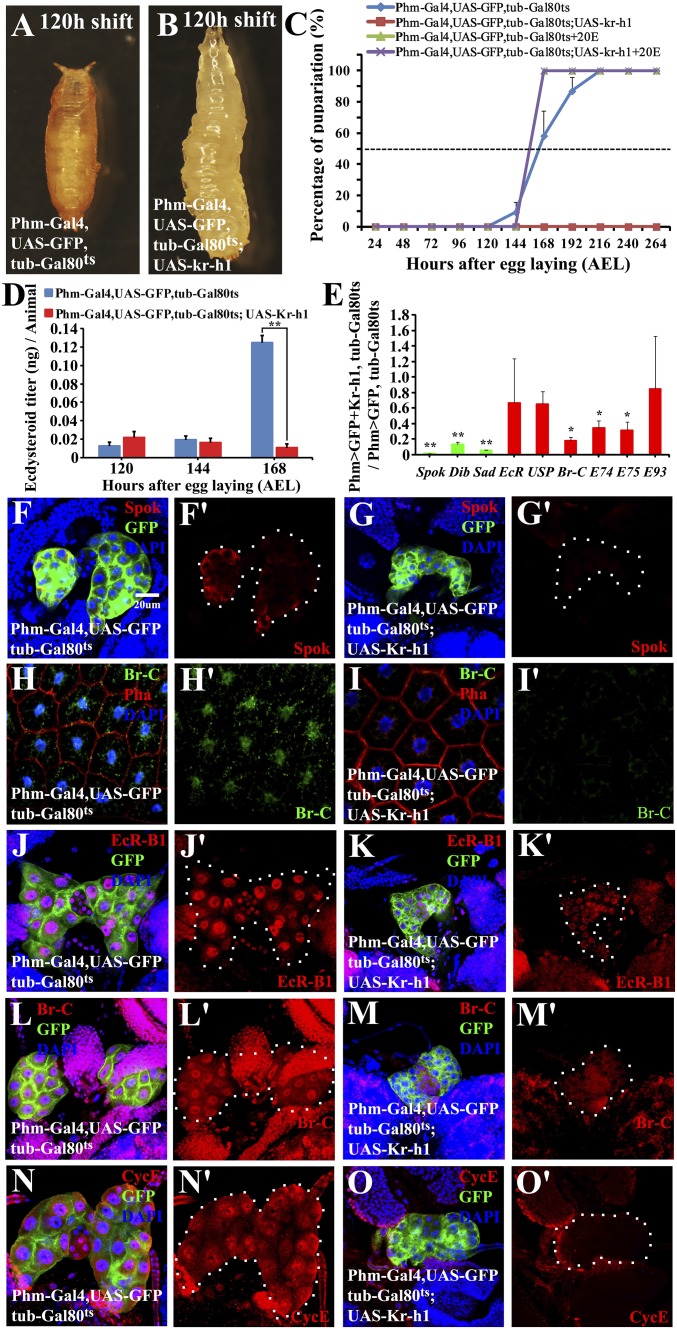
To complement the loss-of-function studies, we tested whether PG-specific Kr-h1 overexpression before the larval-pupal transition is sufficient to repress ecdysone biosynthesis and thus prevent metamorphosis. For this purpose, Phm-Gal4 was combined with the temperature-sensitive Gal80 line Tub-Gal80ts and then used to drive the expression of a UAS-Kr-h1 transgene. Both Phm-Gal4;Tub-Gal80ts and Phm-Gal4;Tub-Gal80ts;UAS-Kr-h1 larvae were first reared at a permissive temperature of 18°C until 120 h after egg laying (AEL) when they reached midthird instar, showing normal larval development. The larvae were then shifted to a restrictive temperature of 29°C. The control animals began to pupariate at 24 h after the shift, whereas the Kr-h1-overexpressing animals were arrested at the third instar, survived about 2 wk with overgrowth phenotypes, and never showed wandering behavior (Fig. 3 A–C). Following the addition of 20E to the diet at 144 h AEL, the Kr-h1–overexpressing animals initiated wandering behavior and pupariated within 24 h (Fig. 3C). At the restrictive temperature, ecdysteroid titers remained low and did not show an increase in the Kr-h1–overexpressing animals (Fig. 3D). At 168 h AEL, expression of Spok, Dib, and Sad was significantly inhibited by Kr-h1 overexpression, and the Spok protein became undetectable in the PG (Fig. 3 E–G′). Moreover, expression of EcR, USP, and the other 20E-primary response genes tested decreased in the whole body of the Kr-h1–overexpressing animals (Fig. 3E), and the Br-C protein levels were undetectable in the fat body (Fig. 3 H–I′).
Fig. 3.
By reducing both steroidogenesis autoregulation and PG size, overexpression of Kr-h1 in the PG decreases and delays ecdysone biosynthesis and prevents metamorphosis. (A and B) Developmental arrest in larvae with PG-specific Kr-h1 overexpression. (C) Developmental timing and percentage of pupariation. Added 20E at 144 h AEL. (D) Ecdysteroid titers. (E) qRT-PCR measurement of gene expression. Fold changes are relative to control. (F–G′) Spok protein level in the PG. Spok (red), GFP (Green), DAPI (blue). (H–I′) Br-C protein level in the fat body. Br-C (green), phalloidin (red), DAPI (blue). (J–O′) EcR-B1, Br-C, and CycE levels in the PG. EcR-B1, Br-C, and CycE (red), GFP (green), DAPI (blue). For the t test: *P < 0.05; **P < 0.01.
These loss-of-function and gain-of-function studies demonstrate that, through Kr-h1, JH prevents metamorphosis by inhibiting ecdysone biosynthesis in the PG. Previous studies identified PTTH and insulin-like peptides (ILPs) as positive regulators of ecdysone biosynthesis in Drosophila PG (6, 40, 41); our work establishes JH as a negative regulator of ecdysone biosynthesis.
JH Signaling in the PG Does Not Affect ILP Biosynthesis.
In agreement with a previous study showing a premature increase in ecdysteroid titers in Aug21-Gal4;UAS-grim larvae (11), we also observed an increase in ecdysteroid titers in both Met27 gce2.5K and Kr-h1K04411 larvae (Fig. S4A). Thus, either the complete absence of JH signaling or the depletion of JH signaling in the PG causes a premature increase in ecdysteroid titers. However, their opposite effects on developmental timing were not fully understood. A number of studies have demonstrated that developmental timing depends on 20E and insulin/ ILP signals, which mainly control growth period and growth rate, respectively. Moreover, triangular interplays might exist among 20E, ILPs, and JH in insects. For example, ILPs promote ecdysone biosynthesis, and 20E inhibits ILP biosynthesis, forming a negative feedback loop (42–44). JH and 20E mutually affect each other’s biosynthesis (9–14). JH and ILPs mutually promote each other’s biosynthesis, forming a positive feedback loop (11, 45–47). Therefore, we compared whether the depletion of JH signaling in the PG and the absence of JH signaling in the whole animals differently affect ILP biosynthesis and thus insulin/ILP signaling [IIS; high expression of InR and 4EBP represents low IIS, and vice versa (11)] during the feeding stages of the third larval instar. Importantly, expression of ILP1-7, 4E-BP, and InR was not altered when Met and gce or Kr-h1 was knocked down in the PG (Fig. S4 B and C). Thus, when JH signaling is only abolished in the PG, ecdysone biosynthesis is enhanced, while ILP biosynthesis and IIS are not affected, resulting in precocious metamorphosis. A previous study has shown increased expression of InR and 4EBP and thus decreased IIS in Aug21-Gal4;UAS-grim during the feeding stages of the third larval instar (11). Importantly, similar observations were detected in Met27 gce2.5K (Fig. S4D). However, expression of ILPs either increased or decreased in this mutant (Fig. S4D). These results show that in the complete absence of JH signaling, ecdysone biosynthesis is enhanced, but IIS is reduced, thus developmental timing is delayed. Besides the PG, there should be another target tissue that mediates JH signaling to regulate growth rate and developmental timing. The data also imply that besides altering expression of ILPs, JH might alter IIS through regulating other physiological processes, such as feeding behavior and nutrient status.
JH Signaling Suppresses Ecdysone Biosynthesis by Reducing Both Steroidogenesis Autoregulation and PG Size.
We next pursued the mechanisms by which JH signaling inhibits ecdysone biosynthesis. It is well documented that a fine regulatory loop exists between ecdysone biosynthesis and 20E signaling in Drosophila and Bombyx (48–52). At least EcR, Br-C, and E75 are involved in the feedback regulation of Halloween gene expression and thus ecdysone biosynthesis in Drosophila PG (48–50). The feedback regulation of ecdysone biosynthesis by 20E signaling is often referred to as steroidogenesis autoregulation, which plays a key role in 20E signaling during metamorphosis (16). We thus wondered whether JH modulates ecdysone biosynthesis at the level of steroidogenesis autoregulation. Indeed, up-regulation of JH signaling by Kr-h1 overexpression resulted in a significant decrease in the protein levels of EcR-B1 and Br-C in the PG (Fig. 3 J–M′). Conversely, knockdown of Met and gce or Kr-h1 in the PG led to an increase in the protein levels of EcR-B1 and Br-C in the PG (Fig. S5). In Drosophila Kc cells, Kr-h1 overexpression directly inhibits expression of EcR, Br-C, and E75, irrespective of whether 20E is absent or present in the medium (Fig. S6). Therefore, through Kr-h1, JH signaling inhibits ecdysone biosynthesis in Drosophila PG by reducing the positive feedback.
Previous studies have shown that the normal PG size is critical for ecdysone biosynthesis (43, 44, 53). The PG size was significantly reduced by Kr-h1 overexpression (Fig. 3 F, G′, and J–O′), accompanied by reduced CycE protein level and cell size (Fig. 3 N–O′ and Fig. S7 A–C). Interestingly, EcR RNAi in the PG did not affect the organ size, CycE protein level, and cell size, but knockdown of EcR in the PG that overexpressed Kr-h1 reduced all three parameters similarly to those in the PG that overexpressed Kr-h1 alone (Fig. S8). Nevertheless, PG size was normal in Aug21-Gal4;UAS-grim, Met27 gce2.5K, and Kr-h1K04411 animals (3, 4, 11, 21, 33, 35, 36). Consistently, PG size was not affected by PG-specific knockdown of Met and gce or Kr-h1 (Figs. S5 and S7D). These results suggest that high JH signaling is able to reduce PG size in a Kr-h1–dependent but 20E-independent manner. The detailed molecular mechanisms of Kr-h1–mediated reduction in PG size warrant further investigation, although CycE protein level is involved in regulating the endocycling of PG cells (53). Hence, JH signaling reduces steroidogenesis autoregulation by suppressing 20E signaling in the PG, while JH signaling reduces PG size independently of 20E signaling.
Taken together, our results show that one of the major target organs of JH signaling is the PG, in which Kr-h1 mediates JH signaling to inhibit ecdysone biosynthesis through a reduction in both steroidogenesis autoregulation and PG size.
20E Prevents JH Biosynthesis in the CA to Permit Metamorphosis.
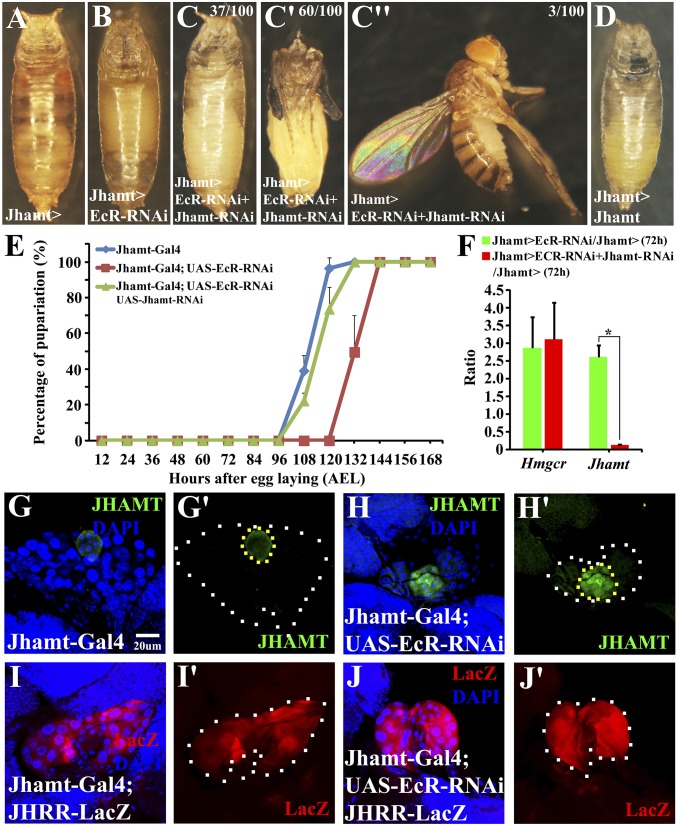
Having demonstrated that JH inhibits ecdysone biosynthesis in the PG, we examined whether vice versa, 20E also targets the CA to regulate JH biosynthesis in Drosophila, as observed in other insects (13, 14). Jhamt-Gal4, which has a more robust CA-specific expression than Aug21-GAL4 (33, 36), was crossed with UAS-EcR-RNAi to specifically reduce EcR expression in the CA by RNAi (Fig. S9 C and D′). Surprisingly, knockdown of EcR in the CA resulted in complete lethality during the pupal stage (Fig. 4 A and B); it also delayed pupariation by ∼24 h (Fig. 4E). At 72 h AEL, knockdown of EcR in the CA induced a two- to threefold increase in the mRNA levels of the two key enzymes (JHAMT and HMGCR) of JH biosynthesis (Fig. 4F). At 96 h AEL, CA-specific knockdown of EcR significantly increased the JHAMT protein level in the CA and JHRR-LacZ expression in the PG (Fig. 4 G–J′), demonstrating elevated JH biosynthesis and JH signaling. Mechanistically, 20E signaling inhibition in the CA attenuates and delays ecdysone biosynthesis in the PG by reducing steroidogenesis autoregulation and PG size (Figs. S7 E and F and S9). Showing elevated JH biosynthesis and JH signaling, knockdown of EcR in the CA led to phenotypic changes similar to but much weaker than those caused by Kr-h1 overexpression in the PG for a period of 48 h during the third larval instar (Fig. 3). Importantly, the lethality caused by knockdown of EcR in the CA was partially rescued by the simultaneous knockdown of Jhamt in the CA (Fig. 4 C and C′′), which strongly supports the conclusion that elevated JH prevents normal development upon knockdown of EcR in the CA. Consistently, low ecdysteroid titers and 20E signaling caused by CA-specific EcR RNAi were rescued by concurrent Jhamt RNAi (Fig. S10). These results suggest that blocking 20E signaling in the CA results in elevated JH biosynthesis, which, in turn, leads to enhanced JH signaling in the PG, thus preventing ecdysone biosynthesis and 20E-induced metamorphosis. This study demonstrates 20E as an extracellular signal to regulate JH biosynthesis in Drosophila.
Fig. 4.
Down-regulation of EcR in the CA increases JH biosynthesis, which decreases and delays ecdysone biosynthesis and prevents metamorphosis. (A–C′′) CA-specific EcR depletion resulted in complete animal lethality at the pupal stage, which was partially rescued by concurrent Jhamt depletion. Note: 60 out of 100 animals were rescued to the pharate adult stage (C′) and three to the adult stage (C′′). (D) CA-specific Jhamt overexpression resulted in complete animal lethality at the pupal stage. (E) Developmental timing and percentage of pupariation. (F) qRT-PCR measurements of gene expression in the brain-RG complex. Fold changes are relative to control. (G–H′) JHAMT protein level in the CA. JHAMT (green), DAPI (blue). (I–J′) JHRR-LacZ (Kr-h1) expression in the PG. JHRR-LacZ (red), DAPI (blue). For the t test: *P < 0.05.
The previous Drosophila white pupal bioassay has shown that topical application of JH analogs on the white prepupae causes pupal lethality (ref. 35 and references therein). To confirm if elevated JH biosynthesis was sufficient to block 20E-induced metamorphosis, Jhamt-Gal4 was crossed with UAS-Jhamt. Jhamt overexpression in the CA to increase JH biosynthesis (54) and JH signaling caused complete lethality during the pupal stage (Fig. 4D and Fig. S11). These data suggest that Drosophila employs 20E signaling to prevent JH biosynthesis in the CA so that the PG produces sufficient ecdysone to ensure the occurrence of metamorphosis. Together, these results purport JH and 20E to be the key factors involved in the reciprocal regulation of ecdysone and JH biosynthesis, respectively, in the RG, thus providing a model of cross-talk between these two hormones in the regulation of insect metamorphosis (Fig. 5).
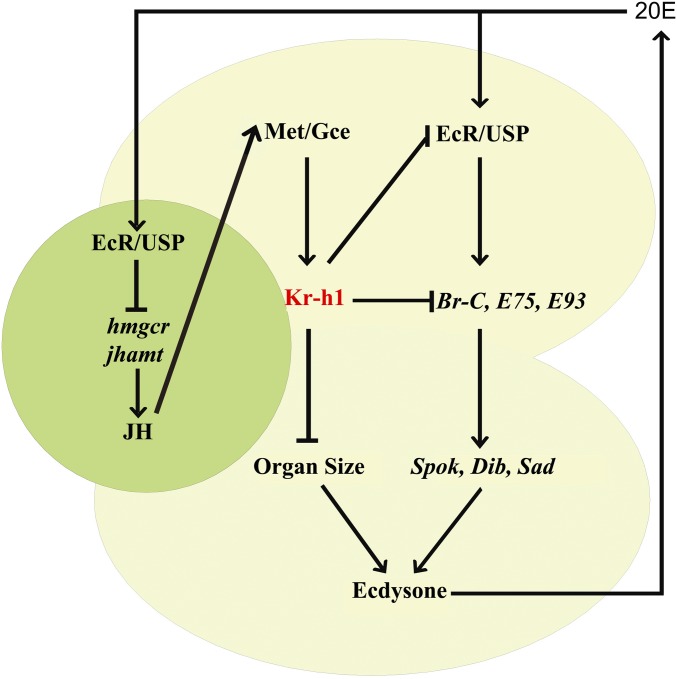
Fig. 5.
A regulatory network of biosynthesis and action of 20E and JH in the RG. Green: CA producing JH; yellow: PG producing ecdysone; CA and PG are two portions of the RG. JH signaling through Kr-h1 inhibits ecdysone biosynthesis in the PG to prevent metamorphosis, while 20E signaling prevents JH biosynthesis in the CA to permit metamorphosis. Thus, antagonistic hormone actions within the RG determine developmental transitions in Drosophila.
In summary, JH signaling through Kr-h1 inhibits ecdysone biosynthesis in the PG to prevent metamorphosis, and vice versa, 20E prevents JH biosynthesis in the CA to ensure the occurrence of metamorphosis. JH and 20E, the two most important insect hormones regulating major developmental transitions, mutually inhibit the biosynthesis of each other, thus forming a regulatory network controlling metamorphosis (Fig. 5). Hence, antagonistic hormone actions within the RG determine developmental transitions in Drosophila. Since Krüppel-like factors are essential effectors of nuclear receptor signaling (55), it will be of great interest to examine whether Krüppel-like factors also mediate sex steroid signals to regulate developmental transitions from the juvenile stage to adulthood in mammals.
Materials and Methods
A detailed description of the materials and methods used in this study is provided in SI Materials and Methods. A number of fly strains and Drosophila genetics were used. Immunostaining, in situ hybridization, and imaging were performed. Developmental timing was analyzed by recording pupariation. Measuring ecdysteroid titers, 20E feeding experiments, cell culture, and qPCR were previously described. See Table S1 for a list of all primers used.
Supplementary Material
Acknowledgments
We thank Drs. Marek Jindra, Stephen S. Tobe, and Yonggang Zheng for helpful comments on this manuscript. We also thank Drs. Michael O’Connor and Tetsu Shinoda for providing important reagents. This study was supported by the National Science Foundation of China (Grant 31620103917), the National Key Research and Development Program of China (Grant 2016YFD0101900), the Chinese Academy of Sciences (Grant 173176001000162007), and the National Science Foundation of China (Grants 31330072 and 31572325) (to S. Li).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716897115/-/DCSupplemental.
References
- 1.Guerriero G. Vertebrate sex steroid receptors: Evolution, ligands, and neurodistribution. Ann N Y Acad Sci. 2009;1163:154–168. doi: 10.1111/j.1749-6632.2009.04460.x. [DOI] [PubMed] [Google Scholar]
- 2.Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam Horm. 2000;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, et al. Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development. 2009;136:2015–2025. doi: 10.1242/dev.033712. [DOI] [PubMed] [Google Scholar]
- 4.Riddiford LM, Truman JW, Mirth CK, Shen YC. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development. 2010;137:1117–1126. doi: 10.1242/dev.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smykal V, et al. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev Biol. 2014;390:221–230. doi: 10.1016/j.ydbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Daimon T, Uchibori M, Nakao H, Sezutsu H, Shinoda T. Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc Natl Acad Sci USA. 2015;112:E4226–E4235. doi: 10.1073/pnas.1506645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 8.Jindra M, Bellés X, Shinoda T. Molecular basis of juvenile hormone signaling. Curr Opin Insect Sci. 2015;11:39–46. doi: 10.1016/j.cois.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Richard DS, Gilbert LI. Reversible juvenile hormone inhibition of ecdysteroid and juvenile hormone synthesis by the ring gland of Drosophila melanogaster. Experientia. 1991;47:1063–1066. doi: 10.1007/BF01923343. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka N, et al. Differential regulation of ecdysteroidogenic P450 gene expression in the silkworm, Bombyx mori. Biosci Biotechnol Biochem. 2007;71:2808–2814. doi: 10.1271/bbb.70420. [DOI] [PubMed] [Google Scholar]
- 11.Mirth CK, et al. Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. Proc Natl Acad Sci USA. 2014;111:7018–7023. doi: 10.1073/pnas.1313058111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogihara MH, Hikiba J, Iga M, Kataoka H. Negative regulation of juvenile hormone analog for ecdysteroidogenic enzymes. J Insect Physiol. 2015;80:42–47. doi: 10.1016/j.jinsphys.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Areiza M, Nouzova M, Rivera-Perez C, Noriega FG. 20-Hydroxyecdysone stimulation of juvenile hormone biosynthesis by the mosquito corpora allata. Insect Biochem Mol Biol. 2015;64:100–105. doi: 10.1016/j.ibmb.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hult EF, Huang J, Marchal E, Lam J, Tobe SS. RXR/USP and EcR are critical for the regulation of reproduction and the control of JH biosynthesis in Diploptera punctata. J Insect Physiol. 2015;80:48–60. doi: 10.1016/j.jinsphys.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Hiruma K, Kaneko Y. Hormonal regulation of insect metamorphosis with special reference to juvenile hormone biosynthesis. Curr Top Dev Biol. 2013;103:73–100. doi: 10.1016/B978-0-12-385979-2.00003-4. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu Rev Entomol. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryoo HD, Baehrecke EH. Distinct death mechanisms in Drosophila development. Curr Opin Cell Biol. 2010;22:889–895. doi: 10.1016/j.ceb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashok M, Turner C, Wilson TG. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA. 1998;95:2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konopova B, Jindra M. Juvenile hormone resistance gene methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci USA. 2007;104:10488–10493. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charles JP, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108:21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minakuchi C, Zhou X, Riddiford LM. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev. 2008;125:91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minakuchi C, Namiki T, Shinoda T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev Biol. 2009;325:341–350. doi: 10.1016/j.ydbio.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Kayukawa T, et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. 2012;109:11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q, et al. Heat shock protein 83 (Hsp83) facilitates methoprene-tolerant (Met) nuclear import to modulate juvenile hormone signaling. J Biol Chem. 2014;289:27874–27885. doi: 10.1074/jbc.M114.582825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozano J, Belles X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci Rep. 2011;1:163. doi: 10.1038/srep00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konopova B, Smykal V, Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS One. 2011;6:e28728. doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, et al. DPP-mediated TGFbeta signaling regulates juvenile hormone biosynthesis by activating the expression of juvenile hormone acid methyltransferase. Development. 2011;138:2283–2291. doi: 10.1242/dev.057687. [DOI] [PubMed] [Google Scholar]
- 28.Belles X, Santos CG. The MEKRE93 (methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem Mol Biol. 2014;52:60–68. doi: 10.1016/j.ibmb.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Ureña E, Manjón C, Franch-Marro X, Martín D. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc Natl Acad Sci USA. 2014;111:7024–7029. doi: 10.1073/pnas.1401478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayukawa T, et al. Krüppel homolog 1 inhibits insect metamorphosis via direct transcriptional repression of Broad-complex, a pupal specifier gene. J Biol Chem. 2016;291:1751–1762. doi: 10.1074/jbc.M115.686121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayukawa T, Jouraku A, Ito Y, Shinoda T. Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc Natl Acad Sci USA. 2017;114:1057–1062. doi: 10.1073/pnas.1615423114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godlewski J, Wang S, Wilson TG. Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem Biophys Res Commun. 2006;342:1305–1311. doi: 10.1016/j.bbrc.2006.02.097. [DOI] [PubMed] [Google Scholar]
- 33.Abdou MA, et al. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem Mol Biol. 2011;41:938–945. doi: 10.1016/j.ibmb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Zeng X, Hou SX. Broad relays hormone signals to regulate stem cell differentiation in Drosophila midgut during metamorphosis. Development. 2012;139:3917–3925. doi: 10.1242/dev.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 2015;11:e1005394. doi: 10.1371/journal.pgen.1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen D, et al. Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLoS Genet. 2015;11:e1005038. doi: 10.1371/journal.pgen.1005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumann AA, et al. Genetic tools to study juvenile hormone action in Drosophila. Sci Rep. 2017;7:2132. doi: 10.1038/s41598-017-02264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ono H, et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol. 2006;298:555–570. doi: 10.1016/j.ydbio.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Danielsen ET, et al. A Drosophila genome-wide screen identifies regulators of steroid hormone production and developmental timing. Dev Cell. 2016;37:558–570. doi: 10.1016/j.devcel.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McBrayer Z, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rewitz KF, Yamanaka N, Gilbert LI, O’Connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science. 2009;326:1403–1405. doi: 10.1126/science.1176450. [DOI] [PubMed] [Google Scholar]
- 42.Colombani J, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 43.Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 46.Tu MP, Yin CM, Tatar M. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen Comp Endocrinol. 2005;142:347–356. doi: 10.1016/j.ygcen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Sheng Z, Palli SR. Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum. PLoS Genet. 2013;9:e1003535. doi: 10.1371/journal.pgen.1003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev Cell. 2002;3:209–220. doi: 10.1016/s1534-5807(02)00204-6. [DOI] [PubMed] [Google Scholar]
- 49.Moeller ME, Danielsen ET, Herder R, O’Connor MB, Rewitz KF. Dynamic feedback circuits function as a switch for shaping a maturation-inducing steroid pulse in Drosophila. Development. 2013;140:4730–4739. doi: 10.1242/dev.099739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parvy JP, et al. Forward and feedback regulation of cyclic steroid production in Drosophila melanogaster. Development. 2014;141:3955–3965. doi: 10.1242/dev.102020. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, et al. 20-Hydroxyecdysone (20E) primary-response gene E93 modulates 20E signaling to promote Bombyx larval-pupal metamorphosis. J Biol Chem. 2015;290:27370–27383. doi: 10.1074/jbc.M115.687293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li K, et al. 20-Hydroxyecdysone (20E) primary response gene E75 isoforms mediate steroidogenesis autoregulation and regulate developmental timing in Bombyx. J Biol Chem. 2016;291:18163–18175. doi: 10.1074/jbc.M116.737072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohhara Y, Kobayashi S, Yamanaka N. Nutrient-dependent endocycling in steroidgenic tissue dictates timing of metamorphosis in Drosophila melanogaster. PLoS Genet. 2017;13:e1006583. doi: 10.1371/journal.pgen.1006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bendena WG, Zhang J, Burtenshaw SM, Tobe SS. Evidence for differential biosynthesis of juvenile hormone (and related) sesquiterpenoids in Drosophila melanogaster. Gen Comp Endocrinol. 2011;172:56–61. doi: 10.1016/j.ygcen.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Knoedler JR, Denver RJ. Krüppel-like factors are effectors of nuclear receptor signaling. Gen Comp Endocrinol. 2014;203:49–59. doi: 10.1016/j.ygcen.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.