In a paper in PNAS (1) we show that, in opossums, endometrial inflammation follows attachment of the embryo to the uterine wall. We argue that this is similar to what occurred in the first live-bearing mammals, and that inflammatory signaling seen at implantation in eutherians evolved from this attachment-induced inflammation. In a letter on our paper, Liu (2) presents evidence of endometrial inflammatory gene expression in mice before embryonic attachment. Liu infers that inflammatory signaling at implantation cannot be a consequence of embryonic attachment because inflammation precedes implantation in mice and humans. Liu alternatively proposes that inflammation during implantation in eutherians has evolved because the embryo coopted L-selectin expression, allowing it to “act” like a leukocyte and facilitate endometrial infiltration. This is an interesting hypothesis, because it may explain why inflammatory signaling is necessary for eutherian implantation.
Liu’s (2) argument reduces to the following inference: In the opossum, endometrial inflammation follows embryo–endometrium interaction. In eutherians, however, inflammatory processes associated with receptivity occur before attachment and, thus, cannot be homologous to the inflammatory reaction in opossums, which follows attachment. Liu finally proposes that “implantation in eutherians is derived from ancestral inflammatory reaction by mimicking the leukocyte capture in blood vessels” (2).
Mimicry of leukocyte capture by the blastocyst is an interesting hypothesis that can explain why inflammation is necessary for implantation (3), but is insufficient to explain the evolutionary origin of inflammatory signaling. The first step of inflammation is local secretion of proinflammatory signals, mainly by macrophages and fibroblasts. These signals recruit leukocytes from the bloodstream, which use L-selectin to invade inflamed tissues. L-selectin–dependent invasion is a reaction to an inflammatory process and supports the progression of inflammation rather than its initiation. Thus, L-selectin expression by the embryo cannot explain the broad inflammatory reaction observed during implantation in eutherians (4). The recruitment, in evolution, of L-selectin to invade the endometrium can only work if there is already a source of inflammatory signals, which was ancestrally due to embryo attachment.
Furthermore, it is not surprising that the ancestral inflammatory signaling is partially assimilated in the normal uterine cycle of eutherians. Genetic assimilation occurs when environmentally induced phenotypes are incorporated into normal development independent of the environmental stimulus (5). This also occurred with endometrial decidualization in primates (6). Applied to early pregnancy, we envision that inflammation occurred so reliably at the beginning of pregnancy that it was incorporated into normal uterine cycling in eutherians, enhancing implantation success. Even with partial assimilation, inflammatory signals are still modulated by the presence of the blastocyst, as demonstrated by Liu (2).
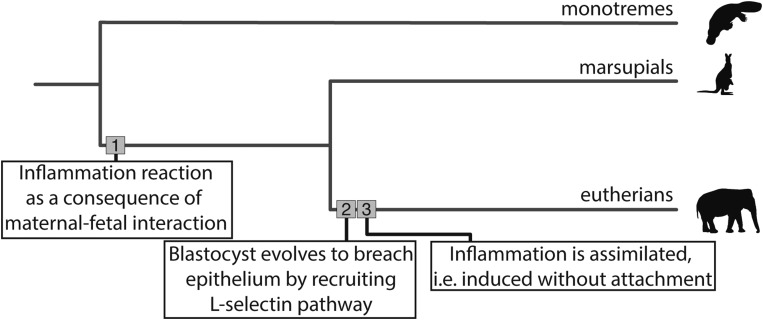
To conclude, we show how Liu’s (2) findings and our results (1) fit into an evolutionary scenario (Fig. 1). The inflammatory reaction evolved as a direct consequence of the first maternal–fetal interaction following the evolution of pregnancy (7). Once inflammation was established as a regular event during gestation, the embryo evolved the ability to invade endometrial tissue by recruiting the invasive machinery used by leukocytes. After that, inflammation became partially independent of embryo attachment by genetic assimilation.
Fig. 1.
Phylogeny of major extant mammalian lineages. We have noted where transformations to the maternal–fetal inflammation response have occurred along branches. 1) Uterine inflammation occurs as a result of the presence of a fetus in utero; this is distinct from the ancestral egg-laying case where uterine inflammation did not occur (1, 4). 2) The blastocyst evolves to invade maternal uterine tissue, perhaps by recruiting the L-selectin pathway and “mimicking” leukocyte cell behavior. 3) The inflammation response is assimilated and induced at some level without attachment, this occurs because of its necessity for the success of implantation and reproduction. Inflammation is a mechanism of receptivity, attracts the blastocyst, and initiates implantation; this explains why uterine biopsies promote implantation success in in vitro fertilization (8, 9).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
References
- 1.Griffith OW, et al. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci USA. 2017;114:E6566–E6575. doi: 10.1073/pnas.1701129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J-L. Implantation in eutherians: Which came first, the inflammatory reaction or attachment? Proc Natl Acad Sci USA. 2017;115:E1–E2. doi: 10.1073/pnas.1716675115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genbacev OD, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 4.Chavan AR, Griffith OW, Wagner GP. The inflammation paradox in the evolution of mammalian pregnancy: Turning a foe into a friend. Curr Opin Genet Dev. 2017;47:24–32. doi: 10.1016/j.gde.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Waddington CH. Genetic assimilation of an acquired character. Evolution. 1953;7:118–126. [Google Scholar]
- 6.Emera D, Romero R, Wagner G. The evolution of menstruation: A new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. BioEssays. 2012;34:26–35. doi: 10.1002/bies.201100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith OW, Wagner GP. The placenta as a model for understanding the origin and evolution of vertebrate organs. Nat Ecol Evol. 2017;1:0072. doi: 10.1038/s41559-017-0072. [DOI] [PubMed] [Google Scholar]
- 8.Barash A, et al. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79:1317–1322. doi: 10.1016/s0015-0282(03)00345-5. [DOI] [PubMed] [Google Scholar]
- 9.Raziel A, et al. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil Steril. 2007;87:198–201. doi: 10.1016/j.fertnstert.2006.05.062. [DOI] [PubMed] [Google Scholar]