Significance
Dopamine signaling modulates important physiological functions such as reward and motor activity through activation of five different receptors. Dopamine D2 receptors (D2R) are critical in the control of motor behavior. D2Rs exist in two isoforms, a long (D2L) and a short (D2S), both expressed in dopamine neurons as well as in cells receiving dopaminergic afferents. Such diversity questions their individual contribution to presynaptic and postsynaptic events. Using isoform-specific knockout mice, we report that while either isoform can mediate the control of basal behaviors, upon pharmacological stimulation of the dopaminergic system, isoform-specific functions are observed at the behavioral and cellular levels. Therapeutic strategies targeting specific D2R isoforms may present treatment for ailments caused by the activation or blockade of both isoforms.
Keywords: dopamine, D2L, D2S, striatum, cocaine
Abstract
The dopamine D2 receptor (D2R) is a major component of the dopamine system. D2R-mediated signaling in dopamine neurons is involved in the presynaptic regulation of dopamine levels. Postsynaptically, i.e., in striatal neurons, D2R signaling controls complex functions such as motor activity through regulation of cell firing and heterologous neurotransmitter release. The presence of two isoforms, D2L and D2S, which are generated by a mechanism of alternative splicing of the Drd2 gene, raises the question of whether both isoforms may equally control presynaptic and postsynaptic events. Here, we addressed this question by comparing behavioral and cellular responses of mice with the selective ablation of either D2L or D2S isoform. We establish that the presence of either D2L or D2S can support postsynaptic functions related to the control of motor activity in basal conditions. On the contrary, absence of D2S but not D2L prevents the inhibition of tyrosine hydroxylase phosphorylation and, thereby, of dopamine synthesis, supporting a major presynaptic role for D2S. Interestingly, boosting dopamine signaling in the striatum by acute cocaine administration reveals that absence of D2L, but not of D2S, strongly impairs the motor and cellular response to the drug, in a manner similar to the ablation of both isoforms. These results suggest that when the dopamine system is challenged, D2L signaling is required for the control of striatal circuits regulating motor activity. Thus, our findings show that D2L and D2S share similar functions in basal conditions but not in response to stimulation of the dopamine system.
The dopamine (DA) D2 receptor (D2R) is a key element of the dopaminergic system. D2Rs have critical presynaptic autoreceptor functions in dopaminergic neurons that enable a finely tuned control of DA synthesis and release (1–4). Postsynaptically, D2Rs control the signaling and firing properties of neurons receiving DA afferents and, acting as heteroreceptors, regulate the release of heterogeneous neurotransmitters (5–8).
D2Rs are composed by two almost identical isoforms, D2 long (D2L) and D2 short (D2S), generated by alternative splicing of the Drd2 gene. The search for cells expressing only one of the isoforms failed and, indeed, at the mRNA level, the two isoforms are both present in each location expressing D2R (9). D2L differs from D2S by the insertion of 29 amino acids in the third intracellular loop of the putative conformation of seven transmembrane domain G protein-coupled receptors family. This region is the site of the receptor’s interaction with signal transduction proteins, thus suggesting that D2L and D2S might interact and regulate different proteins and signaling pathways. In agreement with this hypothesis, we found that in the pituitary, D2L-mediated signaling inhibits the AKT pathway, while D2S is instead required for the activation of the ERK pathway (10, 11). In addition, studies performed in cell culture suggested that D2L and D2S might be segregated in different neuronal compartments and, therefore, differentially respond to DA stimulation (12).
In the striatum, in vivo studies performed using mice lacking D2L (D2L−/−) (13) showed that this isoform is required for the cataleptic effect of haloperidol, the prototype of typical antipsychotics, as well as for the inhibition of the AKT pathway (14). These findings, together with an intact control of DA synthesis and release in D2L−/− mice, led us to propose that in vivo D2S might have a preponderant presynaptic role in DA neurons, while D2L might have postsynaptic functions (15). However, using viral-mediated reexpression of D2L or D2S in dopaminergic neurons of D2R−/− mice, it was proposed that D2S could not operate as the exclusive autoreceptor since it was insufficient to account for drug-induced plasticity in these neurons (16).
Thus, the question of whether endogenous D2S has equal or different functions than D2L in vivo has yet to be directly explored. Using mice with the selective deletion of individual D2R isoforms, we have been able to study the behavioral and cellular characteristics of D2L−/− with that of D2S−/− mice compared with WT littermates. We characterized the impact of loss of either D2 isoform on motor behavior under basal conditions and in response to pharmacological challenge with D2 agonists and antagonists. Interestingly, in striking contrast with the strong impairment of motor activity observed in the absence of D2R signaling either in the constitutive (17) or striatal medium spiny neurons (MSNs)-specific knockout (2), each of the D2 isoform mutants does not differ from WT littermates under basal conditions. These results indicate that the presence of either D2L or D2S is sufficient to guarantee the control of striatal neurons and normal behavior. This observation suggests also that the two isoforms can compensate for each other in MSNs and possibly other D2R expressing neurons. However, upon pharmacological stimulation either by D2-specific ligands or by cocaine, we observed different responses between isoform-specific mutants and a strong degree of specialization in the response of each isoform to presynaptic and postsynaptic functions.
Therapeutics targeting a selective isoform might constitute an effective strategy to treat D2L- or D2S-specific alterations and alleviate side effects due to activation/blockade of both isoforms.
Results
Absence of D2S Does Not Affect D2L Expression and Number of Sites in the Striatum.
D2S−/− mice were generated by removal of the exon 6 and flanking introns and replacement of this region with a fragment of the D2L cDNA (11). To assess whether the absence of D2S would affect D2L expression in the striatum, we performed RT-PCR analyses of striatal mRNAs. Analyses of PCR products show the presence of bands corresponding to both D2R isoforms in WT striata, while the selective expression of either only D2L (245-bp band) or of only D2S (158 bp) was observed in samples from D2S−/− and D2L−/− striata, respectively (Fig. 1A). Quantification by qRT-PCR of the overall expression levels of the Drd2 gene in the striatum of WT, D2S−/−, and D2L−/− mice showed similar levels of D2R mRNA expression in all genotypes (Fig. 1B). To get information at the protein level, we analyzed the pharmacological characteristics of the D2 binding sites in D2S−/− striatal extracts using 3H-spiperone; also at this level, D2S−/− did not differ from their WT littermates (Fig. 1C).
Fig. 1.
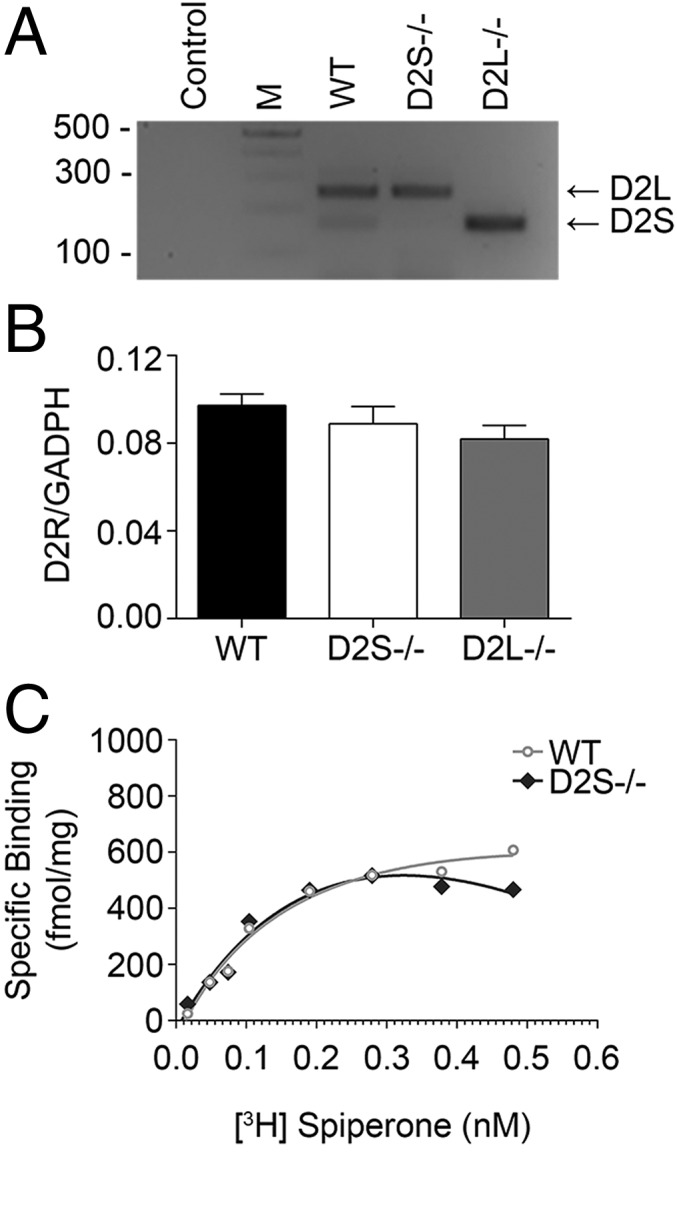
Characterization of D2S−/− mice. (A) RT-PCR analyses of D2R expression in striatal mRNAs from WT, D2S−/−, and D2L−/− mice. The upper band (245 bp) corresponds to D2L, whereas the lower (158 bp) to D2S, as indicated by arrows. (B) qRT-PCR–based quantifications of D2R expression in striatal extracts of WT, D2S−/−, and D2L−/− mice, using primers common to both isoforms (exon 2), using GAPDH as control. Bars are mean ± SEM of D2R/GAPDH ratios (n = 4 per genotype). (C) Saturation isotherm analyses for binding of the D2R antagonist [3H]-Spiperone to striatal membranes from WT (circles) (Bmax = 720 ± 85 fmol/mg protein; Kd = 30 ± 2 pM) and D2S−/− mice (diamonds) (Bmax = 600 ± 67 fmol/mg protein; Kd = 28 ± 1 pM) (n = 3 per genotype).
These results indicate that in D2S−/− mice there is a complete transition from the expression of the two isoforms into a single one, in this case D2L. Nevertheless, the number of D2 binding sites in both D2 isoform mutants remains the same as in WT striata (Fig. 1C) (13).
The Basal Motor Activity of D2S−/− Does Not Differ from That of D2L−/− or WT Littermates.
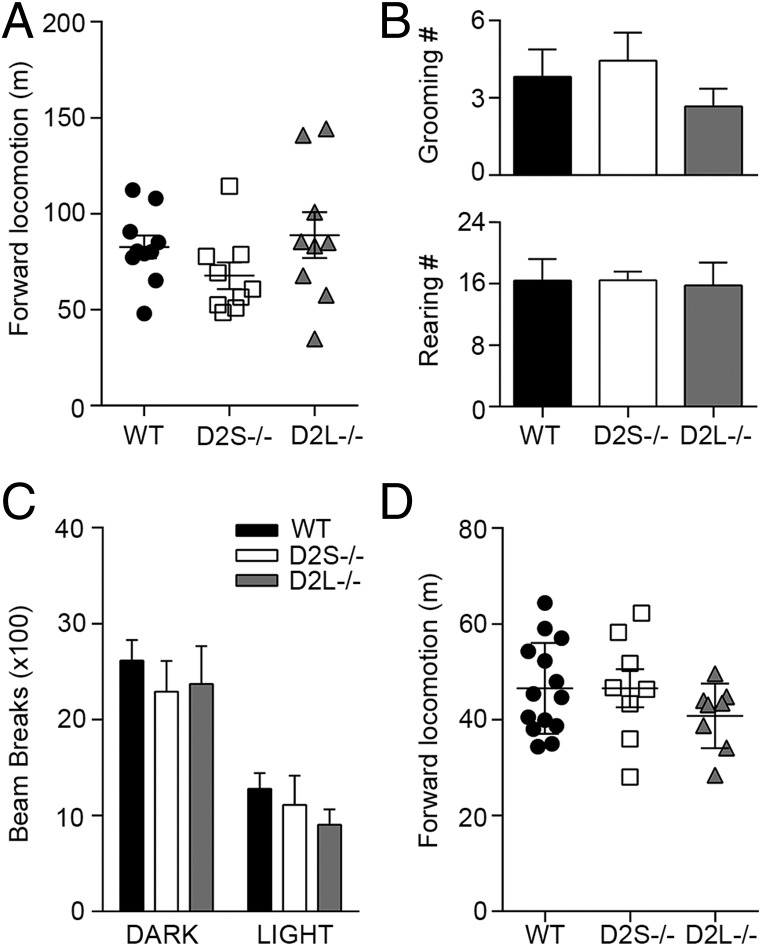
In the striatum, D2Rs are expressed by the medium spiny neurons that form the indirect pathway (iMSNs) (18). We have shown that D2R-mediated signaling in iMSNs is essential in the control of motor behavior, as demonstrated by analyses performed using conditional iMSN-D2RKO mice (2) that perfectly recapitulate the motor phenotype of the constitutive D2R−/− mutants (17). In agreement with the importance of D2R signaling in iMSNs for motor activity, D2R ablation either in dopaminergic neurons (DA-D2RKO mice) (2) or in striatal cholinergic interneurons (ChI-D2RKO mice) (19) does not affect the basal locomotor activity. Importantly, absence of D2L in D2L−/− mice did not result into motor abnormalities in basal conditions (13, 20). To assess whether D2S is principally involved in the control of motor behavior, we analyzed and compared the forward locomotion of D2S−/− mice in a novel home cage (NHC) to that of D2L−/− mice and WT littermates. Interestingly, these analyses show that absence of D2S in mice does not affect basal locomotor activity; indeed, D2S−/− mice behaved similarly to WT and D2L−/− mice [F(2, 25) = 1.583; P = 0.2253] (Fig. 2A). The analysis of stereotypies (rearing and grooming) in the three groups also suggests that the removal of either D2L or D2S does not affect these behaviors (Fig. 2B) [grooming: F(2, 26) = 0.78 P = 0.46; rearing: F(2, 28) = 0.018 P = 0.98]. In addition, forward locomotion either during the light/dark cycle (Fig. 2C) [time × genotype: F(2, 22) = 0.22, P = 0.8] or in the open field (Fig. 2D) [F(2, 27) = 1.102, P = 0.34] does not differ between WT and D2S−/− or D2L−/− mice.
Fig. 2.
Basal motor activity is unaffected by deletion of either D2L or D2S. (A) Scatter plot representation of WT, D2S−/−, and D2L−/− motor activity during 1 h in NHC (n = 9–10 per genotype) [F(2, 25) = 1.583; P = 0.2253]. (B) Quantification of grooming or rearing activity during 1 h in NHC (n = 9–13 per genotype) [F(2, 26) = 0.78 P = 0.46; and F(2, 28) = 0.018 P = 0.98 for grooming and rearing, respectively]. (C) Motor activity in actimetric cages during the dark/light cycle (n = 6–14 per genotype) [F(2, 22) = 0.22, P = 0.8]. (D) Scatter plot representation of motor activity in the open field of WT, D2L−/−, and D2S−/− mice during 30 min (n = 8–13 per genotype) [F(2, 27) = 1.102, P = 0.34]. Values are mean ± SEM.
These results show that D2S−/− mice perform as WT littermates indicating that, in contrast to D2R−/− or iMSN-D2RKO mice, the presence of either D2L or D2S is sufficient to maintain a normal control of motor activity.
Absence of D2S Affects D2 Autoreceptor-Mediated Functions.
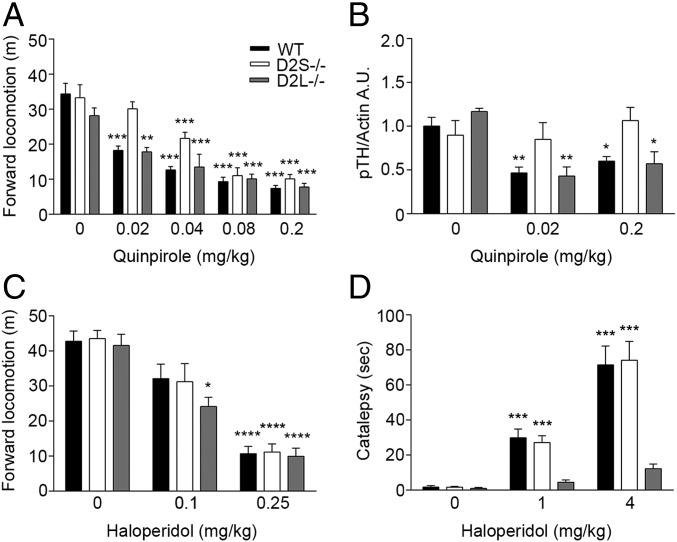
Next, we analyzed the presynaptic versus postsynaptic functions of the D2 isoforms in response to pharmacological stimulations. The administration of low doses of quinpirole (a specific D2R agonist) activates D2 autoreceptors in DA neurons, reducing DA release and, consequently, motor activity (13). Thus, we performed a dose–response analysis of quinpirole and analyzed the effects on forward locomotion comparing D2S−/−, D2L−/−, and their WT littermates. Mice of the three genotypes were administered (i.p.) quinpirole at 0.02, 0.04, 0.08, and 0.2 mg/kg (Fig. 3A). Interestingly, the results of these experiments revealed a statistically significant difference in the effects elicited by quinpirole in D2S−/− compared with WT and D2L−/− mice (Fig. 3A) [genotype × treatment: F(8, 136) = 2.508, P < 0.01; genotype: F(2, 136) = 10.81, P < 0.0001; treatment: F(4, 136) = 64.35, P < 0.0001]. The most relevant difference was observed at the lowest dose (0.02 mg/kg) tested. At this dose, quinpirole was ineffective in decreasing motor activity in D2S−/− mice while it did in the other genotypes. The different responses of D2S−/− mice compared with WT and D2L−/− mice at low concentration of quinpirole support previous conclusions of a specific role of D2S as presynaptic receptor in DA neurons (13).
Fig. 3.
Locomotor activity induced by quinpirole and haloperidol. (A) Quinpirole effect on locomotor activity in WT, D2S−/−, and D2L−/− mice [genotype: F(2, 136) = 10.81 P < 0.0001] **P < 0.01, ***P < 0.001 vs. saline-treated mice. (B) Quantifications of Western blot analyses of TH phosphorylation on Ser40 in protein extracts from the DS of WT, D2S−/−, and D2L−/− mice injected with saline or 0.02 and 0.2 mg/kg quinpirole. Two-way ANOVA, genotype × treatment: F(4, 28) = 2.589; P = 0.0584, n = 3–5 samples/treatment per genotype. *P < 0.05, **P < 0.01, vs. saline treated mice. (C) Motor activity recorded for 1 h in NHC of WT, D2S−/−, and D2L−/− mice injected with either saline or haloperidol (0.1, 0.25 mg/kg). Two-way ANOVA, treatment: F(2, 91) = 92.71, P < 0.0001; genotype: F(2, 91) = 1.057, P = 0.3517, n = 7–16. *P < 0.05, ****P < 0.0001 vs. saline-treated mice. (D) Quantification of the cataleptic behavior upon saline or haloperidol (1 and 4 mg/kg) treatment in WT, D2S−/−, and D2L−/− mice. Two-way ANOVA, genotype × treatment: F(4, 84) = 14.45, P < 0.0001, n = 7–15. ***P < 0.001 vs. saline-treated mice.
To further assess this point, we tested the level of phosphorylation of tyrosine hydroxylase (TH) in D2 mutants and their WT siblings (Fig. 3B). TH is the rate-limiting enzyme in DA synthesis; its activity is regulated by activation of the cAMP/PKA pathway through phosphorylation of the serine 40 (Ser40) (21). Activation of D2Rs inhibits the cAMP pathway and reduces Ser40 phosphorylation (2). Thus, Ser40 TH phosphorylation (p-TH) was analyzed in D2S−/−, D2L−/−, and WT littermates at the lowest and highest dose of quinpirole used (0.02 and 0.2 mg/kg) in behavioral studies. Mice of the three genotypes were administered either quinpirole or saline and euthanized 30 min later. Striatal tissue punches were obtained from the dorsal striatum (DS). While efficiently decreasing TH phosphorylation in WT and D2L−/− mice, our analyses showed that quinpirole, at both doses, was unable to elicit a decrease in TH phosphorylation in D2S−/− mice (Fig. 3B) (DS, genotype × treatment: F(4, 28) = 2.589; P = 0.0584). Similar results were obtained analyzing tissue punches from the ventral striatum (VS) (Fig. S1) [genotype × treatment: F(4, 29) = 3.603; P = 0.0167].
These results indicate that the presynaptic effect of D2R on DA synthesis in dopaminergic neurons is mostly exerted by D2S. Postsynaptic effects might explain the similar motor response of D2S−/− mice to the other genotypes at higher quinpirole concentrations (Fig. 3A).
The D2R-Mediated Postsynaptic Signaling in Striatal Neurons Mostly Requires D2L.
Administration of haloperidol, a D2R antagonist, reduces mouse locomotor activity in a dose-dependent manner and leads to catalepsy or inability to perform voluntary movements. We tested haloperidol effects on both parameters by analyzing motor activity in a NHC for 1 h and, subsequently, the mice performance in the bar test. The results of these tests showed that while motor activity was similarly impaired by haloperidol in all genotypes [treatment: F(2, 91) = 92.71, P < 0.0001; genotype: F(2, 91) = 1.057, P = 0.3517] (Fig. 3C), catalepsy was only observed in WT and D2S−/− mice, but not in D2L−/− mice [genotype × treatment: F(4, 84) = 14.45, P < 0.0001], as previously reported (13) (Fig. 3D). Thus, absence of D2S does not affect the response to haloperidol, further confirming the significant postsynaptic role of D2L in striatal neurons.
Heterosynaptic Role of D2L in Cocaine-Evoked Motor Activity.
We and others have recently reported a critical role of iMSNs in the psychomotor response to cocaine (22, 23). Indeed, absence of D2R in iMSNs abolishes the cocaine-dependent induction of both motor activity and the immediate early gene (c-fos) expression in the MSNs of the direct pathway expressing D1R (dMSNs). Thus, stimulation of D2R enables dMSN-mediated responses to cocaine. D2R in striatal neurons has a heterosynaptic role that governs the release of heterogeneous neurotransmitters such as GABA from iMSNs (24). Previous experiments showed that the cocaine phenotype of iMSN-D2RKO mice could be reversed by intrastriatal injections of bicuculline, a GABAA receptor antagonist, suggesting a heterosynaptic role of D2R in iMSNs.
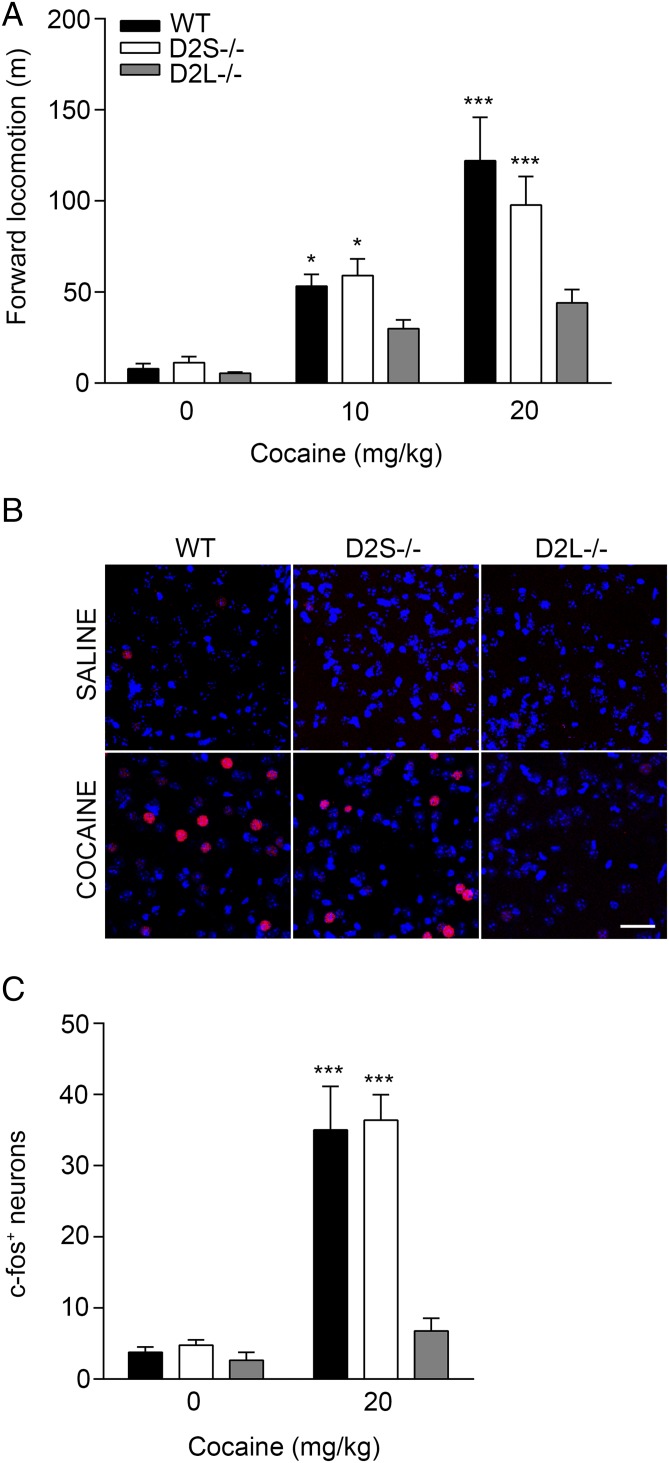
Next, to gain further insight into the respective role of the D2R isoforms as heteroreceptors, we investigated the impact of absence of D2S or D2L signaling on cocaine-mediated behavioral and cellular responses. Upon habituation to a NHC, WT, D2S−/−, and D2L−/− mice were administered either saline or cocaine (10 mg/kg and 20 mg/kg) and their locomotion was recorded for the following hour. Interestingly, while WT and D2S−/− mice showed a similar significant motor response to cocaine, D2L−/− mice did not [genotype × treatment: F(4, 78) = 2.93, P = 0.02; treatment: F(2, 78) = 32.62, P < 0.0001; genotype: F(2, 78) = 7.99, P < 0.001] (Fig. 4A) These results show that absence of D2L strongly impairs the response to cocaine, indicating a preponderant role of D2L in enabling the dMSN-mediated motor response to the drug.
Fig. 4.
Different motor and cellular responses to cocaine in D2 isoform mutants. (A) Motor activity in NHC of WT, D2S−/−, and D2L−/− mice administered either saline or cocaine (10 and 20 mg/kg) recorded for 1 h. Two-way ANOVA, genotype × treatment: F(4, 78) = 2.93, P = 0.02, n = 7–14 mice/treatment per genotype.*P < 0.05, ***P < 0.001 vs. saline-treated mice. (B) Representative images of immunofluorescence analyses using anti c-fos antibodies (red) and Draq7-labeled nuclei (blue) in the DS of WT, D2S−/−, and D2L−/− mice following saline or cocaine treatment (20 mg/kg), as indicated. (Scale bar: 30 µm.) (C) Quantifications of the number of c-fos–positive neurons in same experiments. Values are mean ± SEM of c-fos+ neurons/region of interest (385.7 × 385.7 µm). Two-way ANOVA, genotype: F(2, 14) = 11.30, P = 0.0012 genotype × treatment: F(2, 14) = 8.99, P = 0.003, n = 3–4 mice/treatment per genotype). ***P < 0.001 vs. saline-treated mice.
Analyses of cocaine (20 mg/kg)-mediated c-fos induction in WT, D2S−/−, and D2L−/− mice showed the expected c-fos expression in dMSNs in WT and D2S−/− mice, but not in D2L−/− mice [genotype × treatment: F(2, 14) = 8.99, P = 0.003] (Fig. 4 B and C), suggesting a critical role of the D2L isoform as heterosynaptic receptors in iMSNs.
Bicuculline Reestablishes the Motor and Cellular Response to Cocaine of D2L−/− Mice.
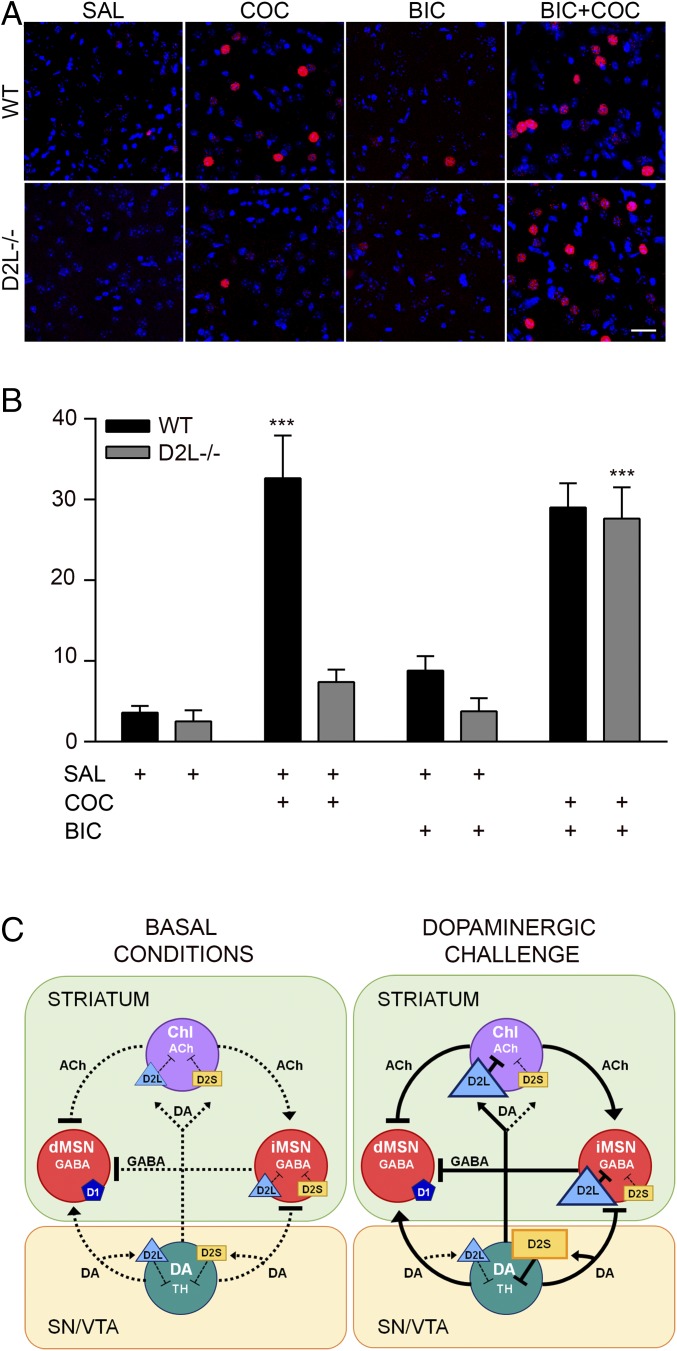
Intrastriatal injection of bicuculline, a GABAA receptor antagonist, is able to restore cocaine-mediated c-fos induction in iMSN-D2RKO mice to WT levels. Thus, we tested whether a similar effect would also be obtained in the striatum of bicuculline-injected D2L−/− mice. For this purpose, cannulas were implanted in the DS of D2L−/− and WT littermates, followed 1 wk later by the administration of either saline or bicuculline (0.01 μg/0.3 μL per side). Cocaine was given systemically 5 min after bicuculline or saline administration. Brains were collected 1 h after cocaine, and c-fos induction was quantified by immunofluorescence analyses. Bicuculline alone did not induce c-fos expression in either genotype (Fig. 5A). Importantly, when administered before cocaine, bicuculline fully restored c-fos induction in the dMSNs of D2L−/− mice to WT levels (Fig. 5B), compared with D2L−/− only treated with cocaine [genotype × treatment: F(3, 21) = 7.894; P = 0.001] (Fig. 5B). These results support a heterosynaptic role of D2L in the control of cocaine-mediated effects.
Fig. 5.
Bicuculline treatment restores cocaine-mediated c-fos induction in D2L−/− mice. (A) Representative images of immunofluorescent analyses using c-fos antibodies (red) and Draq7 (blue) labeled nuclei on striatal sections from WT and D2L−/− mice following saline (SAL) or cocaine (COC) (20 mg/kg, i.p.) or bicuculline (BIC i.c.) or bicuculline plus cocaine (BIC+COC) treatments as indicated. (Scale bar: 30 µm.) (B) Quantifications of the number of c-fos+ cells/region of interest of the experiments shown in A. Values are mean ± SEM. Two-way ANOVA, genotype: F(1, 21) = 11.48, P = 0.0028; genotype × treatment: F(3, 21) =7.894; P = 0.001, n = 3 mice/treatment per genotype). ***P < 0.001 vs. WT or D2L −/− saline-treated mice. (C) Schematic representation of the findings obtained by the analysis of D2L−/− and D2S−/− mice. Collectively, our data suggest that under basal conditions, signaling from either D2R isoforms is sufficient to control D2-mediated functions (i.e., presynaptic control of DA synthesis and postsynaptic ACh and GABA release) as shown by the normal motor behavior (Left: Basal Conditions). However, upon dopaminergic challenge, while D2S signaling in DA neurons is critical for the inhibition of TH phosphorylation and DA synthesis, D2L signaling appears required for the inhibition of iMSNs collaterals to dMSNs, which is critically involved in the psychomotor response to cocaine (22, 23) as well as c-fos induction (22) (Right: Dopaminergic Challenge). The preferential presynaptic and postsynaptic involvement of D2S and D2L, respectively, upon challenge is indicated by the enlargement of the rectangle (D2S) or triangle (D2L). The dashed lines represent the basal striatal tone of neurotransmitters while the solid line is the striatal tone upon dopaminergic stimulation.
Discussion
The presence of two almost identical receptors for D2R, D2L and D2S, which differ only by the presence of an additional small stretch of 29 amino acids in D2L, has generated interest in unraveling the biological significance of such diversity in vivo. Studies performed in cultured cells have shown that both isoforms have similar affinity for dopamine and canonical signaling pathways (15). To analyze the roles and properties of the D2 isoforms in vivo, we have generated D2L and D2S isoform-specific mutants, either by removing exon 6 (D2L−/− mice; ref. 13) or by replacing the genomic sequence containing exons 5, 6, and 7 with the cDNA of the corresponding region of D2L (D2S−/− mice; ref. 11). D2L−/− and D2S−/− mice express a similar number of D2 receptors binding sites as WT mice, although formed by only one isoform instead of two. Studies performed in the pituitary gland of D2L−/− and D2S−/− mice showed that D2L and D2S signaling differentially affects the ERK and AKT pathways (11). These results suggest that in vivo D2L and D2S might play different roles also in the striatum, where D2Rs are largely expressed by iMSNs (18) and cholinergic interneurons (19). Absence of striatal D2R affects motor outputs (2, 22, 23), although we found that the presence either D2L or D2S is sufficient to ensure a normal basal motor activity. This experimental evidence indicates that D2L and D2S share common basal signaling essential to various critical functions; however, the presence of compensatory mechanisms in each of the isoform-specific knockout animals cannot be excluded.
Nevertheless, this possibility seems less likely as the response of the two mutants is different when either receptor is challenged by D2-specific drugs. Indeed, when D2L−/− and D2S−/− mice are challenged with D2-specific agonists or antagonists, we observed some relevant differences. Administration of quinpirole, a D2-specific agonist, at low doses induces a decrease of motor activity. This effect depends on activation of presynaptic dopamine autoreceptors and the consequent reduction of DA release and activation of postsynaptic receptors. Interestingly, the lowest dose of quinpirole in D2S−/− mice was totally ineffective in reducing motor activity compared with the effect in WT and D2L−/− mice. These results suggest that autoreceptors functions are compromised by the absence of D2S. In support of this conclusion, we observed that the D2-mediated inhibition of TH phosphorylation on Ser40 is prevented by absence of D2S in quinpirole-treated D2S−/−, but not in WT or D2L−/− mice. Interestingly, TH phosphorylation in D2S−/− mice was not inhibited even at quinpirole concentrations that induced a reduction of motor activity in mice of all genotypes by acting on postsynaptic receptors. These results strongly support the view that D2S has major presynaptic functions compared with D2L.
In contrast, absence of D2S does not interfere with haloperidol-induced catalepsy. Indeed, by difference with D2L−/− mice (Fig. 3D) (13), D2S−/− and WT mice showed a dose-dependent cataleptic response to haloperidol.
The different responses of D2L−/− and D2S−/− mice to quinpirole or haloperidol raise the questions of whether differences arise from the higher affinity for either D2L or D2S of these drugs or from unique isoform-mediated signaling in specific cell types upon pharmacological challenge. Although we cannot exclude minor pharmacological differences of either D2S or D2L for D2 ligands, as previously observed using cell lines (25), we are more in favor of the second hypothesis. Indeed, despite the difference on motor behavior between D2S and D2L mutants at low quinpirole concentration, a clear D2S-mediated cell-specific effect on the inhibition of TH phosphorylation is observed at any dose tested. Haloperidol, while similarly affecting horizontal locomotion, is not able to induce catalepsy in D2L−/− mice, suggesting similar affinity for both isoforms.
Interestingly, we have recently shown that absence of D2R-mediated control of cholinergic interneurons abolishes haloperidol-induced catalepsy through a mechanism involving acetylcholine stimulation of iMSNs (19). This implies that the haloperidol effect is due to the absence of D2L signaling, which either plays a critical role in the regulation of acetylcholine in cholinergic interneurons or in the control of iMSNs responses or in both. Future studies should be aimed at generating mouse lines with a striatal cell-specific deletion of D2L to assess these possibilities.
Importantly, the response to acute cocaine challenges of D2S−/− and D2L−/− mice with respect to WT animals was also different. Indeed, while D2S−/− mice showed a dose-dependent increase of motor activity in response to cocaine (paralleling WT mice), D2L−/− mice did not. Interestingly, c-fos induction by cocaine also was absent in D2L−/− compared with D2S−/− and WT littermates. The blunted response to cocaine of D2L−/− mice is reminiscent of that of D2RKO and iMSN-D2RKO mice (22, 26) and suggests that absence of D2L in iMSNs might be responsible for it. This suggestion is supported by the rescue of the motor and cellular responses to cocaine induced by the intracellular injection of bicuculline, as previously shown in D2RKO and iMSN-D2RKO mice (22). In conclusion, our findings assign to D2L a critical role in the response to drugs of abuse (possibly mediated by absence of D2L signaling in iMSNs). Therefore, the analysis of D2 isoform-specific knockout mice (see scheme in Fig. 5C) shows a clear impact of D2S as presynaptic autoreceptor on dopaminergic neurons. Conversely, D2L appears to possess postsynaptic functions responsible for the regulation of striatal acetylcholine and activity of iMSNs.
Materials and Methods
Mice.
The D2S−/− mouse line was generated by a knock-in strategy aimed at preventing alternative splicing of exon 6, through replacement of the Drd2 genomic region containing part of exon 5, exon 6, and part of exon 7 with the corresponding cDNA of D2L, thus allowing the synthesis of only D2L in vivo (27). The D2L−/− mouse line was generated by the replacement of exon 6 with a neomycin cassette, thus generating mice expressing only D2S (13). Eight- to 12-wk-old D2L−/− (13), D2S−/− (11) and WT littermates were used. Mice were maintained in standard conditions (12-h light/dark cycle) with food and water ad libitum. For genotyping D2S−/−, D2L−/−, and WT littermates, genomic DNA was extracted from tail biopsies and analyzed by PCR, as previously described (11). All protocols were approved by the Institutional Animal Care and Use Committee in accordance with the NIH guidelines.
Drugs.
Quinpirole (Sigma), cocaine hydrochloride (Sigma), and bicuculline methiodide (Fluka) were dissolved in saline (0.9% NaCl). Haloperidol (Sigma) was dissolved in a drop of glacial acetic acid; pH was adjusted to 6 and the solution brought to volume with saline (13).
RNA Extraction and PCR.
Total RNA was extracted from the striatum using TRIzol (Invitrogen). One microgram of total RNA was retrotranscribed using the iScript cDNA Synthesis kit (Bio-Rad). qRT-PCR were performed on striatal cDNAs to quantify D2R expression using the iQ SYBR Green Supermix (Bio-Rad) and the CFX96 Real-Time System (Bio-Rad). GAPDH was used as internal control. The primers used were as follows: D2R forward (5′-AGTGGCCCCACTGCCCCAAT-3′) and reverse (5′-TCCAGATAGACGACCCAGGGC-3′); GAPDH forward (5′-AGGTCGGTGTGAACGGATTTG-3′) and reverse (5′-TGTAGACCATGTAGTTGAGGTCA-3′). qRT-PCR was run for 40 cycles, 10 s at 95 °C followed by 30 s at 60 °C.
We also performed RT-PCR followed by analyses of the PCR products on 2% agarose gel (Fig. 1A). PCR buffer contained 4 mM MgCl2, 0.2 mM of each dNTP, 2 µL of Taq DNA polymerase (1 U/μL), and 0.8 µM of the following primers: D2R forward (exon 5), 5′-CCTTCATCGTCACCCTGCTGG-3′ and reverse (exon 7), 5′-CTCCATTTCCAGCTCCTGAG-3′. Cycles were 94 °C for 3 min, and 40 cycles of 40 s at 94 °C, 40 s at 57 °C, and 1 min at 72 °C, with a final extension period of 10 min at 72 °C.
Binding Analyses.
Striatal membranes and ligand binding analyses for D2S−/− and WT mice were performed as previously described (13, 17). Twenty micrograms of membrane extracts per sample were used in binding assays using 3H-Spiperone (84.8 Ci/mmol; Perkin-Elmer) at concentrations ranging from 0.01 to 0.6 nM; nonspecific binding was determined in the presence of 1.3 µM (±)-butaclamol. Binding assays were performed in triplicates and repeated three times. Results were analyzed with GraphPad Prism 6.
Behavioral Analyses.
To analyze motor behavior, mice were handled (5 min/d) for 2 d, and basal locomotor activity in the new home cage (NHC, 20 × 30 cm transparent plastic box, 1 h) or open field (white square box, 30 × 30 cm; 70 lx, 30 min) was recorded using a video-tracking system (Viewpoint). Rearing and grooming were measured in NHC and scored for 60 s every 10 min for a 1-h period. Activity during 24 h was measured in an actimetric rack (individual cages 10 × 20 cm; Viewpoint). The effect of quinpirole (0.02–0.2 mg/kg, i.p.) and haloperidol (0.1–0.25 mg/kg, i.p.) on locomotor activity was measured for 30 min or 1 h, respectively, in the NHC. Cocaine effect was tested for 1 h after 2-h habituation to the NHC.
Bar Test.
The bar test was performed as previously described (2), 1 h after haloperidol administration. The time spent immobile (cutoff 120 s) was measured during three consecutive trials (13, 28). The average of the three trials was used for the statistical analyses.
Western Blot Analyses.
Mice were killed 30 min after saline or quinpirole (0.02 and 0.2 mg/kg) administration and processed as previously described (2). Protein content was determined using the BCA kit (Thermo Scientific). Thirty micrograms of extracts were loaded onto 10% SDS/PAGE and transferred to nitrocellulose membranes (Bio-Rad). Antibodies directed against phosphorylated TH-Ser40 (1/1,000 Millipore) followed by the appropriate secondary antibodies were used. Bands were revealed using the ECL Plus reagent (Millipore). Actin was used as control of loaded quantities (anti-actin antibody 1/5,000; Millipore). Quantifications were done using ImageJ (version 1.42q) software. The value of the pTH/actin ratio obtained in WT saline-treated mice was arbitrarily set to 1.
Immunofluorescence.
Mice were anesthetized with Euthasol and transcardially perfused with 4% PFA. Thirty-micrometer vibratome coronal striatal sections were used for immunofluorescence experiments to detect c-fos induction by cocaine. Tissue sections were washed three times in PBS permeabilized for 15 min in PBS with 0.5% Triton X-100 and then incubated in PBS with 5% horse serum (HS) for 1 h at room temperature followed by incubations using c-fos antibody (1/2,000; Santa Cruz) in PBS 1% HS overnight at 4 °C. Sections were rinsed three times in PBS for 10 min. Secondary antibodies were incubated for 1 h in PBS 1% HS. c-fos positive neurons were quantified in the DS in 387.5 × 387.5 µm regions of interest from three to four mice/treatment per genotype.
Statistical Analyses.
All values are mean ± SEM. Statistical analyses were made using GraphPad Prism 6. Data were analyzed by two-way ANOVA followed by Bonferroni’s post hoc or Student’s t test, as appropriate; P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank all members of the E.B.’s laboratory for interest, help, and discussions; in particular Maria Ramos, Claudia De Mei, Hyuna Lee, and Tam Phan. M.C. is supported by Associazione per la Ricerca sul Cancro. This work was funded by National Institute on Drug Abuse DA033554 and INSERM.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717194115/-/DCSupplemental.
References
- 1.Rouge-Pont F, et al. Changes in extracellular dopamine induced by morphine and cocaine: Crucial control by D2 receptors. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anzalone A, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32:9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bello EP, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBoer P, Abercrombie ED. Physiological release of striatal acetylcholine in vivo: Modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther. 1996;277:775–783. [PubMed] [Google Scholar]
- 6.Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centonze D, et al. Cocaine and amphetamine depress striatal GABAergic synaptic transmission through D2 dopamine receptors. Neuropsychopharmacology. 2002;26:164–175. doi: 10.1016/S0893-133X(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 8.Centonze D, et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montmayeur JP, et al. Differential expression of the mouse D2 dopamine receptor isoforms. FEBS Lett. 1991;278:239–243. doi: 10.1016/0014-5793(91)80125-m. [DOI] [PubMed] [Google Scholar]
- 10.Iaccarino C, et al. Control of lactotrop proliferation by dopamine: Essential role of signaling through D2 receptors and ERKs. Proc Natl Acad Sci USA. 2002;99:14530–14535. doi: 10.1073/pnas.222319599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radl D, De Mei C, Chen E, Lee H, Borrelli E. Each individual isoform of the dopamine D2 receptor protects from lactotroph hyperplasia. Mol Endocrinol. 2013;27:953–965. doi: 10.1210/me.2013-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tirotta E, et al. Signaling by dopamine regulates D2 receptors trafficking at the membrane. Cell Cycle. 2008;7:2241–2248. doi: 10.4161/cc.7.14.6307. [DOI] [PubMed] [Google Scholar]
- 13.Usiello A, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 14.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: Presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9:53–58. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gantz SC, et al. Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium. Elife. 2015;4:e09358. doi: 10.7554/eLife.09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baik JH, et al. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 18.Delle Donne KT, Sesack SR, Pickel VM. Ultrastructural immunocytochemical localization of the dopamine D2 receptor within GABAergic neurons of the rat striatum. Brain Res. 1997;746:239–255. doi: 10.1016/s0006-8993(96)01226-7. [DOI] [PubMed] [Google Scholar]
- 19.Kharkwal G, et al. Parkinsonism driven by antipsychotics originates from dopaminergic control of striatal cholinergic interneurons. Neuron. 2016;91:67–78. doi: 10.1016/j.neuron.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindgren N, et al. Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur J Neurosci. 2001;13:773–780. doi: 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- 22.Kharkwal G, Radl D, Lewis R, Borrelli E. Dopamine D2 receptors in striatal output neurons enable the psychomotor effects of cocaine. Proc Natl Acad Sci USA. 2016;113:11609–11614. doi: 10.1073/pnas.1608362113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbs LK, et al. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron. 2016;90:1100–1113. doi: 10.1016/j.neuron.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centonze D, et al. Differential contribution of dopamine D2S and D2L receptors in the modulation of glutamate and GABA transmission in the striatum. Neuroscience. 2004;129:157–166. doi: 10.1016/j.neuroscience.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Castro SW, Strange PG. Differences in the ligand binding properties of the short and long versions of the D2 dopamine receptor. J Neurochem. 1993;60:372–375. doi: 10.1111/j.1471-4159.1993.tb05863.x. [DOI] [PubMed] [Google Scholar]
- 26.Welter M, et al. Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA. 2007;104:6840–6845. doi: 10.1073/pnas.0610790104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montmayeur JP, Borrelli E. Transcription mediated by a cAMP-responsive promoter element is reduced upon activation of dopamine D2 receptors. Proc Natl Acad Sci USA. 1991;88:3135–3139. doi: 10.1073/pnas.88.8.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulay D, et al. Haloperidol-induced catalepsy is absent in dopamine D(2), but maintained in dopamine D(3) receptor knock-out mice. Eur J Pharmacol. 2000;391:63–73. doi: 10.1016/s0014-2999(99)00916-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.