Significance
Diapedesis is a key step in the innate immune response. Emerging evidence suggests that mechanical forces exerted by leukocytes and/or vascular endothelial cells (VECs) could play an important role during diapedesis. However, it is unclear how these forces physically pull the VEC junctions apart and drive the transmigration of the leukocyte. In this study, we quantify the sequence of mechanical events involved in paracellular diapedesis and experimentally demonstrate that the forces exerted by leukocytes can modulate the VEC tensions to open junctional gaps, and subsequently push into the underlying matrix to thrust an invasive protrusion. We also show that diapedesis can be mechanically facilitated by proinflammatory signals that increase VEC contractility, leading to spatial heterogeneities in VEC monolayer tensions.
Keywords: leukocyte, vascular endothelial cell, diapedesis, 3D traction force
Abstract
Leukocyte transmigration across vessel walls is a critical step in the innate immune response. Upon their activation and firm adhesion to vascular endothelial cells (VECs), leukocytes preferentially extravasate across junctional gaps in the endothelial monolayer (paracellular diapedesis). It has been hypothesized that VECs facilitate paracellular diapedesis by opening their cell–cell junctions in response to the presence of an adhering leukocyte. However, it is unclear how leukocytes interact mechanically with VECs to open the VEC junctions and migrate across the endothelium. In this study, we measured the spatial and temporal evolution of the 3D traction stresses generated by the leukocytes and VECs to elucidate the sequence of mechanical events involved in paracellular diapedesis. Our measurements suggest that the contractile stresses exerted by the leukocytes and the VECs can separately perturb the junctional tensions of VECs to result in the opening of gaps before the initiation of leukocyte transmigration. Decoupling the stresses exerted by the transmigrating leukocytes and the VECs reveals that the leukocytes actively contract the VECs to open a junctional gap and then push themselves across the gap by generating strong stresses that push into the matrix. In addition, we found that diapedesis is facilitated when the tension fluctuations in the VEC monolayer were increased by proinflammatory thrombin treatment. Our findings demonstrate that diapedesis can be mechanically regulated by the transmigrating leukocytes and by proinflammatory signals that increase VEC contractility.
The innate immune response involves the extravasation of leukocytes from blood vessels to sites of tissue damage, infection, or inflammation, a process known as diapedesis (1–3). This complex process is regulated spatiotemporally by many receptor molecules in both leukocytes and vascular endothelial cells (VECs). Diapedesis is initiated by the chemotactic activation of leukocytes and VECs in response to cytokines (IL-1 and TNF-α) and chemokines (CXC and IL-8) (4–6). Upon activation, leukocytes bind to the selectin molecules on VECs that facilitate rolling on and adhesion to VEC membranes. The expression of intercellular adhesion molecule-1 (ICAM-1) and VEC adhesion molecule (VCAM-1) on the surface of VECs then triggers the firm adhesion of leukocytes to the VECs via integrins and other transmembrane receptors (1–3). Firm adhesion is followed by transmigration across the VEC monolayer, a process that takes place mainly in postcapillary venules, where the shear stresses due to blood flow are small enough to be withstood by the adhesions between leukocytes and the VECs (7).
Leukocyte transmigration across the vascular endothelium can occur either at VEC junctions (paracellular migration) or through individual VECs (transcellular migration) (3, 8). While the specific factors determining the selection of these routes are not fully understood, in vitro and in vivo experiments have shown that paracellular migration is the preferred route for leukocyte diapedesis, occurring over 90% of the time (9). It has also been shown that diapedesis takes place preferentially at tricellular junctions in vivo or across VEC junctions that have been loosened by chemical or inflammatory mediators (10, 11). Upon firm adhesion, the cross-linking of VEC’s ICAM-1 with leukocyte integrins activates endothelial myosin light chain kinase, leading to actin–myosin fiber contraction in VECs, and the opening of the intercellular junctions (3). These findings suggest that paracellular migration could be the preferred diapedesis route because of the active loosening of the cell–cell junctions in VECs in response to the presence of an adhering leukocyte. However, we cannot rule out the alternative explanation that VEC cell–cell junctions passively provide the locations of reduced mechanical resistance for leukocytes to burrow through. In vitro studies have shown that in both paracellular and transcellular diapedesis clusters of VEC ICAM-1 accumulate around adherent leukocytes, forming specific endothelial “docking structures” which anchor and embrace the leukocytes. These ICAM-1 projections are actin-rich and have been associated with the opening and resealing of junctional gaps that control endothelial permeability during leukocyte diapedesis (12–16). It has also been speculated that these docking structures may facilitate diapedesis by generating 3D contractile stresses that pull the leukocytes into and across the endothelial monolayer (13, 14). These findings have given rise to the hypothesis that diapedesis is initiated by the 3D invasive mechanical forces generated by leukocytes but is mainly driven by the 3D contractile stresses exerted by VECs in response to the presence of adhering leukocytes (10–16).
The above-mentioned studies clarified some aspects of the mechanics of diapedesis, but the specific manner in which leukocytes and VECs interact mechanistically during this process remains largely unknown. There is a paucity of experimental and analytical tools to precisely determine the spatiotemporal evolution of the 3D forces transmitted between leukocytes and VECs during extravasation. Existing measurements of leukocyte traction forces are scarce, and most of them are 2D measurements on flat hydrogels (17–19). So far, the mechanical forces involved in diapedesis have been characterized using endothelial monolayers cultured on micropost arrays (17, 18). These studies revealed that large variations in traction stresses occur immediately underneath the diapedesis site. However, the micropost arrays could not measure the component of force in the direction normal to the VEC monolayer, which is essential in driving the cell invasion (20).
In the present study, we used 3D traction force microscopy (3DTFM) (21) to investigate the role that VECs play in the process of leukocyte diapedesis. We decoupled the forces exerted by leukocytes and VECs by performing experiments where VECs engulf and transport inert particles across the VEC monolayer and by performing experiments where leukocytes invade a substrate in the absence of VECs. Additionally, we manipulate the VEC permeability by using the acute inflammatory mediator thrombin and by activating the endothelial Rho kinase pathway to investigate how inflammatory processes modify the 3D traction stresses needed for leukocyte diapedesis. Our results suggest that upon the firm adhesion of leukocytes to the endothelium, leukocytes generate 3D forces on VECs to first perturb junctional tensions to cause the opening of a gap between adjacent VECs, thus facilitating the subsequent squeezing and invasion through VECs. We show that the 3D traction stresses required for producing this tension-driven opening of VEC’s junctional gaps are highly reduced during inflammation, demonstrating that inflammation effectively softens the physical barrier to leukocyte transmigration, thus facilitating diapedesis.
Materials and Methods
Cell Culture.
Human vascular umbilical endothelial vein cells (VECs) (purchased from Cell Application) were cultured in medium M199 supplemented with 10% (vol/vol) endothelial cell growth medium (Cell Application), 10% (vol/vol) FBS (Lonza), 1% sodium pyruvate, 1% l-glutamine, and 1% penicillin–streptomycin (Gibco) until they formed a confluent monolayer. HL-60 cells (ATCC) were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin. See SI Materials and Methods for additional details on materials and methods.
Results
Dynamics of the Opening of VEC Junctional Gaps in Paracellular Diapedesis.
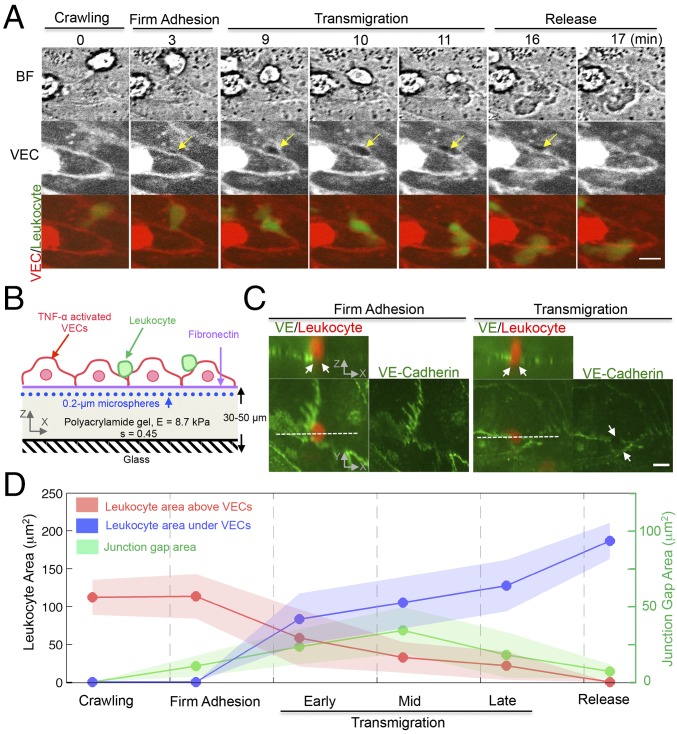
Paracellular diapedesis involves the formation of VEC’s junctional gaps (3). To examine the dynamic interaction of the VEC and the extravasating leukocyte we imaged the VEC junctions and fluorescently labeled leukocytes during diapedesis (Fig. 1 A and B). We analyzed these image sequences by splitting the process into four main sequential stages: (i) crawling, (ii) firm adhesion, (iii) transmigration, and (iv) release. During the crawling stage, the leukocyte adheres to and migrates over the surface of VECs. We used this initial stage as a reference to analyze the mechanical changes taking place during diapedesis. The crawling stage concludes when the leukocyte becomes firmly adhered to a VEC junction. Once the leukocyte positions itself in between two adjacent VECs, the size of the junctional gap increases and the cell begins to transmigrate. The transmigration stage lasts until the leukocyte has completely crossed the monolayer, and this is ensued by a release stage in which the VEC intercellular gap recedes.
Fig. 1.
Three-dimensional endothelial deformation is associated with the remodeling of VEC junctional gaps during leukocyte paracellular diapedesis. (A) Epifluorescent live-cell imaging of VEC junctions stained by the CY5 cell membrane dye and a leukocyte stained by the FITC dye. The yellow arrow indicates the location where an intercellular gap is formed during diapedesis. Merged-color images show the diapedesis stages: crawling, firm adhesion, transmigration, and final release. (Scale bar, 10 μm.) (B) The experimental setup for 3DTFM during leukocyte diapedesis. (C) Three-dimensional confocal images of leukocytes fixed during diapedesis as described in Materials and Methods. Orthogonal (XZ) projection demonstrates a leukocyte is crossing the VEC border. White arrows indicate that a VE-Cadherin gap associated with the extravasating leukocyte. (Scale bar, 10 μm.) (D) Time evolution of the area projection (XY plane) of a leukocyte and VEC junctional gap during diapedesis. Data are representative of three independent experiments with two to three fields in each experiment group. Data are expressed as mean ± SEM.
We imaged the transition between the firm adhesion and transmigration stages using 3D confocal microscopy in fixed immunostained cells (Fig. 1C). These data suggest that there may be appreciable 3D deformations of the endothelium underneath the leukocyte during the firm adhesion stage, even before the VEC–VEC junctional gap is formed. Specifically, VE-Cadherin staining was intact at this site but it shows bending in the cross-sectional plane (Fig. 1C, Left). After the VEC–VEC gap is formed the leukocyte begins to transmigrate while the gap size keeps increasing (Fig. 1 A and C, Right).
We further investigated the dynamics of diapedesis by concurrently measuring the evolution of the projected areas of the leukocyte and the size of the VEC junctional gap (Fig. 1D). These measurements show that the size of the junctional gap increases significantly during the firm adhesion phase, before the leukocyte begins to transmigrate. To better analyze the transmigration phase, we further divided the transmigration phase into early, mid, and late stages, based on the evolution of the projected area of the leukocyte. The early stage was defined as when the leukocyte begins to pass through the VEC’s junctional gap and less than 25% of its body is located below the VECs. The mid and late stages were defined as when ∼50% and 75% of the leukocyte body is below the VECs, respectively. We found that the junctional gap size keeps increasing during early diapedesis, peaks at mid diapedesis, and decreases during late diapedesis. We also observed that the gap remains open from few seconds up to 1 min during the release phase, after the leukocyte has fully transmigrated under the VECs (Fig. 1A). These results suggest the existence of a time lag between the opening of endothelial junctional gaps and the initiation of leukocyte transmigration, and also between the closing of endothelial junctional gaps and the termination of leukocyte transmigration. Importantly, our findings indicate that diapedesis involves 3D contractile traction stresses exerted by both leukocytes and VECs.
Traction Stress Patterns During Leukocyte Diapedesis.
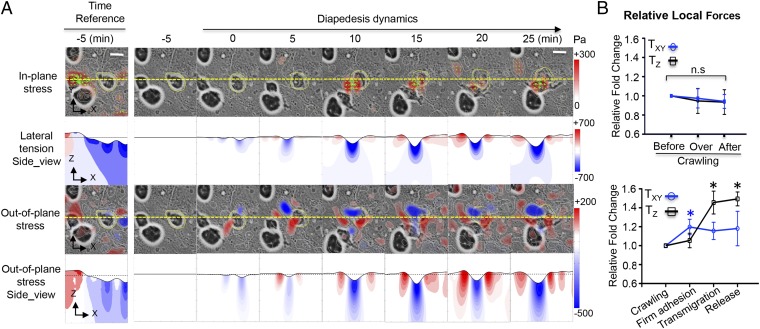
Leukocyte motility during diapedesis involves firm adhesion to the endothelium and the generation of contractile forces that are transmitted to the endothelium and the underlying substrate. These forces together with the contractile forces generated by VECs modify the distribution of intracellular tension in the endothelium, which is associated with endothelial junction integrity and permeability (22, 23). We mapped the traction stresses generated by the VECs and leukocyte on the substrate during the different phases of diapedesis. To study the dynamics of diapedesis we tracked the variations in these stresses and compared them with the corresponding values measured during the crawling stage. The results show that both the in-plane (parallel to the plane of the monolayer) and the out-of-plane (perpendicular to the monolayer) traction stresses changed markedly during diapedesis (Fig. 2A), whereas they remained relatively unaltered during crawling (Fig. S3). The change in in-plane stress vectors during diapedesis showed an inward pattern that colocalized with the transmigrating leukocyte and converged toward the invasion site. The pattern formed by the out-of-plane stresses was colocalized underneath the invading leukocyte and consisted of a central rounded region of downward pushing surrounded by a weaker rim of upward pulling.
Fig. 2.
Spatiotemporal evolution of the 3D stresses during leukocyte crawling, firm adhesion, and diapedesis. (A) Live-cell imaging of a leukocyte transmigrating across VECs at indicated time points. The stress magnitude is represented by pseudocolor maps according to the color bars (pascals). (Scale bar, 10 μm.) The first row shows the in-plane traction stress () overlaid on the bright-field cell image, with the green arrows indicating the orientation of the in-plane stress vector. The second row shows the lateral tension () in an XZ plane section of the matrix that intersects the endothelial monolayer along the yellow dashed line shown in the first row (blue represents lateral compression and red, lateral extension). The third row shows the out-of-plane () traction stresses overlaid on the bright-field cell image (blue represents downward pushing and red, upward pulling). The fourth row shows the vertical stress () in an XZ plane section of the matrix that intersects the endothelial monolayer along the yellow dashed line shown in the third row (blue represents downward pushing and red, upward pulling). In the second and fourth rows the vertical deflection of the substrate has been scaled up by a factor of 4 to aid visualization. The leftmost panel shows the absolute values of stresses and tensions at the end of the crawling stage, and it is used as the reference state for calculating the temporal changes of 3D stresses. (B) Quantification of the relative changes of local 3D stresses in the process of crawling (Upper) and diapedesis (Lower). Data are mean ± SEM of local absolute values of the stresses (blue tangential in-plan; black normal out-of-plane) normalized with the corresponding values measured at the crawling stage. Data are expressed as mean ± SEM. *P < 0.05. Data are representative of six independent experiments.
The temporal evolutions of the in-plane and out-of-plane traction stresses were statistically different (Fig. 2B). Specifically, the in-plane traction stresses (τxz and τyz) increased significantly during the firm adhesion stage and did not vary significantly afterward. However, the out-of-plane stresses (τzz) varied little during the firm adhesion phase but raised significantly during transmigration and remained elevated after the leukocyte completely crossed the monolayer.
Decoupling the Forces Generated by the Transmigrating Leukocytes and the Endothelial Cells During Diapedesis.
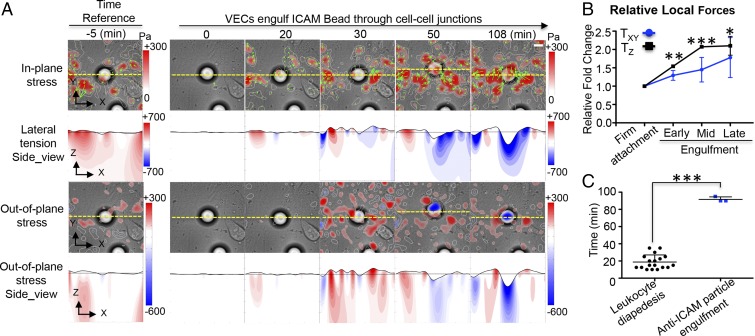
The traction stress patterns observed during diapedesis are similar to those observed in isolated leukocytes invading into 3D matrices without endothelial cells (Fig. S4). This similarity suggests that leukocytes actively contract on the VECs to squeeze through junctional gaps. However, this does not rule out that the measured traction stresses could also be at least in part generated by the VECs. To shed light on this question, we prepared 20-μm-diameter anti-ICAM-coated inert microspheres (SI Materials and Methods) and deposited them onto inflamed VEC monolayers. Remarkably, a substantial fraction (60%) of the coated microspheres became engulfed into the monolayer through openings in VEC junctions (Fig. S5). Given that the microspheres were inert, the engulfment process must have been driven by VEC-generated forces. However, the 3D traction stresses generated by the VECs during microsphere engulfment (Fig. 3A) differ from those observed during leukocyte diapedesis (Fig. 2A). In particular, the stresses are less focused and generate lower substrate compression at the site of the microsphere inclusion. These data confirm that leukocytes do exert significant additional contractile stresses on the VEC monolayer to migrate through it. Consistent with this notion, the in-plane traction stresses increase gradually during microsphere inclusion (Fig. 3B), whereas they increase early and remain elevated during leukocyte diapedesis (Fig. 2B, Lower). The patterns formed by the out-of-plane stresses were similar with those from the leukocyte invasion and diapedesis, where they colocalized underneath the microsphere and consisted of a central rounded region of downward pushing surrounded by a weaker rim of upward pulling (Fig. 3A). Furthermore, the endothelial engulfment of passive microspheres takes significantly longer time than the diapedesis of contractile leukocytes (Fig. 3C).
Fig. 3.
Spatiotemporal evolution of 3D stresses during the engulfment of an anti-ICAM-coated microsphere by an endothelial monolayer. (A) Live imaging of a 20-μm anti-ICAM-coated inert microsphere firmly attached to and engulfed by VECs at indicated time points. Maps showing representative spatial distributions of in-plane stress, out-of-plane stress, and local endothelial tension as describe in Fig. 2A. (Scale bar, 10 μm.) (B) Quantification of the relative changes of the local 3D stresses during the process of endothelial engulfment of anti-ICAM-coated microspheres into the endothelium. (C) The time taken for leukocyte diapedesis and for endothelial engulfment of the inert microspheres. The data are plotted as mean ± SEM of local absolute stress values normalized with the corresponding values measured at the stage of firm attachment. *P < 0.05; **P < 0.01; ***P < 0.001. The data are representative of at least three independent experiments in each case.
Leukocyte Diapedesis Through VEC Monolayers with Altered Contractility and Internal Tension.
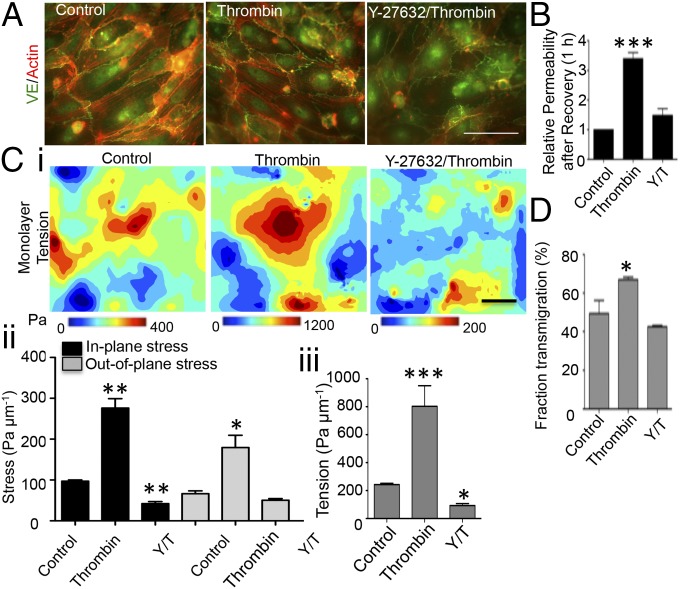
To further investigate the role of VEC mechanical tension in the dynamics of leukocyte diapedesis we performed experiments with VEC monolayers that had been treated with thrombin 1 h before the experiment. Thrombin is known to promote Rho-mediated cell contractility, leading to VEC junction remodeling and increased endothelial permeability (24). Consistent with previously reported data, we observed that thrombin induced formation of thicker stress fibers, temporally localized increases of contractile forces, and permeable intercellular junctions (Fig. 4 A and B and Figs. S6 and S7). Of note, the thrombin-pretreated VECs exerted significantly higher traction stresses than controls, leading to a distribution of monolayer tension that exhibited significantly more pronounced fluctuations (Fig. 4C). Rho kinase inhibitor can repress thrombin-induced cell contractility (24). These changes were reversed after 30 min when the Rho kinase inhibitor Y-27632 treatment was followed by the treatment with thrombin. In addition, the rates of leukocyte diapedesis were significantly higher in thrombin-treated VEC monolayers than in thrombin+Y-27632–treated monolayers and in untreated monolayers (Fig. 4D).
Fig. 4.
Thrombin treatment increases heterogeneity in the distribution of mechanical tension within the VEC monolayer and facilitates leukocyte diapedesis through Rho kinase pathway. (A) F-actin (red) and VE-Cadherin (green) staining of VECs. VECs were preincubated for 30 min in the absence or the presence of 30 μM of Y-27632 and followed for 1 h with the thrombin stimulation. (Scale bar, 50 μm.) (B) Permeability response after the junctional recovery is given as 1 h after thrombin treatment. T, thrombin; Y/T, Y-27632/thrombin. (C) Three-dimensional traction stresses and mechanical tension within the monolayer. (i) Maps showing representative spatial distributions of monolayer tension. (Scale bar, 50 μm.) (ii) Quantification of 3D stresses. (iii) Quantification of monolayer tensions. (D) Quantification of leukocyte diapedesis rates at the end point of the diapedesis assay. Bars represent average fraction of transmigrated cells. The data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. The data are representative of four independent experiments with more than 100 cells per group.
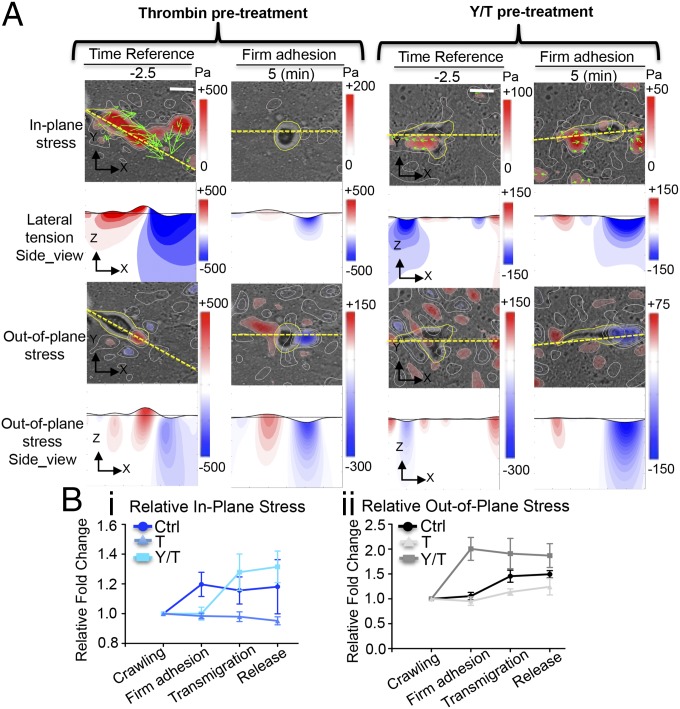
Overall, our data suggest that endothelial monolayer permeability is enhanced by localized transient changes in the intracellular tension of the VEC, and that these changes can be elicited by both the contractility of VECs and the external forces applied by the transmigrating leukocytes. The dynamics of the 3D traction stresses measured during transmigration of leukocytes through VECs pretreated with thrombin and with thrombin+Y-27632 agree with this notion. In thrombin-treated endothelia with enhanced VEC contractility, the interaction between the transmigrating leukocytes and VECs caused small changes in the in-plane traction stresses and the lateral axial stress of the substrate, compared with the already elevated values of these variables before diapedesis (Fig. 5). Furthermore, the stress vectors did not form an inward pattern convergent toward the center of the invading leukocyte as observed in control conditions (Fig. 2A). Instead, the pattern formed by these vectors was similar to that measured around monolayer gaps in thrombin-treated VEC monolayers without invading leukocytes (Fig. S6D). However, in the thrombin+Y-27632–treated endothelium (Fig. 5A), the variations of the in-plane stresses during diapedesis exhibited a traction stress vector pattern more similar to that found in untreated endothelium, although it occurred at a later stage (Fig. 5B). These results suggest that VEC contractility played a less important role in opening gaps in these monolayers. The out-of-plane stresses measured on the substrate increased during diapedesis and showed approximately the same pattern for all cases studied. The results indicate that these stresses originated mainly by the substrate indentation caused by the invading leukocyte. Consistent with this notion, these stresses underwent earlier and larger relative changes for leukocytes invading through thrombin+Y-27632–treated monolayers, whereas the opposite occurred for leukocytes invading through thrombin-treated monolayers.
Fig. 5.
Spatiotemporal evolution of the 3D stresses during diapedesis across thrombin-treated VEC monolayers, and across monolayers treated with thrombin plus Rho kinase inhibitor. Live-cell images and stress maps of a leukocyte crawling and firmly adhered on VEC monolayers pretreated with thrombin and thrombin+Y-27632 (A). (Scale bar, 10 μm.) (B) Quantification of the relative changes of local 3D stresses in the process diapedesis of the control, thrombin, and Y/T groups. The data are plotted as mean ± SEM of local absolute stress values normalized to the crawling stage. Each data point is representative of at least five independent experiments.
Discussion
In vitro and in vivo studies have shown that paracellular diapedesis is the predominant extravasation route for leukocytes (10). Paracellular diapedesis requires the generation of a junctional gap between neighboring VECs to open up a passage through which leukocytes can transmigrate. Adhesion of leukocytes to VECs triggers the activation of signaling molecules that mediate the disassembly of cadherin/catenin complexes at the VEC junctions (3). Endothelial ICAM-1 triggers the formation of an actin-rich docking structure, and subsequently the migrating leukocyte inserts a pseudopod through the junctional gap, which allows transmigration while minimizing plasma leakage (25–27). Previous 2D TFM studies have suggested that acto-myosin contractile forces could play an important role in leukocyte diapedesis (13, 14). However, little is known about how these forces physically pull the cell junctions apart and drive the transmigration of the leukocyte. Particularly, it is unclear whether junctional gaps are mediated by VEC contraction or by the invading protrusions from leukocytes. Inhibition of leukocyte contractility has been shown to decrease diapedesis efficiency; however, leukocytes are still able to invade between neighboring VECs with deficiencies only in the retraction of their tails for completing diapedesis (28). Furthermore, diapedesis is inherently dynamical and 3D, and there is a scarcity of 3D time-resolved data about the mechanics of this process.
Using 3DTFM (21) in a realistic in vitro setup, we simultaneously quantified the evolution of the 3D mechanical forces, cell position, and cell shape during leukocyte diapedesis. These data showed that a junctional gap was formed before the formation of an invading leukocyte protrusion (Fig. 1 A and D). Also before transmigration, the in-plane traction stresses under the VEC monolayer increased significantly at the invasion site, showing a vector pattern that was directed inward toward the junction and locally compressed the endothelium (Fig. 2). We posit that this transient change in endothelial mechanical loading could induce a local perturbation of intracellular tensions (29) to cause a rearrangement of F-actin at cell–cell junctions that promotes the disruption of the junctional linkage (30). In comparison, we did not observe dynamical changes in traction stresses when a leukocyte was crawling over the endothelium without initiating the invasion process (Fig. 2B and Fig. S3).
In the later stages of diapedesis the traction stress pattern became more 3D with a large out-of-plane component. The stresses pulled away from the matrix at several locations along the periphery of the leukocyte, while pushing downward into the matrix at one single location under its center. Given that all of the forces must be in equilibrium, this stress pattern considerably amplifies the magnitude of the downward-pushing stresses, a mechanism that had been previously observed for invasive cancer cells and named “stress focusing” (20). We also found that the monolayer gaps resealed by contractile VEC stresses after the leukocyte completely passed through the gap and became positioned underneath the VEC monolayer (Fig. 1 A and D). Similar outward traction stresses have been previously reported for closing gaps by other investigators (31). The observation of inward-directed cell motion and outward-directed traction suggests that these stresses are not caused by the relative sliding motion between the monolayer and the substrate. We note that this traction stress distribution is associated with positive hoop (purse string) tension inside the monolayer, which could provide a mechanism for gap closure. These data are consistent with previous speculations that the generation of VEC contractile forces and VEC membrane mobility are not only necessary for the formation of junctional gaps (the firm adhesion stage) but also for the maintenance of the barrier integrity during leukocyte diapedesis (the transmigration stage) (26, 32).
The dynamical changes in 3D stresses we observed during diapedesis had the same pattern we measured for leukocytes invading into ECM, suggesting that the leukocytes actively generate mechanical forces to open junctional gaps and transmigrate across the endothelium. Consistent with this idea, we observed that the endothelial engulfment of micrometer-sized, anti-ICAM-coated inert particles required significantly longer times than leukocyte diapedesis (Fig. 3C). Engagement of ICAM-1 clusters has been shown to trigger VEC Rho signaling and induce traction stress generation (14, 25, 33), and we did measure a significant increase in traction stress during endothelial engulfment of anti-ICAM-coated particles (Fig. 3). Altogether, these findings point to the importance of both leukocyte contraction and VEC contraction as regulators of endothelial permeability during diapedesis. Applying mechanical forces on ICAM-1 clusters has been shown to enhance VEC Rho activation and MLC phosphorylation (33), which suggests that the contractile forces exerted by VECs and leukocytes could be orchestrated to facilitate diapedesis using ICAM-1 as a force sensor.
Leukocytes migrate through VECs at sites where endothelial junctions are looser, suggesting that the biomechanical properties of the VEC monolayer are a crucial determinant for diapedesis (10, 11). In vivo, leukocyte transmigration increases significantly at converging venular regions, where VECs morphologically differ from straight regions and form more tricellular junctions. These observations have been the basis for the hypothesis that leukocytes follow the path of least resistance during diapedesis. Motivated by this hypothesis, we conducted a series of experiments with VECs treated with thrombin to alter their junctional integrity. Thrombin causes endothelial barrier disruption by inducing fast acto-myosin contractility and large paracellular gaps (34). The endothelial junctions remained unstable and loose even after these gaps were resealed, causing a prolonged increase in endothelial permeability and leukocyte diapedesis rate (Fig. 4 B and D and Figs. S6 and S7). This state was associated with higher traction stresses and more fluctuations in the intracellular mechanical tensions of the VECs, consistent with previous findings (35–37). Reducing acto-myosin contractility by Rho kinase inhibition reversed the thrombin-induced increases in endothelial tension fluctuations, endothelial permeability, and leukocyte diapedesis rate (Fig. 4).
When leukocytes were transmigrating through thrombin-treated VECs there were no appreciable dynamical changes of the in-plane traction stresses (Fig. 5 and Fig. S8), suggesting that unstable VEC junctions permit leukocytes to traverse the endothelium without exerting contractile forces on the endothelium to modulate its mechanical tension. In addition, the out-of-plane stress increased significantly only after diapedesis (Fig. 5 and Fig. S8), presumably when the leukocyte was directly in contact with the matrix and possibly being pushed down by the endothelial monolayer, which was resealing above it. Consonantly, inhibition of Rho kinase in the VECs restored the dynamics of the process and the 3D stress patterns measured in control conditions (Fig. 5 and Fig. S8). Overall, the behaviors observed in different conditions of endothelial permeability provide evidence that leukocytes may choose the path of least physical resistance when crossing the VEC monolayer.
In summary, applying 3DTFM in an in vitro model of leukocyte transendothelial migration, we have decoupled the forces exerted by the transmigrating leukocytes and the VECs during diapedesis. We have demonstrated that the 3D contractile stresses exerted by the orchestrated interaction between leukocyte and VEC causes the disassembly of cell–cell junctions before the initiation of leukocyte invasion into the endothelium. We have shown that after their firm adhesion to VEC the leukocytes actively contract before the opening VEC’s junctional gaps and subsequently thrust themselves through by generating a pattern of contraction that amplifies the magnitude of the stresses that push into the matrix at the invasion site. Furthermore, we found that diapedesis can be separately facilitated when the tension fluctuations in the VEC monolayer were increased by thrombin treatment. These findings provide evidence for the complementary interplay between transmigrating leukocytes and VECs in the well-orchestrated process of transendothelial migration. The leukocytes play an initiating role in starting the diapedesis, while the VECs play a concluding role in closing the junctional gaps.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants GM084227 (to J.C.L. and J.C.d.Á.), HL108735, HL121365, and HL106579 (to S.C.); the dragon-gate project MOST-106-2633-B-009-001/105-2321-B-400-007 (J.-J.C.); and American Heart Association Grant 17POST33460467 (to Y.-T.Y.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717489115/-/DCSupplemental.
References
- 1.Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol. 2014;184:886–896. doi: 10.1016/j.ajpath.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 4.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellicome SM, et al. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990;144:2558–2565. [PubMed] [Google Scholar]
- 6.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 7.Nourshargh S, Marelli-Berg FM. Transmigration through venular walls: A key regulator of leukocyte phenotype and function. Trends Immunol. 2005;26:157–165. doi: 10.1016/j.it.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodfin A, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns AR, et al. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol. 1997;159:2893–2903. [PubMed] [Google Scholar]
- 11.Martinelli R, et al. Probing the biomechanical contribution of the endothelium to lymphocyte migration: Diapedesis by the path of least resistance. J Cell Sci. 2014;127:3720–3734. doi: 10.1242/jcs.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 13.Rabodzey A, Alcaide P, Luscinskas FW, Ladoux B. Mechanical forces induced by the transendothelial migration of human neutrophils. Biophys J. 2008;95:1428–1438. doi: 10.1529/biophysj.107.119156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Sniadecki NJ, Chen CS. Mechanical forces in endothelial cells during firm adhesion and early transmigration of human monocytes. Cell Mol Bioeng. 2010;3:50–59. doi: 10.1007/s12195-010-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One. 2008;3:e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petri B, et al. Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood. 2011;117:942–952. doi: 10.1182/blood-2010-02-270561. [DOI] [PubMed] [Google Scholar]
- 17.Smith LA, Aranda-Espinoza H, Haun JB, Dembo M, Hammer DA. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys J. 2007;92:L58–L60. doi: 10.1529/biophysj.106.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakes PW, et al. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–1395. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yip AK, Chiam KH, Matsudaira P. Traction stress analysis and modeling reveal that amoeboid migration in confined spaces is accompanied by expansive forces and requires the structural integrity of the membrane-cortex interactions. Integr Biol. 2015;7:1196–1211. doi: 10.1039/c4ib00245h. [DOI] [PubMed] [Google Scholar]
- 20.Aung A, et al. 3D traction stresses activate protease-dependent invasion of cancer cells. Biophys J. 2014;107:2528–2537. doi: 10.1016/j.bpj.2014.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Álamo JC, et al. Three-dimensional quantification of cellular traction forces and mechanosensing of thin substrata by fourier traction force microscopy. PLoS One. 2013;8:e69850. doi: 10.1371/journal.pone.0069850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazellières E, et al. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbajal JM, Gratrix ML, Yu CH, Schaeffer RC., Jr ROCK mediates thrombin’s endothelial barrier dysfunction. Am J Physiol Cell Physiol. 2000;279:C195–C204. doi: 10.1152/ajpcell.2000.279.1.C195. [DOI] [PubMed] [Google Scholar]
- 25.van Buul JD, et al. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heemskerk N, et al. F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signalling. Nat Commun. 2016;7:10493. doi: 10.1038/ncomms10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goswami D, Vestweber D. How leukocytes trigger opening and sealing of gaps in the endothelial barrier. F1000 Res. 2016;5:2321. doi: 10.12688/f1000research.9185.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroka KM, Hayenga HN, Aranda-Espinoza H. Human neutrophil cytoskeletal dynamics and contractility actively contribute to trans-endothelial migration. PLoS One. 2013;8:e61377. doi: 10.1371/journal.pone.0061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hur SS, et al. Roles of cell confluency and fluid shear in 3-dimensional intracellular forces in endothelial cells. Proc Natl Acad Sci USA. 2012;109:11110–11115. doi: 10.1073/pnas.1207326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowotarski SH, Peifer M. Cell biology: A tense but good day for actin at cell-cell junctions. Curr Biol. 2014;24:R688–R690. doi: 10.1016/j.cub.2014.06.063. [DOI] [PubMed] [Google Scholar]
- 31.Kocgozlu L, et al. Epithelial cell packing induces distinct modes of cell extrusions. Curr Biol. 2016;26:2942–2950. doi: 10.1016/j.cub.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinelli R, et al. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. J Cell Biol. 2013;201:449–465. doi: 10.1083/jcb.201209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lessey-Morillon EC, et al. The RhoA guanine nucleotide exchange factor, LARG, mediates ICAM-1-dependent mechanotransduction in endothelial cells to stimulate transendothelial migration. J Immunol. 2014;192:3390–3398. doi: 10.4049/jimmunol.1302525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huveneers S, et al. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavara N, et al. Thrombin-induced contraction in alveolar epithelial cells probed by traction microscopy. J Appl Physiol (1985) 2006;101:512–520. doi: 10.1152/japplphysiol.00185.2006. [DOI] [PubMed] [Google Scholar]
- 36.Valent ET, van Nieuw Amerongen GP, van Hinsbergh VW, Hordijk PL. Traction force dynamics predict gap formation in activated endothelium. Exp Cell Res. 2016;347:161–170. doi: 10.1016/j.yexcr.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Beckers CM, et al. ROCK2 primes the endothelium for vascular hyperpermeability responses by raising baseline junctional tension. Vascul Pharmacol. 2015;70:45–54. doi: 10.1016/j.vph.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.