Phosphorus (P) is required by all living organisms for the synthesis of genetic and cellular components, metabolism, and energy transfer. The phosphate ion (PO43-, P valence +5), also referred to as inorganic phosphate (Pi), is typically the most easily assimilated P species but is also a limiting nutrient in many ecosystems. Microorganisms can also obtain Pi from inorganic and organic forms of reduced P compounds (P valence +1, +2, +3), including phosphonates (organophosphorus compounds with a direct C-P bond), phosphite (HPO32-), and hypophosphite (1). Reduced P compounds have long been known to exist in the environment, although only recently has it become clear that these compounds make up a significant proportion of the P available to microbial organisms (2–4). DNA sequencing studies have revealed the corresponding enormous diversity of microorganisms that can utilize or synthesize reduced P compounds (5, 6). Therefore, the microbial transformations of reduced P compounds are expected to have previously unrecognized roles in the ecology and biogeochemistry of ecosystems. Figueroa et al. (7) explore how microorganisms in an anoxic environment obtain all of the energy necessary for growth by oxidizing phosphite to Pi, thereby contributing to the production of new biomass and to the P redox cycle. Their findings contribute to emerging evidence that the P redox cycle is an intrinsic part of the microbial cycling of carbon, energy, and other nutrients.
In oceanic waters, P redox pathways are an active component of the P cycle (8) and are of primary importance because they supply additional P to organisms when Pi is scarce (9, 10). In fact, two of the most widespread and abundant photosynthetic microorganisms in nutrient-poor oceanic waters, Prochlorococcus and Trichodesmium, possess genes to supplement their P requirements by oxidizing phosphite (11, 12) in a process known as assimilatory phosphite oxidation (APO). Figueroa et al. (7) investigate a phosphite-oxidizing bacterium inhabiting a drastically different environment, the anaerobic sludge in waste-water treatment plants. Unlike APO-performing organisms, the newly discovered organism (Candidatus Phosphitivorax anaerolimi) depends completely on phosphite oxidation, not only to obtain P, but to generate sufficient energy to power cellular growth, a metabolic strategy known as dissimilatory phosphite oxidation (DPO).
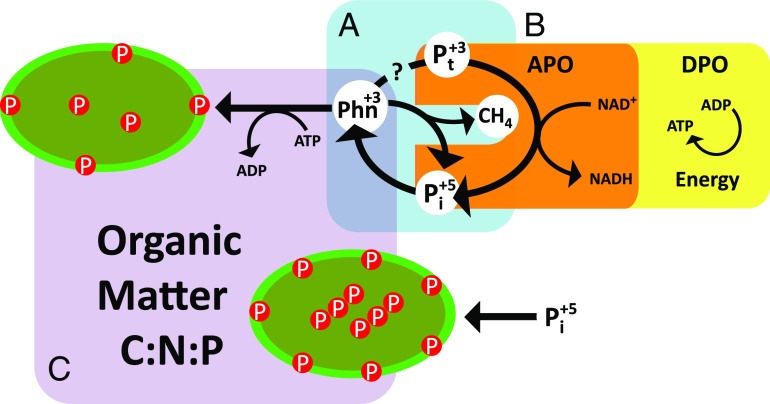
A key feature that distinguishes DPO from APO is the ability to conserve the energy derived from phosphite (Fig. 1). The organism investigated by Figueroa et al. (7) draws electrons from phosphite to reduce CO2, and in this way fix carbon into new biomass. However, Candidatus Phosphitivorax anaerolimi lacked a known biochemical pathway for carbon fixation and it was unclear how it could conserve the energy obtained by oxidizing phosphite to sustain growth. DNA sequencing revealed the presence of all of the genes necessary to complete a potentially novel CO2 fixation pathway (13) and a viable mechanism by which the organism can harness energy efficiently, namely through the regeneration of electron carriers and the production of ATP, the energy currency of the cell (Fig. 1). These findings illustrate the potential of yet undiscovered niches and environments in which microbial metabolism may be solely reliant on P redox reactions.
Fig. 1.
Links between P redox metabolism and the biogeochemistry of organic matter. Phosphonates (Phn) play a central role in the P redox cycle. (A) Microorganisms obtain phosphate (Pi) or potentially phosphite (Pt), from the degradation of phosphonates in organic matter. The degradation of phosphonates can produce hydrocarbons—methylphosphonate produces methane, for example—that can be oxidized or assimilated by some organisms. (B) Phosphite derived from phosphonate metabolism or geochemical processes can be used during DPO or APO. DPO organisms conserve energy in the form of NADH and ATP to drive cellular growth. APO organisms expend ATP to import phosphite but may partially conserve some of this energy by regenerating NADH. Phosphonate-degrading organisms also invest ATP to transport phosphonates into the cell. (C) The energetic balance of reduced P metabolism may result in changes in the nutrient content of biomass compared with growth primarily on phosphate.
Only one other DPO-capable organism has been identified so far (14). This organism can couple DPO to nitrate and sulfate reduction, in addition to CO2 reduction (14, 15). This metabolic versatility may allow DPO-performing organisms to occupy diverse niches. DPO may be prevalent in low-energy environments lacking terminal electron acceptors and with a stable supply of phosphite (16). The membrane proteins that import phosphite into cells operate with such high affinity (17) that they may allow organisms to scavenge sufficient phosphite to perform DPO (or APO for that matter), even when phosphite is present at low concentrations. Among the environments where DPO may occur are the deep sea and subseafloor basaltic crust, where darkness and low inputs of organic matter predominate, and where phosphite may be generated through the reduction of Pi or P-containing minerals during hydrothermal circulation (16). Oxygen-depleted marine regions may also promote DPO because nitrate becomes the predominant electron acceptor and phosphite may be produced during organic matter remineralization. DPO may also proliferate in marine and coastal sediments where phosphite may accumulate due to the biological decomposition of organic matter or industrial and agricultural activities (16). As Figueroa et al. (7, 16) point out, the possibility of phosphite oxidation driving cellular growth may have important implications on primary production and carbon cycling.
The cycling of phosphonates demonstrates how P redox reactions can have diverse ramifications in the biogeochemistry of organic matter and the ecology of microbial communities (Fig. 1). In the ocean, for example, molecular evidence suggests that phosphonates in organic matter are utilized at the same rate as organic phosphate esters, even at depths where Pi is abundant (3). The active cycling of phosphonates therein and evidence that phosphonate metabolism may produce phosphite (2, 18) makes it plausible that phosphonates are an important source of phosphite to microorganisms. Some phosphonates are also metabolized for their reduced carbon (1), thus contributing to the turnover of organic matter. In addition, alkyl phosphonates cleaved by the phosphonate-degrading enzyme C-P lyase release hydrocarbons (1) that can serve as a source of carbon and energy to microorganisms or, in the case of methylphosphonate, contribute to methanogenesis in marine surface waters (4, 19). Finally, microorganisms growing on phosphonates can suffer changes in the composition of their biomass, as was the case for Trichodesmium cultures, which accumulated less P in their cells compared with cultures grown with Pi or phosphate esters (20). Therefore, phosphonate metabolism can sustain an active P redox cycle with energetic and metabolic implications for microbial communities other than simply supplying P for growth and can also alter greenhouse gas balance and the nutrient content of organic matter available to consumers higher in the food web (Fig. 1).
The Figueroa et al. (7) findings expand the role of phosphorus redox chemistry in microbial life and should motivate research on the ecology and bioenergetics of DPO and other reduced P metabolisms in nature. Cultivation-independent methods based on DNA sequencing of environmental samples have proved to be a powerful tool in dissecting these pathways (7, 15). Additional work is needed to identify the sources and inventories of reduced P compounds in natural environments, especially of phosphite in the ocean. Elucidating these and other aspects of P redox metabolism will be critical to fully understand the role of P in the ecology of microorganisms.
Supplementary Material
Footnotes
The author declares no conflict of interest.
See companion article on page E92.
References
- 1.White AK, Metcalf WW. Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol. 2007;61:379–400. doi: 10.1146/annurev.micro.61.080706.093357. [DOI] [PubMed] [Google Scholar]
- 2.Pasek MA, Sampson JM, Atlas Z. Redox chemistry in the phosphorus biogeochemical cycle. Proc Natl Acad Sci USA. 2014;111:15468–15473. doi: 10.1073/pnas.1408134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark LL, Ingall ED, Benner R. Marine phosphorus is selectively remineralized. Nature. 1998;393:426. [Google Scholar]
- 4.Repeta DJ, Ferrón S, Sosa OA, DeLong EF, Karl DM. Marine methane paradox explained by bacterial degradation of dissolved organic matter. Nat Geosci. 2016;9:884–887. [Google Scholar]
- 5.Yu X, et al. Diversity and abundance of phosphonate biosynthetic genes in nature. Proc Natl Acad Sci USA. 2013;110:20759–20764. doi: 10.1073/pnas.1315107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarreal-Chiu JF, Quinn JP, McGrath JW. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front Microbiol. 2012;3:19. doi: 10.3389/fmicb.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa IA, et al. Metagenomics-guided analysis of microbial chemolithoautotrophic phosphite oxidation yields evidence of a seventh natural CO2 fixation pathway. Proc Natl Acad Sci USA. 2017;115:E92–E101. doi: 10.1073/pnas.1715549114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Mooy BAS, et al. Phosphorus cycling. Major role of planktonic phosphate reduction in the marine phosphorus redox cycle. Science. 2015;348:783–785. doi: 10.1126/science.aaa8181. [DOI] [PubMed] [Google Scholar]
- 9.Dyhrman ST, Ammerman JW, Van Mooy BAS. Microbes and marine phosphorus cycle. Oceanography (Wash DC) 2007;20:110–116. [Google Scholar]
- 10.Karl DM. Microbially mediated transformations of phosphorus in the sea: New views of an old cycle. Annu Rev Mar Sci. 2014;6:279–337. doi: 10.1146/annurev-marine-010213-135046. [DOI] [PubMed] [Google Scholar]
- 11.Martínez A, Osburne MS, Sharma AK, DeLong EF, Chisholm SW. Phosphite utilization by the marine picocyanobacterium Prochlorococcus MIT9301. Environ Microbiol. 2012;14:1363–1377. doi: 10.1111/j.1462-2920.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 12.Polyviou D, Hitchcock A, Baylay AJ, Moore CM, Bibby TS. Phosphite utilization by the globally important marine diazotroph Trichodesmium. Environ Microbiol Rep. 2015;7:824–830. doi: 10.1111/1758-2229.12308. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Even A, Noor E, Flamholz A, Milo R. Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes. Biochim Biophys Acta. 2013;1827:1039–1047. doi: 10.1016/j.bbabio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Schink B, Friedrich M. Phosphite oxidation by sulphate reduction. Nature. 2000;406:37. doi: 10.1038/35017644. [DOI] [PubMed] [Google Scholar]
- 15.Poehlein A, Daniel R, Schink B, Simeonova DD. Life based on phosphite: A genome-guided analysis of Desulfotignum phosphitoxidans. BMC Genomics. 2013;14:753. doi: 10.1186/1471-2164-14-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueroa IA, Coates JD. Microbial phosphite oxidation and its potential role in the global phosphorus and carbon cycles. Adv Appl Microbiol. 2017;98:93–117. doi: 10.1016/bs.aambs.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Bisson C, et al. The molecular basis of phosphite and hypophosphite recognition by ABC-transporters. Nat Commun. 2017;8:1746. doi: 10.1038/s41467-017-01226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren S, Williams MR. The acid-catalysed decarboxilation of phosphonoformic acid. J Chem Soc B. 1971;0:618–621. [Google Scholar]
- 19.Karl DM, et al. Aerobic production of methane in the sea. Nat Geosci. 2008;1:473–478. [Google Scholar]
- 20.White AE, Karl DM, Björkman KM, Beversdorf LJ, Letelier RM. Production of organic matter by Trichodesmium IMS101 as a function of phosphorus source. Limnol Oceanogr. 2010;55:1755–1767. [Google Scholar]