Abstract
Background:
Diabetic nephropathy (DN) is the most common and serious microvascular complication of diabetes. To date, the gold standard for identifying DN and nondiabetic renal disease (NDRD) is a renal biopsy; however, there is currently no reliable diagnostic marker to identify DN and NDRD in a noninvasive manner. This study aimed to investigate the different glycopatterns in urine specimens of DN patients and NDRD patients for a differential diagnosis.
Methods:
In total, 19 DN patients and 18 NDRD patients who underwent renal biopsies between March 2015 and March 2016 at the Chinese People's Liberation Army General Hospital were enrolled in this study. A lectin microarray was used to investigate the glycopatterns in the urinary protein of the 37 patients. Ratio analysis and one-way analysis of variance were used to screen altered glycopatterns. Then, the altered glycopatterns between the DN and NDRD groups were verified by a urinary protein microarray among another 32 patients (15 with DN and 17 with NDRD), and receiver operating characteristic (ROC) curve analysis was used to determine the diagnostic value of the altered glycopatterns in differentiating DN and NDRD. Finally, lectin blotting was used to evaluate the altered glycosylation in protein level.
Results:
The result of lectin microarrays revealed that the relative abundance of the (β-1,4)-linked N-acetyl-D-glucosamine (GlcNAc) recognized by lectin Datura stramonium agglutinin (DSA) was significantly higher in urinary protein in DN patients than that in NDRD patients (fold change >1.50, P < 0.001). Subsequently, the results of urinary protein microarrays were consistent with lectin microarrays (P < 0.05). Furthermore, the ROC curve showed that glycopatterns could effectively distinguish DN from NDRD patients (area under the ROC curve = 0.94, P < 0.001). DSA lectin blotting showed that glycoproteins, with a molecular weight of approximately 50,000, demonstrated a difference in urine samples between DN patients and NDRD patients.
Conclusions:
The relative abundance of (β-1,4)-linked GlcNAc recognized by lectin DSA and urinary glycoprotein with a molecular weight of approximately 50,000 are significantly different between DN and NDRD patients, indicating that the glycopatterns could be used as potential biomarkers for a differential diagnosis.
Keywords: Diabetic Nephropathy, Glycopattern, Nondiabetic Renal Disease, Urinary Protein
INTRODUCTION
Diabetic nephropathy (DN) is a common microvascular complication that results from diabetes. The main pathological manifestation of DN is an increase in glomerular and renal tubular extracellular matrix proteins; this increase leads to glomerular mesangial proliferation and fibrosis.[1] DN is a risk factor for death in the maintenance of hemodialysis patients and increases the risk of death in patients with type 2 diabetes. Nondiabetic renal disease (NDRD) refers to diabetes with other kidney diseases, such as IgA nephropathy (IgAN) or membranous nephropathy (MN). The pathogenesis, clinical manifestations, treatment, and prognosis are different between DN and NDRD. However, to our knowledge, renal biopsy is still the gold standard to differentiate DN from NDRD.[2,3,4,5] In consideration of some disadvantages such as invasiveness, it is very important to discover a new noninvasive diagnostic indicator. Protein glycosylation is an important posttranslational modification that occurs predominantly in the endoplasmic reticulum and the Golgi apparatus. Abnormal glycosylation is also known to be associated with a variety of diseases, such as cancer, inflammation, and neurotransmitter diseases.[6,7] In 2016, Suzuki et al.[8] analyzed the glycan profile of urinary protein and showed that the expression of IgA1 was elevated in IgAN patients with galactose deficiency and that urinary Gd-IgA1 levels were associated with proteinuria. The glycopattern of (β-1,4)-linked N-acetyl-D-glucosamine (GlcNAc) identified by Datura stramonium agglutinin (DSA) commonly emerged in N-glycan. In 2010, Abbott et al.[9,10] reported that the expression of this glycopattern was elevated in ovarian cancer tissue compared to normal mouse ovary. As reported by Saravanan et al.,[11] the expression level of DSL-reactive glycoprotein was upregulated in healed corneas compared to the normal controls. Those findings suggested that the altered glycopattern was associated with the tumor or inflammatory microenvironment. However, little is known about the expression of this glycopattern in urinary protein among patients with DN or NDRD. This study aimed to investigate the altered glycopatterns in urine specimens between patients with DN and NDRD for a differential diagnosis.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Chinese People's Liberation Army General Hospital (No. S2014-012-01). Informed written consent was obtained from all patients prior to their enrollment in this study.
Subjects
Patients who underwent renal biopsies from March 2015 to March 2016 at the Chinese People's Liberation Army General Hospital were included in the study. The renal biopsy standard was consistent with the guideline for the 2008 Kidney Disease Outcomes Quality Initiative guidelines; the guidelines include the following clinical manifestations for suspected NDRD patients: naked eye/microscopic hematuria, elevated serum creatinine not accompanied by significant proteinuria, persistent large amounts of proteinuria with normal renal function, and no diabetic retinopathy. Patients who had a renal biopsy analysis following the criteria above were screened according to the following inclusion/exclusion criteria. Inclusion criteria were as follows: age between 20 and 70 years of age; diagnosis of type 2 diabetes and persistent urinary protein positivity (urinary micro albumin excretion rate ≥300 mg/24 h or urinary protein excretion rate ≥500 mg/24 h at least twice, and excluding urinary tract infection); serum creatinine <442 μmol/L; and voluntary acceptance of renal biopsy. Exclusion criteria were as follows: diagnosis of kidney disease prior to diagnosis as type 2 diabetes; can be diagnosed as NDRD clinically, including lupus nephritis, Henoch-Schonlein purpura nephritis, family hereditary nephropathy, such as autosomal dominant polycystic kidney disease; and unclear pathological diagnosis. At least two pathology experts and two nephrology doctors were asked to make a diagnosis using renal histopathology. To investigate whether urinary glycoproteins change with the progression of DN, we further divided the DN group into two subgroups according to estimated glomerular filtration rate (eGFR) levels. eGFR is calculated from the modified kidney disease (MDRD) formula. The DN I group is defined as eGFR ≥60 ml·min–1·1.73 m–2, while the DN II group is defined as eGFR <60 ml·min–1·1.73 m–2. In total, 19 patients with DN (nine patients in DN Group I and 10 in DN Group II) and 18 patients with NDRD (10 patients with membranous nephropathy and 8 with IgA nephropathy) were used in the study. The basic clinical information of these patients is shown in Table 1.
Table 1.
Detailed clinical information for the DN and NDRD patients used for lectin microarray analysis
| Characteristics | DN (n = 19) | NDRD (n = 18) | ||
|---|---|---|---|---|
| DN group I (n = 9) | DN group II (n = 10) | MN (n = 10) | IgAN (n = 8) | |
| Age (years) | 55.1 ± 8.4 | 60.9 ± 7.7 | 59.6 ± 5.6 | 51.0 ± 6.3 |
| Sex (male/female) | 5/4 | 5/5 | 6/4 | 5/3 |
| BMI (kg/cm2) | 24.3 ± 3.8 | 24.7 ± 2.3 | 24.6 ± 3.1 | 25.4 ± 1.5 |
| Duration of diabetes (months)* | 139.8 (128.1) | 146.6 (143.2) | 58.5 (97.8) | 51.0 (98.3) |
| HbA1c (%) | 7.0 ± 1.1 | 7.3 ± 1.3 | 7.2 ± 2.0 | 6.9 ± 1.4 |
| Glucose (mmol/L) | 7.9 ± 1.5 | 8.0 ± 1.4 | 7.3 ± 2.5 | 7.1 ± 1.5 |
| Systolic blood pressure (mmHg) | 141.3 ± 15.4 | 143.2 ± 20.4 | 131.2 ± 21.6 | 133.4 ± 19.8 |
| Diastolic blood pressure (mmHg) | 87.3 ± 11.4 | 85.2 ± 12.5 | 76.7 ± 9.7 | 77.8 ± 8.7 |
| Urinary protein (g/24 h) | 1.9 ± 1.2 | 3.2 ± 2.0 | 3.0 ± 1.7 | 1.7 ± 1.0 |
| Blood urea nitrogen (mmol/L)* | 6.5 (3.2) | 9.3 (4.3) | 6.2 (2.3) | 6.7 (3.9) |
| Creatinine (µmol/L)* | 88.1 (34.2) | 134.1 (30.3) | 63.0 (43.9) | 97.8 (111.0) |
| eGFR (ml·min−1·1.73 m−2)* | 75.5 (26.7) | 41.7 (13.3) | 104.4 (32.8) | 78.8 (46.8) |
Variables following normal distribution are expressed with mean ± SD. *Variables following nonnormal distribution are expressed with the median (interquartile range). NDRD: Nondiabetic renal disease; DN: Diabetic nephropathy; SD: Standard deviation; eGFR: Estimated glomerular filtration rate; BMI: Body mass index; MN: Membranous nephropathy; IgAN: IgA nephropathy; HbA1c: Glycated hemoglobin.
Sample processing and preparation of the lectin microarray
First, a 15-ml midstream of the patients’ morning urine was collected. The urine sample was centrifuged at 5000 ×g at 4°C for 15 min. The precipitate was discarded, and the supernatant was collected following the precipitation of urine protein using the trichloroacetic acid/acetone precipitation method.[12] After centrifugation, the supernatant was mixed with trichloroacetic acid/acetone (1:9, v/v) and stored overnight at −80°C, followed by collection of the precipitate after centrifugation at 10,000 ×g and 4°C for 15 min. The precipitate was resuspended in precold acetone, and the sample was centrifuged at 10,000 ×g for 10 min; then, the supernatant was discarded, and the precipitate was placed on ice to volatilize the acetone. Subsequently, the precipitate was resuspended in PBST buffer containing 0.5% Tween-20, and the proteins were fully dissolved by sonication for 15 min. The sample was quantified using a BCA protein assay kit (Pik-day Institute of Biotechnology, China) and stored at −80°C after aliquoted. Urinary proteins from 37 patients (19 with DN, 18 with NDRD) were collected; the clinical information of patients is shown in Table 1. To eliminate the variations between individuals, each group's urinary protein was pooled and then divided into three biological replications. For each replication, 80-μg of urinary protein was mixed with an equal volume of sodium carbonate (pH 9.3); 5-μl of Cy3 fluorescent dye (GE Healthcare, Piscataway, NJ) was added into the mixture and then the mixture was incubated for 3 h in darkness. After that, 30 μl of 4 mol/L hydroxylamine hydrochloride was added to the mixture and placed on ice for 10 min to quench any superfluous fluorescence. The labeled urinary protein was isolated by a Sephadex G-25 column (GE Healthcare, New Jersey, CA, USA) and quantified by a nano-photometer (IMPLEN, Germany).
Preparation of the lectin microarray and data analysis
The glycan profiles of urinary protein from DN and NDRD patients were analyzed by a lectin microarray as described previously.[13,14] Briefly, the lectin microarray was blocked with a blocking solution containing 3% BSA for 1 h. Subsequently, 4 μg of Cy3-labeled urinary protein was incubated with the lectin microarray for 3 h, followed by washing and drying. Then, the microarray was scanned by a laser confocal scanner 4000B (AXON Instruments, Weatherford, TX, USA), and the fluorescence signal values were extracted using GenePix 6.0 software (AXON Instruments, Weatherford, TX, USA). To eliminate the effects of nonspecific adsorption, the signal intensity of lectin lower than the mean + standard deviation (SD) was considered to be invalid and was not included in the subsequent data analysis. The data were normalized by global normalization. After normalization, the altered glycopatterns were screened according to the following criteria: fold changes ≥1.50 or ≤0.67 and P < 0.05 in the pairs, indicating up- or downregulation of certain kind of glycopatterns, respectively.
Fabrication of urinary protein microarray and data analysis
To validate altered glycopatterns of urinary protein between DN and NDRD, urinary proteins from another 32 patients (15 with DN, 17 with NDRD) were collected to prepare the urinary protein microarray. The clinical information of patients is shown in Supplementary Table 1. The preparation and incubation process of the urinary protein microarray was similar to that of a lectin microarray. Urinary proteins were spotted on the surface of an epoxy modified glass slide using a Smart Arrayer 48 (Capital Bio Corp. Beijing, China). A total of 15 patients with DN (seven patients with DN I and eight with DN II) and 17 patients with NDRD (nine patients with MN and eight patients with IgAN) were enrolled. According to the result of the lectin microarrays, the selected lectin was labeled with Cy3 and incubated with the urinary protein microarray. Finally, the incubation image and the fluorescence signals were recorded using GenePix 6.0 software (Axon, Union City, CA, USA).
Supplementary Table 1.
Detailed clinical information for the DN and NDRD patients used for urinary protein microarray analysis
| Characteristics | DN (n = 15) | NDRD (n = 17) | ||
|---|---|---|---|---|
| DN group I (n = 7) | DN group II (n = 8) | MN (n = 9) | IgAN (n = 8) | |
| Age (years) | 58.9 ± 10.4 | 59.4 ± 9.4 | 57.6 ± 6.0 | 54.0 ± 10.4 |
| Sex (male/female) | 4/3 | 5/3 | 5/4 | 5/3 |
| BMI (kg/cm2) | 23.8 ± 2.0 | 25.7 ± 4.2 | 24.6 ± 3.2 | 24.2 ± 3.3 |
| Duration of diabetes (months)* | 121.0 (43.1) | 144.1 (78.1) | 69.2 (109.3) | 58.9 (88.3) |
| HbA1c (%) | 6.9 ± 1.2 | 7.2 ± 2.1 | 7.1 ± 2.0 | 6.7 ± 1.9 |
| Glucose (mmol/L) | 7.5 ± 1.3 | 7.8 ± 2.2 | 7.6 ± 1.7 | 7.1 ± 2.5 |
| Systolic blood pressure (mmHg) | 140.2 ± 18.9 | 144.6 ± 13.1 | 130.1 ± 12.0 | 134.2 ± 9.5 |
| Diastolic blood pressure (mmHg) | 81.9 ± 11.6 | 81.1 ± 10.1 | 81.6 ± 6.5 | 88.8 ± 9.7 |
| Urinary protein (g/24 h) | 1.9 ± 1.4 | 2.8 ± 1.6 | 3.3 ± 2.2 | 2.0 ± 1.8 |
| Blood urea nitrogen (mmol/L)* | 7.0 (2.9) | 8.7 (3.4) | 5.0 (2.1) | 7.0 (4.9) |
| Creatinine (µmol/L)* | 95.0 (38.0) | 154.5 (47.7) | 67.0 (35.7) | 105.8 (74.8) |
| eGFR (ml·min−1·1.73 m−2)* | 80.6 (30.7) | 41.8 (19.8) | 99.9 (29.5) | 58.8 (50.1) |
Variables following normal distribution are expressed with mean ± SD. *Variables following nonnormal distribution are expressed with the median. NDRD: Nondiabetic renal disease; DN: Diabetic nephropathy; SD: Standard deviation; MN: Membranous nephropathy; IgAN: IgA nephropathy; eGFR: Estimated glomerular filtration rate; BMI: Body mass index; HbA1c: Glycated hemoglobin.
Lectin blotting
To further verify the altered glycopatterns between DN and NDRD patients, lectin blotting was used to characterize the differences of glycosylation in protein level. First, 30 μg of urinary protein from DN and NDRD groups was separated by 10% SDS-PAGE. The separated protein was then transferred to the PVDF membrane (Millipore, Bed-ford, MA, USA) using a transfer system (Bio-rad, Hercules, CA). After that, the PVDF membrane was blocked by 10 ml of carbo-free blocking solution (Vectorlabs, Burlingame, CA) for 1 h. Then, 100 μl of Cy5-labeled lectin DSA was incubated with PVDF membrane overnight at 4°C. The PVDF membrane was then washed three times with TBST for 10 min per wash. The images were acquired using a STORM scanner (Molecular Dynamics, Piscataway, NJ), and the protein strip gray value of selected protein bands was measured by ImageJ (1.41v, US National Institutes of Health, USA).
Statistical analysis
Data are shown as mean ± SD or median (interquartile range), and GraphPad Prism Software (Vision 6, San Diego, CA, USA) was used for data analysis. Ratio analysis and one-way analysis of variance were used to compare average normalized fluorescence intensities between DN and NDRD and the altered glycopatterns in urinary protein associated with DN were screened. The original data from the urinary protein microarray were analyzed by scatter plot, and the significance between different groups was assessed by Kruskal–Wallis test. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the ability of the altered glycopatterns to differentiate DN and NDRD. In all tests, P < 0.05 was considered significantly different.
RESULTS
Differences of glycopatterns on urinary protein between diabetic nephropathy and nondiabetic renal disease patients
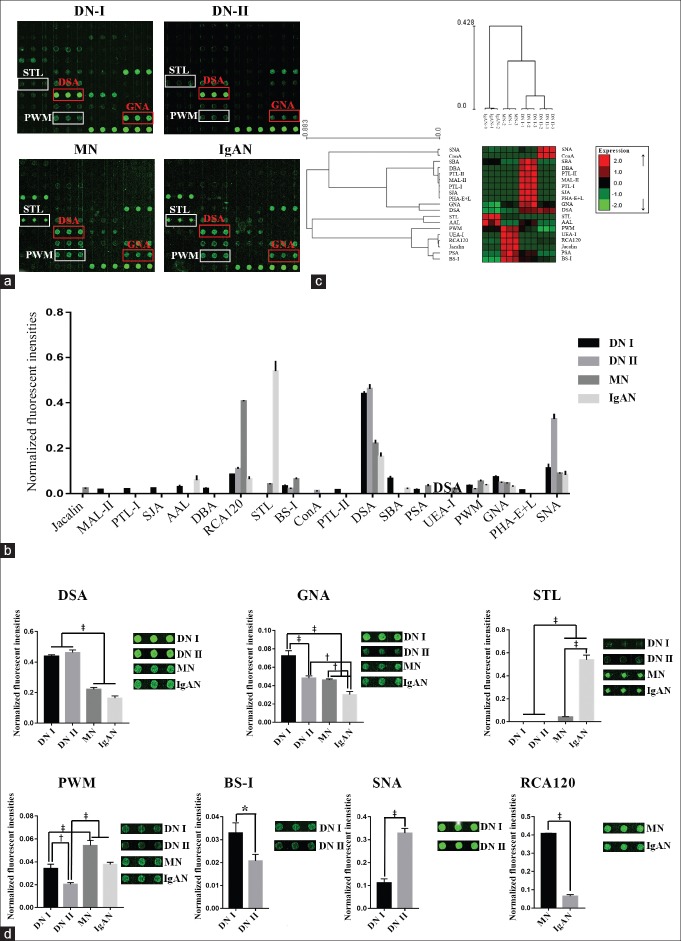
The glycan profile of urinary protein from DN and NDRD patients was analyzed by a lectin microarray. The results showed that the glycan profile exhibited a difference between DN and NDRD groups [Figure 1a]. A total of 15, 7, 10, and 8 lectins were detected effective binding signals in the urinary proteins from DN I group, DN II group, MN group, and IgA nephropathy group, respectively [Figure 1b and Table 2]. In DN patients, the relative abundance of the glycopatterns of (β-1,4)-linked GlcNAc recognized by lectin DSA was higher than the others. In the MN patients, the relative abundance of glycopatterns from β-Gal identified by lectin RCA120 was higher than the others. In IgAN patients, the relative abundance of the terminal GlcNAc recognized by STL was higher than the others. The results of hierarchical cluster analysis revealed that these lectins could be divided into two groups. The expressions of the glycopatterns identified by 11 lectins such as SNA, Con A, and SBA were increased in DN patients. In contrast, the expressions of the glycopatterns identified by 8 lectins such as STL, AAL, and PWM were decreased in the DN groups. In addition, DN I and DN II group patients can be classified as a class together, while MN and IgAN patients can be identified as another class [Figure 1c]. These results indicated that the abundance of glycopatterns on urinary protein from DN and NDRD patients was different.
Figure 1.
Analysis of the sugar chain spectra of urinary protein samples from patients with DN and NDRD. (a) Lectin chip results of urinary proteins from the DN I, DN II, MN, and IgAN groups. Lectins in the red rectangle are lectins that recognize sugar chains possessing a higher abundance in the DN group; lectins in the white rectangle are lectins that recognize sugar chains possessing a higher abundance in the NDRD group. (b) Comparison of the NFIs of the 19 different kinds of lectins from the four patient groups. The lectin chip data were screened and the effective signals were processed using the global normalization method. The data are shown as mean ± SD. (c) The heat map and hierarchical clustering of the 19 lectins. Glycan profiles of the DN groups (DN I and DN II) and the NDRD groups (MN and IgAN) were clustered (average linkage and correlation similarity). The samples are listed in the columns and the lectins are listed in the rows. Data are shown as median values centered in the row dimension and the color and intensity of each square indicates expression levels relative to the other data in the row: red, high; green, low; black, medium. (d) Lectins showed significant differences in NFIs between DN group and NDRD group. *P < 0.05, †P < 0.01, ‡P < 0.001. NFIs: Normalized fluorescence intensities; NDRD: Nondiabetic renal disease; DN: Diabetic nephropathy; SD: Standard deviation; MN: Membranous nephropathy; IgAN: IgA nephropathy.
Table 2.
The normalized fluorescence values of lectin microarray in DN and NDRD groups
| Lectins | Sugar chain structures | DN (n = 19) | NDRD (n = 18) | ||
|---|---|---|---|---|---|
| DN group I (n = 9) | DN group II (n = 10) | MN (n = 10) | IgAN (n = 8) | ||
| Jacalin | Galβ1-3-GalNAcα-Ser/Thr(T), GalNAcα-Ser/Thr(Tn), GlcNAcβ1-3-GalNAcα-Ser/Thr(Core3), sialyl-T (ST). Not bind to Core2, Core6, and sialyl-Tn (STn) | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.023 ± 0.001 | 0.000 ± 0.000 |
| MAL-II | Siaα-2-3Galβ1-4Glc(NAc)/Glc, Siaα2-3Gal, Siaα2-3, Siaα2-3GalNAc | 0.017 ± 0.001 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| PTL-I | GalNAc, GalNAcα-1,3Gal, GalNAcα-1,3Galβ1,3/4Glc | 0.020 ± 0.001 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| SJA | Terminal in GalNAc and Gal, anti-A and anti-B human blood group | 0.024 ± 0.001 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| AAL | Fucα1-6 GlcNAc (core fucose), Fucα1-3(Galβ1-4) GlcNAc | 0.030 ± 0.006 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.060 ± 0.018 |
| DBA | α-GalNAc, Tn antigen, GalNAcα1-3((Fucα1-2)) Gal (blood group A antigen) | 0.022 ± 0.003 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| RCA120 | β-Gal, Galβ-1,4GlcNAc (type II), Galβ1-3GlcNAc (type I) | 0.084 ± 0.001 | 0.108 ± 0.006 | 0.407 ± 0.002 | 0.064 ± 0.010 |
| STL | Trimers and tetramers of GlcNAc, core (GlcNAc) of N-glycan, oligosaccharide containing GlcNAc and MurNAc | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.042 ± 0.002 | 0.540 ± 0.043 |
| BS-I | α-Gal, α-GalNAc, Galα-1,3Gal, Galα-1,6Glc | 0.033 ± 0.004 | 0.020 ± 0.003 | 0.064 ± 0.005 | 0.000 ± 0.000 |
| ConA | High-Mannose, Manα1-6(Manα1-3) Man, αMannose, αGlc | 0.000 ± 0.000 | 0.012 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| PTL-II | Gal, blood group H, T-antigen | 0.017 ± 0.001 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| DSA | (GlcNAc) 2-4, polyLacNAc and LacNAc (NA3, NA4) | 0.439 ± 0.009 | 0.462 ± 0.017 | 0.221 ± 0.014 | 0.164 ± 0.016 |
| SBA | α- or β-linked terminal GalNAc, (GalNAc) n, GalNAcα1-3Gal, blood-group A | 0.066 ± 0.010 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.022 ± 0.001 |
| PSA | Fucα-1,6GlcNAc, α-D-Man, α-D-Glc | 0.016 ± 0.004 | 0.000 ± 0.000 | 0.033 ± 0.007 | 0.000 ± 0.000 |
| UEA-I | Fucα1-2Galβ1-4Glc (NAc) | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.021 ± 0.003 | 0.000 ± 0.000 |
| PWM | (GlcNAc)n and polyLacNAc | 0.034 ± 0.004 | 0.020 ± 0.002 | 0.054 ± 0.005 | 0.038 ± 0.002 |
| GNA | HighMannose, Manα1-3Man | 0.073 ± 0.006 | 0.048 ± 0.003 | 0.046 ± 0.001 | 0.030 ± 0.003 |
| PHA-E + L | Bisecting GlcNAc, bi-antennary N-glycans, tri- and tetra-antennary complex-type N-glycan | 0.016 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| SNA | Sia2-6Gal/GalNAc | 0.112 ± 0.017 | 0.328 ± 0.021 | 0.090 ± 0.002 | 0.083 ± 0.015 |
The signal intensities obtained for three biological replications were normalized and represented as mean ± SD. NDRD: Nondiabetic renal disease; DN: Diabetic nephropathy; SD: Standard deviation; MN: Membranous nephropathy; IgAN: IgA nephropathy; DSA: Datura stramonium agglutinin.
In the comparison of the glycopatterns of DN and NDRD, the ratio analysis showed that the relative abundance of (β-1, 4)-linked GlcNAc identified by DSA was higher in both DN groups than that in the NDRD groups (fold change >1.50, P < 0.001, F > 12.56). Furthermore, the relative abundance of the Manα1–3Man identified by GNA was significantly higher in the DN groups than that in the IgAN group (fold change >1.57, P < 0.01, F > 54.41); there was no significant difference between the DN II group and the MN group. The glycopatterns of terminal GlcNAc recognized by STL has a uniquely higher relative abundance in NDRD patients. Moreover, the expression of GlcNAc oligomer recognized by PWM was significantly higher in NDRD groups than in the DN II group (P< 0.001, F > 32.62) [Figure 1d].
Fifteen and 7 lectin-detected effective binding signals were found in samples from the DN I and DN II groups, respectively, indicating that the types of glycopatterns in the urinary protein from DN patients with low eGFR were less in the DN patients with high eGFR. There were six lectins including SNA, DSA, and RCA120 that were detected in both DN groups. Of note, the relative abundance of Sia2-6Gal/GalNAc that was recognized by SNA was significantly higher in the DN II group (fold change = 3.95, P < 0.001, F = 195.76). In contrast, the normalized fluorescence intensities of the Manα1–3Manbinder GNA (fold change = 0.67, P < 0.001, F = 48.02), α-Gal binder BS-I (fold change = 0.63, P < 0.05, F = 15.75), and GlcNAc binder PWM (fold change = 0.60, P < 0.01, F = 32.76) were significantly higher in the DN I group than in the DN II group.
To compare the glycopatterns of urinary protein between the NDRD groups, 10 and 8 lectins were detected as effective binding signals in the MN and IgAN groups, respectively. Among these, 6 lectins, such as STL, SNA, and DSA, were detected in both groups. The relative abundance of the terminal GlcNAc structure identified by lectin STL was significantly higher in IgAN patients than in MN patients (fold change = 12.99, P < 0.001, F = 410.01). The relative abundance of Manα1–3Man and β-Gal, which are recognized by GNA (fold change = 0.65, P < 0.01, F = 66.54) and RCA120 (fold change = 0.16, P < 0.001, F = 3676.56), was higher in MN patients than in IgAN patients [Figure 1d].
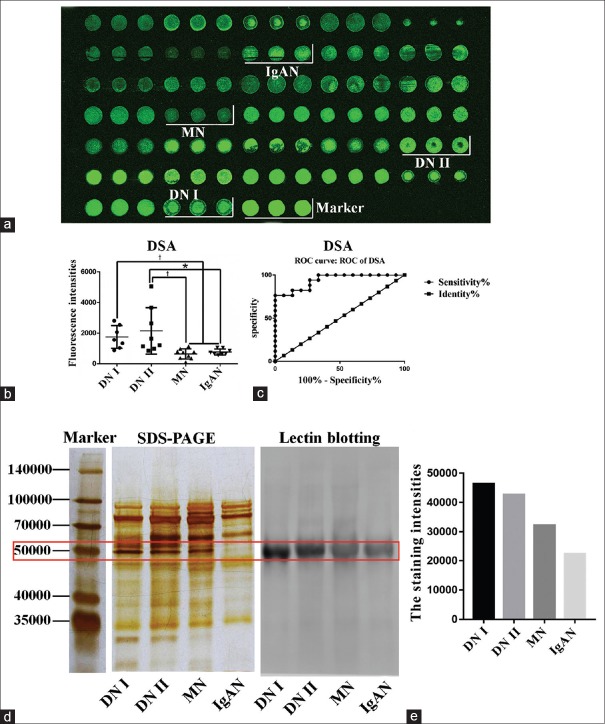
Validation by urinary protein microarray
The lectin microarray revealed that the relative abundance of the (β-1,4)-linked GlcNAc was significantly higher in DN patients than in NDRD patients. To verify this finding, urinary protein was performed to evaluate the difference between DN and NDRD patients. As shown in Figure 2a, the DSA binding intensity was significantly different between DN and NDRD samples (P < 0.05, H > 12.98). In addition, the results showed that the relative abundance of the (β-1,4)-linked GlcNAc was significantly different between the DN and NDRD groups [Figure 2b]. The potential application value of DSA to distinguish between DN and NDRD was evaluated by an ROC curve analysis; the result revealed that the area under the ROC curve was 0.94 (P < 0.001), indicating that the glycopatterns of (β-1,4)-linked GlcNAc have a potential in differentiating DN from NDRD [Figure 2c].
Figure 2.
Results of the urinary protein chip test and the lectin blotting test. (a) Urinary protein chip test results. Results were obtained by incubating Cy3-labeled DSA with the urinary protein microarray and used this to analyze urinary protein samples from 32 patients with DN or NDRD. (b) Scatter diagram analysis of the original data achieved from the urinary protein microarrays. Statistical significance of differences between groups is indicated. *P < 0.05, †P < 0.001. (c) ROC curve analysis of DSA to differentiate DN and NDRD, patients AUC = 0.94, P < 0.0001. (d) SDS-PAGE gel and lectin blot of pooled urinary protein from DN and NDRD groups, using DSA. Lane 1, DN Group I; lane 2, DN Group II; lane 3, patients with MN; lane 4, patients with IgAN. (e) The gray value of the protein band of approximately 50,000, marked with the red frame, was measured by ImageJ software. DSA: Datura stramonium agglutinin; ROC: Receiver operating characteristic; AUC: Area under the ROC curve; NDRD: Nondiabetic renal disease; DN: Diabetic nephropathy; IgAN: IgA nephropathy.
Lectin blotting
In this study, we found that the relative abundance of the (β-1,4)-linked GlcNAc was significantly higher in DN patients compared with NDRD patients. Subsequently, the results of the urinary protein microarray were consistent with the lectin microarray. According to the SDS-PAGE and lectin blotting analyses, a protein band of approximately 50,000 was clearly observed in samples from four groups [Figure 2d]. The gray scale values of the bands in the different groups were measured by ImageJ software; it showed that the staining signal in both DN groups was higher than that in the NDRD groups [Figure 2e]. Consequently, this finding indicated that the abundance of (β-1,4)-linked GlcNAc on 50,000 proteins was higher in urinary protein from DN patients compared with NDRD patients.
DISCUSSION
In this study, a lectin microarray was used to investigate the glycan profile of urinary proteins between DN and NDRD patients. We found a significant difference in glycopatterns on urinary proteins between DN and NDRD patients. The relative abundance of (β-1, 4)-linked GlcNAc is higher in the urinary proteins of DN patients compared with NDRD patients. We then used a urinary protein microarray to analyze the urinary proteins collected from another 32 patients with DN and NDRD. As a consequence, we found that the expression of (β-1,4)-linked GlcNAc was different between DN and NDRD patients. ROC analysis showed that it has a potential clinical application in the clinical diagnosis of DN and NDRD. Thus, our findings indicated that the glycopatterns of (β-1,4)-linked GlcNAc on urinary protein recognized by DSA could be used as a potential clinical biomarker to distinguish DN from NDRD.
Elevated blood glucose is well known as an important feature of diabetes. Some studies indicated that the glycopatterns of α2-6 sialic acid is decreased in the glycodelin-A of patients with gestational diabetes mellitus; this altered glycosylation may impair immunomodulatory activities.[15] (β-1,4)-linked GlcNAc is a part of N-glycan. Keser et al. reported that the complexity of the plasma N-glycan structure was increased in individuals with a higher risk of developing type 2 diabetes and a poorer regulation of blood glucose levels.[16] IgG is a highly abundant protein in plasma. The diversity of IgG N-glycome is associated with clinical risk factors of type 2 diabetes, such as age, body mass index, smoking, and dyslipidemia. As reported by Lemmers et al., five types of N-glycans (FA2B, A2BG1, FA2[3]G1, FA2[6]BG1, and FA2[3]BG1) that all contained (β-1,4)-linked GlcNAc increased in the IgG of patients with type 2 diabetes compared to controls.[17] In addition, previous studies showed that high levels of glucose can promote hexosamine biosynthetic pathways and lead to the formation of excess GlcNAc and an increase of O-GlcNAc modifications.[18] Degrell et al. found that O-GlcNAc glycosylation of proteins was significantly higher in the glomeruli and renal tubules of DN patients than in healthy controls. Interestingly, this may be important as O-GlcNAc glycosylation may promote the progression of DN.[19] In addition, Barrios et al. further showed that GlcNAc expression was elevated in IgG combined with renal impairment.[20] Combined with our findings, we postulate that hyperglycemia in DN patients can induce excessive production of GlcNAc by activating the hexose amidose pathway, which can cause an increase of O-GlcNAc modifications. With the pathological progression of DN, the abundance of GlcNAc glycopatterns increases as a result of an increase in urinary protein levels. Moreover, we found that with the development of DN, the glomerular filtration rate decreases, and the urinary protein glycopattern types similarly decrease as well. In support of this finding, Inoue et al. found that these types of urinary proteins were gradually reduced during the development of DN after conducting a comparative analysis of glycopattern profiles of the urinary proteins from patients with different degrees of DN.[21]
In summary, glycomic techniques were utilized to investigate the difference of glycopatterns on urinary proteins between DN and NDRD patients. In particular, we found a significant difference in the abundance of (β-1,4)-linked GlcNAc between them. Furthermore, a urinary protein microarray showed that using these glycopatterns could effectively distinguish DN from NDRD. Our study suggests that the glycopatterns of (β-1,4)-linked GlcNAc are promising diagnostic markers for the noninvasive clinical identification of DN and NDRD. However, our study has some deficiencies, such as the small sample size, and additional types of nephropathy should be enrolled to evaluate the specificity of these glycopatterns. In future studies, we will further assess the properties of (β-1,4)-linked GlcNAc on urinary protein in larger clinical samples to evaluate the potential of these glycopatterns in differential diagnosis of DN and NDRD patients.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by grants from the National Key R&D Program of China (No. 2016YFC1305500), the National Natural Science Foundation of China (No. 61471399; No. 61671479), and the Innovation Nursery Fund of PLA General Hospital (No. 15KMZ04).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imtiaz S, Salman B, Nasir K, Drohlia MF, Ahmad A. Clinical variables differentiating diabetic from nondiabetic kidney disease in patients with diabetes: A single-center study. Saudi J Kidney Dis Transpl. 2017;28:307–12. doi: 10.4103/1319-2442.202794. doi: 10.4103/1319-2442.202794. [DOI] [PubMed] [Google Scholar]
- 3.Prakash J, Lodha M, Singh SK, Vohra R, Raja R, Usha, et al. Diabetic retinopathy is a poor predictor of type of nephropathy in proteinuric type 2 diabetic patients. J Assoc Physicians India. 2007;55:412–6. [PubMed] [Google Scholar]
- 4.Chen H, Wang DG, Yuan L, Liu GL, He HJ, Wang J, et al. Clinical characteristics of patients with diabetic nephropathy on maintenance hemodialysis: A multicenter cross-sectional survey in Anhui province, Eastern China. Chin Med J. 2016;129:1291–7. doi: 10.4103/0366-6999.182832. doi: 10.4103/0366-6999.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang YZ, Wang JW, Wang F, Wu YT, Zhao HY, Chen M, et al. Incidence, development, and prognosis of diabetic kidney disease in China: Design and methods. Chin Med J. 2017;130:199–202. doi: 10.4103/0366-6999.198002. doi: 10.4103/0366-6999.198002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wada Y, Azadi P, Costello CE, Dell A, Dwek RA, Geyer H, et al. Comparison of the methods for profiling glycoprotein glycans – HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study. Glycobiology. 2007;17:411–22. doi: 10.1093/glycob/cwl086. doi: 10.1093/glycob/cwl086. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. doi: 10.1016/j.cell.2006.08.019. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Allegri L, Suzuki Y, Hall S, Moldoveanu Z, Wyatt RJ, et al. Galactose-deficient IgA1 as a candidate urinary polypeptide marker of IgA nephropathy? Dis Markers. 2016;2016:7806438. doi: 10.1155/2016/7806438. doi: 10.1155/2016/7806438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott KL, Nairn AV, Hall EM, Horton MB, McDonald JF, Moremen KW, et al. Focused glycomic analysis of the N-linked glycan biosynthetic pathway in ovarian cancer. Proteomics. 2008;8:3210–20. doi: 10.1002/pmic.200800157. doi: 10.1002/pmic.200800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott KL, Lim JM, Wells L, Benigno BB, McDonald JF, Pierce M, et al. Identification of candidate biomarkers with cancer-specific glycosylation in the tissue and serum of endometrioid ovarian cancer patients by glycoproteomic analysis. Proteomics. 2010;10:470–81. doi: 10.1002/pmic.200900537. doi: 10.1002/pmic.200900537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saravanan C, Cao Z, Head SR, Panjwani N. Analysis of differential expression of glycosyltransferases in healing corneas by glycogene microarrays. Glycobiology. 2010;20:13–23. doi: 10.1093/glycob/cwp133. doi: 10.1093/glycob/cwp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H, Liu M, Yu H, Liu X, Zhong Y, Shu J, et al. Glycopatterns of urinary protein as new potential diagnosis indicators for diabetic nephropathy. J Diabetes Res. 2017;2017:5728087. doi: 10.1155/2017/5728087. doi: 10.1155/2017/5728087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin Y, Zhong Y, Zhu M, Dang L, Yu H, Chen Z, et al. Age-and sex-associated differences in the glycopatterns of human salivary glycoproteins and their roles against influenza A virus. J Proteome Res. 2013;12:2742–54. doi: 10.1021/pr400096w. doi: 10.1021/pr400096w. [DOI] [PubMed] [Google Scholar]
- 14.Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy. Contrib Nephrol. 2011;170:36–47. doi: 10.1159/000324942. doi: 10.1159/000324942. [DOI] [PubMed] [Google Scholar]
- 15.Lee CL, Chiu PC, Pang PC, Chu IK, Lee KF, Koistinen R, et al. Glycosylation failure extends to glycoproteins in gestational diabetes mellitus: Evidence from reduced α2-6 sialylation and impaired immunomodulatory activities of pregnancy-related glycodelin-A. Diabetes. 2011;60:909–17. doi: 10.2337/db10-1186. doi: 10.2337/db10-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keser T, Gornik I, Vučković F, Selak N, Pavić T, Lukić E, et al. Increased plasma N-glycome complexity is associated with higher risk of type 2 diabetes. Diabetologia. 2017;60:2352–60. doi: 10.1007/s00125-017-4426-9. doi: 10.1007/s00125-017-4426-9. [DOI] [PubMed] [Google Scholar]
- 17.Lemmers RF, Vilaj M, Urda D, Agakov F, Šimurina M, Klaric L, et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim Biophys Acta. 2017;1861:2240–9. doi: 10.1016/j.bbagen.2017.06.020. doi: 10.1016/j.bbagen.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Hanover JA, Krause MW, Love DC. Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13:312–21. doi: 10.1038/nrm3334. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 19.Degrell P, Cseh J, Mohás M, Molnár GA, Pajor L, Chatham JC, et al. Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci. 2009;84:389–93. doi: 10.1016/j.lfs.2009.01.007. doi: 10.1016/j.lfs.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Barrios C, Zierer J, Gudelj I, Štambuk J, Ugrina I, Rodríguez E, et al. Glycosylation profile of IgG in moderate kidney dysfunction. J Am Soc Nephrol. 2016;27:933–41. doi: 10.1681/ASN.2015010109. doi: 10.1681/ASN.2015010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue K, Wada J, Eguchi J, Nakatsuka A, Teshigawara S, Murakami K, et al. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS One. 2013;8:e77118. doi: 10.1371/journal.pone.0077118. doi: 10.1371/journal.pone.0077118. [DOI] [PMC free article] [PubMed] [Google Scholar]