Abstract
A 42‐year‐old man with hemochromatosis and cirrhosis developed aplastic anemia. He underwent liver transplantation from a female donor and splenectomy, and his aplastic anemia spontaneously resolved. A bone marrow examination 6 months after the liver transplant showed 17.5% female cells. He did well for 13 years without the need for any blood product support but then developed bone pain and was found to have metastatic hepatocellular carcinoma in the vertebral bodies. Molecular analysis demonstrated that the tumor cells were from his original liver. No primary liver tumor was identified in the explant. The case demonstrates the application of fluorescent in situ hybridization with X and Y chromosome‐specific probes to study chimerism and tumor origin after liver transplantation between individuals of different sex. (Hepatology Communications 2018;2:13–15)
Abbreviations
- CT
computed tomography
- HCC
hepatocellular carcinoma
- WBC
white blood count
The Case
A 42‐year‐old man was referred in 1995 with abnormal liver enzymes. Investigations demonstrated a serum ferritin level of 919 μg/L and transferrin saturation of 83%. There was no history of alcohol abuse, and hepatitis B surface antigen and anti‐hepatitis C virus were negative. Liver biopsy showed cirrhosis with marked iron overload. There was no steatohepatitis. Hepatic iron concentration was 465 μmol/g dry weight (normal <35 μmol/g). He had a nodular liver without focal lesions. Genetic testing for hemochromatosis in 1998 confirmed that he was C282Y homozygous.
The patient underwent 43 weekly 500‐mL phlebotomies until his serum ferritin level was 54 μg/L. He then had maintenance phlebotomy every 3 months with ultrasound every 6 months. In 2004, he developed fatigue and was found to have a hemoglobin of 4 g/dL, white blood count (WBC) of 1.2 × 109/L, and platelets of 10 × 109/L. A bone marrow biopsy was consistent with aplastic anemia (Fig. 1). He was not tested for telomere mutations, and there was no evidence of pulmonary fibrosis or skin rash. He tried a number of treatments for the anemia, including erythropoietin, steroids, androgens, antithymocyte globulin, and cyclosporine, with no improvement. He became dependent on weekly transfusions, and secondary iron overload was developing. At this time, deferoxamine was the only chelator available and it was not used because he was moving toward palliative care. He was considered for combined liver and hemopoietic stem cell transplantation, but the hematologist did not think he could tolerate the procedure.
Figure 1.

Bone marrow biopsy showing hypoplastic bone marrow just prior to liver transplantation (100× magnification).
In October 2004, the patient had a liver transplant and splenectomy 14 months after the diagnosis of aplastic anemia. He was not in liver failure, and the pre‐operative goal was to consider stem cell transplantation after recovery. Pretransplant liver imaging (computed tomography [CT] and ultrasonography) showed no focal liver lesions. Two days before liver transplantation, he had an alpha‐fetoprotein level of 1.2 μg/L (normal <5 μg/L). He received six units of packed red blood cell transfusions and two units of platelets intraoperatively and was treated with tacrolimus, mycophenolate, and prednisone. The removed spleen was 17 × 14 × 6 cm and weighed 595 g. The patient had an improvement in hemoglobin and WBC within 3 days, with a rise in WBC from 1.2 × 109/L to 10.2 × 109/L. The team considered other options, including stem cell transfer of donor cells to the recipient bone marrow (chimerism), extramedullary hematopoiesis, and immunosuppression.
A multifactorial cause of the improvement is a possibility. The donor was female, and we considered sex‐specific cell identification strategies. Our medical center is the home of the Barr body discovery in 1949,1 but we decided to use a more contemporary method of interphase fluorescent in situ hybridization bone marrow analysis, using an X/Y DNA probe. Nine months after liver transplantation, this analysis showed that the bone marrow contained 17.5% female cells and was considered to be hypoplastic. It is widely known that donor cells can spread throughout the body within 48 hours.2 The patient never again needed blood cell support. The explanted liver was examined in 1‐cm sections and showed cirrhosis with transfusional iron overload. There was no evidence of a liver tumor.
In 2017, the patient presented with severe back pain in the lumbar spine. CT and magnetic resonance imaging of the spine demonstrated multiple spinal tumors. Biopsy of the spinal tumor was suggestive of hepatocellular carcinoma (HCC), and the patient's serum alpha‐fetoprotein was 32,390 μg/L (normal <5.0). There were no clear clinical reasons why the tumor occurred 13 years later. The patient had been on stable immunosuppression for many years and had no new concomitant illness. A previous report of late recurrence of HCC (>5 years after transplantation) reported late recurrence in 5.6% of 88 patients, with an average length after recurrence of 8.5 years.3 The tumor stained positive for hepatocyte‐specific antigen (HepPAR1) (Fig. 2). CT and magnetic resonance imaging of the transplanted liver showed no focal lesions. DNA extracted from his original explant was compared to the spinal tumor biopsy, using short tandem repeat analysis, which includes the amelogenin locus. This allowed determination of sex (XY or XX) and showed matching results at multiple (five of five informative loci) genetic sites. Based on population‐based allele frequencies, the chances that these specimens came from two different individuals is approximately 3.5 × 10–6 (U.S. Caucasian data). Interestingly, the tumor showed loss of the Y chromosome allele, likely secondary to spontaneous or tumor‐related clonal loss of the amelogenin Y chromosomal allele or complete or partial loss of the Y chromosome.4, 5 This demonstrated that this metastatic HCC likely came from a small undetectable locus of tumor in his original cirrhotic liver 13 years previously. The patient was treated with stabilization of the spine using metal rods, radiotherapy, and sorafenib.
Figure 2.
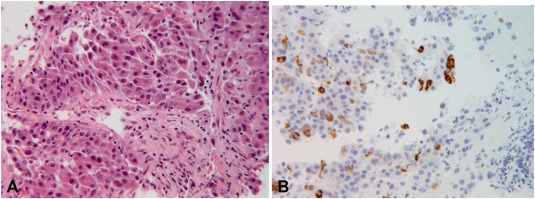
Micrograph of the spinal tumor biopsy. (A) poorly differentiated carcinoma (hematoxylin and eosin stain) and (B) immunohistochemical positivity for hepatocyte‐specific antigen (HepPar1). Tumor cells were also positive for CK20, MOC‐31, poly CEA, and arginase and were negative for PSA, CK7, p63, S100, Melan‐A, HMB45, TTF‐1, and vimentin. Serum alpha‐fetoprotein was 32,390 μg/L. The findings strongly suggested metastatic hepatocellular carcinoma, possibly originating from his native liver. Abbreviations: CK, cytokeratin; HMB, human melanoma black; Melan‐A, melanoma antigen recognized by T cells; MOC‐31, mouse monoclonal epithelial cell adhesion molecule antibody; poly CEA, polyclonal carcinoembryonic antigen; PSA, prostate‐specific antigen; TTF‐1, thyroid transcription factor‐1 (100× magnification).
In summary, we report on the use of sex‐specific probes and short tandem repeat analysis in an unusual case of aplastic anemia resolving after liver transplantation along with the late development of HCC 13 years after liver transplantation with no primary tumor identified.6
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Barr ML, Bertram EG. A Morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis. Nature 1949;163:676. [DOI] [PubMed] [Google Scholar]
- 2. Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS, et al. Chimerism after organ transplantation. Curr Opinion Nephro Hypertens 1997;6:292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Ja, Kwee SA, Wong LL. Later recurrence of hepatocellular carcinoma after liver transplantation. Hepatoma Res 2017;3:58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vauhkonen H, Hedman M, Vauhkonen M, Sipponen P, Sajantila A. Typing of XY (male) genotype from malignant neoplastic tissue by the amelogenin‐based sex test. J Forensic Sci 2004;49:222‐226. [PubMed] [Google Scholar]
- 5. Vauhkonen H, Hedman M, Vauhkonen M, Kataja M, Sipponen P, Sajantila A. Evaluation of gastrointestinal cancer tissues as a source of genetic information for forensic investigations by using STRs. Forensic Sci Int 2004;139:159‐167. [DOI] [PubMed] [Google Scholar]
- 6. Chen R, Gao Z, Wu X, Campbell JL, Zhang P, Chen B. Hepatocellular carcinoma presenting as thoracic spinal canal metastasis with no clinical primary foci: a report of a rare case and review of the literature. Oncol Lett 2015;10:2333‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]


