Abstract
Accurate staging of liver fibrosis is crucial to guide therapeutic decisions for patients with nonalcoholic fatty liver disease (NAFLD). Digital image analysis has emerged as a promising tool for quantitative assessment of fibrosis in chronic liver diseases. We sought to determine the relationship of histologic fibrosis stage with fiber amounts quantified in liver biopsy specimens for the better understanding of NAFLD progression. We measured area ratios of collagen and elastin fibers in Elastica van Gieson‐stained biopsy tissues from 289 patients with NAFLD from four hospitals using an automated computational method and examined their correlations with Brunt's fibrosis stage. As a secondary analysis, we performed multivariable logistic regression analysis to assess the associations of the combined area ratios of collagen and elastin with noninvasive fibrosis markers. The combined fiber area ratios correlated strongly with Brunt's stage (Spearman correlation coefficient, 0.78; P < 0.0001), but this relationship was nonlinear (P = 0.007) with striking differences between stage 4 (median area ratios, 12.3%) and stages 0‐3 (2.1%, 2.8%, 4.3%, and 4.8%, respectively). Elastin accumulation was common in areas of thick bridging fibrosis and thickened venous walls but not in areas of perisinusoidal fibrosis. The highest tertile of the combined fiber area ratios was associated with the fibrosis‐4 index and serum type IV collagen 7s domain (7s collagen) levels, whereas the upper two tertiles of the fiber amounts significantly associated with body mass index, aspartate aminotransferase, and 7s collagen in the multivariable analysis. Conclusion: Quantitative fibrosis assessment reveals a nonlinear relationship between fibrosis stage and fiber amount, with a marked difference between stage 4 and stage 3 and much smaller differences among stages 0‐3, suggesting a heterogeneity in disease severity within NAFLD‐related cirrhosis. (Hepatology Communications 2018;2:58–68)
Abbreviations
- 7s collagen
type IV collagen 7s domain
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- EVG
Elastica van Gieson
- FIB‐4
fibrosis‐4
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
Introduction
Because of changing lifestyles, nonalcoholic fatty liver disease (NAFLD) is increasingly a major cause of chronic liver disease worldwide.1, 2 NAFLD is a heterogeneous group of diseases that comprises two clinicopathologic subtypes: nonalcoholic fatty liver and nonalcoholic steatohepatitis (NASH), the latter being a more aggressive form of the disease progressing to cirrhosis over decades.3, 4, 5, 6, 7 Evidence indicates that the assessment of liver fibrosis is crucial in the clinical management of NAFLD/NASH to reduce mortality related to hepatocellular carcinoma, liver failure, and cardiovascular disease.8, 9, 10, 11, 12, 13 Liver biopsy is essential for determining the diagnosis and fibrosis stage of the disease.7, 14, 15 However, current histologic staging systems are not continuous quantitative measures of hepatic fibrosis.16 Previous studies have suggested that noninvasive or minimally invasive biomarkers, including platelet counts, serum levels of the type IV collagen 7s domain (7s collagen) and Wisteria floribunda agglutinin‐positive Mac‐2 binding protein, fibrosis‐4 (FIB‐4) index, and liver stiffness computed by imaging technologies, are surrogate measurements for estimating the degree of liver fibrosis in patients with NAFLD.8, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Emerging evidence attests to a possible role of fibrosis‐targeted therapy against progressive fibrotic diseases, including NAFLD.27, 28, 29, 30 Consequently, to facilitate future clinical trials, there is an increasing need for accurate evaluation methods for liver fibrosis.
The quantification of collagen fiber amounts by digital image analysis has been a powerful tool not only for continuous grading of hepatic fibrosis but also for the prediction of clinical outcomes in chronic liver diseases, including hepatitis C and alcoholic liver disease.16, 31, 32, 33, 34 Previous studies have linked higher levels of accumulation of hepatic collagen and elastin fibers to an increased risk of hepatocellular carcinoma in patients with hepatitis C virus infection.35, 36 However, the potential role of quantitative analysis on hepatic fiber amounts in the assessment of NAFLD remains poorly understood. This current study aimed to measure the amount of collagen and elastin fibers in liver biopsy specimens and to determine its relationship with the histologic fibrosis stage classified by Brunt's criteria. Toward that end, we analyzed 289 liver biopsy specimens from patients with NAFLD collected from four hospitals using a previously established automated computational method to quantify the amount of collagen and elastin fibers present in the specimens. As a secondary exploratory analysis, we assessed the associations between fiber amount and clinicopathologic characteristics of NAFLD, including noninvasive fibrosis markers, to support the validity of our quantitative measurements on fibers.
Patients and Methods
STUDY POPULATIONS AND LIVER BIOPSY SPECIMENS
We reviewed 325 patients with NAFLD who underwent liver biopsies between July 2003 and September 2013 at one of the following four institutions: Ehime University Hospital (Ehime, Japan), Ikeda Municipal Hospital (Osaka, Japan), Kawasaki Medical School Hospital (Okayama, Japan), and Sapporo Kosei General Hospital (Hokkaido, Japan). We excluded patients who had a history of other liver diseases, including hepatitis B virus or hepatitis C virus infection; those taking drugs known to influence the activity of NAFLD, such as tamoxifen or a glucocorticoid; and those who had a history of alcohol abuse (defined as a daily alcohol consumption of ≥20 g). Among the 325 NAFLD cases, 36 did not meet the following criteria for the quality of liver biopsy specimens: specimen ≥15 mm in length and having six or more portal tracts within each tissue section. Consequently, we analyzed 289 NAFLD cases with available quantitative data on collagen and elastin fibers in liver biopsy tissues. Variations in clinicopathologic characteristics of the 289 patients with NAFLD among the four participating institutions are shown in Supporting Table S1. Written informed consent was obtained from all participants. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by each institutional review committee's a priori approval of this study.
QUANTIFICATION OF LIVER FIBROSIS IN ELASTICA
Formalin‐fixed paraffin‐embedded liver biopsy tissues were sliced to a thickness of 4 μm and underwent Elastica van Gieson (EVG) staining using the following procedure: Briefly, deparaffinized and hydrated sections were dipped in 70% ethanol containing 1% hydrogen chloride, incubated in resorcin–fuchsin solution for 60 minutes, and washed in 100% ethanol and in water, followed by counterstaining with van Gieson's solution (saturated picric acid containing 0.09% acid fuchsin) for 5 minutes. EVG staining for all slides was performed at one institute (Department of Pathology, Keio University School of Medicine). We scanned liver biopsy tissue sections to obtain whole‐slide images using a NanoZoomer 2.0HT scanner (Hamamatsu Photonics K.K, Hamamatsu, Japan) with a 20× objective lens. Hepatic fiber amounts were quantified based on a pixel‐by‐pixel (0.46 μm/pixel) evaluation of the ratios of the areas of collagen and elastin fibers to the total area of the biopsy tissue specimens in whole‐slide images, using a previously established automated computational method (Supporting Fig. S1).37, 38 To validate the replicability of our quantitative fibrosis assessment, we selected 12 NAFLD cases to quantify the amounts of fibers for each biopsy specimen, using two consecutive slides that underwent EVG staining with different batches of stain, and we confirmed the reproducibility of the fiber measurements (P = 0.59 for collagen, P = 0.68 for elastin; two‐sided paired t test). There were no significantly positive correlations of biopsy size (length or area) with the area ratios of collagen fiber, elastin fiber, or both fibers combined or with fibrosis stage (Spearman correlation coefficient r < 0.09). In addition, 38 NAFLD cases that had two biopsy cores in each section were analyzed for the amounts of hepatic fiber, and we did not observe any significant differences between cores (P = 0.20 for collagen, P = 0.50 for elastin; two‐sided paired t test). To characterize the clinicopathologic significance of the hepatic fiber amounts, we categorized patients into tertiles according to the combined area ratios of collagen and elastin fibers (tertile 1, 0.677%‐2.805%; tertile 2, 2.806%‐4.777%; tertile 3, 4.778%‐42.953%).
In an exploratory analysis, we conducted Sirius Red staining using van Gieson's solution (saturated picric acid containing 0.3% Sirius Red) on specimens from 6 selected patients with NAFLD to compare results with those of EVG staining and found that the sublocalization of elastin and collagen was generally different, although some areas of collagen were likely covered by elastin staining in EVG‐stained sections (Supporting Fig. S2). These findings might be because of the principle of the EVG staining in which resorcin–fuchsin combines and masks elastin areas to preclude the following collagen stain. Indeed, the area ratios of collagen in Sirius Red‐stained sections were higher than those in EVG‐stained sections (P = 0.012; two‐sided paired t test) but were lower than the combined fiber area ratios by EVG staining (P = 0.004; Supporting Table S2).
HISTOLOGIC EVALUATION OF LIVER BIOPSY SPECIMENS
Histopathologic assessment was performed using Brunt's criteria; NAFLD/NASH was classified according to the necroinflammatory activity (grade 0, absent; grade 1, mild; grade 2, moderate; and grade 3, severe) and the extent of fibrosis with or without architectural remodeling (stage 0, no fibrosis; stage 1, zone 3 fibrosis; stage 2, zone 3 and portal fibrosis; stage 3, zone 3 and portal fibrosis with bridging fibrosis; and stage 4, cirrhosis).39, 40 The degrees of steatosis, ballooning, and lobular inflammation were graded using a NAFLD activity scoring system.41 The histologic evaluation for the current data set was conducted and carefully validated by three board‐certified liver pathologists (M.K., M.S., and H.T.) who were blinded to the clinical data, as described.21
CLINICAL AND LABORATORY DATA
We collected patient information, including age at diagnosis, sex, weight, and height.21 The body mass index (BMI) was calculated as the weight (kg) divided by the square of the height (m). Blood samples were obtained in the morning after overnight fasting either immediately before or no more than 2 months after liver biopsy. We measured biochemical variables using conventional automated analyzers at the respective hospitals. Data were collected for platelet count; prothrombin time (activity); and serum levels of bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma‐glutamyl transpeptidase, albumin, cholesterol, triglyceride, fasting plasma glucose, and 7s collagen.21 The FIB‐4 index was calculated as follows:19
STATISTICAL ANALYSIS
Statistical analyses were conducted using SPSS version 22.0 (Chicago, IL), and all P values were two‐sided. To assess the correlations of the fiber area ratios (i.e., the area ratios of collagen fiber, elastin fiber, and both fibers combined, as continuous variables) with Brunt's fibrosis stage (ranging from 0 to 4, as an ordinal variable), we performed the Spearman correlation test. We conducted the Games–Howell test to compare the distributions of fiber area ratios between adjacent categories of fibrosis stage (0 versus 1, 1 versus 2, 2 versus 3, and 3 versus 4), adjusting the P value for significance to 0.004 (≈0.05/12) by Bonferroni correction for multiple hypothesis testing. To test the linearity of the relationship between fibrosis stage and fiber area ratios, we performed the score test for the proportional odds assumption in the univariable ordinal logistic regression model for Brunt's fibrosis stage (0‐4, as an ordinal outcome variable). To assess the linear relationships between continuous variables, including fiber area ratios and the laboratory data, the Pearson correlation test was performed.
To assess associations of the tertile categories of the combined fiber area ratios with categorical clinicopathologic characteristics (except for lobular inflammation for which Fisher's exact test was performed), the chi‐square test was performed. To compare continuous clinical data, analysis of variance was performed. Because we examined the associations of the combined fiber amount with 21 clinicopathologic characteristics, we adjusted the two‐sided α level to 0.002 (≈0.05/21) by simple Bonferroni correction.
Multivariable binary logistic regression analyses were performed to assess the association between noninvasive fibrosis biomarkers (predictor) and the combined fiber amount (outcome). We tested two models using two different cutoff levels for the combined fiber area ratios as an outcome variable (tertiles 1/2 versus tertile 3 and tertile 1 versus tertiles 2/3). In the multivariable logistic regression analysis, we analyzed 204 cases with full sets of laboratory data. The multivariable model initially included the age at diagnosis (continuous), sex (female versus male), smoking history (current/past versus negative versus missing [12.1%]), BMI (continuous), platelet count (continuous), prothrombin time (continuous), bilirubin (continuous), AST (continuous), ALT (continuous), gamma‐glutamyl transpeptidase (continuous), albumin (continuous), cholesterol (continuous), triglyceride (continuous), fasting plasma glucose (continuous), 7s collagen (continuous), and the FIB‐4 index (continuous). Backward elimination with a threshold of P = 0.05 was used to select covariates for the final model. Because we examined the 16 predictor variables in each binary logistic regression analysis model, we adjusted the two‐sided α level to 0.003 (≈0.05/16) for multiple hypothesis testing.
Results
Using an automated computational method, we measured the area ratios of collagen and elastin fibers in EVG‐stained whole‐slide images of liver biopsy specimens from 289 patients with NAFLD. The area ratios of collagen fiber (range, 0.6%‐31.3%; median, 3.2%; mean, 4.7%), the area ratios of elastin fiber (range, 0.1%‐12.2%; median, 0.4%; mean, 1.0%), and the combined area ratios of the two fibers (range, 0.7%‐43.0%; median, 3.6%; mean, 5.7%) had skewed distributions (Supporting Fig. S3). The area ratios of collagen fiber, elastin fiber, and the two fibers combined all correlated positively with Brunt's fibrosis stage by the Spearman correlation test (Spearman correlation coefficients r = 0.76, 0.60, and 0.78, respectively; all P < 0.0001). The relationships between the area ratios of fiber deposition and the histologic fibrosis stage of NAFLD are illustrated in Fig. 1. The association of the combined area ratio of collagen and elastin fibers with Brunt's fibrosis stage appeared to be nonlinear with a striking difference in the combined fiber amounts between fibrosis stage 4 (median of the combined fiber area ratios, 12.3%) and stage 3 (median area ratio, 4.8%; P < 0.0001); the differences among stages 0‐3 (median area ratios, 2.1%, 2.8%, 4.3%, and 4.8%, respectively) were much smaller (Fig. 1A). The relationship of the fibrosis stage with the collagen fiber amount was similar to its association with the combined fiber area ratios (Fig. 1B). There was a significant difference in the area ratio of elastin fiber between stage 4 (median area ratio, 2.1%) and stages 0‐3 (median area ratios, 0.2%‐0.5%; Fig. 1C). Although there was a trend toward higher amounts of fiber in stage 3 (median of the combined area ratios, 4.8%) than in stage 2 (median area ratio, 4.3%), we did not observe any statistically significant differences in fiber area ratios (P = 0.70 for collagen fiber, P = 0.034 for elastin fiber, P = 0.16 for both fibers combined; Games–Howell test; Fig. 1) with the adjusted α level of 0.004 for multiple hypothesis testing. The nonlinearity of the association between the combined fiber area ratios and Brunt's fibrosis stage was confirmed by the score test for the proportional odds assumption (P = 0.007) in the univariable ordinal logistic regression model for the fibrosis stage (0‐4, as an ordinal outcome variable).
Figure 1.
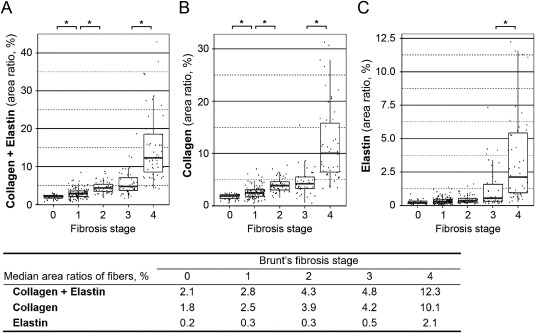
Scattergrams and box plots showing the relationships of Brunt's fibrosis stage with the combined area ratio of collagen and elastin fibers (A), the area ratio of collagen fiber (B), or that of elastin fiber (C). Asterisks indicate significant differences in the amount of fibers between fibrosis stages (all P < 0.0001 by the Games–Howell test). The ends of the vertical lines (whiskers) indicate the minimum and maximum values, unless outliers are present in which case the whiskers extend to a maximum of 1.5 times the inter‐quartile range (boxes). The table (bottom) shows the median area ratios of fibers for each fibrosis stage.
Histologic review of liver biopsy slides was performed to clarify the localization of collagen and elastin fiber deposition in different parts of the liver acinus (Fig. 2). With the current quantitative analysis system, native collagen fibers in the liver parenchyma were detected as the main component of the extracellular matrix in portal tracts and in the walls of the hepatic vein (Supporting Fig. S4). Small amounts of native elastin fiber were also detected by the image analysis system in portal stroma and in the elastic lamina of blood vessels (Supporting Fig. S4). Fibrotic areas in NAFLD liver biopsy specimens mainly consisted of collagen fiber deposition (Fig. 2). Zone 3 perisinusoidal deposition of collagen fibers was a characteristic finding of stage 1 NAFLD and was also observed across stages 2‐4. Wide variations in the severity of bridging fibrosis were observed in advanced NAFLD (stages 3/4); thin and/or focal bridging fibrosis was a common finding in stage 3, whereas thick and extensive bridging (so‐called fibrous septa) was frequent in stage 4. Increased elastin deposition was often found in areas of thick bridging fibrosis and thickened venous walls but was uncommon in areas of perisinusoidal fibrosis.
Figure 2.
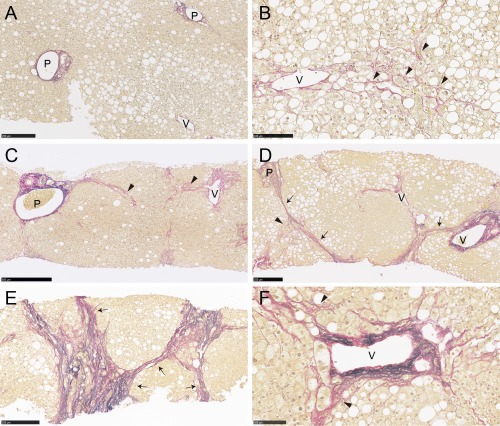
Histologic localization of collagen and elastin fibers in liver biopsy tissues from patients with NAFLD. Collagen and elastin fibers were stained red and black, respectively, by EVG staining. Fibrotic areas in liver biopsy specimens were mainly composed of collagen fibers. (A) Native collagen fibers were found in portal tracts and hepatic veins. (B) Zone 3 perisinusoidal fibrosis (arrowheads) was a characteristic finding of stage 1 NAFLD and was also seen in stages 2‐4. (C) Patients with zone 3 and portal fibrosis were categorized as stage 2. (D,E) Bridging fibrosis (arrows) that links vascular structures is a hallmark of advanced NAFLD (stages 3/4). Thick and extensive bridging fibrosis was infrequent in stage 3 but was common in stage 4. (F) Increased elastin deposition was often found in areas of thick bridging fibrosis and thickened venous walls but was uncommon in areas of perisinusoidal fibrosis. Stage 0 (A), 1 (B), 2 (C), 3 (D), and 4 (E); EVG stain (A‐F). Scale bars, 500 μm (C), 250 μm, (A,D,E), 100 μm (B,F). Abbreviations: P, portal tract; V, hepatic vein.
To characterize the clinicopathologic significance of the hepatic fiber amounts, we categorized patients into tertiles according to the combined area ratios of collagen and elastin fibers. The clinical and pathologic features according to the combined fiber amount in 289 patients with NAFLD are summarized in Table 1. A higher combined amount of fiber deposition was significantly associated with a higher age (P < 0.0001), higher BMI (P = 0.0007), lower platelet count (P < 0.0001), prolonged prothrombin time (P < 0.0001), higher serum levels of AST (P < 0.0001) and 7s collagen (P < 0.0001), lower serum level of albumin (P < 0.0001), higher FIB‐4 index (P < 0.0001), higher Brunt's activity grade (P < 0.0001), higher degrees of lobular inflammation (P < 0.0001) and ballooning (P < 0.0001) as established by the NAFLD activity scoring system, and higher Brunt's fibrosis stage (P < 0.0001). The combined fiber amount was not significantly associated with the other characteristics examined (P > 0.002; with an adjusted α level of 0.002; Table 1). In addition, we calculated Pearson correlation coefficients to assess the linear relationships between the combined fiber area ratios (as a continuous variable) and laboratory data (as continuous variables; Supporting Table S3). We found moderate linear correlations with 7s collagen (Pearson correlation coefficient r = 0.53; Fig. 3A) and FIB‐4 index (r = 0.43; Fig. 3B) and weak inverse correlations with prothrombin time (r = −0.39), platelet count (r = −0.38), and serum levels of albumin (r = −0.28) and cholesterol (r = −0.24).
Table 1.
CLINICOPATHOLOGIC CHARACTERISTICS ACCORDING TO THE COMBINED FIBER AMOUNT IN LIVER BIOPSY SPECIMENS FROM 289 PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE
| Combined Area Ratios of Collagen and Elastin Fibers in Liver Biopsy Specimensa | |||||
|---|---|---|---|---|---|
| Characteristicsb |
Total No. (n = 289) |
Tertile 1 (n = 96) |
Tertile 2 (n = 96) |
Tertile 3 (n = 97) |
P Valuec |
| Median area ratio of fibers, % | |||||
| Collagen + Elastin | 3.6 | 2.1 | 3.6 | 8.2 | |
| Collagen | 3.2 | 1.8 | 3.2 | 6.4 | |
| Elastin | 0.4 | 0.2 | 0.4 | 1.2 | |
| Age, years | 54.8 ± 14.6 | 52.2 ± 16.1 | 52.0 ± 14.2 | 60.0 ± 11.9 | <0.0001 |
| Sex | 0.08 | ||||
| Male | 159 (55%) | 52 (54%) | 61 (64%) | 46 (47%) | |
| Female | 130 (45%) | 44 (46%) | 35 (36%) | 51 (53%) | |
| Smoking history | 0.56 | ||||
| Negative | 172 (68%) | 62 (72%) | 52 (66%) | 58 (65%) | |
| Current/Past | 82 (32%) | 24 (28%) | 28 (34%) | 31 (35%) | |
| BMI, kg/m2 | 27.6 ± 4.7 | 26.2 ± 3.8 | 28.6 ± 5.1 | 28.1 ± 4.8 | 0.0007 |
| Platelet count, 109/L | 18.9 ± 6.8 | 20.8 ± 6.4 | 20.6 ± 6.3 | 15.4 ± 6.4 | <0.0001 |
| Prothrombin time, % | 99.3 ± 16.7 | 106.7 ± 17.3 | 100.9 ± 11.8 | 90.2 ± 16.2 | <0.0001 |
| Bilirubin, mg/dL | 1.0 ± 0.6 | 0.9 ± 0.5 | 0.9 ± 0.4 | 1.1 ± 0.8 | 0.008 |
| AST, U/L | 61.4 ± 48.9 | 42.4 ± 24.1 | 65.8 ± 54.4 | 75.9 ± 55.4 | <0.0001 |
| ALT, U/L | 85.5 ± 68.9 | 66.0 ± 52.7 | 97.3 ± 72.1 | 93.2 ± 75.9 | 0.003 |
| GGT, U/L | 92.3 ± 89.9 | 86.7 ± 99.3 | 89.2 ± 84.4 | 101.1 ± 85.6 | 0.50 |
| Albumin, g/dL | 4.2 ± 0.4 | 4.3 ± 0.4 | 4.2 ± 0.4 | 4.0 ± 0.5 | <0.0001 |
| Cholesterol, mg/dL | 195.4 ± 41.1 | 202.7 ± 44.2 | 196.5 ± 34.1 | 186.8 ± 43.0 | 0.028 |
| Triglyceride, mg/dL | 144.4 ± 77.2 | 136.2 ± 69.8 | 153.5 ± 89.3 | 143.4 ± 70.5 | 0.30 |
| FPG, mg/dL | 115.2 ± 38.4 | 112.4 ± 35.9 | 111.5 ± 39.2 | 121.7 ± 39.7 | 0.14 |
| 7s collagen, ng/mL | 5.5 ± 2.2 | 4.4 ± 1.3 | 5.0 ± 1.5 | 6.9 ± 2.6 | <0.0001 |
| FIB‐4 index | 2.5 ± 2.0 | 1.7 ± 1.4 | 2.0 ± 1.6 | 3.7 ± 2.3 | <0.0001 |
|
Activity grade (Brunt's criteria) |
<0.0001 | ||||
| 0 | 43 (15%) | 33 (34%) | 9 (9%) | 1 (1%) | |
| 1 | 96 (33%) | 37 (39%) | 40 (42%) | 19 (20%) | |
| 2 | 70 (24%) | 17 (18%) | 26 (27%) | 27 (28%) | |
| 3 | 80 (28%) | 9 (9%) | 21 (22%) | 50 (52%) | |
| Steatosis | 0.028 | ||||
| 5%‐33% | 64 (22%) | 31 (32%) | 17 (18%) | 16 (16%) | |
| > 33%‐66% | 125 (43%) | 40 (42%) | 45 (47%) | 40 (41%) | |
| > 66% | 100 (35%) | 25 (26%) | 34 (35%) | 41 (42%) | |
| Lobular inflammation | <0.0001 | ||||
| No foci | 10 (3%) | 7 (7%) | 3 (3%) | 0 (0%) | |
| < 2 foci per 200× field | 130 (45%) | 64 (67%) | 42 (44%) | 24 (25%) | |
| 2‐4 foci per 200× field | 133 (46%) | 22 (23%) | 48 (50%) | 63 (65%) | |
| >4 foci per 200× field | 16 (6%) | 3 (3%) | 3 (3%) | 10 (10%) | |
| Ballooning | <0.0001 | ||||
| None | 43 (15%) | 33 (34%) | 9 (9%) | 1 (1%) | |
| Few balloon cells | 96 (33%) | 37 (39%) | 40 (42%) | 19 (20%) | |
| Many cells/prominent | 150 (52%) | 26 (27%) | 47 (49%) | 77 (79%) | |
|
Fibrosis stage (Brunt's criteria) |
<0.0001 | ||||
| 0 | 35 (12%) | 33 (34%) | 2 (2%) | 0 (0%) | |
| 1 | 113 (39%) | 52 (54%) | 49 (51%) | 12 (12%) | |
| 2 | 49 (17%) | 5 (5%) | 29 (30%) | 15 (15%) | |
| 3 | 41 (14%) | 6 (6%) | 14 (15%) | 21 (22%) | |
| 4 | 51 (18%) | 0 (0%) | 2 (2%) | 49 (51%) | |
Patients were categorized into tertiles according to the combined area ratios of collagen and elastin fibers in liver biopsy specimens, i.e., tertile 1 (range, 0.677%‐2.805%), tertile 2 (2.806%‐4.777%), and tertile 3 (4.778%‐42.953%).
Continuous patient data are expressed as means ± SD. Percentages for categorical variables indicate the proportion of cases with a specific cliniopathologic feature among patients in each tertile of the combined fiber area ratios.
The chi‐square test was used to assess associations between the combined fiber amount and categorical data (except for lobular inflammation for which Fisher's exact test was performed). To compare continuous variables, an analysis of variance was performed. We adjusted the two‐sided α level to 0.002 (≈0.05/21) by simple Bonferroni correction for multiple hypothesis testing.
Abbreviations: FPG, fasting plasma glucose; GGT, gamma‐glutamyl transpeptidase.
Figure 3.
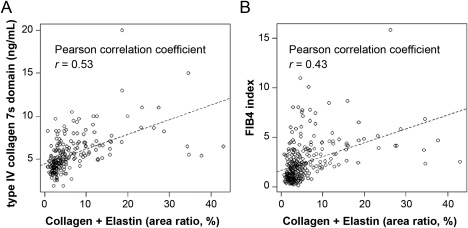
Scatterplots with regression lines showing the correlation of the combined fiber area ratios with the (A) serum concentration of 7s collagen or (B) the FIB‐4 index.
In our exploratory analysis, we further conducted multivariable binary logistic regression analysis to assess the association of noninvasive fibrosis markers with the combined fiber amounts (Table 2), with the use of two different cutoff levels for the combined fiber area ratios as an outcome variable. In the model for the combined fiber area ratios (tertiles 1/2 versus tertile 3, as a binary outcome variable), the highest tertile of the combined fiber area ratios was significantly associated with a higher serum level of 7s collagen (odds ratio [OR], 1.76; 95% confidence interval [CI], 1.31‐2.36; P = 0.0002) and a higher FIB‐4 index (OR, 1.68; 95% CI, 1.23‐2.30; P = 0.001), adjusting the P value for significance to 0.003 for multiple hypothesis testing. The multivariable binary logistic regression analysis model for the combined fiber area ratios (tertile 1 versus tertiles 2/3, as a binary outcome variable) revealed that a higher BMI (OR, 1.15; 95% CI, 1.06‐1.26; P = 0.002) and higher levels of serum AST (OR, 1.38; 95% CI, 1.17‐1.63; P = 0.0001) and 7s collagen (OR, 1.85; 95% CI, 1.30‐2.65; P = 0.0007) were independent factors to correlate with the upper two tertiles of the combined fiber area ratios.
Table 2.
MULTIVARIABLE BINARY LOGISTIC REGRESSION ANALYSIS TO CALCULATE ADJUSTED ODDS RATIOS FOR ASSOCIATIONS OF VARIABLES WITH THE COMBINED FIBER AMOUNT (OUTCOME) IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE
| Variables Remaining in the Final Multivariable Model | Multivariable OR (95% CI) | P Valuec |
|---|---|---|
| Model for tertiles 1/2 vs tertile 3 of the combined fiber area ratios as a binary outcome variable (n = 204)a | ||
| BMI (for a unit of 1 kg/m2 increase) | 1.08 (1.00‐1.16) | 0.040 |
| AST (for a unit of 10 U/L increase) | 0.82 (0.67‐1.01) | 0.06 |
| ALT (for a unit of 10 U/L increase) | 1.15 (1.02‐1.30) | 0.022 |
| 7s collagen (for a unit of 1 ng/mL increase) | 1.76 (1.31‐2.36) | 0.0002 |
| FIB‐4 index (for a unit of 1 increase) | 1.68 (1.23‐2.30) | 0.001 |
| Model for tertile 1 vs tertiles 2/3 of the combined fiber area ratios as a binary outcome variable (n = 204)b | ||
| Female (vs male) | 2.48 (1.08‐5.71) | 0.032 |
| BMI (for a unit of 1 kg/m2 increase) | 1.15 (1.06‐1.26) | 0.002 |
| Platelet count (for a unit of 5 ×109/L decrease) | 1.49 (1.00‐2.23) | 0.05 |
| Prothrombin time (for a unit of 10% decrease) | 1.29 (0.97‐1.70) | 0.08 |
| AST (for a unit of 10 U/L increase) | 1.38 (1.17‐1.63) | 0.0001 |
| 7s collagen (for a unit of 1 ng/mL increase) | 1.85 (1.30‐2.65) | 0.0007 |
| FIB‐4 index (for a unit of 1 increase) | 0.76 (0.55‐1.05) | 0.09 |
Adjusted ORs were calculated using a multivariable binary logistic regression model for the combined fiber area ratios (tertiles 1/2 versus tertile 3, as a binary outcome variable). The multivariable binary logistic regression analysis model initially included age, sex, smoking history, BMI, platelet count, prothrombin time, bilirubin, AST, ALT, gamma‐glutamyl transpeptidase, albumin, cholesterol, triglyceride, fasting plasma glucose, 7s collagen, and FIB‐4 index. Backward elimination with a threshold of P = 0.05 was used to select the variables for the final model.
Adjusted ORs were calculated using a multivariable binary logistic regression model for the combined fiber area ratios (tertiles 1 versus tertiles 2/3, as a binary outcome variable). The multivariable binary logistic regression analysis model initially included the same set of variables as described above, and we selected variables for the final model by backward elimination with a threshold of P = 0.05.
Because we examined the 16 predictor variables for each model, we adjusted the two‐sided α level to 0.003 (≈0.05/16) by Bonferroni correction for multiple hypothesis testing.
Discussion
Quantitative fibrosis analysis revealed a strong but nonlinear relationship between the histologic fibrosis stage and hepatic fiber amount and found a striking difference in fiber accumulation between stage 3 and stage 4 and much smaller differences among stages 0‐3, indicating the heterogeneity in disease severity within NAFLD‐related cirrhosis. Although histologic staging for NAFLD/NASH is the gold standard assessment of liver fibrosis, current semiquantitative staging systems are not continuous quantitative measures of hepatic fibrosis and have no stage beyond cirrhosis (stage 4).16 Liver cirrhosis is not a “single disease state,” as fibrosis progression and parenchymal remodeling continue even though the final fibrotic stage has been reached.32, 42 These lines of evidence, together with our findings, likely underscore the importance of subclassification of cirrhosis for the development of a continuous staging system for NAFLD.
The extent of liver fibrosis in NASH has been frequently discussed as defining two risk groups: early fibrosis (stages1/2) and advanced fibrosis (stages 3/4).9, 43, 44 Previous evidence has shown that advanced fibrosis is associated with increased risk of all‐cause and liver‐related mortality.10, 45 Intriguingly, our quantitative analysis did not show any significant differences in fiber area ratios between stages 2 and 3. A histologic difference between the two stages is the presence or absence of bridging fibrosis. Although there were wide variations in the severity of bridging within advanced NAFLD, we found relatively limited areas of bridging fibrosis at stage 3, which likely helps to explain the absence of a statistically significant increase in the amount of fiber between stages 2 and 3. Because bridging fibrosis links vascular structures, leads to alteration of the regional blood flow, and may thereby promote liver injury, the detection of the architectural histologic feature of bridging fibrosis by pathologists is noteworthy.45 Nonetheless, the retrospective study with a large sample size (n = 619) has demonstrated an increased mortality risk for patients with NAFLD with each successive fibrosis stage (ranging from 0 to 4),9 suggesting that the binary staging system (early versus advanced fibrosis) is likely arbitrary. Taken together, an accurate fibrosis assessment of liver biopsy specimens based on the semiquantitative histologic grading of structural changes in combination with quantitative fiber measurements may be valuable for the improvement of clinical staging for NAFLD/NASH.
Evidence suggests that fibrosis can progress but it may also regress during the disease course of NAFLD.45 Considering the biochemically stable nature of elastin compared with collagen,46 elastin accumulation in the liver may contribute to the irreversibility of the disease. However, the potential role of elastin fiber deposition in the irreversibility of chronic liver diseases remains poorly understood. Our quantitative fibrosis data suggest that elastin fiber is a minor fibrous component in the early stages of fibrosis but may accumulate in areas of thick bridging fibrosis, fibrous septa, or venous walls at the cirrhosis stage of NAFLD, a finding that is consistent with studies on various chronic liver diseases.46, 47 Our previous study attested to the positive association of higher elastin area ratios (≥3.6%) with the risk of hepatocellular carcinoma in patients with hepatitis C virus infection.36 In the current NAFLD cohort, 22 (24%) of 92 patients with advanced NAFLD (4 of 41 with stage 3; 18 of 51 with stage 4) had elastin area ratios greater than or equal to 3.6%, whereas none of 162 patients with early fibrosis with stages 1/2 harbored this amount of elastin in liver biopsy specimens. Future studies may clarify the clinical significance of elastin deposition in NAFLD in relation to disease reversibility as well as clinical outcomes, including patient mortality, the development of liver cancer, and therapeutic responses.
In our current analysis, the serum level of type IV collagen 7s domain (7s collagen) was the sole significant factor consistently associated with fiber amounts in the binary logistic regression analysis models using two different cutoff levels for the combined area ratios of collagen and elastin fibers. In addition, among the laboratory data examined, the serum concentration of 7s collagen showed the strongest linear association with the combined fiber area ratios. Previous studies have shown that serum 7s collagen is useful for predicting the severity of liver fibrosis in patients with NASH,20, 48 which is consistent with our findings and may support the reliability of our quantitative measurements on fibers. Previous immunohistochemical analysis revealed increased expression of type IV collagen in the perisinusoidal spaces and extended fibrous areas within fibrotic livers.49 These findings suggest that 7s collagen in the circulation may reflect a rapid turnover of basement membrane, which is mainly composed of laminin and type IV collagen, during extracellular matrix remodeling in hepatic fibrogenesis.50 Therefore, serum 7s collagen appears to be a rational biomarker for the accurate estimation of fibrosis levels in patients with NAFLD.
One limitation of the current study is its cross‐sectional nature. Hence, we were not able to assess changes in the amount of hepatic fiber over the time course of NAFLD progression or determine a critical point of irreversibility for the disease. Future prospective studies on patients with NAFLD are needed to address these issues. We recognize the limitations in the semiquantitative staging method of liver fibrosis. The semiquantitative histologic assessments were performed and validated by three liver pathologists21 with the use of the fibrosis staging system proposed by Brunt's criteria, which is a globally accepted standardized method in pathology practice.7, 14, 15 In addition, we found a strong correlation between Brunt's fibrosis stage and the quantitative data on the combined fiber area ratios (Spearman correlation coefficient r = 0.78; P < 0.0001), supporting the validity of our semiquantitative fibrosis assessments. Another limitation is possible variations in disease severity of patients with NAFLD between hospitals. We collected samples from four different hospitals across Japan to generalize and empower our analyses. Nonetheless, histopathologic assays, including EVG staining and automated quantification of hepatic fibers, were performed at one institution (Department of Pathology, Keio University School of Medicine) to reduce institutional bias. Biochemical analyses were done at the respective hospitals; however, all the laboratory data were generated by conventional automated analyzers.
The strengths of this study include the automated nature of the computational image analysis of hepatic fiber accumulation in liver biopsy specimens.37 This provided us with highly reproducible data. Additionally, our quantitative fibrosis analysis used a reasonably large sample size (n = 289) with comprehensive patient data, allowing us to rigorously investigate the association of the clinicopathologic features of NAFLD with hepatic fiber amounts, controlling for potential confounders.
In conclusion, computer‐assisted image analysis on collagen and elastin fibers has uncovered a nonlinear relationship between fibrosis stage and fiber amount in liver biopsy specimens from patients with NAFLD. Upon validation, our quantitative assessment of hepatic fibrosis may provide a better understanding of NAFLD progression and can likely inform translational research and clinical practice on the development of management strategies for patients with NAFLD/NASH.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1121/full.
Supporting Information 1
Supporting Information S1
Supporting Information S2
Supporting Information S3
Supporting Information S4
Acknowledgment
This research was performed by the Hepatitis Glyco‐Biomarker Study Group.
Potential conflict of interest: Dr. Hino was funded by Merck Sharp & Dohme, Bristol Meyers Squibb, Ajinomoto Pharmaceutical, Gilead Sciences, and Otsuka Pharmaceutical. Dr. Mizokami served as a consultant to Gilead Sciences and was funded by Gilead Sciences and Sysmex Co. This study was not funded by any of these companies. The other authors have nothing to report.
Supported by a Grant‐in Aid for Scientific Research (B) (26293179) from the Japan Society for the Promotion of Science to K.H. and by the Research Program on Hepatitis (16fk0210116h0001) from the Japan Agency for Medical Research and Development to K.H.
REFERENCES
- 1. Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016;64:19‐22. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 3. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing‐steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148‐1155. [DOI] [PubMed] [Google Scholar]
- 4. Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, et al.; LIDO Study Group . A systematic review of follow‐up biopsies reveals disease progression in patients with non‐alcoholic fatty liver. J Hepatol 2013;59:550‐556. [DOI] [PubMed] [Google Scholar]
- 5. Calzadilla Bertot L, Adams LA. The natural course of non‐alcoholic fatty liver disease. Int J Mol Sci 2016;17:pii:E774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kleiner DE, Makhlouf HR. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children. Clin Liver Dis 2016;20:293‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakamoto M, Tsujikawa H, Effendi K, Ojima H, Harada K, Zen Y, et al. Pathological findings of nonalcoholic steatohepatitis and nonalcoholic fatty liver disease. Pathol Int 2017;67:1‐7. [DOI] [PubMed] [Google Scholar]
- 8. Rinella ME, Sanyal AJ. Management of NAFLD: a stage‐based approach. Nat Rev Gastroenterol Hepatol 2016;13:196‐205. [DOI] [PubMed] [Google Scholar]
- 9. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 11. Unalp‐Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology 2017;66:84‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams LA, Anstee QM, Tilg H, Targher G. Non‐alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138‐1153. [DOI] [PubMed] [Google Scholar]
- 13. Francque SM, van der Graaff D, Kwanten WJ. Non‐alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol 2016;65:425‐443. [DOI] [PubMed] [Google Scholar]
- 14. 1Brunt EM . Nonalcoholic fatty liver disease: pros and cons of histologic systems of evaluation. Int J Mol Sci 2016;17:pii.E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bedossa P. Pathology of non‐alcoholic fatty liver disease. Liver Int 2017;37(Suppl. 1):85‐89. [DOI] [PubMed] [Google Scholar]
- 16. Almpanis Z, Demonakou M, Tiniakos D. Evaluation of liver fibrosis: “Something old, something new...”. Ann Gastroenterol 2016;29:445‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hannah WN Jr, Harrison SA. Noninvasive imaging methods to determine severity of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2016;64:2234‐2243. [DOI] [PubMed] [Google Scholar]
- 18. Vallet‐Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin‐Venier V, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 19. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network . Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoneda M, Mawatari H, Fujita K, Yonemitsu K, Kato S, Takahashi H, et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J Gastroenterol 2007;42:375‐381. [DOI] [PubMed] [Google Scholar]
- 21. Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin‐positive Mac‐2 binding protein and the fibrosis stage of non‐alcoholic fatty liver disease. J Gastroenterol 2015;50:776‐784. [DOI] [PubMed] [Google Scholar]
- 22. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non‐invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016;65:1006‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mizuno M, Shima T, Oya H, Mitsumoto Y, Mizuno C, Isoda S, et al. Classification of patients with nonalcoholic fatty liver disease using rapid immunoassay of serum type IV collagen compared with that using liver histology and other fibrosis markers. Hepatol Res 2016:Mar 20. [DOI] [PubMed] [Google Scholar]
- 24. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Unalp‐Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology 2016;63:1170‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:782‐789.e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017:pii:S0169‐409X(17)30063‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oseini AM, Sanyal AJ. Therapies in non‐alcoholic steatohepatitis (NASH). Liver Int 2017;37(Suppl. 1):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barry‐Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase‐like‐2 impedes the development of a pathologic microenvironment. Nat Med 2010;16:1009‐1017. [DOI] [PubMed] [Google Scholar]
- 30. Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 2013;8:e83481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manousou P, Burroughs AK, Tsochatzis E, Isgro G, Hall A, Green A, et al. Digital image analysis of collagen assessment of progression of fibrosis in recurrent HCV after liver transplantation. J Hepatol 2013;58:962‐968. [DOI] [PubMed] [Google Scholar]
- 32. Tsochatzis E, Bruno S, Isgro G, Hall A, Theocharidou E, Manousou P, et al. Collagen proportionate area is superior to other histological methods for sub‐classifying cirrhosis and determining prognosis. J Hepatol 2014;60:948‐954. [DOI] [PubMed] [Google Scholar]
- 33. Calvaruso V, Burroughs AK, Standish R, Manousou P, Grillo F, Leandro G, et al. Computer‐assisted image analysis of liver collagen: relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology 2009;49:1236‐1244. [DOI] [PubMed] [Google Scholar]
- 34. Pirhonen J, Arola J, Sadevirta S, Luukkonen P, Karppinen SM, Pihlajaniemi T, et al. Continuous grading of early fibrosis in NAFLD using label‐free imaging: a proof‐of‐concept study. PLoS One 2016;11:e0147804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang Y, de Boer WB, Adams LA, MacQuillan G, Bulsara MK, Jeffrey GP. Image analysis of liver biopsy samples measures fibrosis and predicts clinical outcome. J Hepatol 2014;61:22‐27. [DOI] [PubMed] [Google Scholar]
- 36. Yasui Y, Abe T, Kurosaki M, Higuchi M, Komiyama Y, Yoshida T, et al. Elastin fiber accumulation in liver correlates with the development of hepatocellular carcinoma. PLoS One 2016;11:e0154558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abe T, Hashiguchi A, Yamazaki K, Ebinuma H, Saito H, Kumada H, et al. Quantification of collagen and elastic fibers using whole‐slide images of liver biopsy specimens. Pathol Int 2013;63:305‐310. [DOI] [PubMed] [Google Scholar]
- 38. Murakami Y, Abe T, Hashiguchi A, Yamaguchi M, Saito A, Sakamoto M. Color correction for automatic fibrosis quantification in liver biopsy specimens. J Pathol Inform 2013;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467‐2474. [DOI] [PubMed] [Google Scholar]
- 40. Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012;32:3‐13. [DOI] [PubMed] [Google Scholar]
- 41. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 42. Garcia‐Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Guideline C: National Institute for Health and Care Excellence: Guidance In: Non‐Alcoholic Fatty Liver Disease: Assessment and Management. London, UK: National Institute for Health and Care Excellence (UK) 2016. [PubMed] [Google Scholar]
- 44. Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brunt EM. Nonalcoholic fatty liver disease and the ongoing role of liver biopsy evaluation. Hepatol Commun 2017;1:370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanta J. Elastin in the liver. Front Physiol 2016;7:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakayama H, Itoh H, Kunita S, Kuroda N, Hiroi M, Matsuura H, et al. Presence of perivenular elastic fibers in nonalcoholic steatohepatitis fibrosis stage III. Histol Histopathol 2008;23:407‐409. [DOI] [PubMed] [Google Scholar]
- 48. Sakugawa H, Nakayoshi T, Kobashigawa K, Yamashiro T, Maeshiro T, Miyagi S, et al. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol 2005;11:255‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mak KM, Chen LL, Lee TF. Codistribution of collagen type IV and laminin in liver fibrosis of elderly cadavers: immunohistochemical marker of perisinusoidal basement membrane formation. Anat Rec (Hoboken) 2013;296:953‐964. [DOI] [PubMed] [Google Scholar]
- 50. Mak KM, Mei R. Basement membrane type IV collagen and laminin: an overview of their biology and value as fibrosis biomarkers of liver disease. Anat Rec (Hoboken) 2017;300:1371‐1390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1121/full.
Supporting Information 1
Supporting Information S1
Supporting Information S2
Supporting Information S3
Supporting Information S4


