Abbreviations
- CEACAM1
carcinoembryonic antigen‐related cell adhesion molecule 1
- FASN
fatty acid synthase
- GLP‐1
glucagon‐like peptide‐1
- HFD
high‐fat diet
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PPARγ
peroxisome proliferator‐activated receptor gamma
Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent liver diseases in industrialized countries, with approximately 30%‐40% of adults suffering from NAFLD. Of those, 15%‐20% will develop to its progressive entity nonalcoholic steatohepatitis (NASH), a condition that may further progress to liver fibrosis and cirrhosis.1 Because insulin resistance and visceral obesity are major contributors in the pathogenesis of NAFLD/NASH, weight loss, exercise, and insulin‐sensitizing drugs are considered as primary treatment regimens. Obeticholic acid, a farnesoid X nuclear receptor agonist, previously approved for the treatment for primary biliary cholangitis, is currently in a phase III trial for patients with NASH fibrosis. In the United States, no effective treatments for NAFLD/NASH have been approved by the U.S. Food and Drug Administration.
Potential therapeutic agents for NAFLD include antidiabetic medications, such as pioglitazone, a peroxisome proliferator‐activated receptor gamma (PPARγ) agonist, and exenatide, a long‐acting glucagon‐like peptide‐1 (GLP‐1) receptor agonist. GLP‐1 is derived from the proglucagon molecule. In pancreatic α cells, the proglucagon molecule is processed to glucagon, which increases blood glucose levels. In the gut, GLP‐1 and GLP‐2 are produced from the same proglucagon molecule. Interestingly, GLP‐1 suppresses blood glucose levels by stimulating pancreatic β cells to secrete insulin, which is in contrast to glucagon.2, 3 Because the mechanism of action of GLP‐1 receptor agonists is to stimulate insulin secretion to improve insulin resistance and sensitivity, exenatide has been shown to reverse steatohepatitis and is thus a potential therapeutic agent.2, 4 The protease dipeptidyl peptide‐4 has been shown to degrade native GLP‐1. Notably, exenatide degrades dipeptidyl peptide‐4 to maintain the levels of endogenous GLP‐1. Although GLP‐1‐mediated insulin secretion in pancreatic β cells has been well documented, the role of GLP‐1 signaling and exenatide's mechanism of action, which is thought to include the induction of carcinoembryonic antigen‐related cell adhesion molecule 1 (CEACAM1), in hepatocytes is poorly understood. CEACAM1 expression is transcriptionally regulated by insulin and lipids, and CEACAM1 regulates insulin clearance in hepatocytes. A better understanding of the underlying mechanisms of hepatic insulin clearance by the GLP‐1–CEACAM1 axis would be highly relevant to targeting and ultimately preventing the progression of NAFLD.
Insulin is released in a pulsatile manner by pancreatic β cells. When insulin reaches the liver through portal circulation, the insulin receptor tyrosine kinase in hepatocytes is phosphorylated and then initiates the phosphorylation of its substrates, such as CEACAM1. Once phosphorylated, CEACAM1 promotes receptor‐mediated insulin uptake into clathrin‐coated vesicles in hepatocytes to be degraded, leading to an extraction of 50% of insulin.1 Previous studies have shown that phosphorylated and internalized CEACAM1 binds fatty acid synthase (FASN), an enzyme that catalyzes the formation of palmitic acid from malonyl‐coenzyme A in de novo lipogenesis.1, 5 By binding to FASN, CEACAM1 decreases FASN enzymatic activity and severely restricts hepatic de novo lipogenesis. Studies have also shown that under hyperinsulinemic conditions, the pulsatility of insulin secretions decreases, in effect limiting insulin signaling and downstream CEACAM1 phosphorylation. Subsequently, the suppressive effect of FASN is removed, leading to hyperinsulinemia‐driven lipogenesis.1, 5
In the present issue of Hepatology Communications, Ghadieh et al.6 provide new evidence that a GLP‐1 agonist, exenatide, improves hepatic steatosis by regulating hepatic insulin clearance through induction of CEACAM1 in mice.
This study first presented that high‐fat diet (HFD) feeding reduced expression of CEACAM1 in hepatocytes. Because CEACAM1 contributes to hepatic insulin clearance, this finding suggests that NAFLD is associated with reduced insulin clearance. Ghadieh et al. demonstrate that exenatide treatment dramatically increased expression of CEACAM1 in hepatocytes in mice fed a HFD diet and induced elevation of insulin internalization in hepatocytes from wild‐type mice; this induced elevation of insulin uptake was not observed in CEACAM1–/– hepatocytes. Their results strongly suggest that exenatide treatment upregulates insulin uptake and subsequent clearance through CEACAM1 induction. The authors further performed a deeper analysis of the regulatory mechanism of exenatide‐mediated CEACAM1 induction and found that exenatide increased CEACAM1 promoter activity through an increase in PPARγ. Their chromatin immunoprecipitation assay clearly demonstrated that ligated PPARγ bound to the CEACAM1 promoter region in cells treated with rosiglitazone, a PPARγ agonist, or exenatide, indicating that PPARγ or exenatide‐induced PPARγ contributes to up‐regulation of CEACAM1 promoter activity and transcription. Interestingly, insulin and exenatide synergistically increased Ceacam1 promoter activity, while exenatide plus rosiglitazone did not show synergistic action in Ceacam1 promoter activity. This suggests that exenatide‐induced Ceacam1 transcription is mediated through PPARγ (Fig. 1).
Figure 1.
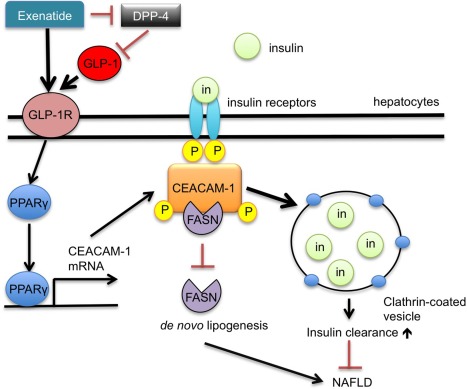
Schematic of the role of exenatide and CEACAM1 in insulin uptake and de novo lipogenesis. Exenatide binding to the GLP‐1 receptor (GLP‐1R) activates GLP‐1R signaling, initiating PPARγ‐mediated transcription of CEACAM1 mRNA. CEACAM1 activation simultaneously inhibits de novo lipogenesis by binding FASN and increases insulin uptake and clearance, preventing progression to NAFLD. Abbreviations: DPP‐4, dipeptidyl peptidase‐4; GLP‐1R, GLP‐1 receptor; in, insulin; mRNA, messenger RNA; P, phosphorylation.
The effect of exenatide on CEACAM1 induction and insulin clearance in primary hepatocytes has been confirmed by an in vivo animal model. In both wild‐type and CEACAM1–/– mice, exenatide treatment suppressed food intake and induced acute‐phase insulin secretion, which were also observed in both regular and HFD feeding conditions. These findings suggest that CEACAM1 is not important in pancreatic β cells and the central nervous system and that the role of CEACAM1 seems to be limited in hepatocytes, which further suggests that hepatic CEACAM1 does not influence food intake and insulin secretion from β cells. Another explanation is that the dysfunction of insulin clearance did not affect GLP‐1‐mediated insulin secretion and reduction of body weight. This may require further study to investigate whether these events are truly independent. Consistently, exenatide treatment recovered hepatic CEACAM1 expression along with its phosphorylation, which resulted in full clearance of insulin. This recovered insulin clearance by exenatide was blunted in CEACAM1–/– mice, demonstrating that exenatide‐mediated in vivo insulin clearance is dependent on induction of CEACAM1. Importantly, worsened insulin and glucose tolerance tests by HFD feeding were mitigated by exenatide treatment. Increased hepatic triglyceride levels and steatosis by HFD feeding were also improved by exenatide treatment. One of the molecular mechanisms that Ghadieh and co‐authors identified is that induced CEACAM1 forms a complex with FASN to decrease FASN activity, which suppresses hepatic steatosis. These therapeutic effects of exenatide were abolished in CEACAM1–/– mice, demonstrating the importance of CEACAM1 in exenatide‐mediated improvement of the systemic metabolic phenotype and NAFLD. Additionally, exenatide showed an anti‐inflammatory effect by demonstrating lowered messenger RNA levels of interleukin‐1β, interleukin‐6, and interferon‐γ.
Exenatide has been shown to suppress appetite and increase glucose‐stimulated insulin secretion. These effects are mediated through activation of GLP‐1 receptor signaling in the central nervous system and pancreatic β cells. An important finding of this study is the positive effect that exenatide has on insulin clearance in hepatocytes, which has not been demonstrated previously. Moreover, exenatide can suppress hepatic de novo lipogenesis through binding of CEACAM1 to FASN, which inhibits FASN activity. In addition to the improvement of insulin secretion and sensitivity, enhanced insulin clearance and inhibition of de novo lipogenesis by exenatide cumulatively participate in preventing the development of NAFLD. Although CEACAM1 plays an important role in insulin clearance, the reduced hepatic triglyceride levels by CEACAM1 are mediated through inhibition of FASN activity. This might be independent of CEACAM1‐mediated promotion of insulin clearance.
GLP‐1 receptor activation has been established as a powerful incretin and anorexigenic peptide in the pancreas and central nervous system; however, this study investigated GLP‐1 receptor signaling in hepatocytes. Although the authors revealed that GLP‐1‐mediated enhancement of insulin clearance is associated with the improvement of NAFLD, the precise mechanism of how improved insulin clearance improves hepatic steatosis and inflammation in NAFLD has not been well studied. Ghadieh and colleagues showed exenatide improved hepatic steatosis through CEACAM1, which is induced through PPARγ. However, PPARγ is also known to enhance lipogenesis.7 This discrepancy might be explained by the fact that PPARγ has different effects between liver and adipose tissues. A better understanding of the molecular mechanisms of hepatic insulin clearance may provide novel therapies that affect the development of NAFLD; this may potentially provide various options for treating NAFLD in patients.
Potential conflict of interest: Nothing to report.
Supported by National Institutes of Health grants through the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. R01DK085252 to E.S.) and the National Institute on Alcohol Abuse and Alcoholism (grant no. R21AA025841 to E.S.); and by the 2017 Winnick Research award from Cedars‐Sinai Medical Center (to E.S.).
REFERENCES
- 1. Heinrich G, Ghadieh HE, Ghanem SS, Muturi HT, Rezaei K, Al‐Share QY, et al. Loss of hepatic CEACAM1: a unifying mechanism linking insulin resistance to obesity and non‐alcoholic fatty liver disease. Front Endocrinol (Lausanne) 2017;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hurtado V, Roncero I, Blazquez E, Alvarez E, Sanz C. Glucagon‐like peptide‐1 and its implications in obesity In: Fedele M, ed. Hot Topics in Endocrine and Endocrine‐Related Diseases. InTech; [open access]. 2013:165‐195. [Google Scholar]
- 3. Lee YS, Jun HS. Anti‐diabetic actions of glucagon‐like peptide‐1 on pancreatic beta‐cells. Metabolism 2014;63:9‐19. [DOI] [PubMed] [Google Scholar]
- 4. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin‐4, a glucagon‐like protein‐1 (GLP‐1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006;43:173‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Najjar SM, Yang Y, Fernstrom MA, Lee SJ, Deangelis AM, Rjaily GA, et al. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab 2005;2:43‐53. [DOI] [PubMed] [Google Scholar]
- 6. Ghadieh HE, Muturi HT, Russo L, Marino CC, Ghanem SS, Khuder SS, et al. Exenatide induces carcinoembryonic antigen‐related cell adhesion molecule 1 expression to prevent hepatic steatosis. Hepatol Commun 2017; doi: 10.1002/hep4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity‐induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol 2017;13:36‐49. [DOI] [PubMed] [Google Scholar]


