Abstract
Carcinogenesis is known to be primarily associated with gene mutations. Recently, increasing evidence has suggested that epigenetic events also serve crucial roles in tumor etiology. Environmental factors, including nutrition, toxicants and ethanol, are involved in carcinogenesis through inducing epigenetic modifications, such as DNA methylation, histone deacetylase and miRNA regulation. Studying epigenetic mechanisms has facilitated the development of early diagnostic strategies and potential therapeutic avenues. Modulation at the epigenetic level, including reversing epigenetic modifications using targeted drugs, has demonstrated promise in cancer therapy. Therefore, identifying novel epigenetic biomarkers and therapeutic targets has potential for the future of cancer therapy. The present review discusses the environmental factors involved in epigenetic modifications and potential drug candidates for cancer therapy.
Keywords: epigenetic modification, environmental factor, promising drug, cancer therapy
1. Introduction
Currently, cancer is a major threat human health worldwide. Carcinogenesis is a multi-step process resulting mainly from the activation of oncogenes and the deactivation of tumor-suppressor genes. Etiologically, emerging evidences have demonstrated that epigenetic mechanisms are equally vital to carcinogenesis (1), including the chemical modifications of DNA and histone proteins, post-transcriptional regulation of microRNAs (miRNA)s and associated signaling pathways (2). Epigenetic modifications have been suggested to be a nearly event in carcinogenesis, and maybe useful as potential targets for early diagnosis, cancer treatment and prognosis evaluation (3). Based on the increasing number of studies, the focus of investigations of carcinogenesis mechanisms have also shifted from the genetic to epigenetic (4). Epidemiologically, epigenetic mechanisms are stressed by foreign substances, including xenobiotics and environmental conditions (5). Identifying an association between environmental factors and tumorigenesis may enable the development of personalized epigenetic medicines. In the present review, the environmental factors involved in epigenetic actions of carcinogenesis and the recent advancements in epigenetic drugs for cancer treatment are summarized.
2. Epigenetic modifications
Epigenetic modifications are defined as heritable alterations of gene expression levels induced by environment-gene interactions, including DNA methylation, DNA hydroxy methylation, histone modifications, non-coding RNA and miRNA (1). The manifestations of epigenetic alterations are various post-translational modifications (PTMs), including acetylation, methylation, phosphorylation and ADP-ribosylation (6). PTMs drive local changes in chromatin structure and allow for selective access of transcriptional machinery to the DNA. They can also induce various types of signals, subsequently activating mechanisms that induce specific cellular responses to the environment (7).
3. Environment factors and the how to influence epigenetic modifications
Epidemiologically, the majority of environmental factors, including geographical regions, stress, nutrition and toxicants, affect malignant diseases by inducing epigenetic modifications (8). Additionally, the environmental factors include race, climate, life style, diet, nutritional factors (9), airborne polycyclic aromatic hydrocarbons (10), toxicants (e.g., cocaine) (11), alcohol (5), fungicides or pesticides (e.g., dicofol and vinclozolin) (12), aflatoxin (13), bacteria (e.g., Helicobacter Pylori), viruses (e.g., hepatitis virus) (14), heavy metal exposure (e.g., cadmium, arsenic) (15) and endocrine disruptors (e.g., bisphenol-A) (16).
Previous studies have demonstrated that the majority of environmental factors have the ability to interfere with DNA methylation by altering the availability of the methyl donor or the activity of DNA methyltransferases (DNMTs) (17). Compounds in the environment, including the endocrine disruptors (e.g., diethylstilbestrol), tobacco and ethanol, may induce epigenetic modification (18). Dysplasia and sudden exposure in the critical stage (e.g., early development) to environmental factors promotes disease occurrence in adults (19). Environmental factors may permanently change the epigenetic genome and gene expression levels, and result in alterations of phenotypes and susceptibility to disease (19).
Evidence from liver cancer tissue samples revealed that ethanol altered the methylation status of histone H3 at two lysine residues (e.g., lys-4/9) and increased the phosphorylation of histone H3 at two serine residues (e.g., ser-10/28) (5). Chronic ethanol uptake may result in upregulation of certain miRNAs (miR-34a, miR-107 and miR-122), which can also alter the methylation pattern of DNA in liver tumors, thereby affecting gene expression levels (20). Taken together, histone modification, DNA methylation and miRNA may produce a synergistic effect in ethanol-associated tumors. It was reported that the hepatitis B virus X protein may induce aberrant epigenetic modifications in human hepatocellular carcinoma by inducing the DNA hypermethylation of tumor suppression genes (21), promotion-associated gene-specific DNA hypomethylation, histone acetylation or deacetylationand alterations of miRNAs (22).
Epigenetic modifications serve an important role in cancer development; the deregulation of this has been identified as a feature of cancer initiation (3). Investigating the underlying mechanisms may aid the development of specific therapeutic targets and personalized epigenetic medicines (23). Epigenetic drugs have emerged as potential agents for cancer treatment (Tables I and II).
Table I.
The pattern of DNMT inhibitors and their applications.
| Compound | Group | DNMT specificity | FDA approved application | Other applications in malignant tumors | (Refs.) |
|---|---|---|---|---|---|
| Azacytidine | Nucleoside | DNMT 1, DNMT3A, DNMT3B | Myelodysplastic syndrome | Acute myeloid leukemia, lung, esophageal, liver, breast, pancreatic, colon, ovarian, prostate, cervical and gastric cancer | (26) |
| Decitabine | Nucleoside | DNMT 1, DNMT3A, DNMT3B | Myelodysplastic syndrome | Acute myeloid leukemia, lung, esophageal, liver, breast, pancreatic, colon, ovarian, prostate, cervical, gastric, glioblastoma and head and neck cancer | (27) |
| 5-Fluoro-2 deoxycytidine | Nucleoside | Undetermined | None | Colon cancer | (28) |
| Zebularine | Nucleoside | DNMT 1, DNMT3A, DNMT3B | None | Cholangiocarcinoma, colon, liver, acute lymphoblastic leukemia, prostate, lung, breast and head and neck cancer | (29) |
| SGI-110 | Nucleoside | DNMT 1 | None | Ovarian, acute myeloid leukemia and lung cancer | (30) |
| Mahanine | Non-nucleoside | Undetermined | None | Lung, glioblastoma, cervical, prostate and colon cancer | (31) |
| Hydralazine | Non-nucleoside | DNMT 1, DNMT3A, DNMT3B | None | Myelodysplastic syndrome, cutaneous t-cell lymphoma, prostate and cervical cancer | (32) |
| Procaine | Non-nucleoside | DNMT 1 | None | Lung, nasopharyngeal and liver cancer | (33) |
| Procainamide | Non-nucleoside | DNMT 1 | None | Lung and breast cancer | (33) |
| SGI-1027 | Non-nucleoside | DNMT 1, DNMT3A, DNMT3B | None | None | (34) |
| Curcumin | Non-nucleoside | DNMT 1 | None | Colon, pancreatic, oral, prostate, breast, cervical, ovarian, lung and liver cancer | (35) |
| RG108 | Non-nucleoside | DNMT 1 | None | Prostate, promyelocytic leukemia and breast cancer | (36) |
| 3,6-dihydroxyflavone | Non-nucleoside | DNMT 1 | None | Breast, cervical and prostate cancer | (37) |
| Epigallocatechin gallate Parthenolide | Non-nucleoside | DNMT 1 | None | Lung, liver, colon, pancreatic, ovarian, breast, prostate, oral, chronic myeloid leukemia and head and neck cancer | (38) |
| Apigenin | Non-nucleoside | DNMT 1 | None | Osteosarcoma, bladder, breast, colon, ovarian and pancreatic cancer | (39) |
| PRIMA-1 | Non-nucleoside | DNMT 1, DNMT3A, DNMT3B | None | Pancreatic, lung, thyroid cancer, Ewing sarcoma, breast and ovarian cancer | (40) |
| Genistein | Non-nucleoside | DNMT 1, DNMT3A, DNMT3B | None | Breast, colon, prostate, acute myeloid leukemia, cervical, oral and liver cancer | (41) |
| Parthenolide | Non-nucleoside | DNMT 1, DNMT3A, DNMT3B | None | Intracranial glioma, oral, colon and breast cancer | (42) |
DNMT, DNA methyltransferase; FDA, Food and Drug Administration.
Table II.
Pattern of HDAC inhibitors and their applications.
| Compound | Group | HDAC specificity | FDA approved application | Other applications in malignant tumor | (Refs.) |
|---|---|---|---|---|---|
| Vorinostat | Hydroxamate | Class I, II | Cutaneous T cell lymphoma | Pancreatic cancer, neuroblastoma, acute myeloid leukemia, breast, osteosarcoma, pleural mesothelioma and lung cancer | (44) |
| Panobinostat | Hydroxamate | Class I, II | Multiple myeloma | Non-small cell lung cancer, diffuse intrinsic pontine glioma, acute myeloid leukemia, glioblastoma, anaplastic glioma, pancreatic and colon cancer | (45) |
| Trichostatin A | Hydroxamate | Class I, II | None | Gastric cancer, chondrosarcoma, bladder, esophageal and breast cancer | (46) |
| Dacinostat | Hydroxamate | Class I, II | None | Acute myeloid leukemia | (47) |
| Tubacin | Hydroxamate | Class IIb | None | Burkitt's lymphoma, neuroblastoma, urothelial cancer, acute lymphoblastic leukemia and breast cancer | (48) |
| Belinostat | Hydroxamate | Class I, IIb | Peripheral T Cell Lymphoma | Cancer of unknown primary site, renal cancer, cutaneous T-cell lymphoma, thymic epithelial tumor and acute myeloid leukemia | (49) |
| Romidepsin | Cyclic tetrapeptide | Class I | Cutaneous T cell lymphoma; T-cell lymphoma | Anaplastic glioma, renal, colon, prostate small-cell lung, head and neck and gastric cancer | (50) |
| Entinostat | Benzamide | Class I | None | Breast cancer, lung cancer, B-cell lymphoma, acute myeloid leukemia, myelodysplastic syndrome and hepatocellular cancer | (51) |
| Valproic acid | Short-chain fatty acid | Class I, IIa | None | Acute myeloid leukemia, primary chronic lymphocytic leukemia, gastric, pancreatic, ovarian and renal cancer | (52) |
| Sodium 4-phenyl-butyrate | Short-chain fatty acid | Class I, IIa | None | Bladder, breast and colon cancer and Burkitt's lymphoma | (53) |
| Trifluoromethylketone | Electrophilicketones | Class II | None | Hepatocellular cancer and neuroblastoma | |
| Mocetinostat | Miscellaneous compounds | Class I | None | Pancreatic cancer, B-cell chronic lymphocytic leukemia, small cell lung cancer, colon cancer, multiple myeloma and Hodgkin's lymphoma | (54) |
| EX-527 | Other | Class III | None | Pancreatic cancer, melanoma, hepatocellular, breast and gastric cancer | (55) |
| Cambinol | Other | Class III | None | Lung, pancreatic, breast, colon cancer and Burkitt's lymphoma | (56) |
| I-7ab | Other | Class I | None | Prostate cancer and acute myelocytic | (57) |
| SB939 | Other | Class I, II, IV | None | Leukemia, myelofibrosis, ovarian and colon cancer | (58) |
HDAC, histone deacetylase; FDA, Food and Drug Administration.
4. Epigenetic modifications and inhibitors
Evidence has demonstrated that histone modifications together with DNA methylation constitute an ‘epigenetic code’, which regulates transcriptional status and disruptscode writing or interpretation (23). These aberrant alterations to the code may activate the expression of oncogenes, including c-Myc, which promotes the development of specific small molecule modulators of histone binding proteins (24). A few of these compounds have been used in clinical development for tumor therapy, Tables I and II summarized the current reported epigenetic inhibitors.
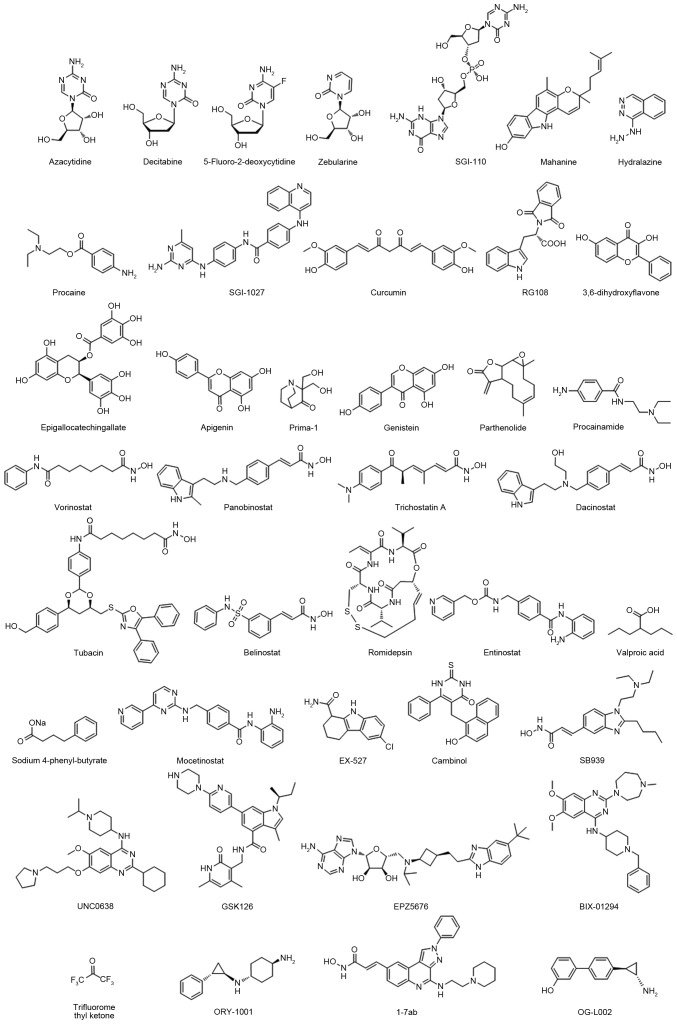
DNMTs, including DNMT1, DNMT3A and DNMT3B, catalyze a methyl group transformation from the methyl donor S-adenosylmethionine to the C-5 of cytosine in DNA. In malignant cells, hypermethylation at the CpG island induces suppression of numerousvital tumor suppressor genes, including p16 (25). Thus, small molecules targeting DNMTs may potentially reverse epigenetic silencing of cancer suppressor genes in a number of different cancer types. The DNMT inhibitors were used in tumor clinical treatments, including azacitidine, decitabine and SGI-110 (others are presented in Table I and Fig. 1) (26–42). These compounds demonstrated good anti-proliferative effects in various cancer cell lines, including breast, prostate, lung, pancreas, liver and leukemia (23). However, the practical utility in clinics has been limited by systemic toxicity and off-target effects, including in certain heme malignancies.
Figure 1.
The structural features of promising epigenetic inhibitors.
The other major category is the histone deacetylase (HDACs) inhibitor, which enables the catalysis of N-acetyl residues hydrolysis in histones and activation of histone acetyl transferases. A previous study revealed that HDACs serve roles as crucial mediators in tumor survival and progression (43). A total of four HDAC inhibitors were approved by the Food and Drug Administration (FDA): Vorinostat, belinostat, panobinostat and romidepsin (details are presented in Table II and Fig. 1) (44–58).
Following the development of epigenetic drugs, second-generation epigenetic inhibitors emerged, including histone methyltransferase inhibitors, euchromatic histone lysine methyltransferase 2 (G9a) inhibitors, enhancer of zeste 2 polycomb repressive complex 2 subunit inhibitors, DOT1 like histone lysine methyltransferase inhibitors, histone demethylases and Jumonji C inhibitors (Table III and Fig. 1) (59–64). These epigenetic clinical agents have intrinsically greater binding specificity to their molecular targets and may be developed as drugs for malignant disease.
Table III.
Promising epigenetic inhibitors.
| Drug | Inhibitor Type | Targets | (Refs.) |
|---|---|---|---|
| BIX-01294 | HMT(G9a) | H3K9me2 | (59) |
| UNC0638 | HMT(G9a) | H3K9me2 | (60) |
| GSK126 | HMT(EZH2) | H3K27 | (61) |
| EPZ5676 | HMT(DOT1L) | H3K79 | (62) |
| OG-L002 | HMT(LSD1) | MAO-A and B | (63) |
| ORY-1001 | HMT(LSD1) | LSD1 | (64) |
| ---- | HMT (Jumonji C) | LSD1 | (65) |
HMT, histone methyltransferases; LSD, lysine-specific demethylase; EZH2, enhancer of zeste 2 polycomb repressive complex 2 subunit; DOT1L, DOT1 like histone lysine methyltransferase.
Valproic acid (VPA; valproate), an acidic chemical compound, was mainly used in the treatment of epilepsy, bipolar mania and migraine prophylaxis previously (65). In 1996, Cinatl et al (66), reported the inhibiting effect of VPA on N-myconco protein expression in human neuroblastoma cells, suggesting that VPA may have anticancer properties. In the past few decades, great effort has been made to study its epigenetic mechanism in various types of cancer, the majority of which focused on transcriptionally activating chromatin structures (67). Recently a phase I/II clinical trial headed by Iwahashi et al (68) demonstrated that S-1 (an oral fluoropyrimidine derivative consisting of the 5-fluorouracil prodrug tegafur combined with VPA for patients with pancreatobiliary tract cancer, had a manageable safety profile and preliminary antitumor activity. Sugimoto et al (69), reported that combined VPA with PEG-interferon (IFN)-α increased caspase-3/7 activity, induced IFN-α and -β receptor subunit (IFNAR)1 and IFNAR2 expression and increased the expression levels of IFN-α receptor and IFN regulatory factor 8 in pancreatic cancer, which revealed that VPA may be useful for the treatment of pancreatic cancer via enhancing the function of IFN-α.
5. Novel drug exploration using the old-fashioned ‘Drug repositioning’ method
Increasing interest has been drawn to the idea of ‘drug repositioning’. Although it is a costly approach to novel drug development, the clinical value is low as the majority of the drugs have not passed the phase I trial. Therefore, certain existing drugs have been re-examined (70). A typical and successful example is Viagra, which had high expectations for use in the treatment of cardiovascular disease, but serves a role in the treatment of male sexual dysfunction. Another example is vorinostat, which was initially designed for cutaneous T-cell lymphoma but facilitated a breakthrough in HIV treatment by disturbing HIV's latency in stationary phase patients (71). Due to the potential effects and characteristics of targeted treatment for epigenetic-associated disease, epigenetic drugs are making progress and attracting attention for cancer therapy (72). The FDA approved the aforementioned epigenetic drugs, including the DNMT inhibitors azacitidine and decitabine, which were revealed to be effective in myelody splastic syndrome therapy (73). The HDAC inhibitors, vorinostat, romidepsin and belinostat, also acquired recognition in the treatment of cutaneous and peripheral T cell lymphoma (74). Emerging evidence demonstrated that azacitidine and decitabine also possessed anticancer effects on liver cancer, pancreatic cancer and breast cancer cells (75). It is reasonable to speculate that combining azacitidine and decitabine with other anticancer drugs, including platinum compounds and monoclonal antibodies may produce a stronger anticancer effect (76). Furthermore, vorinostat and romidepsin were also popular for gastric and lung cancer therapy (77,78). Novel drug development also requires investigation using cutting-edge technology, including gene sequencing.
Sanger sequencing, first-generation sequencing that markedly impacted gene research has now evolved into next generation sequencing (NGS), which has a lower cost, higher speed and improved throughput. Recently, an epigenetic study used NGS and achieved a novel understanding of ependymoma in children. The previous study investigated DNA methylation patterns and defined a tumor CpG island methylator phenotype for infant nervous system malignancy, using whole genome sequencing and whole-exome sequencing (79). They revealed that the development of posterior fossa ependymomas group A (PFA), which had a poor prognosis, occurred primarily in infants and was associated with epigenetic modifications. The PFA exhibits an increased number of methylated CpG sites, an increased number of genes with CpG methylation and an increased number of genes that are transcriptionally silenced by CpG hypermethylation in tumor development and maintenance. The in vivo data demonstrated that treatment with decitabine and Gsk343 is able to attenuate the proliferation of PFA cells. This may further support the concept of ‘drug repositioning’. Widely applicable in modern cancer clinical research (80), NGS has begun to elucidate the underlying epigenetic mechanisms; however, there is a large amount of data. Methodological improvement is required for convenient clinical application.
6. Summary
Epigenetics provides a molecular and etiological mechanism for the incidence of malignant cancer. Early ectogenic exposure can program later life physiology and adult onset disease due to the replication of the epigenome during somatic cell mitosis, during which ‘epigenetic transgenerational inheritance’ initiates. Although an increasing number of approved antitumor drugs have emerged, the outcomes of clinical trials have been unsatisfactory. This may be due to the lack of specificity and the combination with environmental exposure. In view of the critical roles of ectogenic cues on tumorigenesis, comprehensive analysis and treatment is required for early diagnosis, standardized and personalized treatment. The presence of epigenetic factors is associated with gene abnormality in premalignant cancer, and its potential reversibility indicated that epigenetic alterations may be promising biomarkers and potential novel mechanism-based strategies for tumor early diagnosis and treatment.
Previous clinical trials revealed that first generation inhibitors, including DNMTs and HDACs, have been observed to have limited utility due to toxicity and off-target effects. However, second generation compounds have been suggested to have more promise. These clinical agents have greater selectivity for their molecular target and may be a robust driver or key mediator at safe doses in malignancies. Additionally, drug repositioning still requires further enhancement and study. The improvement of epigenetic therapeutic strategies needs to be combined with cytotoxic factors, immunotherapy, targeted kinase inhibitors, NSG and the possible environmental cues.
Acknowledgements
The present study was supported by the National Natural Science Fund of China (grant nos. 31570509 and 81702326), the Gansu Province Science Foundation for Distinguished Young Scholars (grant no. 1606RJDA317) and Science and Technology Program of Lanzhou City (grant no. 2015-3-93).
Glossary
Abbreviations
- PTM
post translational modification
- HDAC
histone deacetylase
- DNMT
DNA methyltransferase
- VPA
valproic acid
- NGS
next generation sequencing
- HMT
histone methyltransferases
- LSD
lysine-specific demethylase
References
- 1.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Ramirez MA, Nicoli S. Role of miRNAs and epigenetics in neural stem cell fate determination. Epigenetics. 2014;9:90–100. doi: 10.4161/epi.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graça I, Pereira-Silva E, Henrique R, Packham G, Crabb SJ, Jerónimo C. Epigenetic modulators as therapeutic targets in prostate cancer. Clin Epigenetics. 2016;8:98. doi: 10.1186/s13148-016-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do C, Shearer A, Suzuki M, Terry MB, Gelernter J, Greally JM, Tycko B. Genetic-epigenetic interactions in cis: A major focus in the post-GWAS era. Genome Biol. 2017;18:120. doi: 10.1186/s13059-017-1250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla SD, Lim RW. Epigenetic effects of ethanol on the liver and gastrointestinal system. Alcohol Res. 2013;35:47–55. doi: 10.35946/arcr.v35.1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothbart SB, Strahl BD. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 2014;1839:627–643. doi: 10.1016/j.bbagrm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkins BJ, Rall NA, Ostwal Y, Kruitwagen T, Hiragami-Hamada K, Winkler M, Barral Y, Fischle W, Neumann H. A cascade of histone modifications induces chromatin condensation in mitosis. Science. 2014;343:77–80. doi: 10.1126/science.1244508. [DOI] [PubMed] [Google Scholar]
- 8.Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod Toxicol. 2013;36:104–116. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4:e4488. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3:e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiménez-Chillarón JC, Nijland MJ, Ascensão AA, Sardão VA, Magalhães J, Hitchler MJ, Domann FE, Oliveira PJ. Back to the future: Transgenerational transmission of xenobiotic-induced epigenetic remodeling. Epigenetics. 2015;10:259–273. doi: 10.1080/15592294.2015.1020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y. Detection of epigenetic aberrations in the development of hepatocellular carcinoma. Methods Mol Biol. 2015;1238:709–731. doi: 10.1007/978-1-4939-1804-1_37. [DOI] [PubMed] [Google Scholar]
- 14.Tian Y, Yang W, Song J, Wu Y, Ni B. Hepatitis B virus X protein-induced aberrant epigenetic modifications contributing to human hepatocellular carcinoma pathogenesis. Mol Cell Biol. 2013;33:2810–2816. doi: 10.1128/MCB.00205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu HW, Lee DH, Won HR, Kim KH, Seong YJ, Kwon SH. Influence of toxicologically relevant metals on human epigenetic regulation. Toxicol Res. 2015;31:1–9. doi: 10.5487/TR.2015.31.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan D, Ahmed SA. Epigenetic regulation of non-lymphoid cells by Bisphenol A, a model endocrine disrupter: Potential implications for immunoregulation. Front Endocrinol (Lausanne) 2015;6:91. doi: 10.3389/fendo.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerrero-Bosagna C, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of phenotype and disease. Mol Cell Endocrinol. 2012;354:3–8. doi: 10.1016/j.mce.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfrey KM, Costello PM, Lillycrop KA. The developmental environment, epigenetic biomarkers and long-term health. J Dev Orig Health Dis. 2015;6:399–406. doi: 10.1017/S204017441500121X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dippold RP, Vadigepalli R, Gonye GE, Patra B, Hoek JB. Chronic ethanol feeding alters miRNA expression dymanics during liver regeneration. Alcohol Clin Exp Res. 2013;37:E59–E69. doi: 10.1111/j.1530-0277.2012.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pogribny IP, Rusyn I. Role of epigenetic aberrations in the development and progression of human hepatocellular carcinoma. Cancer Lett. 2014;342:223–230. doi: 10.1016/j.canlet.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei X, Xiang T, Ren G, Tan C, Liu R, Xu X, Wu Z. miR-101 is down-regulated by the hepatitis B virus X protein and induces aberrant DNA methylation by targeting DNA methyltransferase 3A. Cell Signal. 2013;25:439–446. doi: 10.1016/j.cellsig.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Dhanak D, Jackson P. Development and classes of epigenetic drugs for cancer. Biochem Biophys Res Commun. 2014;455:58–69. doi: 10.1016/j.bbrc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Patel DJ. Small molecule epigenetic inhibitors targeted to histone lysine methyltransferases and demethylases. Q Rev Biophys. 2013;46:349–373. doi: 10.1017/S0033583513000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enjuanes A, Albero R, Clot G, Navarro A, Beà S, Pinyol M, Martín-Subero JI, Klapper W, Staudt LM, Jaffe ES, et al. Genomewide methylation analyses identify a subset of mantle cell lymphoma with a high number of methylated CpGs and aggressive clinicopathological features. Int J Cancer. 2013;133:2852–2863. doi: 10.1002/ijc.28321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller AM, Florek M. 5-Azacytidine/5-Azacitidine. Recent Results Cancer Res. 2014;201:299–324. doi: 10.1007/978-3-642-54490-3_19. [DOI] [PubMed] [Google Scholar]
- 27.Momparler RL. Epigenetic therapy of cancer with 5-aza-2′-deoxycytidine (decitabine) Semin Oncol. 2005;32:443–451. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Q, Fan J, Hong W, Li L, Wu M. Inhibition of cancer cell proliferation by 5-fluoro-2′-deoxycytidine, a DNA methylation inhibitor, through activation of DNA damage response pathway. Springerplus. 2012;1:65. doi: 10.1186/2193-1801-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquez VE, Kelley JA, Agbaria R, Ben-Kasus T, Cheng JC, Yoo CB, Jones PA. Zebularine: A unique molecule for an epigenetically based strategy in cancer chemotherapy. Ann N Y Acad Sci. 2005;1058:246–254. doi: 10.1196/annals.1359.037. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Taverna P, Karpf AR, Griffiths EA. Immunomodulatory action of the DNA methyltransferase inhibitor SGI-110 in epithelial ovarian cancer cells and xenografts. Epigenetics. 2015;10:237–246. doi: 10.1080/15592294.2015.1017198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal S, Amin KS, Jagadeesh S, Baishay G, Rao PG, Barua NC, Bhattacharya S, Banerjee PP. Mahanine restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in prostate cancer cells. Mol Cancer. 2013;12:99. doi: 10.1186/1476-4598-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dueñas-Gonzalez A, Coronel J, Cetina L, González-Fierro A, Chavez-Blanco A, Taja-Chayeb L. Hydralazine-valproate: A repositioned drug combination for the epigenetic therapy of cancer. Expert Opin Drug Metab Toxicol. 2014;10:1433–1444. doi: 10.1517/17425255.2014.947263. [DOI] [PubMed] [Google Scholar]
- 33.Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong M, Zhi X, Jablons DM, You L. Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol Rep. 2009;22:1479–1484. doi: 10.3892/or_00000590. [DOI] [PubMed] [Google Scholar]
- 34.Rilova E, Erdmann A, Gros C, Masson V, Aussagues Y, Poughon-Cassabois V, Rajavelu A, Jeltsch A, Menon Y, Novosad N, et al. Design, synthesis and biological evaluation of 4-amino-N-(4-aminophenyl) benzamide analogues of quinoline-based SGI-1027 as inhibitors of DNA methylation. ChemMedChem. 2014;9:590–601. doi: 10.1002/cmdc.201300420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramasamy TS, Ayob AZ, Myint HH, Thiagarajah S, Amini F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: Insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015;15:96. doi: 10.1186/s12935-015-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graça I, Sousa EJ, Baptista T, Almeida M, Ramalho-Carvalho J, Palmeira C, Henrique R, Jerónimo C. Anti-tumoral effect of the non-nucleoside DNMT inhibitor RG108 in human prostate cancer cells. Curr Pharm Des. 2014;20:1803–1811. doi: 10.2174/13816128113199990516. [DOI] [PubMed] [Google Scholar]
- 37.Lee E, Jeong KW, Jnawali HN, Shin A, Heo YS, Kim Y. Cytotoxic activity of 3,6-dihydroxyflavone in human cervical cancer cells and its therapeutic effect on c-Jun N-terminal kinase inhibition. Molecules. 2014;19:13200–13211. doi: 10.3390/molecules190913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakrabarty S, Ganguli A, Das A, Nag D, Chakrabarti G. Epigallocatechin-3-gallate shows anti-proliferative activity in HeLa Cells targeting tubulin-microtubule equilibrium. Chem Biol Interact. 2015;242:380–389. doi: 10.1016/j.cbi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Shukla S, Gupta S. Apigenin: A promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiang W, Jin T, Yang Q, Liu W, Liu S, Ji M, He N, Chen C, Shi B, Hou P. PRIMA-1 selectively induces global DNA demethylation in p53 mutant-type thyroid cancer cells. J Biomed Nanotechnol. 2014;10:1249–1258. doi: 10.1166/jbn.2014.1862. [DOI] [PubMed] [Google Scholar]
- 41.Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Genistein and cancer: Current status, challenges, and future directions. Adv Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyrębska A, Gach K, Janecka A. Combined effect of parthenolide and various anti-cancer drugs or anticancer candidate substances on malignant cells in vitro and in vivo. Mini Rev Med Chem. 2014;14:222–228. doi: 10.2174/1389557514666140219113509. [DOI] [PubMed] [Google Scholar]
- 43.Minami J, Suzuki R, Mazitschek R, Gorgun G, Ghosh B, Cirstea D, Hu Y, Mimura N, Ohguchi H, Cottini F, et al. Histone deacetylase 3 as a novel therapeutic target in multiple myeloma. Leukemia. 2014;28:680–689. doi: 10.1038/leu.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richon VM. Targeting histone deacetylases: Development of vorinostat for the treatment of cancer. Epigenomics. 2010;2:457–465. doi: 10.2217/epi.10.20. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Zhang J, Xie Y, Jiang Y, Yingjie Z, Xu W. Progress of HDAC inhibitor panobinostat in the treatment of cancer. Curr Drug Targets. 2014;15:622–634. doi: 10.2174/1389450115666140306152642. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Xu J, Wang H, Wu L, Yuan W, Du J, Cai S. Trichostatin A, a histone deacetylase inhibitor, reverses epithelial-mesenchymal transition in colorectal cancer SW480 and prostate cancer PC3 cells. Biochem Biophys Res Commun. 2015;456:320–326. doi: 10.1016/j.bbrc.2014.11.079. [DOI] [PubMed] [Google Scholar]
- 47.Ganai SA. Strategy for enhancing the therapeutic efficacy of histone deacetylase inhibitor dacinostat: The novel paradigm to tackle monotonous cancer chemoresistance. Arch Pharm Res. 2015 doi: 10.1007/s12272-015-0673-9. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 48.Xing LF, Wang DT, Yang Y, Pan SY. Effect of HDAC-6 on PD cell induced by lactacystin. Asian Pac J Trop Med. 2015;8:855–859. doi: 10.1016/j.apjtm.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Kirschbaum MH, Foon KA, Frankel P, Ruel C, Pulone B, Tuscano JM, Newman EM. A phase 2 study of belinostat (PXD101) in patients with relapsed or refractory acute myeloid leukemia or patients over the age of 60 with newly diagnosed acute myeloid leukemia: A California Cancer Consortium study. Leuk Lymphoma. 2014;55:2301–2304. doi: 10.3109/10428194.2013.877134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertino EM, Otterson GA. Romidepsin: A novel histone deacetylase inhibitor for cancer. Expert Opin Investig Drugs. 2011;20:1151–1158. doi: 10.1517/13543784.2011.594437. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz R, Raez LE, Rolfo C. Entinostat (SNDX-275) for the treatment of non-small cell lung cancer. Expert Opin Investig Drugs. 2015;24:1101–1109. doi: 10.1517/13543784.2015.1056779. [DOI] [PubMed] [Google Scholar]
- 52.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: Preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34:206–222. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Tsunedomi R, Iizuka N, Harada S, Oka M. Susceptibility of hepatoma-derived cells to histone deacetylase inhibitors is associated with ID2 expression. Int J Oncol. 2013;42:1159–1166. doi: 10.3892/ijo.2013.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nielsen TK, Hildmann C, Riester D, Wegener D, Schwienhorst A, Ficner R. Complex structure of a bacterial class 2 histone deacetylase homologue with a trifluoromethylketone inhibitor. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63:270–273. doi: 10.1107/S1744309107012377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao YF, Lin F, Yan XY, Gao XG, Teng F, Fu ZR, Wang ZX. Galectin-9 in combination with EX-527 prolongs the survival of cardiac allografts in mice after cardiac transplantation. Transplant Proc. 2015;47:2003–2009. doi: 10.1016/j.transproceed.2015.04.091. [DOI] [PubMed] [Google Scholar]
- 56.Mahajan SS, Scian M, Sripathy S, Posakony J, Lao U, Loe TK, Leko V, Thalhofer A, Schuler AD, Bedalov A, Simon JA. Development of pyrazolone and isoxazol-5-one cambinol analogues as sirtuin inhibitors. J Med Chem. 2014;57:3283–3294. doi: 10.1021/jm4018064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Liang Q, Shen K, Ma L, An N, Deng W, Fei Z, Liu J. A novel class I histone deacetylase inhibitor, I-7ab, induces apoptosis and arrests cell cycle progression in human colorectal cancer cells. Biomed Pharmacother. 2015;71:70–78. doi: 10.1016/j.biopha.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 58.Eigl BJ, North S, Winquist E, Finch D, Wood L, Sridhar SS, Powers J, Good J, Sharma M, Squire JA, et al. A phase II study of the HDAC inhibitor SB939 in patients with castration resistant prostate cancer: NCIC clinical trials group study IND195. Invest New Drugs. 2015;33:969–976. doi: 10.1007/s10637-015-0252-4. [DOI] [PubMed] [Google Scholar]
- 59.Cui J, Sun W, Hao X, Wei M, Su X, Zhang Y, Su L, Liu X. EHMT2 inhibitor BIX-01294 induces apoptosis through PMAIP1-USP9X-MCL1 axis in human bladder cancer cells. Cancer Cell Int. 2015;15:4. doi: 10.1186/s12935-014-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu L, Yan FX, An XR, Hou J. Effects of the histone methyltransferase inhibitor UNC0638 on histone H3K9 dimethylation of cultured ovine somatic cells and development of resulting early cloned embryos. Reprod Domest Anim. 2014;49:e21–e25. doi: 10.1111/rda.12277. [DOI] [PubMed] [Google Scholar]
- 61.Tiffen JC, Gunatilake D, Gallagher SJ, Gowrishankar K, Heinemann A, Cullinane C, Dutton-Regester K, Pupo GM, Strbenac D, Yang JY, et al. Targeting activating mutations of EZH2 leads to potent cell growth inhibition in human melanoma by derepression of tumor suppressor genes. Oncotarget. 2015;6:27023–27036. doi: 10.18632/oncotarget.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horiuchi KY, Eason MM, Ferry JJ, Planck JL, Walsh CP, Smith RF, Howitz KT, Ma H. Assay development for histone methyltransferases. Assay Drug Dev Technol. 2013;11:227–236. doi: 10.1089/adt.2012.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maes T, Mascaró C, Ortega A, Lunardi S, Ciceri F, Somervaille TC, Buesa C. KDM1 histone lysine demethylases as targets for treatments of oncological and neurodegenerative disease. Epigenomics. 2015;7:609–626. doi: 10.2217/epi.15.9. [DOI] [PubMed] [Google Scholar]
- 64.Qian S, Wang Y, Ma H, Zhang L. Expansion and functional divergence of Jumonji C-containing histone demethylases: Significance of duplications in ancestral angiosperms and vertebrates. Plant Physiol. 2015;168:1321–1337. doi: 10.1104/pp.15.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verrotti A, Carelli A, di Genova L, Striano P. Epilepsy and chromosome 18 abnormalities: A review. Seizure. 2015;32:78–83. doi: 10.1016/j.seizure.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 66.Cinatl J, Jr, Cinatl J, Scholz M, Driever PH, Henrich D, Kabickova H, Vogel JU, Doerr HW, Kornhuber B. Antitumor activity of sodium valproate in cultures of human neuroblastoma cells. Anticancer Drugs. 1996;7:766–773. doi: 10.1097/00001813-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Woodworth AM, Holloway AF. The role of epigenetic regulation in transcriptional memory in the immune system. Adv Protein Chem Struct Biol. 2017;106:43–69. doi: 10.1016/bs.apcsb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Iwahashi S, Utsunomiya T, Imura S, Morine Y, Ikemoto T, Arakawa Y, Saito Y, Ishikawa D, Shimada M. Effects of valproic acid in combination with S-1 on advanced pancreatobiliary tract cancers: Clinical study phases I/II. Anticancer Res. 2014;34:5187–5191. [PubMed] [Google Scholar]
- 69.Sugimoto K, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Iwahashi S. Valproic acid enhances the anti-tumor effect of pegylated interferon-α towards pancreatic cancer cell lines. Anticancer Res. 2014;34:3403–3409. [PubMed] [Google Scholar]
- 70.Bastos LF, Coelho MM. Drug repositioning: Playing dirty to kill pain. CNS Drugs. 2014;28:45–61. doi: 10.1007/s40263-013-0128-0. [DOI] [PubMed] [Google Scholar]
- 71.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Costanzo A, Del Gaudio N, Migliaccio A, Altucci L. Epigenetic drugs against cancer: An evolving landscape. Arch Toxicol. 2014;88:1651–1668. doi: 10.1007/s00204-014-1315-6. [DOI] [PubMed] [Google Scholar]
- 73.Mummaneni P, Shord SS. Epigenetics and oncology. Pharmacotherapy. 2014;34:495–505. doi: 10.1002/phar.1408. [DOI] [PubMed] [Google Scholar]
- 74.Glaser KB. HDAC inhibitors: Clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Mei Q, Nie J, Fu X, Han W. Decitabine: A promising epi-immunotherapeutic agent in solid tumors. Expert Rev Clin Immunol. 2015;11:363–375. doi: 10.1586/1744666X.2015.1002397. [DOI] [PubMed] [Google Scholar]
- 76.Garrido-Laguna I, McGregor KA, Wade M, Weis J, Gilcrease W, Burr L, Soldi R, Jakubowski L, Davidson C, Morrell G, et al. A phase I/II study of decitabine in combination with panitumumab in patients with wild-type (wt) KRAS metastatic colorectal cancer. Invest New Drugs. 2013;31:1257–1264. doi: 10.1007/s10637-013-9947-6. [DOI] [PubMed] [Google Scholar]
- 77.Zhou C, Ji J, Shi M, Yang L, Yu Y, Liu B, Zhu Z, Zhang J. Suberoylanilide hydroxamic acid enhances the antitumor activity of oxaliplatin by reversing the oxaliplatin–induced Src activation in gastric cancer cells. Mol Med Rep. 2014;10:2729–2735. doi: 10.3892/mmr.2014.2548. [DOI] [PubMed] [Google Scholar]
- 78.Karthik S, Sankar R, Varunkumar K, Ravikumar V. Romidepsin induces cell cycle arrest, apoptosis, histone hyperacetylation and reduces matrix metalloproteinases 2 and 9 expression in bortezomib sensitized non-small cell lung cancer cells. Biomed Pharmacother. 2014;68:327–334. doi: 10.1016/j.biopha.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Mack SC, Witt H, Piro RM, Gu L, Zuyderduyn S, Stütz AM, Wang X, Gallo M, Garzia L, Zayne K, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445–450. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tabatabaeifar S, Kruse TA, Thomassen M, Larsen MJ, Sørensen JA. Use of next generation sequencing in head and neck squamous cell carcinomas: A review. Oral Oncol. 2014;50:1035–1040. doi: 10.1016/j.oraloncology.2014.08.013. [DOI] [PubMed] [Google Scholar]