Abstract
Cyclohexanone (C6H10O, CAS No. 108-94-1) is a colorless oily liquid obtained through the oxidation of cyclohexane or dehydrogenation of phenol. It is used in the manufacture of adhesives, sealant chemicals, agricultural chemicals, paint and coating additives, solvent, electrical and electronic products, paints and coatings, photographic supplies, film, photochemicals, and as an intermediate in nylon production. Owing to the lack of information on repeated inhalation toxicity of cyclohexaone, in this study, we aimed to characterize the subacute inhalation toxicity. B6C3F1 mice were exposed to 0, 50, 150, and 250 ppm of cyclohexanone for 6 hr/day, 5 days/week for 4 weeks via whole-body inhalation in accordance with the OECD Test Guideline 412 (subacute inhalation toxicity: 28-day study). Mortality, clinical signs, body weights, food consumption, hematology, serum biochemistry, organ weights, as well as gross and histopathological findings were evaluated between the control and exposure groups. No mortality or remarkable clinical signs were observed during the study. No adverse effects on body weight, food consumption, hematology, serum biochemistry, and organ weights, gross or histopathological lesions were observed in any male or female mice in any of the exposure groups, although some statistically significant changes were observed in organ weights. We concluded that no observable adverse effect level (NOAEL) is above 250 ppm in mice exposed to cyclohexanone for 6 hr/day for 5 days/week.
Keywords: Cyclohexanone, NOAEL, Subacute inhalation toxicity, Whole-body inhalation
INTRODUCTION
Cyclohexanone (C6H10O, CAS No. 108-94-1) is a colorless oily liquid obtained through the oxidation of cyclohexane or dehydrogenation of phenol and its vapor pressure is 5 mm Hg at 25°C (1). It is used in the manufacture of adhesives, sealant chemicals, agricultural chemicals, paint and coating additives, solvent, electrical and electronic products, paints and coatings, photographic supplies, film, photochemicals, and as an intermediate in nylon production (1,2). In 2014, cyclohexanone production at work place was 256,931 ton and 8,399 workers were exposed to cyclohhexanone in Korea (3). The occupational exposure limit for cyclohexanone is estimated to be 25 ppm as time-weighted average (TWA) and 50 ppm as the short-term exposure limit (STEL) (4).
The toxicity of cyclohexanone has been rarely documented in humans and experimental animals. Human exposure by inhalation causes irritation to the respiratory system (5). Inhalation of large quantities of cyclohexanone can cause nervous system depression, and irritate the eye and skin (1). Cyclohexanone toxicological experiments were conducted to study the acute inhalation toxicity of cyclohexanone, and the result demonstrated an LD50 value > 2,000 ppm in rats (6). In addition, inhalation study of cyclohexanone revealed central nervous system depression as well as liver and kidney degeneration in rabbit and monkey (7). Cyclohexanone is widely used in the manufacturing industry, and its exposure via inhalation can cause adverse effects. Thus, exposure of workers to cyclohexanone leads to the potential health problem. As there is a lack of information on repeated inhalation toxicity, in this study, we aimed to evaluate the potential subacute inhalation toxicity of whole-body exposure to cyclohexanone in B6C3F1 mice in accordance with the Good Laboratory Practice guideline.
MATERIALS AND METHODS
Animal husbandry and maintenance
Six-week-old specific-pathogen-free B6C3F1 mice of both sexes were obtained from Japan SLC Inc. (Shizuoka, Japan) and acclimated for 1 week. The room was maintained at a temperature of 22 ± 3°C, humidity of 50 ± 20%, 12 : 12 hr light:dark cycle, and fresh air ventilation (10~15 times/hour). The mice were allocated individually in stainless steel wire mesh cages (W 750 mm × L 220 mm × H 180 mm) and they had free access to a commercial rodent diet (LabDiet®, St. Louis, MO, USA) and filtered tap water. The study was approved by the Institutional Animal Care and Use Committee (IACUC-1607).
Test chemical and exposure system
Cyclohexanone (lot no. 4C3WCMJ) was purchased from Tokyo chemical industry Co. LTD (Tokyo, Japan). A whole-body exposure chamber (WITC-00-M, HCT Co., Icheon, Korea), including a gas generator (LVg-04-A, HCT Co., Icheon, Korea), was used to expose the mice to cyclohexanone at 0, 50, 150 and 250 ppm for 6 hr/day, 5 days/week for 4 weeks. Exposures were conducted in accordance with test No. 412 (Subacute Inhalation Toxicity, 2009) by the Organization for Economic Co-operation and Development (OECD) (8). Inhalation exposure procedures were conducted from 10:00 to 16:00. The experimental design was based on the major exposure route for the test substance and the routine working schedule of employees.
Conditions in the inhalation chambers
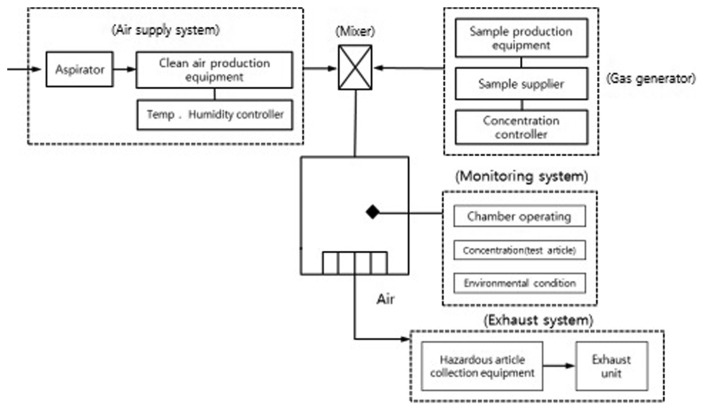
The chamber conditions, including temperature, relative humidity, pressure, and air ventilation, were recorded using an environmental controller (WITC-00-M, HCT, Icheon, Korea). The concentrations of cyclohexanone in the chambers were calibrated with a standard chemical (RIGAS, Daejeon, Korea). Cyclohexanone was analyzed by an Infrared Spectrophotometer (FT-IR, IR-GAS, CIC phtonics, Albuquerque, NM, USA) using a deuterated triglycine sulfate detector module with spectral range of 485 to 8,500 cm−1 and VCSEL solid state 760 nm laser in gas cell with path length: 6m and volume: 0.6 L. The temperature and pressure in gas cell were 40°C and 760 Torr, respectively. The vapor concentrations of cyclohexanone in the chambers were monitored every 10 s during the exposures, and kept within ± 5% of the target concentration. The mean concentration measured every day was obtained as the value on a given day. This was then averaged over the 4 week exposure period to evaluate the mean and standard deviation. The chamber condition including gas generator was illustrated in Fig. 1.
Fig. 1.
Illustration of whole body chamber condition.
Experimental groups
Forty mice (20 males and 20 females) were allocated randomly to the following four groups (5 per sex per group): control (0 ppm), low dose (50 ppm), middle dose (150 ppm), and high dose (250 ppm). All mice were euthanized after 4 weeks of treatment. The following cyclohexanone concentrations were selected: the lowest dose of cyclohexanone was selected based on the 0.2 mg/L/6 hr (50 ppm), the highest dose of classification 1; the highest dose of cyclohexanone was selected based on 1.0 mg/L/6 hr (250 ppm), the highest dose of classification 2; the middle dose (150 ppm) of cyclohexanone was selected on the basis of the average concentration of lowest and highest doses, according to the standard of classification for specific target organ toxicity (repeat exposure) in the standard for Classification and Labeling of Chemicals and Material Safety Data Sheets(Public notice No. 2016-19 of the Ministry of Employment and Labor) (9). A concentration of 3 times the reference value did not apply to the selected doses, as recommended in the 28-day test in Public Notice No. 2016-19, because 250 ppm of cyclohexanone was the maximum vapor concentration that could be generated.
Clinical signs, body weight, and food consumption
All animals were recorded once daily for mortality and clinical signs, and weighed immediately before inhalation exposure on day 1 and once per week thereafter. Daily food consumption was noted before the initiation of inhalation and once per week thereafter. Food consumption was evaluated by subtracting remaining feed from the total feed supplied.
Hematology
All animals were fasted for 4 hr before necropsy and blood collection. The blood samples were collected from the abdominal vena cava using a syringe with a 26-gauge needle under isoflurane anesthesia (Ilsung Pharm, Seoul, Korea) and collected into vacutainers containing EDTA-3K (Becton Dickinson, Franklin Lakes, NJ, USA). The samples were analyzed within 20 min of collection using an automated hematology analyzer (Hemavet 950, Drew Scientific, Waterbury, CT, USA). The following parameters were determined in this study: total erythrocyte counts (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), platelet count (PLT), whole leukocyte counts (WBC), neutrophils (NEU, %), eosinophils (EOS, %), basophils (BASO, %), lymphocytes (LYM, %) and monocytes (MONO, %).
Serum biochemistry
The blood samples were centrifuged at 3,000 rpm for 10 min within 1 hr after collection. The sera were stored at −80°C before analysis. The following serum biochemistry parameters were assessed using an automated analyzer (TBA-120FR, Toshiba Medical Systems, Tochigi, Japan): total protein (TP), albumin (ALB), blood urea nitrogen (BUN), creatinine (CREA), total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glucose (GLU), total cholesterol (TCHO), and triglycerides (TG).
Necropsy, organ weight, and histopathological evaluations
Gross examinations of organs in the cranial, thoracic, and abdominal cavities of the mice were conducted. The absolute and relative (organ to body weight) weights of the brain, thymus, lung, heart, liver, spleen, kidneys, adrenal glands, testes, and ovaries were measured. The following tissues were removed from each animal at necropsy: the liver, kidney, adrenal gland, heart, lung, cerebrum, cerebellum, olfactory bulb, pituitary, spleen, seminal vesicle, prostate, testis, epididymis, ovary, uterus, vagina, tongue, trachea, esophagus, thymus, thyroid, stomach, duodenum, urinary bladder, small/large intestine, eye/Harderian gland, skeletal muscle, sciatic nerve, pancreas, aorta, mesenteric lymph node, femur, larynx, and nasal cavity. The nasal cavity was sectioned at three levels: 1, posterior to the upper incisors; 2, incisive papilla; 3, first molar teeth. The eyes and the testes were fixed in Davidson’s solution before being transferred to 10% neutral buffered formalin. The other organs were fixed in 10% neutral buffered formalin. All organs were processed routinely, embedded in paraffin, sectioned at 3~4 μm, stained with hematoxylin and eosin, and examined microscopically under low- to high- power field.
Statistical analyses
Differences in the measured parameters among groups were evaluated using SPSS (ver. 19.0, IBM, Chicago, IL, USA). The homogeneity of variance was analyzed by Levene’s test, followed by either one-way analysis of variance for samples with homogenous variance or the Kruskal-Wallis test for samples with heterogeneous variance. Dunnett’s multiple range test was conducted to compare the result of each experimental group with that of the control group if the first statistical result was significant.
RESULTS
Chamber monitoring
The ranges of chamber conditions was 21.2~23.0°C, 44.5~61.3% relative humidity, −78.4~18.17 pa pressure, 20.50~21.42% O2 concentration and 11.65~12.26 air change/h. The average concentrations of cyclohexanone in the study were 50.8 ± 1.38, 152.2 ± 4.22, and 248.1 ± 10.21 ppm for the low, medium, and high concentration groups, respectively. The daily mean chamber concentration was within ± 5% of the target concentration (Table 1).
Table 1.
Concentration of cyclohexanone by exposure groups
| Groups | Concentration (ppm) | |||
|---|---|---|---|---|
|
| ||||
| Establishment | Lower | Upper | Mean ± SD | |
| Groups 1 | 0 | 0.63 | 4.72 | 2.07 ± 0.28 |
| Groups 2 | 50 | 47.49 | 57.47 | 50.81 ± 1.38 |
| Groups 3 | 150 | 146.57 | 157.31 | 152.20 ± 4.22 |
| Groups 4 | 250 | 230.14 | 257.65 | 248.07 ± 10.21 |
Clinical signs
No deaths or adverse clinical signs were observed in any mice exposed to any concentration of cyclohexanone.
Body weight and food consumption
Body weights and food consumption did not changed significantly in any male or female mice exposed to any concentration of cyclohexanone (data not shown).
Hematology
No statistically significant hematological changes were observed in any groups exposed to cyclohexanone compared to the control group (data not shown).
Serum biochemistry
No statistically significant serum biochemical changes were observed in any of groups exposed to cyclohexanone compared to the control group (data not shown).
Organ weight
No statistically significant changes in male and female absolute organ weight and male relative organ weight were observed in any of the cyclohexanone groups compared with the control group (data not shown). However, the relative adrenal glands weight increased significantly in female mice exposed to 50 ppm cyclohexanone group compared to that in the control group (p < 0.05) (Tables 2 and 3).
Table 2.
Relative organ weights of male mice exposed to cyclohexanone for 4 weeks
| Organ weights (%) | Groups (ppm) | |||
|---|---|---|---|---|
|
| ||||
| G1 (0) | G2 (50) | G3 (150) | G4 (250) | |
| Body weight(g) | 29.18 ± 1.71 | 30.29 ± 0.92 | 29.90 ± 1.04 | 30.57 ± 1.69 |
| Adrenal glands | 0.029 ± 0.013 | 0.021 ± 0.040 | 0.028 ± 0.012 | 0.014 ± 0.004 |
| Brain | 1.618 ± 0.047 | 1.492 ± 0.155 | 1.608 ± 0.047 | 1.532 ± 0.075 |
| Heart | 0.450 ± 0.013 | 0.440 ± 0.040 | 0.438 ± 0.032 | 0.435 ± 0.033 |
| Kidneys | 1.512 ± 0.076 | 1.495 ± 0.078 | 1.553 ± 0.043 | 1.513 ± 0.105 |
| Testes | 0.780 ± 0.034 | 0.729 ± 0.064 | 0.808 ± 0.125 | 0.768 ± 0.190 |
| Liver | 5.089 ± 0.151 | 5.075 ± 0.368 | 5.187 ± 0.128 | 5.177 ± 0.162 |
| Spleen | 0.271 ± 0.024 | 0.274 ± 0.030 | 0.271 ± 0.029 | 0.249 ± 0.013 |
| Lung | 0.529 ± 0.030 | 0.539 ± 0.034 | 0.599 ± 0.120 | 0.540 ± 0.036 |
| Thymus | 0.182 ± 0.026 | 0.161 ± 0.018 | 0.182 ± 0.014 | 0.194 ± 0.027 |
All values are expressed as mean ± standard deviation.
Table 3.
Relative organ weights of female mice exposed to cyclohexanone for 4 week
| Organ weights (%) | Groups (ppm) | |||
|---|---|---|---|---|
|
| ||||
| G1 (0) | G2 (50) | G3 (150) | G4 (250) | |
| Body weight(g) | 23.89 ± 0.75 | 25.37 ± 1.62 | 25.67 ± 1.06 | 24.89 ± 0.86 |
| Adrenal glands | 0.051 ± 0.004 | 0.037 ± 0.005* | 0.047 ± 0.001 | 0.045 ± 0.001 |
| Brain | 2.085 ± 0.086 | 1.940 ± 0.059 | 1.992 ± 0.133 | 2.000 ± 0.091 |
| Heart | 0.523 ± 0.030 | 0.477 ± 0.038 | 0.499 ± 0.027 | 0.481 ± 0.021 |
| Kidneys | 1.428 ± 0.025 | 1.340 ± 0.042 | 1.400 ± 0.057 | 1.406 ± 0.070 |
| Liver | 4.670 ± 0.413 | 4.888 ± 0.264 | 4.962 ± 0.416 | 4.957 ± 0.269 |
| Spleen | 0.393 ± 0.019 | 0.430 ± 0.044 | 0.422 ± 0.049 | 0.424 ± 0.048 |
| Lung | 0.677 ± 0.099 | 0.630 ± 0.029 | 0.793 ± 0.199 | 0.670 ± 0.060 |
| Thymus | 0.253 ± 0.033 | 0.252 ± 0.035 | 0.280 ± 0.031 | 0.265 ± 0.008 |
All values are expressed as mean ± standard deviation.
Significant differences as compared with the control group:
p<0.05.
Gross and histopathological evaluations
No gross lesion except for alopecia in the abdominal skin in a male mouse exposed to the control or 150 ppm cyclohexanone and in a female mouse exposed to 50 or 250 ppm cyclohexanone group, were observed in any cylohexanone group. No histopathological finding other than the alopecia of the abdominal skin were observed in any organ of the control or any cylohexanone-exposed group.
DISCUSSION
This study was performed to evaluate the potential subacute toxicity of cyclohexanone by repeated inhalation exposure to B6C3F1 mice at concentrations of 0, 50, 150, and 250 ppm for 4 weeks. Although we found some statistically significant differences in organ weights in cyclohexanone exposed mice when compared to the organ weights of the control group, as well as alopecia in some tested animals, no adverse effects were observed.
No clinical signs were observed in any mice exposed to any concentration of cyclohexanone. Previous reports indicated that cyclohexanone exposure caused irritation to the respiratory system, central nervous system, eyes, and skin, showing clinical signs in humans (1,5). Differences in species and exposure routes or periods may have contributed to the differences between the previous findings and those of our study.
Body weights, food consumption, hematology, and serum chemistry did not changed significantly in any male or female mice exposed to cyclohexanone.
There were statistically significant increases in the relative organ weights of the adrenal glands in female mice of the 50 and 250 ppm groups. However, these changes were not considered to be adverse effects because they were not dose-dependent nor accompanied by an increase in the absolute organ weights of the adrenal glands; they also were not associated with histopathological alterations of the adrenal glands (10).
Histopathological examination of alopecia of the skin revealed normal histological appearance. This change was not considered to be adverse because it was not dose-dependent and was observed also in the control group. Generally, alopecia in rodent is caused by over-grooming, external trauma, inflammatory or degenerative processes that deteriorate formation of new hair shafts, and improperly constructed feeder openings or watering devices (11,12). However, it has been reported that the cause of thoracic and abdominal alopecia of B6C3F1 mice is unknown (13). Workers exposed to cyclohexanone for more than 5 years showed liver damage (14). In addition, subchronic inhalation of cyclohexanone caused central nervous system depression as well as liver and kidney degeneration in rabbits and monkeys (7). Nevertheless, those lesions were not observed in this study. As mentioned earlier, species differences and exposure periods likely contributed to the differences between the results of the prior studies and ours.
In conclusion, a 4-week repeated whole-body inhalation exposure of mice to 50, 150, and 250 ppm of cyclohexanone did not reveal toxic effects. Therefore, the no observable adverse effect level (NOAEL) for cyclohexanone was considered to be above 250 ppm in mice exposed for 6 hr/day, 5 days/week. The present study provides the concentration to be used for subsequent subchronic inhalation studies and useful information on the inhalation toxicity of cyclohexanone.
ACKNOWLEDGMENTS
This work was supported by the Korea Occupational Safety and Health Agency, Ministry of Labor, Republic of Korea, and a Grant-in-Aid for chemical hazard assessment, 2016.
REFERENCES
- 1.National Center for Biotechnology Information. Pubchem: open chemistry data base. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/7967#section=Top/
- 2.MSDS of Cyclohexanone. Available from: https://cameochemicals.noaa.gov/chemical/3044/
- 3.Korea occupational safety and health agency. Work environment survey in 2014. 2014 Available from: http://www.kosha.or.kr/content.do?menuId=10260/
- 4.National Institute for Occupational Safety and Health (NIOSH) Cycloheanone in international chemical safety cards. 2004 Available from: https://www.cdc.gov/niosh/ipcsneng/neng0425.html/
- 5.New Jersey department of health. Hazardous substance fact sheet. 2009 Available from: http://www.nj.gov/health/eoh/rtkweb/documents/fs/0164.pdf/
- 6.Organisation for Economic Co-operation and Development (OECD) OECD screening information data set (SIDS) – cyclohexanone. 1996 Available from: http://www.inchem.org/documents/sids/sids/108941.pdf/
- 7.National Institute for Occupational Safety and Health (NIOSH) Cyclohexaonone in occupational health guidelines for chemical hazards. 1988;3 DHHS (NIOSH), Publication No. 81-123. Available from: https://www.cdc.gov/niosh/docs/81-123/pdfs/0166.pdf/ [Google Scholar]
- 8.Organisation for Economic Co-operation and Development (OECD) OECD guidelines for the testing of chemicals 412, subacute inhalation toxicity (28-day study) 2009 Available from: http://www.oecd-ilibrary.org/docserver/download/9741201e.pdf?expires=1458631443&id=id&accname=guest&checksum=D1112C41DA570B17CD0404910E9BBB4D/
- 9.The Ministry of Employment and Labor. Public notice No. 2016–19 of the Ministry of employment and labor. 2016 Available from: https://www.moel.go.kr/view.jsp?cate=4&sec=4&smenu=2&bbs_cd=116&seq=1459902724642&div_cd=&mode=view/
- 10.Lewis RW, Billington R, Debryune E, Gamer A, Lang B, Carpanini F. Recognition of adverse and nonadverse effects in toxicity studies. Toxicol Pathol. 2002;30:66–74. doi: 10.1080/01926230252824725. [DOI] [PubMed] [Google Scholar]
- 11.Jackson Laboratory. Alopecia (loss of hair) in C57BL/6J and related strains. 1987 Available from: https://www.jax.org/news-and-insights/1987/october/alopecia-in-c57bl-6-and-related-mouse-strains/
- 12.Mecklenburg L, Kusewitt D, Kolly C, Treumann S, Adams ET, Diegel K, Yamate J, Kaufmann W, Müller S, Danilenko D, Bradley A. Proliferative and non-proliferative lesions of the rat and mouse integument. Toxicol Pathol. 2013;26:27S–57S. doi: 10.1293/tox.26.27S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burek JD, Molello JA, Warner SD. In: Selected nonneoplastic disease in The Mouse in Biomedical Research (Vol. 2: Disease) Forster HL, Small JD, Fox JG, editors. Academic press; New York: 1982. pp. 425–440. [Google Scholar]
- 14.International agency for research on cancer (IARC) Monographs on the Evaluation of the carcinogenic risk of chemicals to humans. Vol. 47. World Health Organization, International Agency for Research on Cancer; Geneva: 1989. p. 164. Available from: http://monographs.iarc.fr/ENG/Classification/index.php/ [Google Scholar]