Abstract
Cholangiocarcinoma (CCA) is a rare and fatal tumor. In previous decades, there has been a steady increase in the incidence and mortality rates of this tumor worldwide. Metastasis is regarded as the major factor that contributes to poor prognosis in CCA patients. Studies therefore aim to develop novel therapeutic targets to control CCA metastasis. Fyn is known to enhance expression and promote metastasis in various cancers, including pancreatic cancer, prostate cancer and colorectal cancer. However, the exact function and mechanism of Fyn in CCA metastasis remains unclear. In the present study, mRNA and protein expression levels of Fyn, AMP-activated protein kinase (AMPK), phosphorylated (p-)AMPK, mammalian target of rapamycin (mTOR) and p-mTOR were measured, using the reverse transcription-quantitative polymerase chain reaction and western blot analysis, in CCA tissues and cell lines. In addition, Transwell assays were used to determine the migratory and invasive abilities of human CCA QBC939, following transfection. In the present study, it was found that Fyn was overexpressed in CCA cell lines. Fyn knockdown inhibited CCA cell migration and invasion. Furthermore, it was demonstrated that Fyn knockdown induces phosphorylation of AMPK, inhibits downstream phosphorylation of mTOR, and activate the AMPK/mTOR signaling pathway. Compound C, an AMPK inhibitor, inhibited the AMPK/mTOR signaling pathway, and reversed the effect of Fyn knockdown on migration and invasion of CCA cells. In conclusion, the present study suggests that Fyn knockdown inhibits cell migration and invasion by regulating the AMPK/mTOR signaling pathway in CCA cell lines and that Fyn knockdown is a potential target for anti-CCA therapy.
Keywords: Fyn, cholangiocarcinoma, migration, invasion, AMPK, mTOR
Introduction
Cholangiocarcinoma (CCA) is the second most common primary liver malignancy after hepatocellular carcinoma in 2012 worldwide (1). It is defined as a rare and fatal tumor, which in previous decades has demonstrated a steady increase in the incidence and mortality rate worldwide (2). The early clinical symptoms of CCA are challenging to identify, resulting in a lack of accurate diagnosis at the early stage, and inability to control the incidence rate (3). Patients with early stage CCA are usually recommended to undergo surgical resection, which is considered to be the most favorable therapy to achieve good prognosis and, to a certain extent, prolong the survival of CCA patients (4,5). While the prognosis of advanced CCA patients remains poor, metastasis is regarded as the major factor that contributes to their poor outcome (5–7). Tumor metastasis is a complex and multistep biological process, wherein metastasis-promoting genes and inhibition of metastasis suppressors play critical roles during the entire metastatic process (8). A number of genes can affect the tumor metastatic process, and therefore, researchers aim to develop novel therapeutic targets to control CCA metastasis.
Fyn, a member of the Src tyrosine kinase family (SFK), is known to be involved in several biological activities associated with cancer. Fyn expression is upregulated in multiple human cancers including prostate cancer, glioblastoma, chronic myelogenous leukemia, melanoma and squamous cell carcinoma (9–13). Fyn expression is also associated with tumor metastasis. Chen et al (14,15) have found that Fyn expression is upregulated in pancreatic cancer and that Fyn requires HnRNPA2B1 and Sam68 to coordinate and regulate metastasis in pancreatic cancer. In advanced prostate cancer, Fyn was found to enhance the neuroendocrine phenotype and increase visceral metastasis (16). In colorectal cancer, Fyn is induced by cellular prion protein accelerates colorectal cancer metastasis (17). In breast cancer, Fyn expression is induced by Ras/PI3K/Akt signaling and is essential for enhanced breast cancer migration and invasion (18).
The exact function and mechanism of Fyn in CCA metastasis remains unclear. In the present study, the expression of Fyn in CCA cell lines and the association with CCA cell migration and invasion were investigated. The results showed that Fyn is upregulated in CCA cell lines and that downregulation of Fyn suppresses CCA cell migration and invasion. Mechanistic studies revealed that Fyn inhibited AMPK/mTOR signaling, which promoted CCA cell migration and invasion. The present results confirmed that Fyn blockade is a potential target for anti-CCA therapy.
Materials and methods
Cell culture
The normal bile duct HIBEC cell line and the human CCA QBC939, RBE, CCLP1 and HCCC-9810 cell lines were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified atmosphere containing 5% CO2.
Fyn knockdown
Suppression of Fyn (NM_002037.5) expression was achieved using short hairpin RNA (shRNA) lentiviral transduction particles. The short hairpin RNA (shRNA) of Fyn (NM_002037.5; shFyn) was amplified from normal human genomic DNA using the following primers (Genewiz, Inc., Suzhou, China): shRNA forward (BamHI), 5′-GATCCCACAGGTGGCTGCAGGAATGGTCAAGAGCCATTCCTGCAGCCACCTGTGTTTTTTTG-3′ and reverse, (EcoRI), 5′-AATTCCAAAAAAACACAGGTGGCTGCAGGAATGGCTCTTGACCATTCCTGCAGCCACCTGTGG-3′. The shFyn was cloned into pLVX-shRNA1 vectors (Clontech Laboratories, Inc., Mountain View, CA, USA), termed pLVX-shFyn. Recombinant pLVX-shFyn and pHelper 1.0 and 2.0 plasmids (Clontech Laboratories, Inc.) were generated by the transient transfection of HEK 293T cells (2×106 cells; Cell Bank of Chinese Academy of Sciences), using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol, and the lentivirus was packaged in accordance with a previous study (19). For stable transfection, QBC939 cells were infected with the virus supernatant fluid along with 8 µg/ml polybrene and selected in 0.5 µg/ml puromycin for one week, followed by 0.2 µg/ml puromycin for one month. The stable cells were harvested 24 or 48 h after transfection for additional analysis.
Cell groups and treatment
The stable QBC939 cells expressing shFyn were termed shFyn. The QBC939 cells transfected with lentivirus expressing negative control shRNA were termed shNC. To validate that Fyn promotes CCA metastasis through the activated AMPK/mTOR signaling pathway, AMPK inhibitor compound C (10 µM; Calbiochem, San Diego, CA, USA) was used to inhibit the AMPK/mTOR signaling and 1% dimethyl sulphoxide (DMSO) was used as negative control (NC). In the present study, 2×105 shFyn-transfected QBC939 cells were cultured at 37°C for 24 h in a humidified atmosphere containing 5% CO2, and subsequently with 10 µl compound C or DMSO at 37°C for 24 or 48 h. Then, the shFyn-transfected QBC939 cells were harvested for additional analysis.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cultured QBC939 cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions, and the RNA was reverse transcribed into cDNA at 42°C for 15 min using AffinityScript QPCR cDNA Synthesis kit (Agilent Technologies, Inc., Santa Clara, CA, USA). Fyn RNA expression was detected by RT-qPCR using Brilliant II SYBR Green QPCR Master Mix kit (Agilent Technologies, Inc.). Amplification was performed using the following PCR conditions: Preheating at 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 10 sec. Fyn and 18srRNA (internal control) primers were synthesized by Sangon Biotech (Shanghai, China). The following primer sequences were used: Fyn forward, 5′-GGGTGCTAATGTGGAGACTG-3′ and reverse, 5′-GCTTTGATGCTGACTTGCAG-3′; and 18srRNA forward, 5′-CCTGGATACCGCAGCTAGGA-3′ and reverse, 5′-GCGGCGCAATACGAATGCCCC-3′. RT-qPCR was performed on an Applied Biosystems 7500 system (Thermo Fisher Scientific, Inc.) with melt curve analysis. Gene expression was measured in triplicate. The data were analyzed using the 2−ΔΔCq method (20). Quantitative analysis was performed using the ratio of the target gene to 18srRNA, and normalized to the control.
Western blotting
Protein expression of Fyn, AMPK, phosphorylated AMPK (p-AMPK), mTOR and phosphorylated mTOR (p-mTOR) was detected by western blot analysis. The normal bile duct HIBEC cell line and human CCA QBC939, RBE, CCLP1 and HCCC-9810 cell lines were harvested for Fyn protein expression analysis. QBC939 cells, stably expressing shNC or shFyn, were harvested for Fyn, AMPK, p-AMPK, mTOR and p-mTOR analysis. All harvested cells were lysed using radioimmunoprecipitation assay buffer (Takara Biotechnology, Co., Ltd., Dalian, China), followed by protein quantification using BCA Protein Assay kit (Beyotime Institute of Biotechnology, Haimen, China). Approximately 30 µg of protein sample was loaded per lane. Proteins were separated on 10% SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane. The membrane was blocked in Tris-buffered saline with 1% Tween-20 buffer containing 5% skimmed dried milk and incubated at 4°C overnight. The primary antibodies, consisting of rabbit monoclonal anti-Fyn (dilution, 1:1,000; catalog no. ab125016), mouse monoclonal anti-AMPK (dilution, 1:500; catalog no. ab80039), rabbit monoclonal anti-p-AMPK (dilution, 1:2,000; catalog no. ab133448), rabbit polyclonal anti-mTOR (dilution, 1:2,000; catalog no. ab2732), rabbit polyclonal anti-p-mTOR (dilution, 1:1,000; catalog no. ab109268) (all from Abcam, Cambridge, MA, USA) and anti-GAPDH (dilution, 1:10,000; catalog no. KC-5G5; Kangchen Biotech Co., Ltd., Shanghai, China), were added and the mixture was incubated overnight at 4°C on a rocking platform. This was followed by incubation of the membrane with goat anti-mouse or goat anti-rabbit IgG (H+L) secondary antibody conjugated to horseradish peroxidase (1:5,000; catalog no. 1034-05 or 4050-05, respectively; Southern Biotech, Birmingham, AL, USA) for 2 h. The proteins were visualized using Pierce™ ECL Western Blotting Substrate (Pierce; Thermo Fisher Scientific, Inc.) and were exposed on X-ray film. Gray scale images were then analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The expression levels of proteins of interest were normalized to the expression of GAPDH.
Transwell migration and invasion assays
Cell migration and invasion were assessed using a Transwell migration assay. For the migration assay, the suspension (200 µl) containing 1×105 QBC939 cells, stably expressing shNC or shFyn, was dispensed into the upper chamber (8 µm pore size; BD Biosciences, San Diego, CA, USA) and RPMI-1640 medium containing 10% FBS was added to the lower chamber of the Transwell. The chamber was incubated at 37°C for 48 h in a humidified atmosphere containing 5% CO2. Following, cells were fixed with 4% paraformaldehyde for 15 min at 25°C and stained with 0.1% crystal violet in 20% ethanol for 10 min at 25°C. Images were captured using a LEICA light microscope (magnification, ×200). The number of migrating cells in the center and five surrounding independent fields were counted, and average counts were calculated as the migrating cell numbers. For the invasion assay, the artificial substrate Matrigel (BD Biosciences) was layered in the Transwell chamber and 1×105 QBC939 cells from each group were dispensed into the upper chamber followed by incubation at 37°C for 48 h in a humidified atmosphere containing 5% CO2. The subsequent treatment procedures followed those used for the migration assays. Each experiment was performed with three wells and the same experiment was measured in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Quantitative data are presented as the mean ± standard deviation. Fisher's least significant difference test was used to control for the multiple comparisons of comparing all CCA cell lines to the same HIBEC control. The differences between shNC and shFyn groups were analyzed by the Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Fyn was upregulated in CCA tissues and cell lines
To investigate the role of Fyn in the regulation of CCA cell migration and invasion, the present study determined Fyn expression levels in the normal bile duct HIBEC cell line and in human CCA QBC939, RBE, CCLP1, and HCCC-9810 cell lines using RT-qPCR and western blot analysis. The results showed that Fyn expression was significantly decreased in CCA cell lines compared with HIBEC cells, particularly QBC939 cells (Fig. 1). Based on these results, QBC939 cells were used for subsequent experiments.
Figure 1.
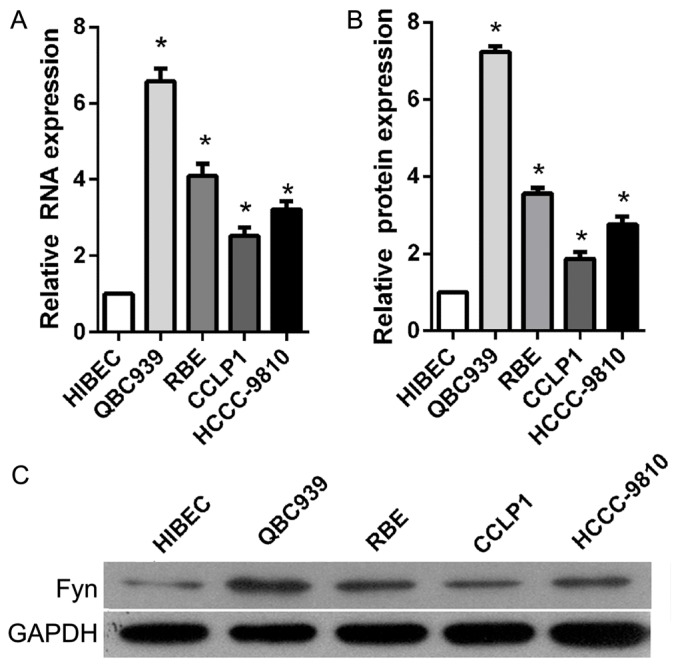
Fyn is overexpressed in CCA cell lines. (A) Fyn mRNA expression in the normal bile duct HIBEC cell line and in the human CCA QBC939, RBE, CCLP1 and HCCC-9810 cell lines using reverse transcription-quantitative polymerase chain reaction. GAPDH served as the internal control. (B) Fyn protein expression in the normal bile duct HIBEC cell line and in human CCA QBC939, RBE, CCLP1 and HCCC-9810 cell lines was assessed using western blot analysis. GAPDH served as internal control for western blotting. (C) Representative images of levels of Fyn protein as detected by western blot analysis. Data are represented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. HIBEC. CCA, cholangiocarcinoma.
Fyn knockdown inhibited CCA cell migration and invasion
To investigate the role of Fyn in the regulation of CCA cell migration and invasion, shFyn was transfected into QBC939 cells. After 48 h of transfection, QBC939 cells were harvested for western blot analysis. The results showed that transfection of QBC939 cells with shFyn effectively inhibited Fyn expression (Fig. 2A). Furthermore, in order to measure QBC939 cell migration and invasion, Transwell assays were used subsequent to transfection of cells with shFyn or shNC for 48 h. The number of migrating cells after transfection with shNC or shFyn was 223±9 and 144±13, respectively (P<0.05; Fig. 2B), and the number of invading cells was 134±17 and 89±10, respectively (P<0.05) (Fig. 2C). The Transwell migration assay showed that the ability of migration through the membrane into the lower chamber was significantly inhibited in cells transfected with shFyn compared with shNC-transfected cells (P<0.05; Fig. 2D). The Transwell invasion assay showed that the ability of cells to invade a Matrigel-coated membrane and migrate into the lower chamber was significantly decreased in cells transfected with shFyn compared with shNC-transfected cells (P<0.05; Fig. 2E).
Figure 2.
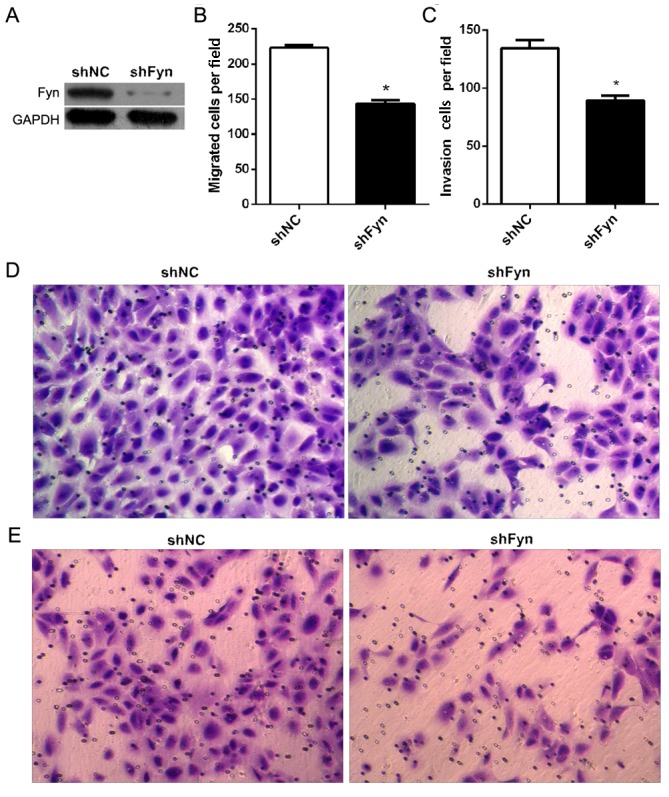
Fyn knockdown inhibited cholangiocarcinoma cell migration and invasion. (A) Fyn expression in QBC939 cells was examined by western blotting after transfection with shRNA for 48 h. GAPDH served as an internal control. (B) The mean number of migrating cells per field for indicated experimental groups. (C) The mean number of invading cells per field for indicated experimental groups. (D) Migration assays of QBC939 cells. Magnification, ×200. (E) Invasion assays of QBC939 cells. Magnification, ×200. Data represent the mean ± standard deviation of three independent experiments. *P<0.05 vs. shNC. shNC, control short hairpin RNA; shFyn, short hairpin RNA against Fyn.
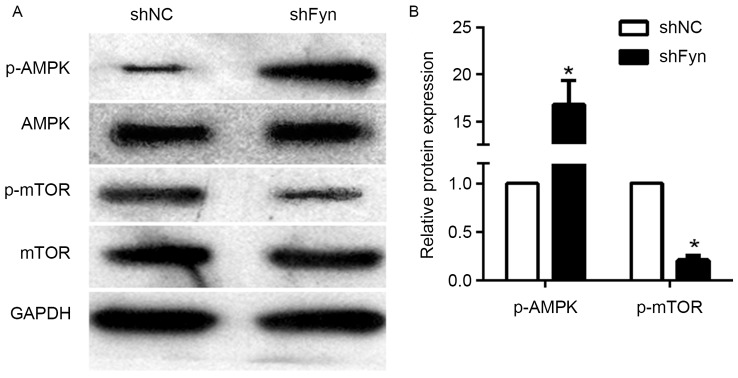
Effects of Fyn inhibition on AMPK/mTOR signaling pathway
The AMPK/mTOR signaling pathway has an important role in tumor development and metastasis. To investigate the potential mechanism of Fyn in regulation of CCA cell migration and invasion, the present study examined the effects of Fyn on AMPK/mTOR signaling. The expression of p-AMPK, AMPK, p-mTOR and mTOR in the QBC939 cells transfected with shFyn and shNC was analyzed by western blotting (Fig. 3A). The results showed that phosphorylated AMPK levels in QBC939 cells transfected with shFyn were significantly increased compared with QBC939 cells transfected with shNC. By contrast, the expression of phosphorylated mTOR was decreased, but the expression of AMPK and mTOR were not significantly different in both groups (Fig. 3B). Thus, Fyn knockdown can activate the AMPK/mTOR signaling pathway.
Figure 3.
Fyn knockdown promoted p-AMPK expression and inhibited p-mTOR expression, suggesting that Fyn knockdown activated AMPK/mTOR signaling. (A) Expression of p-AMPK, AMPK, p-mTOR and mTOR in the cholangiocarcinoma cell line QBC939 transfected with shFyn and shNC was analyzed by western blotting. GAPDH served as an internal control. (B) Levels of p-AMPK and p-mTOR proteins in QBC939 cells transfected with shFyn and shNC. Data represent the mean ± standard deviation for three independent experiments. *P<0.05 vs. shNC. AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; p-, phosphorylated; shNC, control short hairpin RNA; shFyn, short hairpin RNA against Fyn.
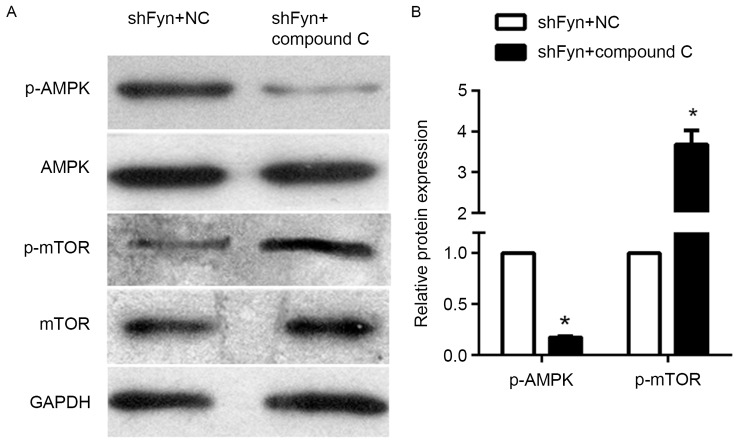
Compound C can reverse the effect of AMPK/mTOR signaling
The expression of p-AMPK, AMPK, p-mTOR and mTOR in the CCA QBC939 cell line treated with shFyn plus compound C or NC were analyzed by western blotting (Fig. 4A). The results of western blotting experiments showed that levels of phosphorylated AMPK in QBC939 cells treated with shFyn plus compound C were significantly decreased compared with QBC939 cells treated with shFyn plus NC. By contrast, the levels of phosphorylated mTOR were increased, whereas the expression of AMPK and mTOR were not significantly different between both the groups (Fig. 4B). Thus, compound C can reverse the effect the effect of Fyn shRNA on AMPK/mTOR signaling pathway in QBC939 cells.
Figure 4.
Compound C inhibited the AMPK/mTOR signaling pathway. (A) Expression of p-AMPK, AMPK, p-mTOR and mTOR in the cholangiocarcinoma cell line QBC939 treated with shFyn plus compound C or NC were analyzed by western blotting. GAPDH acted as an internal control. (B) Levels of phosphorylated AMPK and mTOR protein expression in QBC939 cells treated with shFyn plus compound C or NC. Data represent mean ± standard deviation of three independent experiments. *P<0.05 vs. shFyn+NC. AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; p-, phosphorylated; shNC, control short hairpin RNA; shFyn, short hairpin RNA against Fyn.
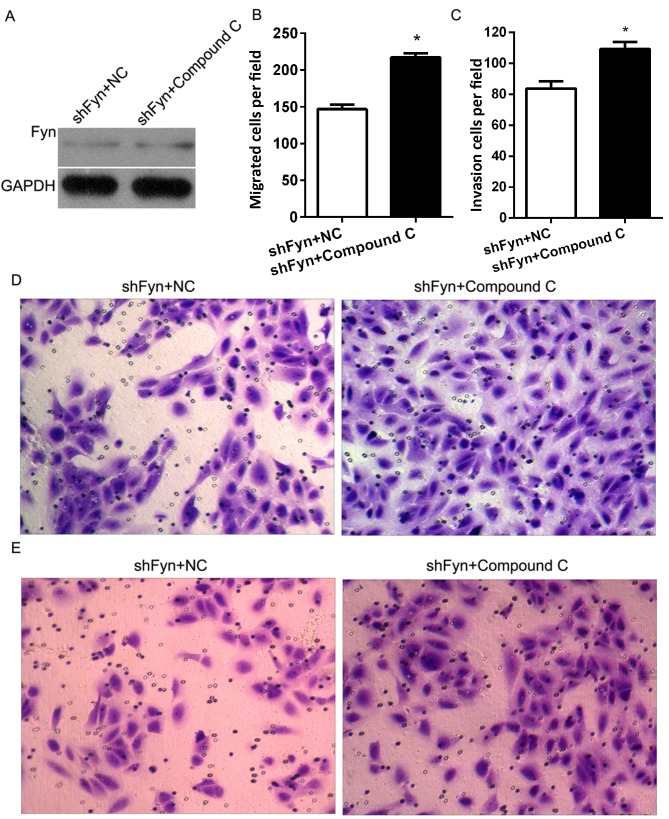
Inhibition of AMPK/mTOR promoted CCA cell migration and invasion
In order to investigate the role of Fyn and AMPK/mTOR signaling in the regulation of CCA cell migration and invasion, the present study cultured QBC939 cells treated with shFyn plus compound C or NC. After 48 h of culture, Fyn expression was detected by western blotting. The results showed no differences in Fyn expression between the QBC939 cells treated with shFyn plus compound C and the QBC939 cells treated with shFyn plus NC (Fig. 5A). In addition, Transwell assays were used to estimate the migration and invasion of QBC939 cells treated with shFyn plus compound C or NC. After 48 h of culture, the migration numbers of QBC939 cells treated with shFyn plus compound C or NC were 147±16 and 217±15, respectively (P<0.05; Fig. 5B), and the invasion numbers of QBC939 cells treated with shFyn plus compound C or NC were 83±11 and 109±11, respectively (P<0.05; Fig. 5C). The Transwell migration assay showed that the ability of the cells to migrate through the membrane into the lower chamber was significantly inhibited in the shFyn plus compound C group compared to the shFyn plus NC group (P<0.05; Fig. 5D). The Transwell invasion assay showed that the ability of the cells to pass through a Matrigel-coated membrane into the lower chamber was significantly decreased in shFyn plus compound C group compared to shFyn plus NC group (P<0.05; Fig. 5E).
Figure 5.
Compound C promoted cholangiocarcinoma cell migration and invasion. (A) Fyn expression in QBC939 cells transfected with shFyn was examined by western blotting after culturing with compound C or NC for 48 h. GAPDH served as an internal control. (B) The average number of migrating cells per field for the indicated experimental groups. (C) The average number of invading cells per field for the indicated experimental groups. (D) Migration assays for QBC939 cells. Magnification, ×200. (E) Invasion assays for QBC939 cells. Magnification, ×200. Data represent the mean ± standard deviation for three independent experiments. *P<0.05 vs. shFyn+NC.
Discussion
CCA is a rare and fatal tumor with steadily increasing incidence and mortality rates worldwide over previous decades (1). Metastasis is regarded as the major factor that contributes to the poor prognosis of CCA patients (5). Therefore, researchers aim to develop novel therapeutic targets to control CCA metastasis. Fyn, a member of the SFK, has been found to enhance expression and promote tumor metastasis in various cancers, including pancreatic, prostate and colorectal cancers (15–17). In the present study, the function of Fyn in the regulation of CCA cell migration and invasion was investigated. The present results provide in vitro evidence that Fyn is overexpressed in CCA cell lines and that silencing Fyn inhibits CCA cell migration and invasion. These results reveal the oncogenic potential of Fyn in CCA in a manner similar to other cancers.
Little is known about the mechanisms by which Fyn induces migration and invasion of CCA cell lines. AMPK is a ubiquitous serine/threonine protein kinase that regulates tumor occurrence, development and chemoresistance through negative regulation of mTOR. Li et al (21) showed that vitamin D3 potentiates the growth inhibitory effects of metformin in human prostate DU145 cancer cells through activation of p-AMPK with subsequent inhibition of downstream mTOR signaling. Wu et al (22) demonstrated that activation of AMPK/mTOR signaling pathway is involved in autophagy-mediated cisplatin resistance in lung adenocarcinoma. AMPK/mTOR signaling also has an important role in tumor metastasis. In human non-small cell lung carcinoma (NSCLC), activated AMPK/mTOR signaling pathway suppressed the invasion and migration of NSCLC cells (23). In the present study, the effects of Fyn on AMPK/mTOR signaling were investigated. These findings demonstrate that Fyn knockdown can activate the phosphorylation of AMPK, inhibit downstream phosphorylation of mTOR, and activate the AMPK/mTOR signaling pathway.
Furthermore, the present study cultured CCA cells treated with shFyn plus compound C or NC to investigate the role of Fyn and AMPK/mTOR signaling in the regulation of CCA cell migration and invasion. As expected, the AMPK inhibitor compound C suppressed the AMPK phosphorylation and increased mTOR phosphorylation, without significantly affecting Fyn expression. In addition, shFyn plus compound C promoted the migration and invasion of CCA cells compared with shFyn plus NC. Thus, compound C could reverse the effect of Fyn shRNA on migration and invasion of CCA cells.
In conclusion, Fyn knockdown inhibits cell migration and invasion by activating the AMPK/mTOR signaling pathway in CCA cells. The present data only provide in vitro evidence regarding the role of Fyn in CCA cells. In vivo studies are required to further confirm the oncogenic role of Fyn and to help establish a therapeutic strategy based on Fyn targeting.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Tyson GL, Ilyas JA, Duan Z, Green LK, Younes M, El-Serag HB, Davila JA. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59:3103–3110. doi: 10.1007/s10620-014-3276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinese Chapter of International Hepato-Pancreato-Biliary Association. Liver Surgery Group. Surgical Branch of the Chinese Medical Association. Cai JQ, Cai SW, Cong WM, Chen MS, Chen P, Chen XP, Chen YL, Chen YF, Dai CL, Huang Q, et al. Diagnosis and treatment of cholangiocarcinoma: A consensus from surgical specialists of China. J Huazhong Univ Sci Technolog Med Sci. 2014;34:469–475. doi: 10.1007/s11596-014-1301-5. [DOI] [PubMed] [Google Scholar]
- 4.Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: A large single-center cohort study. J Gastrointest Surg. 2014;18:562–572. doi: 10.1007/s11605-013-2447-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Yang H, Shen C, Luo J. Cholangiocarcinoma: Prognostic factors after surgical resection in China. Int J Clin Exp Med. 2015;8:5506–5512. [PMC free article] [PubMed] [Google Scholar]
- 6.Yubin L, Chihua F, Zhixiang J, Jinrui O, Zixian L, Jianghua Z, Ye L, Haosheng J, Chaomin L. Surgical management and prognostic factors of hilar cholangiocarcinoma: Experience with 115 cases in China. Ann Surg Oncol. 2008;15:2113–2119. doi: 10.1245/s10434-008-9932-z. [DOI] [PubMed] [Google Scholar]
- 7.Zheng-Rong L, Hai-Bo Y, Xin C, Chuan-Xin W, Zuo-Jin L, Bing T, Jian-Ping G, Sheng-Wei L. Resection and drainage of hilar cholangiocarcinoma: An 11-year experience of a single center in mainland China. Am Surg. 2011;77:627–633. [PubMed] [Google Scholar]
- 8.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posadas EM, Al-Ahmadie H, Robinson VL, Jagadeeswaran R, Otto K, Kasza KE, Tretiakov M, Siddiqui J, Pienta KJ, Stadler WM, et al. FYN is overexpressed in human prostate cancer. BJU Int. 2009;103:171–177. doi: 10.1111/j.1464-410X.2008.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu KV, Zhu S, Cvrljevic A, Huang TT, Sarkaria S, Ahkavan D, Dang J, Dinca EB, Plaisier SB, Oderberg I, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69:6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ban K, Gao Y, Amin HM, Howard A, Miller C, Lin Q, Leng X, Munsell M, Bar-Eli M, Arlinghaus RB, Chandra J. BCR-ABL1 mediates up-regulation of Fyn in chronic myelogenous leukemia. Blood. 2008;111:2904–2908. doi: 10.1182/blood-2007-05-091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Asawa T, Takato T, Sakai R. Cooperative roles of Fyn and cortactin in cell migration of metastatic murine melanoma. J Biol Chem. 2003;278:48367–48376. doi: 10.1074/jbc.M308213200. [DOI] [PubMed] [Google Scholar]
- 13.Kim JE, Roh E, Lee MH, Yu DH, Kim DJ, Lim TG, Jung SK, Peng C, Cho YY, Dickinson S, et al. Fyn is a redox sensor involved in solar ultraviolet light-induced signal transduction in skin carcinogenesis. Oncogene. 2016;35:4091–4101. doi: 10.1038/onc.2015.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZY, Cai L, Bie P, Wang SG, Jiang Y, Dong JH, Li XW. Roles of Fyn in pancreatic cancer metastasis. J Gastroenterol Hepatol. 2010;25:293–301. doi: 10.1111/j.1440-1746.2009.06021.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZY, Cai L, Zhu J, Chen M, Chen J, Li ZH, Liu XD, Wang SG, Bie P, Jiang P, et al. Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis. 2011;32:1419–1426. doi: 10.1093/carcin/bgr088. [DOI] [PubMed] [Google Scholar]
- 16.Gururajan M, Cavassani KA, Sievert M, Duan P, Lichterman J, Huang JM, Smith B, You S, Nandana S, Chu GC, et al. SRC family kinase FYN promotes the neuroendocrine phenotype and visceral metastasis in advanced prostate cancer. Oncotarget. 2015;6:44072–44083. doi: 10.18632/oncotarget.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Qian J, Wang F, Ma Z. Cellular prion protein accelerates colorectal cancer metastasis via the Fyn-SP1-SATB1 axis. Oncol Rep. 2012;28:2029–2034. doi: 10.3892/or.2012.2025. [DOI] [PubMed] [Google Scholar]
- 18.Yadav V, Denning MF. Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration. Mol Carcinog. 2011;50:346–352. doi: 10.1002/mc.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JL, Zheng W, Chen W, Qian Y, Ouyang YM, Fan CY. Lentivirus-mediated microRNA-124 gene-modified bone marrow mesenchymal stem cell transplantation promotes the repair of spinal cord injury in rats. Exp Mol Med. 2017;49:e332. doi: 10.1038/emm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Li HX, Gao JM, Liang JQ, Xi JM, Fu M, Wu YJ. Vitamin D3 potentiates the growth inhibitory effects of metformin in DU145 human prostate cancer cells mediated by AMPK/mTOR signalling pathway. Clin Exp Pharmacol Physiol. 2015;42:711–717. doi: 10.1111/1440-1681.12409. [DOI] [PubMed] [Google Scholar]
- 22.Wu T, Wang MC, Jing L, Liu ZY, Guo H, Liu Y, Bai YY, Cheng YZ, Nan KJ, Liang X. Autophagy facilitates lung adenocarcinoma resistance to cisplatin treatment by activation of AMPK/mTOR signaling pathway. Drug Des Devel Ther. 2015;9:6421–6431. doi: 10.2147/DDDT.S95606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang JI, Hong JY, Lee HJ, Bae SY, Jung C, Park HJ, Lee SK. Anti-tumor activity of yuanhuacine by regulating AMPK/mTOR signaling pathway and actin cytoskeleton organization in non-small cell lung cancer cells. PLoS One. 2015;10:e0144368. doi: 10.1371/journal.pone.0144368. [DOI] [PMC free article] [PubMed] [Google Scholar]