Abstract
The WHO 2010 classification divides gastrointestinal neuroendocrine neoplasms (GI-NENs) into neuroendocrine tumor (NET) G1, NET G2, neuroendocrine carcinoma (NEC) and mixed adenoendocrine carcinoma (MANEC) groups. A total of 136 cases of GI-NENs diagnosed at our hospitals as gastrointestinal carcinoids, endocrine cell carcinomas and NENs over the last 11 years, using the WHO 2010 classification were assessed. Among the 136 cases, 88.2% (120/136) were classified into the NET group (NET G1/G2) and 11.8% (16/136) were classified into the NEC group (NEC/MANEC). The incidences of lymphatic and venous invasions were higher in the NEC group compared with in the NET group (P<0.0001 and P=0.0021, respectively). The immunohistochemical staining of cluster of differentiation 73 (CD73) was evaluated in GI-NENs. CD73 is a potentially useful molecule in tumor immunity. In general, CD73 on the tumor cell membrane converts adenosine monophosphate to adenosine, which restrains the production of interferon-γ and cytocidal activity. Although the association between stem cells of pancreatic NENs and CD73 has been reported, few studies have reported on CD73 expression in GI-NENs. Immunohistochemical CD73 expression on the cytomembrane of neuroendocrine cells was detected in 27.2% (37/136) of the GI-NENs. The positive ratio of CD73 was significantly higher in the NEC group compared with in the NET group (P=0.0015). CD73 is also considered as a potential biomarker of anti-programmed death-1 (PD-1) therapy. The expression of programmed death-ligand 1 (PD-L1) on the cytomembrane of GI-NENs was assessed. The positive ratio of PD-L1 was higher in the NEC group compared with in the NET group (P=0.0011). Furthermore, CD73 expression status was significantly correlated with PD-L1 expression (P<0.0001). These results indicate that CD73 may be an interesting candidate for a biomarker for certain prognostic factors and therapeutics concerning PD-1 therapy.
Keywords: gastrointestinal neuroendocrine neoplasms, WHO2010 classification, immunohistochemical staining, cluster of differentiation 73, programmed death-1
Introduction
Neuroendocrine neoplasms (NENs) were described by Langhans in 1867 (1). The tumors were referred to as carcinoid (karzinoide) by Oberndorfer in 1907 (2). They were considered benign tumors. However, presently, these tumors are recognized as malignant. NENs arise in most organs of the body. In general, they are concentrated tumors of a specific organ system, such as the lungs, pancreas, and gastrointestinal tract (3,4). On microscopic assessment, NENs are composed of round or ovoid cells with a granular cytoplasm and nuclei that have a ‘salt and pepper’ appearance. The cells often form nests or may form small follicles or gland-like structures.
NENs are labeled by immunohistochemical neuroendocrine markers, including synaptophysin, chromogranin A (CGA), cluster of differentiation 56 (CD56), and neuron-specific enolase (NSE). Synaptophysin is considered the most sensitive neuroendocrine marker, whereas CGA is the most specific marker (5). Therefore, both synaptophysin and CGA are recommended for use in routine pathological diagnosis of NENs. There are few reports that reveal the association between immunohistological CGA and synaptophysin expressions and therapeutics and prognosis.
In Japan, gastrointestinal NENs (GI-NENs) are not diagnosed according to a unified classification. Many pathologists in Japan diagnose GI-NENs according to the original classification depending on each organ. For example, a colorectal neuroendocrine tumor (NET) was diagnosed according to the Japanese Classification of Colorectal Carcinoma (JCCC) (6). In recent years, many pathologists globally diagnose GI-NENs according to the WHO 2010 classification.
Owing to our understanding of tumor biology and immunity in recent years, cancer immunotherapy has made remarkable advances. Cluster of differentiation 73 (CD73), also known as ecto-5′-nucleotidase, is a pivotal molecule that enzymatically dephosphorylates and converts extracellular adenosine monophosphate (AMP) into adenosine and inorganic phosphate (7). Adenosine restrains the production of INF-γ and cytocidal activity through the adenosine A2A receptor (A2AAR) on T cells and natural killer cells (8–11). In mice with defective A2AAR and CD73, antitumor immunity is enhanced, which reduces the tumor growth (11–13). Recent studies have revealed that CD73 is identified as not only a unique biomarker for pancreatic NENs (pNENs) cancer stem cells but also a novel molecular target for pNENs therapy (14). We hypothesized that CD73 is expressed on the cytomembrane of GI-NENs with high malignant potential. CD73 is also considered as a potential biomarker for anti-programmed death-1 (PD-1) therapy (15).
The first aim of the present study was to reassess 136 cases of GI-NENs according to the WHO 2010 classification. Additionally, we retrospectively examined the relationship between diagnosis and clinical characteristics, including patient sex, patient age, tumor location, and presence of lymphatic and venous invasions. The second aim of the present study was to examine the CD73 expression status of GI-NENs. Moreover, we evaluated the immunohistochemical expression of PD-L1 on the cytomembrane of GI-NENs, which is a key antigen for PD-1 therapy. The final aim of the present study was to examine the relationship between the expressions of PD-L1 and CD73.
Materials and methods
Patient and clinical data
We retrospectively studied 136 cases of GI-NENs that were pathologically diagnosed at Showa University Hospital (Tokyo, Japan) and Showa University Fujigaoka Hospital (Yokohama, Japan) between January 2005 and December 2015. The collected clinicopathological information included patient age, patient sex, tumor location, sampling methods (surgical, endoscopic, or autopsy), and pathological diagnosis. The study used human material and was approved by the Institutional Ethics Committee of Showa University (no. 2044, 2016).
Evaluation of GI-NENs according to the WHO 2010 classification
All cases of GI-NENs were reassessed according to the WHO 2010 classification. The WHO 2010 classification involves the following groups: G1 (mitotic count <2 per 10 high power fields (HPFs) and/or Ki-67 labeling index of ≤2%); G2 (mitotic count of 2-20/10 HPFs and/or Ki-67 labeling index of 3–20%); and G3 neuroendocrine carcinoma (NEC) (mitotic count exceeding 20/10 HPFs and/or Ki-67 labeling index of >20%) (16). Mixed adenoendocrine carcinoma (MANEC) has a phenotype that is morphologically recognizable as both gland-forming epithelial and NEC with at least 30% of either component.
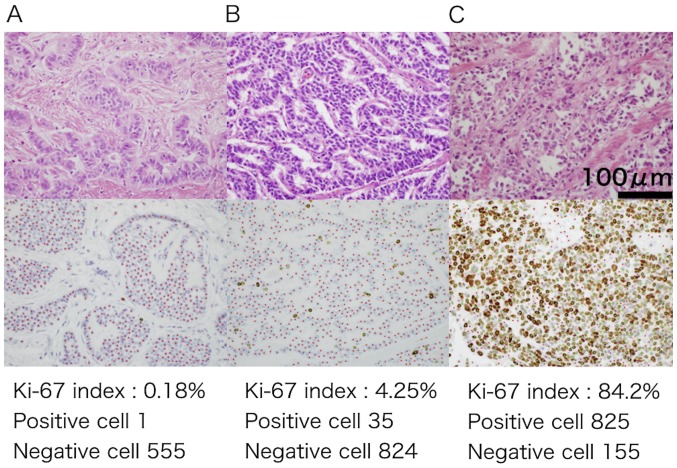
In the present study, we excluded cases in which we could not count >2,000 tumor cells. The Ki-67 labeling index was calculated using e-count (e-path Institute Inc., Fujisawa, Japan), which is an automatic measuring instrument. It is able to calculate the index with accuracy and efficiency. Positive cells were marked with green points, and negative cells were marked with red points (Fig. 1). We assessed lymphatic and venous invasions in cases (n=102) involving endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), surgery, and autopsy. We classified NET G1/G2 into the NET group and NEC/MANEC into the NEC group.
Figure 1.
(A-C) The Ki-67 labeling index was calculated using an automatic measuring instrument in the present study. Positive and negative cells are marked with green and red points, respectively (magnification, ×400). Top: hematoxylin and eosin staining; bottom: immunohistochemical Ki-67.
Evaluation of hematoxylin and eosin (H&E) and immunohistochemical staining for GI-NENs
Nine serial sections of formalin-fixed, paraffin-embedded specimens (3 µm) were used for H&E staining and immunohistochemical analysis with a panel of monoclonal antibodies (Table I). We diagnosed GI-NENs according to specific histological findings on H&E staining and the expressions of CD56, CGA, cytokeratin (CK) AE1/AE3, NSE, and synaptophysin. Additionally, we evaluated the Ki-67 labeling index and expressions of CD73 and PD-L1 in GI-NENs. According to a previous study, CD73 expression levels were graded on a scale of 0–3 on the basis of cytomembrane staining intensity and the proportion of positive tumors (17). The staining was graded as 0 when all cancer cells were negative, 1 when staining was weakly positive in less than one-third of the cancer cytomembrane, 2 when staining was weakly positive in greater than two-third of the cancer cytomembrane or strongly positive in greater than one-third of the cancer cytomembrane, and 3 when staining was weakly positive in most of the cancer cytomembrane or strongly positive in greater than two-third of the cancer cytomembrane. The immunohistochemical CD73 expression status in GI-NENs was classified as negative (grade 0) or positive (grade 1–3). With regard to PD-L1 expression, we considered a case as positive even if the expression was weak or partially positive in the cancer cytomembrane.
Table I.
Antibodies used for immunohistochemistry.
| Antigen | Animal | Clone | Source | Processing method | Dilution | Automatic stainer |
|---|---|---|---|---|---|---|
| Ki-67 | Mouse | MIB-1 | DAKO Cytomation, Glostrup, Denmark | HT | 1:100 | Nichirei, Tokyo, Japan |
| CD56 | Mouse | CD564 | Novocastra, Newcastle upon Tyne, UK | HT | 1:50 | Nichirei, Tokyo, Japan |
| Chromogranin A | Rabbit | Poly | DAKO Cytomation, Glostrup, Denmark | HT | 1:500 | Nichirei, Tokyo, Japan |
| CK AE1/AE3 | Mouse | AE1/AE3 | Novocastra, Newcastle upon Tyne, UK, | HT-RTU | 1:100 | Nichirei, Tokyo, Japan |
| NSE | Rabbit | Poly | Nichirei, Tokyo, Japan | Nichirei, Tokyo, Japan |
Method Stain after deparaffinization and activation of the antigen Stainer Histostainer 36A (Nichirei, Tokyo, Japan). CD, cluster of differentiation; CK, cytokeratin; NSE, neuron-specific enolase.
Two expert pathologists (ES and TY), who were blinded to the clinical records of the patients, graded the expressions of CD73 and PD-L1 on the cytomembrane.
Statistical analysis
The χ2 good-of-fit test or Fisher's exact test was used to analyze categorical data. All analyses were performed using JMP 12 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Reassessment of GI-NENs based on the WHO 2010 classification
The clinical characteristics of the patients enrolled in the present study are summarized in Table II. The study included 136 patients, who were diagnosed with gastrointestinal carcinoids, endocrine cell carcinomas, and NENs. There were 54 female (39.7%) and 82 male (60.3%) patients. The median age of the patients was 65.5 years (range, 24–85 years). The tumor locations were the stomach (n=24), duodenum (n=19), small intestine (n=2), appendix (n=4), colon (n=26), and rectum (n=61). The sampling methods included endoscopic biopsy (n=34), EMR (n=43), ESD (n=23), surgery (n=35), and autopsy (n=1). The cases were classified into the following four groups based on the WHO 2010 classification: NET G1 (n=113), NET G2 (n=7), NEC (n=12), and MANEC (n=4) (Table III). We found that the NET group mainly involved the rectum and colon (P=0.0014), and the NEC group mainly involved the stomach (P=0.0003). The incidences of lymphatic and venous invasions were higher in the NEC group than in the NET group (P<0.0001 and P=0.0021, respectively; Table IV).
Table II.
Clinical characteristics of the patients enrolled in the present study (n=136).
| Characteristic | Value |
|---|---|
| Age, years | |
| Median, range | 65.5 (24–85) |
| Sex, n (%) | |
| Male | 82 (60.3) |
| Female | 54 (39.7) |
| Location, n (%) | |
| Stomach | 24 (17.7) |
| Duodenum | 19 (14.0) |
| Small intestine | 2 (1.3) |
| Appendix | 4 (2.9) |
| Colon | 26 (19.1) |
| Rectum | 61 (45.0) |
| Sampling methods, n (%) | |
| Biopsy | 34 (25.0) |
| EMR | 43 (31.7) |
| ESD | 23 (16.9) |
| Surgery | 35 (25.7) |
| Autopsy | 1 (0.7) |
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.
Table III.
Association between tumor location and the WHO2010 classification among the 136 patients with gastrointestinal neuroendocrine neoplasms.
| WHO 2010 classification | ||||
|---|---|---|---|---|
| Location | NET G1 (n=113) | NET G2 (n=7) | NEC (n=12) | MANEC (n=4) |
| Stomach | 15 | 1 | 8 | 0 |
| Duodenum | 16 | 2 | 1 | 0 |
| Small Intestine | 2 | 0 | 0 | 0 |
| Appendix | 1 | 0 | 1 | 2 |
| Colon | 21 | 1 | 2 | 2 |
| Rectum | 58 | 3 | 0 | 0 |
| Total | 113 | 7 | 12 | 4 |
NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MANEC, mixed adenoendocrine carcinoma.
Table IV.
Association between clinicopathological characteristics and the WHO 2010 classification among patients with gastrointestinal neuroendocrine neoplasms.
| WHO 2010 classification | ||||
|---|---|---|---|---|
| Clinicopathological feature | No. | NET group (G1/G2) | NEC group (NEC/MANEC) | P-value |
| Age, years | 0.6394 | |||
| <70 | 92 | 82 | 10 | |
| ≥70 | 44 | 38 | 6 | |
| Sex | 0.2789 | |||
| Male | 82 | 70 | 12 | |
| Female | 54 | 50 | 4 | |
| Location (stomach or not) | 0.0003 | |||
| Stomach | 24 | 16 | 8 | |
| Except stomach | 112 | 104 | 8 | |
| Location (colorectum or not) | 0.0014 | |||
| Colorectum | 87 | 83 | 4 | |
| Except colorectum | 49 | 37 | 12 | |
| Lymphatic invasion | <0.0001 | |||
| + | 17 | 10 | 7 | |
| − | 85 | 82 | 3 | |
| Venous invasion | 0.0021 | |||
| + | 34 | 26 | 8 | |
| − | 68 | 66 | 2 | |
NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MANEC, mixed adenoendocrine carcinoma.
CD73 expression in the cytomembrane of GI-NENs
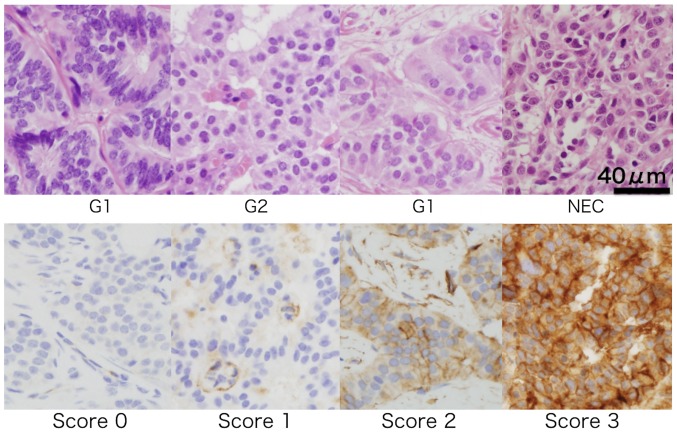
We examined the immunohistochemical expression status of CD73 on the cytomembrane of GI-NENs (Fig. 2). We found that 27.2% of the patients (n=37) showed CD73 expression (from grade 1 to grade 3). There was a trend that patients who were diagnosed with a highly malignant type of GI-NENs expressed a high grade of CD73 (Table V). The positive ratio of CD73 was significantly higher in the NEC group than in the NET group (P=0.0015). Additionally, the positive ratio of CD73 was higher in the duodenum than in other gastrointestinal organs (P<0.0001).
Figure 2.
Representative immunohistochemical cluster of differentiation 73 expression status in gastrointestinal neoplasms with H&E staining. Staining was graded as 0 when all cancer cells were negative, 1 when staining was weakly positive in less than one-third of the cancer cytomembrane, 2 when staining was weakly positive in greater than two-third of the cancer cytomembrane or strongly positive in greater than one-third of the cancer cytomembrane, and 3 when staining was weakly positive in most of the cancer cytomembrane or strongly positive in greater than two-third of the cancer cytomembrane (magnification, ×400). Top: H&E; bottom: immunohistochemical Ki-67. NEC, neuroendocrine carcinoma; H&E, hematoxylin and eosin.
Table V.
IHC staining for CD73 and the relationship with the WHO 2010 classification.
| CD73 IHC grade | n (%) | NET G1 (%) | NET G2 (%) | NEC (%) | MANEC (%) |
|---|---|---|---|---|---|
| 0 | 99 (72.8) | 77.9 | 71.4 | 41.7 | 25 |
| 1 | 7 (5.1) | 1.8 | 14.3 | 25 | 25 |
| 2 | 8 (5.9) | 6.2 | 0 | 8.3 | 0 |
| 3 | 22 (16.2) | 14.1 | 14.3 | 25 | 50 |
NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MANEC, mixed adenoendocrine carcinoma; CD, cluster of differentiation; IHC, immunohistochemical.
PD-L1 expression in the cytomembrane of GI-NENs
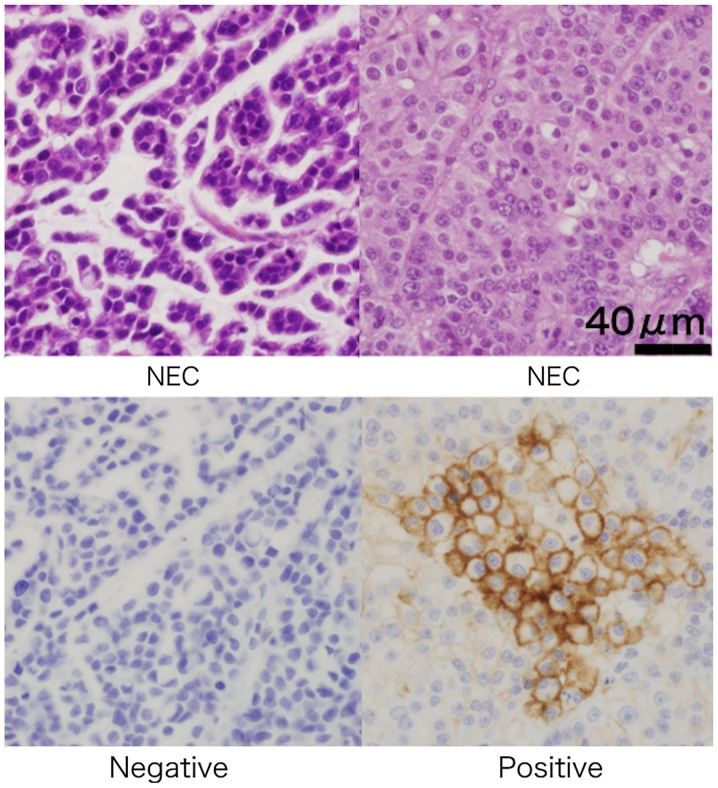
We examined the PD-L1 expression on the cytomembrane of GI-NENs (Fig. 3). The positive ratio of PD-L1 was 9.56% (13/136) in GI-NENs. The positive ratio of PD-L1 was higher in the NEC group than in the NET group (P=0.0011). Additionally, the positive ratio of PD-L1 was higher in the CD73-positive group than in the negative group (P<0.0001). There were no statistically significant associations between PD-L1 expression and lymphatic (P=0.3871) and venous invasions (P=0.1732) (Table VI).
Figure 3.
Representative immunohistochemical programmed death-ligand 1 expression status in gastrointestinal neoplasms with H&E staining. We considered a case as positive even if the expression was weak or partially positive in the cancer cytomembrane (magnification, ×400). Top: H&E; bottom: immunohistochemical Ki-67. NEC, neuroendocrine carcinoma; H&E, hematoxylin and eosin.
Table VI.
Associations between clinicopathological characteristics and CD73 and PD-L1 expressions on gastrointestinal neuroendocrine neoplasms.
| CD73 expression | PD-L1 expression | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological feature | No. | Positive | Negative | P-value | Positive | Negative | P-value |
| Age, years | 0.0038 | 0.3299 | |||||
| <70 | 92 | 18 | 74 | 7 | 85 | ||
| ≥70 | 44 | 19 | 25 | 6 | 38 | ||
| Sex | 0.7855 | 0.0759 | |||||
| Male | 82 | 23 | 59 | 11 | 71 | ||
| Female | 54 | 14 | 40 | 2 | 52 | ||
| Location (Duodenum or not) | <0.0001 | 0.0031 | |||||
| Duodenum | 19 | 13 | 6 | 6 | 13 | ||
| Except duodenum | 117 | 24 | 93 | 7 | 110 | ||
| Location (Colorectum or not) | 0.0074 | 0.0020 | |||||
| Colorectum | 87 | 17 | 70 | 3 | 84 | ||
| Except colorectum | 49 | 20 | 29 | 10 | 39 | ||
| WHO 2010 classification | 0.0015 | 0.0011 | |||||
| NET group (G1, G2) | 120 | 27 | 93 | 7 | 113 | ||
| NEC group (NEC, MANEC) | 16 | 10 | 6 | 6 | 10 | ||
| Invasion | |||||||
| Lymphatic invasion | 0.2435 | 0.3871 | |||||
| + | 17 | 7 | 10 | 3 | 14 | ||
| − | 85 | 23 | 62 | 8 | 77 | ||
| Venous invasion | 0.6448 | 0.1732 | |||||
| + | 34 | 9 | 25 | 6 | 28 | ||
| − | 68 | 21 | 47 | 5 | 64 | ||
| CD73 expression | − | <0.0001 | |||||
| + | − | − | − | 11 | 26 | ||
| − | − | − | − | 2 | 97 | ||
CD, cluster of differentiation; PD-L, programmed death-ligand; NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MANEC, mixed adenoendocrine carcinoma.
Discussion
The primary site of GI-NENs in Japan is different from that in other countries. It has been reported that the locations of GI-NENs varied, with 26.1, 3.6, and 70.3% in the foregut, midgut, and hindgut, respectively, and that 6.7% of GI-NENs were NEC in Japan (18). The most common location of GI-NENs among patients in the United States varied, with 11, 38, 16, 34, and 1% in the stomach, small intestine, colon, rectum, and unknown sites, according to analysis of the Surveillance Epidemiology End Results database (19). In Western nations, 30–60% of GI-NENs are derived from the midgut (20–22). In our study, the locations of GI-NENs varied, with 17.7, 14.0, 1.3, 2.9, 19.1, and 45.0% in the stomach, duodenum, small intestine, appendix, colon, and rectum, respectively. These results are consistent with the findings of a previous Japanese report (18). Sixteen cases of GI-NENs belonged to NEC group in our study. Furthermore, 8 cases had NEC in the stomach. Notably, the number of case showing NECs of stomach was significant (P=0.003); therefore, we have used the expression ‘stomach or not’ in Table IV.
We reassessed 136 cases of GI-NENs according to the WHO 2010 classification. Because assessments of the Ki-67 labeling index and mitotic cells depend on respective pathologists, there is a discrepancy in reporting among institutions (6,23–27). The mitotic count should be calculated from the most active areas (or hot spots), which are recognized by scanning the sample under intermediate magnification. A minimum of 50 HPFs should be carefully evaluated to precisely determine the mitotic count. Recently, a mitosis-specific marker, phosphohistone H3 (PHH3), was introduced for the assessment of mitotic counts in NENs. Mitotic counts determined by PHH3 staining and H&E staining showed a high concordance rate, but the results need to be validated using many cases (28). For counting with regard to the Ki-67 labeling index, strong, dark-brown nuclear staining is recommended, whereas cytoplasmic staining or weak nuclear staining should not be counted (29). Ki-67 staining also depends on the paraffin sectioning method. The time for clamping of vessels and surgical resection of tissues decreases the mitotic count abruptly. Therefore, grading with the Ki-67 labeling index is always higher than grading with mitosis (30). Thus, it is important to accurately calculate the Ki-67 labeling index. In the present study, we were able to normalize the evaluation of the Ki-67 labeling index by using an automatic measuring instrument (e-count; e-path Institute Inc.). In the reassessment, 88.2% (120/136) of GI-NENs were classified into the NET group and 11.8% (16/136) were classified into the NEC group.
Immunohistological CD73 expression does not connect with the diagnosis for GI-NENs, such as CGA or synaptophysin, but with the tumor growth associated with A2AAR. Recent studies identified CD73 as not only a unique biomarker for pNENs cancer stem cells but also a novel molecular target for pNENs therapy (14). CD73 expression is also associated with poor prognosis in several types of tumors (31), including colorectal cancer (32), gastric cancer (33), gallbladder cancer (34), serous ovarian cancer (35), triple negative breast cancer (36), and malignant melanoma (37). In endometrial tumors, no differences in the CD73 expression among tumors were observed with enzyme assays in either tissue slices or tissue homogenates (38). To the best of our knowledge, there has been no report on CD73 expression in GI-NENs. Furthermore, a significant association between CD73 and CGA and synaptophysin has not been revealed. We hypothesized that CD73 is expressed on the membrane of GI-NENs with high malignant potential. In the present study, we also examined the expression of CD73 on the cytomembrane of GI-NENs using immunohistochemical staining. The ratio of immunohistochemical CD73 expression was higher in the NEC group than in the NET group. Additionally, elevated CD73expression is associated with an increased malignant potential of GI-NENs in the present study.
It has been reported that lymphatic and venous invasions are independent predictive factors of lymph node metastasis in colorectal NENs (39). Moreover, cases involving tumors <10 mm in size with lymphatic and venous invasions are at high risk of lymph node metastasis with regard to colorectal NENs (39). It has also been reported that the immunohistochemical CD73 expression status was significantly correlated with invasion into adjacent organs in pNENs (14). In the present study, lymphatic and venous invasions were more frequent in the NEC group than in the NET group. However, there was no relationship between immunohistochemical CD73 expression and lymphatic and venous invasions in our study. It is assumed that lymphatic and venous invasions are not associated with the pathway related to CD73.
CD73 is considered as a potential biomarker for PD-1 therapy (15). Targeted blockade of CD73 can enhance the therapeutic activity of anti-PD-1 and anti-CTLA-4 monoclonal antibodies and may thus potentiate therapeutic strategies targeting immune checkpoint inhibitors in general (40). PD-1 is a checkpoint molecule on T cells that plays a vital role in limiting adaptive immune responses and preventing autoimmune and auto-inflammatory reactivity in a normal host. PD-L1, its primary ligand, is variably expressed on cancer cells and antigen-presenting cells within tumors. It is clearly associated with a positive outcome for treatment with PD-1/PD-L1 blocking antibodies, which are able to reduce tumor growth (9,41). In PD-L1 immunohistochemical assay, the rabbit monoclonal antibody 28-8 can specifically detect the PD-L1 plasma membrane protein expressed in cancer cells. The antibody has been investigated as a potential biomarker to predict the clinical response to nivolumab in clinical settings in the lungs (42). Clone 22C3 is also able to detect the PD-L1 plasma membrane protein. The efficacy of pembrolizumab for lung adenocarcinoma is associated with staining of clone 22C3 in the lungs (43). We also assessed PD-L1 expression on the cytomembrane of GI-NENs. A strong correlation between the expressions of CD73 and PD-L1 on the cytomembrane of GI-NENs was indicated.
The primary limitation of the present study was that we did not examine the influence of CD73 on the clinical course. The assessment of the relationship between CD73 expression and the prognosis of GI-NENs is valuable.
In conclusion, our analysis suggests that CD73 may be an interesting candidate for a biomarker for certain prognostic factors and therapeutics concerning PD-1 therapy. However, because only 16 cases of NEC were evaluated in the present study, accumulation of more cases is needed to validate the clinical use of evaluating immunohistochemical CD73 expression in GI-NENs.
Acknowledgements
We would like to thank T. Nagai and Y. Sasaki of the Department of Pathology and Laboratory Medicine, Showa University School of Medicine (Tokyo, Japan) for providing valuable assistance with the immunohistochemical analysis of the tissue samples.
This manuscript was presented in the 106th annual meeting of the Japanese Society of Pathology, held on April 27–29, 2017 in Tokyo, Japan.
References
- 1.Langhans TH. Ueber einen Drüsenpolyp im ileum. Virchows Arch Euro J Pathol. 1867;38:pp559–560. doi: 10.1007/BF02114017. [DOI] [Google Scholar]
- 2.Oberndorfer S. Karzinoide tumoren des dunndarms. Frankf Z Pathol. 1907;1:426–432. [Google Scholar]
- 3.Modlin IM, Shapiro MD, Kidd M, Eick G. Siegfried Oberndorfer and the evolution of carcinoid disease. Arch Surg. 2007;142:187–197. doi: 10.1001/archsurg.142.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 5.Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gönen M, Jensen RT, Kidd M, Kulke MH, Lloyd RV, et al. Pathology reporting of neuroendocrine tumors: Application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300–313. doi: 10.1097/PAS.0b013e3181ce1447. [DOI] [PubMed] [Google Scholar]
- 6.Japanese society for cancer of the colon and rectum: General rules for clinical and pathological studies on cancer of the colon, rectum and anus. 8th edn. Tokyo: Japanese society for cancer of the colon and rectum; 2013. [Google Scholar]
- 7.Yegutkin GG. Nucleotide- and nucleoside converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci USA. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsuta E, Tanaka S, Mogushi K, Shimada S, Akiyama Y, Aihara A, Matsumura S, Mitsunori Y, Ban D, Ochiai T, et al. CD73 as a therapeutic target for pancreatic neuroendocrine tumor stem cells. Int J Oncol. 2016;48:657–669. doi: 10.3892/ijo.2015.3299. [DOI] [PubMed] [Google Scholar]
- 15.Beavis PA, Slaney CY, Milenkovski N, Henderson MA, Loi S, Stagg J, Kershaw MH, Darcy PK. CD73: A potential biomarker for anti-PD-1 therapy. Oncoimmunology. 2015;4:e1046675. doi: 10.1080/2162402X.2015.1046675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rindi G, Arnold R, Bosman FT. WHO Classification of Tumors of the Digestive System. IARC; Lyon: 2010. Nomenclature and classification of neuroendocrine neoplasms of the digestive system; pp. 13–14. [Google Scholar]
- 17.Oh HK, Sin JI, Choi J, Park SH, Lee TS, Choi YS. Overexpression of CD73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory T cell infiltration. J Gynecol Oncol. 2012;23:174–281. doi: 10.3802/jgo.2012.23.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: A nationwide survey analysis. J Gastroenterol. 2015;50:58–64. doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 19.Mocellin S, Nitti D. Gastrointestinal carcinoid: Epidemiological and survival evidence from a large population-based study (n=25 531) Ann Oncol. 2013;24:3040–3044. doi: 10.1093/annonc/mdt377. [DOI] [PubMed] [Google Scholar]
- 20.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after ‘carcinoid’: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 21.Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R. Barcelona Consensus Conference participants: ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 22.Oberg K. Diagnosis and treatment of carcinoid tumors. Expert Rev Anticancer Ther. 2003;3:863–877. doi: 10.1586/14737140.3.6.863. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda K, Kojima M, Saito N, Sakuyama N, Koushi K, Watanabe T, Sugihara K, Akimoto T, Ito M, Ochiai A. Current status of the histopathological assessment, diagnosis, and reporting of colorectal neuroendocrine tumors: A web survey from the Japanese society for cancer of colon and rectum. Pathol Int. 2016;66:94–101. doi: 10.1111/pin.12388. [DOI] [PubMed] [Google Scholar]
- 24.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumors of the digestive system, 4th edition. Lyon. Int Agency Res Cancer. 2010;3:pp417. [Google Scholar]
- 25.Soga J. The term ‘carcinoid’ is a misnomer: The evidence based on local invasion. J Exp Clin Cancer Res. 2009;28:15. doi: 10.1186/1756-9966-28-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkins CS, Darling JL, Gill SS, Revesz T, Thomas DG. Cell proliferation in serial biopsies through human malignant brain tumours: Measurement using Ki67 antibody labelling. Br J Neurosurg. 1991;5:289–298. doi: 10.3109/02688699109005189. [DOI] [PubMed] [Google Scholar]
- 27.Torp SH, Alsaker M. Ki-67 immunoreactivity, basic fibroblastic growth factor (bFGF) expression, and microvessel density as supplementary prognostic tools in low-grade astrocytomas. An immunohistochemical study with special reference to the reliability of different Ki-67 antibodies. Pathol Res Pract. 2002;198:261–265. doi: 10.1016/S0344-0338(04)70251-4. [DOI] [PubMed] [Google Scholar]
- 28.Voss SM, Riley MP, Lokhandwala PM, Wang M, Yang Z. Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol. 2015;39:13–24. doi: 10.1097/PAS.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 29.Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: Current concepts and controversies. Endocr Pathol. 2014;25:65–79. doi: 10.1007/s12022-013-9295-2. [DOI] [PubMed] [Google Scholar]
- 30.Cross SS, Start RD, Smith JH. Does delay in fixation affect the number of mitotic figures in processed tissue? J Clin Pathol. 1990;43:597–599. doi: 10.1136/jcp.43.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue Y, Yoshimura K, Kurabe N, Kahyo T, Kawase A, Tanahashi M, Ogawa H, Inui N, Funai K, Shinmura K, et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget. 2017;8:8738–8751. doi: 10.18632/oncotarget.14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu XR, He XS, Chen YF, Yuan RX, Zeng Y, Lian L, Zou YF, Lan N, Wu XJ, Lan P. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106:130–137. doi: 10.1002/jso.23056. [DOI] [PubMed] [Google Scholar]
- 33.Lu XX, Chen YT, Feng B, Mao XB, Yu B, Chu XY. Expression and clinical significance of CD73 and hypoxia-inducible factor-1α in gastric carcinoma. World J Gastroenterol. 2013;19:1912–1918. doi: 10.3748/wjg.v19.i12.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong L, Wen Y, Miao X, Yang Z. NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res. 2014;355:365–374. doi: 10.1007/s00441-013-1752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DM, Haibe-Kains B, Karn T, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75:4494–4503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- 36.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci USA. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Aliagas E, Vidal A, Texidó L, Ponce J, Condom E, Martín-Satué M. High expression of ecto-nucleotidases CD39 and CD73 in human endometrial tumors. Mediators Inflamm. 2014;2014:509027. doi: 10.1155/2014/509027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojima M, Ikeda K, Saito N, Sakuyama N, Koushi K, Kawano S, Watanabe T, Sugihara K, Ito M, Ochiai A. Neuroendocrine tumors of the large intestine: Clinicopathological features and predictive factors of lymph node metastasis. Front Oncol. 2016;6:173. doi: 10.3389/fonc.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 41.Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: Current status and future directions. Cancer Immunol Immunother. 2017;66:551–564. doi: 10.1007/s00262-017-1954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips T, Simmons P, Inzunza HD, Cogswell J, Novotny J, Jr, Taylor C, Zhang X. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23:541–549. doi: 10.1097/PAI.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangachari D, VanderLaan PA, Shea M, Le X, Huberman MS, Kobayashi SS, Costa DB. Correlation between classic driver oncogene mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 ≥50% expression in lung adenocarcinoma. J Thorac Oncol. 2017;12:878–883. doi: 10.1016/j.jtho.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]