Significance
Ultrasound-induced microbubble oscillation can lead to cell injury or mechanotransduction via calcium signaling processes such as intracellular calcium waves (ICWs). However, the mechanisms by which microbubbles stimulate ICWs remain unknown. Using a microfluidic system with highly controlled bubble−cell interaction, we identified two distinct types of ICWs: a fast response correlating with significant membrane poration, and a slow response triggered by calcium influx through stretch-activated ion channels. The fast ICWs, distinguished from those under physiological conditions, are associated with cell injuries. We further elicited ICWs without cell injury by displacing integrin-binding beads on the cell membrane under mild cavitation conditions. This study provides mechanistic insights into ICWs for guiding ultrasound therapy in tissue modification, drug delivery, and cell mechanotransduction.
Keywords: intracellular calcium wave, cavitation bioeffects, single-cell analysis, cell injury, mechanotransduction
Abstract
One of the earliest events in cellular mechanotransduction is often an increase in intracellular calcium concentration associated with intracellular calcium waves (ICWs) in various physiologic or pathophysiologic processes. Although cavitation-induced calcium responses are believed to be important for modulating downstream bioeffects such as cell injury and mechanotransduction in ultrasound therapy, the fundamental mechanisms of these responses have not been elucidated. In this study, we investigated mechanistically the ICWs elicited in single HeLa cells by the tandem bubble-induced jetting flow in a microfluidic system. We identified two distinct (fast and slow) types of ICWs at varying degrees of flow shear stress-induced membrane deformation, as determined by different bubble standoff distances. We showed that ICWs were initiated by an extracellular calcium influx across the cell membrane nearest to the jetting flow, either primarily through poration sites for fast ICWs or opening of mechanosensitive ion channels for slow ICWs, which then propagated in the cytosol via a reaction−diffusion process from the endoplasmic reticulum. The speed of ICW (CICW) was found to correlate strongly with the severity of cell injury, with CICW in the range of 33 μm/s to 93 μm/s for fast ICWs and 1.4 μm/s to 12 μm/s for slow ICWs. Finally, we demonstrated that micrometer-sized beads attached to the cell membrane integrin could trigger ICWs under mild cavitation conditions without collateral injury. The relation between the characteristics of ICW and cell injury, and potential strategies to mitigate cavitation-induced injury while evoking an intracellular calcium response, may be particularly useful for exploiting ultrasound-stimulated mechanotransduction applications in the future.
Cavitation can produce a broad and diverse range of bioeffects during ultrasound therapy, including blood–brain barrier opening (1), tissue ablation and antitumor immune response (2–4), targeted drug and gene delivery (5, 6), shock wave lithotripsy (SWL) (7), and histotripsy (8). Although cavitation-induced calcium responses have been reported during sonoporation (5, 9–12), ultrasonic neuromodulation (13), and with laser-generated cavitation bubbles (14, 15), the mechanism whereby the calcium ion (Ca2+) transient is initiated, its propagation characteristics, and relationship to downstream bioeffects such as cell injury and mechanotransduction have not been carefully examined (16), especially at the single-cell level. For example, it is unclear how the Ca2+ transients produced during sonoporation, with or without membrane poration, differ from each other quantitatively, and whether different mechanisms are involved (9, 17). Particularly, there is growing evidence linking excessive Ca2+ entry and high cytoplasmic Ca2+ concentration with cytotoxicity and associated apoptotic or necrotic cell death during sonication (12, 16, 18). In addition, mechanotransduction applications such as sonogenetics have gained increasing attention as a noninvasive method for neuromodulation where microbubbles are required to facilitate the cellular response (13). Despite the growing interest and potential, the role of cavitation-induced Ca2+ transients in such mechanotransduction processes is also not well understood. Moreover, minimum injury and membrane poration are desirable in sonogenetics and other ultrasonic mechanotransduction applications, e.g., stimulation of stem cell proliferation and differentiation (19, 20). Altogether, a fundamental understanding of the mechanisms underpinning cavitation-induced Ca2+ response and associated bioeffects is critical for exploiting the full potential of ultrasound in targeted molecular delivery, tissue modification, and sonogenetics through mechanosensory responses (13) that can produce the intended therapeutic outcome with minimal adverse effects (16).
In biology, it is well known that a number of extracellular stimuli, such as hormones, neurotransmitters, and physical signals such as mechanical stress, can be transduced via intracellular Ca2+ signaling to regulate a variety of important downstream processes, including exocytosis, contraction, transcription, fertilization, and proliferation (21, 22). Ca2+-mediated signaling can be triggered when extracellular Ca2+ influxes into the cell through plasma membrane, or when Ca2+ is released from intracellular stores, such as the endoplasmic reticulum (ER). This signal transduction is often accompanied by an intracellular Ca2+ wave (ICW), which may further propagate across cell junctions to neighboring cells to trigger intercellular Ca2+ waves for integrative, organ-level response (23, 24). Although Ca2+ signaling has been well investigated in biology (25, 26) regarding the role of ion channels and intracellular release, limited work has been carried out on the Ca2+ response to membrane poration and cell injury, which occurs frequently in ultrasound therapy with exposure to cavitation. In particular, cavitation can generate impulsive shear flows, and high-strain-rate cell membrane deformation that may result in transient membrane poration and lethal to sublethal cell injury (27–29). Therefore, from the biological point of view, it would be important to investigate cavitation-induced Ca2+ signaling and other cell response subjected to such high-strain-rate mechanical loading.
However, challenges exist for using current techniques of ultrasound-generated cavitation bubbles to dissect the complex bubble(s)−cell interaction due to the randomness in bubble generation and dynamics. Therefore, the mechanisms responsible for such bioeffects are largely unclear at the fundamental level. Furthermore, bubble−bubble interaction or bubble collapse near a boundary with cells can lead to jet formation (30, 31), which is common in therapeutic ultrasound such as SWL and high-intensity focused ultrasound. We have previously developed a microfluidic platform (28, 32) with laser-generated tandem bubbles (TBs), and the resultant jetting flow was directed to single patterned cells at different standoff distances (Sd). Such a unique experimental system offers precise control of the dynamic bubble(s)−cell interactions, thus allowing us to dissect various mechanisms responsible for cavitation-induced bioeffects. However, the transient intracellular Ca2+ response, especially in relationship to membrane poration produced by cavitation, has not been investigated.
In this study, we examined the intracellular Ca2+ response in single HeLa cells produced by the directional jetting flow from TB at different Sd, corresponding to various degrees of membrane deformation. We quantified the propagation speed of ICW (CICW), assessed its correlation with cell membrane poration, and further identified two distinct types of intracellular Ca2+ responses. In addition, the mechanisms responsible for each type of Ca2+ response were investigated by selectively modulating extracellular Ca2+ concentration, intracellular Ca2+ release, or Ca2+ influx from stretch-activated ion channels on the cell membrane. More importantly, we have demonstrated that intracellular Ca2+ response can be induced at large Sd without injury by attaching micrometer-sized beads to the cell membrane through the Arg−Gly−Asp (RGD)–integrin link. The observed relation between the characteristics of ICW and cell injury, and potential strategies to mitigate cavitation-induced injury while evoking an intracellular Ca2+ response, may be particularly useful for exploiting sonogenetics and neuromodulation applications in the future.
Results
Directional Perturbation of Single Cells from TB-Generated Jetting Flow.
We utilized a unique microfluidic platform with surface patterning to generate TBs with control of the bubble location, size, orientation, and Sd to an individual HeLa cell, captured and grown on an H-shaped island (32 × 32 μm) covered with fibronectin. The cell pattern can minimize the influence of cell size and adhesion characteristics on bubble(s)−cell interaction (Fig. 1 A and B). TBs (maximum diameter Dmax = 50 ± 2 μm) of antiphase oscillation are produced by two pulsed Neodymium-doped yttrium aluminum garnet (Nd:YAG) laser beams (λ = 532 nm, 5-ns pulse duration) with an interpulse delay of about 2.5 μs. These two laser beams are focused onto a pair of gold dots (15 nm thick and 6 μm in diameter, with a separation distance of dIB = 40 μm) patterned on the glass substrate of a microfluidic channel (H = 25 μm, Fig. 1A). Because the two bubbles are oscillating out of phase, they repel each other by the secondary Bjerknes forces (33), resulting in an outward directional jet formation between 3.0 μs and 5.0 μs (Fig. 1D). This directional jetting flow imposes a highly localized shear stress and stress gradient onto the target cell grown nearby (with a normalized standoff distance γ = Sd/Rmax = 1.2 to 2.4). By varying Sd of the patterned island to the TB, we can modulate the strength of mechanical stimulations imposed on individual cells and examine their intracellular Ca2+ response and other bioeffects under well-controlled experimental conditions. We have previously shown that, when γ < 1 (e.g., Sd = 10 μm to 20 μm), a pinpoint membrane poration with significant uptake of membrane impermeant propidium iodide (PI) could be produced, leading to necrosis or apoptosis in majority of the cells treated. In contrast, at γ = 1.2 to 1.6 (e.g., Sd = 30 μm to 40 μm), repairable to negligible membrane poration with increased cell survival was observed (28). In this work, we focus on Ca2+ response produced in the sublethal range of γ = 1.2 to 2.4 (Sd = 30 μm to 60 μm) with repairable or no detectable membrane poration (28, 32), which are most relevant to cavitation-mediated macromolecular delivery and sonogenetics applications.
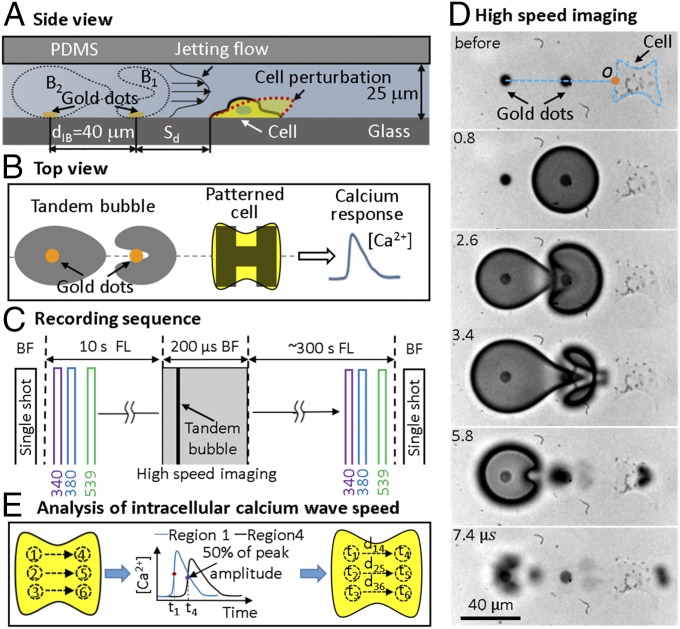
Fig. 1.
Schematic diagrams of experimental design and data analysis for spatiotemporal measurements of intracellular Ca2+ transients stimulated by TB interactions. (A) Side view and (B) top view of TB interaction with a single cell grown nearby on an H-shaped pattern in a microfluidic channel. (C) Recording sequences for BF imaging of cell morphology, simultaneous monitoring of intracellular Ca2+ transients (340-, 380-nm excitation) and membrane poration (539-nm excitation) through FL imaging, and high-speed imaging of tandem bubble interaction with jet formation. (D) Example of high-speed image sequence showing the interaction of laser-generated tandem bubble from gold dots with an H-patterned HeLa cell (1-µm RGD–integrin binding beads were attached on cell surface for visualization of membrane deformation). The orange circle (o) represents the intersection of jet axis with cell leading edge (jet impact point), which is used to calculate the original distance of beads to the jet impact point. (E) Protocol used for CICW analysis.
We used sequential fluorescence (FL) imaging to monitor concurrently the intracellular Ca2+ transients (340-, 380-nm excitation of fura-2) and membrane poration (539-nm excitation of PI) produced by TBs. FL imaging was first recorded at baseline level for 10 s before high-speed imaging of the TB interaction and jet formation, followed by a sequence of FL imaging of Ca2+ response and PI uptake in the target cell for 300 s (Fig. 1C). Before and after the TB treatment, changes in cell morphology were also documented by single-shot bright-field (BF) images. Cell injury is assessed by PI uptake and BF cell morphology change. To quantify the propagation of intracellular Ca2+ transient, we have developed a scheme to calculate CICW based on small-region analysis. The Ca2+ response profiles from the cell leading edge (small regions 1, 2, 3) to the trailing edge (small regions 4, 5, 6) are analyzed based on the time of flight in 50% risetime of individual pairs (Fig. 1E; see details in Materials and Methods).
Spatiotemporal Evolution of Intracellular Ca2+ Response and PI Uptake Induced by TB at Various Sd.
Membrane poration has been previously shown to play an important role in the initiation of intracellular Ca2+ response produced by cavitation bubbles, yet a detailed understanding of the process is limited (9, 11). We therefore first carried out a systematic analysis of the correlation between these two critical events in the biological response of single cells to TBs at different Sd. Fig. 2 A−E shows the image sequences of the typical intracellular Ca2+ response (Top) and PI uptake (Bottom) for individual cells treated with TB at Sd = 30 (Fig. 2A), 40 (Fig. 2 B and C), 50 (Fig. 2D), and 60 (Fig. 2E) µm, respectively. BF images of the cell morphology before and after the TB treatment are shown in Fig. 2 A–E, Right. In general, the intensity of the Ca2+ response and PI uptake showed a clear Sd dependency, with both signals initiated at the leading edge of the cell facing the jetting flow (indicated by the white arrows) and propagated toward the trailing edge (Fig. 2 A–C; also see Movie S1). Within the population of individual cells treated by TBs, the probability of an evoked Ca2+ response was found to drop progressively with Sd (circles in Fig. 2F), varying from 100% at Sd = 30 µm to no response at Sd = 60 µm. This Sd dependency in Ca2+ response is similar to TB-induced membrane poration, and may be associated with the exponentially decaying shear stress experienced by the target cells at increased Sd (28, 32). The resultant area strain integral (with time) is shown by the diamonds and squares in Fig. 2F, representing the upper- and lower-bound values. Using a triad of beads in close proximity, the local area strain of the membrane deformation was calculated based on either the principal strains or trigonometry of the triad, as described previously (28).
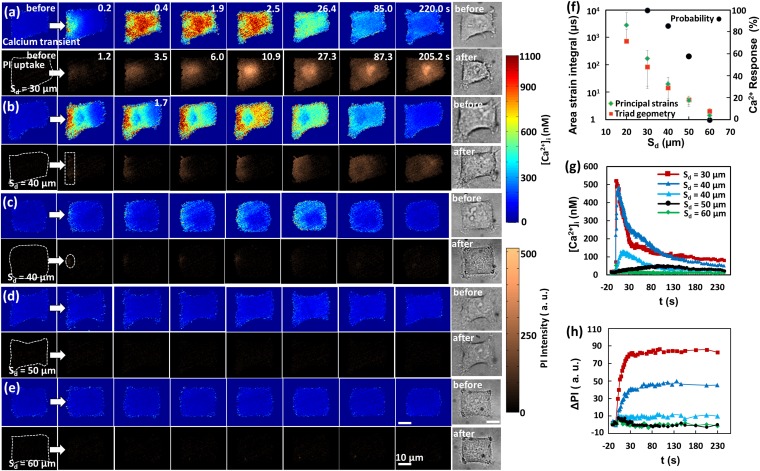
Fig. 2.
Typical intracellular Ca2+ response and PI uptake induced by TB treatment at various Sd. (A–E) (Left) Image sequences showing (Top) intracellular Ca2+ response and (Bottom) PI uptake in five representative individual cells at Sd = 30, 40 (two cells), 50, and 60 µm, respectively. (Right) Single-shot BF images of the cell before and after the treatment. The timing for the image sequences shown in B–E are the same as in A except as otherwise marked on the image. The white arrows indicate the jetting flow direction. The cell contour before treatment is outlined with white dashed lines in the first PI image. Cells were exposed to TBs at t = 0. Rescaled and more visible Ca2+ and PI concentration changes in C and D are shown in Fig. S1. (Scale bars, 10 µm.) (F) Ca2+ response probability (black circles) and area strain integral (shown in green diamonds and red squares) at different Sd (n = 19, 21, 19, and 10 for the Ca2+ response probability at Sd = 30, 40, 50, and 60 µm, respectively). For area strain integral, data at Sd = 20, 30, and 40 µm were taken from previous study (28), while data for Sd = 50 and 60 µm were interpolated. G and H are the temporal profiles of averaged intracellular Ca2+ concentration and PI uptake, respectively, inside each individual cell shown in A–E. The colors and symbols used for different Sd are the same in G and H.
Furthermore, the spatiotemporal distributions in the Ca2+ response and PI uptake were found to vary significantly with Sd. The average [Ca2+]i and PI uptake (ΔPI) within the individual cells shown in Fig. 2 A–E are plotted vs. time in Fig. 2 G and H, respectively. At Sd = 30 µm (or γ = 1.2), a fast Ca2+ response with short risetime (tr = 2.2 s) and large [Ca2+]i change (523 nM) was produced, which was accompanied by a high PI uptake (Fig. 2 A, G, and H). In contrast, at Sd = 50 µm (or γ = 2), a slow Ca2+ response with long tr (99 s) and small [Ca2+]i change (38 nM) was observed, associated with negligible PI uptake; see Fig. 2 D, G, and H. At Sd = 40 µm, a variety of responses ranging from fast to slow Ca2+ transients were obtained, corresponding to high to low PI uptake; see Fig. 2 B, C, G, and H. Finally, at Sd = 60 µm, neither Ca2+ response nor PI uptake was observed (Fig. 2 E–H).
We also noticed substantial variations in the detailed features of the Ca2+ response and PI uptake. At Sd = 30 µm (Fig. 2A), a pinpoint rupture with localized and strong PI uptake was often seen at the leading edge of the cell membrane, with PI gradually diffused inside the cytosol, reaching a plateau in about 30 s (red curve in Fig. 2H, without staining the cell nucleus), indicating repairable membrane poration (28). Importantly, such a localized membrane poration was found to coincide with the initiation of an intracellular Ca2+ response from the same region, which propagated radially at t = 0.2 s before spreading out quickly across the cytosol by t = 0.4 s. After initiation, however, the intracellular Ca2+ transient traveled faster than the diffusion of PI in the cytosol. For example, in Fig. 2A, the intracellular Ca2+ transient spread from the leading edge to the trailing edge of the cell in less than 0.4 s, compared with about 27.3 s for PI. This observation suggests that an influx of extracellular Ca2+ at the poration site might trigger the intracellular Ca2+ release from ER based on a reaction−diffusion mechanism known as the “calcium-induced calcium release” (CICR) (34). Such a process can drive the propagation of the Ca2+ transient inside the cell (35), leading to a faster spread of the Ca2+ signal than PI (more details will be provided in Discussion). At Sd = 40 µm, we observed either a relatively homogeneous PI uptake at the cell leading edge with reduced intensity and slow spread (highlighted by the dashed rectangle in Fig. 2B) or a localized yet weak PI uptake with limited diffusion into the cytosol (highlighted by the dashed circle in Fig. 2C). The former was accompanied by a more uniform Ca2+ response, propagating as a plane wave across the cytosol, while the latter was accompanied by a less vigorous Ca2+ response (Fig. 2C and Fig. S1A). In comparison, a slow and weak Ca2+ response with negligible PI uptake was generally observed at Sd = 50 µm (Fig. 2D and Fig. S1B), which might be triggered by a different mechanism compared with the Ca2+ response in cells with significant membrane poration, such as those treated at Sd = 30 µm.
It is worth noting that, at Sd = 30 µm, the cell deformed and shrank significantly after the TB treatment (see the BF cell image), indicating possible membrane injury and cell detachment from the substrate. In contrast, the cells treated at Sd = 40 μm to 60 µm revealed less to insignificant changes in cell morphology, with better recovery and improved survival rates (28, 32). Overall, the reduction in cell spread area after the TB treatment was found to correlate negatively with Sd (Fig. S2C), which, together with the loss of F-actin stress fibers, at Sd = 30 and 40 µm, was confirmed by microscopy (Fig. S2D) (see SI Text for more details). Furthermore, it was found that the reduction in cell spread area generally correlated with the amount of PI uptake (membrane poration) for cells treated at Sd = 30, 40, and 50 µm (Fig. S3). Altogether, these findings suggest that, although the poration site was resealed quickly after TB treatment, a transient gush of Ca2+ influx, such as induced at Sd = 30 µm, might have already triggered the downstream signal transduction pathways for cytoskeleton rearrangement, and, potentially, cell apoptosis (18).
Quantification of Calcium Response and ICW Propagation.
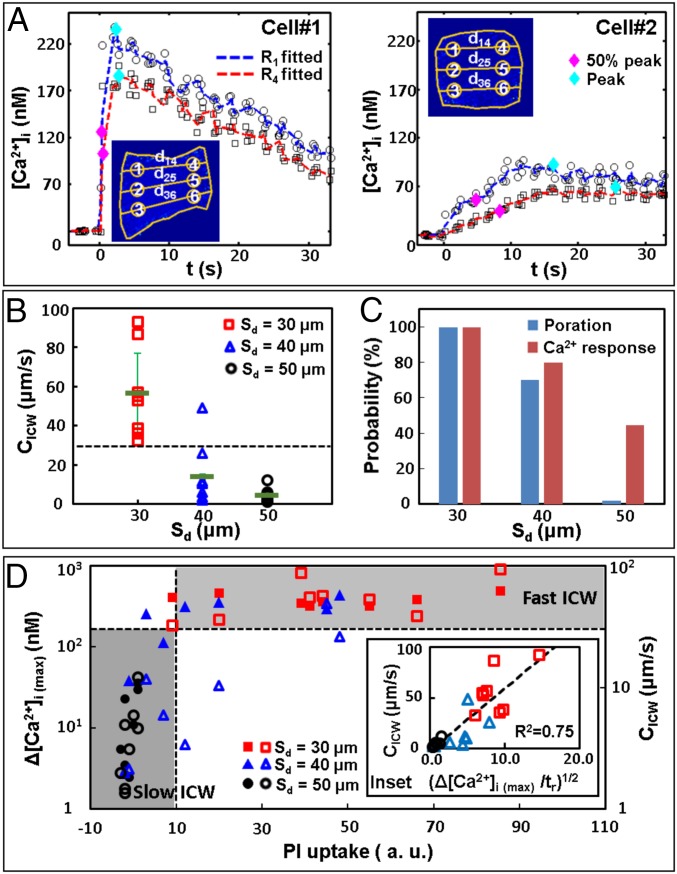
Next, we performed small-region analyses to characterize the spatiotemporal distribution of the evoked intracellular Ca2+ transients, from which CICW was calculated. Fig. 3A shows two representative examples: cell #1 (also shown in Fig. 2A) with a strong Ca2+ response and cell #2 (also shown in Fig. 2C) with a mild Ca2+ response. Three pairs of small regions of interest (ROI) along the ICW propagation path were chosen to cover the leading edge (labeled as 1, 2, 3) and trailing edge (labeled as 4, 5, 6) of the cell, respectively. Based on the separation distance and the time delay corresponding to 50% of the maximum intracellular Ca2+ concentration change Δ[Ca2+]i(max) (36), CICW along each individual propagation path (i.e., 1 → 4, 2 → 5, 3 → 6) was calculated and then averaged to determine the mean of CICW in each cell (Table S1). Overall, CICW (mean ± SD) was found to be 57 ± 21 µm/s at Sd = 30 µm, which is significantly higher than the corresponding values of 14 ± 15 µm/s at Sd = 40 µm and 4 ± 3 µm/s at Sd = 50 µm (Fig. 3B). The corresponding probability of membrane poration and Ca2+ response at different Sd is shown in Fig. 3C. At Sd = 30 µm, the probabilities of Ca2+ response and membrane poration are both 100%. In contrast, at Sd = 50 µm, 44% of the cells showed a Ca2+ response with negligible PI uptake.
Fig. 3.
Quantification of Ca2+ response and ICW propagation. (A) Small-region analysis for determining CICW. Cells #1 and #2 are the cells shown in Fig. 2A (Sd = 30 µm) and Fig. 2C (Sd = 40 µm), respectively. Insets show the schematics for small-region analysis. (B) CICW vs. Sd. The error bars indicate the SD of the calculated averaged uncertainties. (C) Membrane poration and Ca2+ response probability at different Sd. (D) Correlations of the maximum intracellular Ca2+ concentration change Δ[Ca2+]i(max) (filled symbols) and CICW (open symbols) vs. PI uptake in the cytosol at Sd = 30 μm to 50 µm. Results for different Sd are labeled with different symbols and colors. The dashed lines indicate separation of data based on the threshold of PI uptake of 10 (a.u.), and CICW of 30 µm/s. (Inset) CICW vs. the square root of concentration change rate (Δ[Ca2+]i(max)/tr)1/2, where tr is the risetime from baseline to peak concentration. The dashed line represents a linear fitting of the data (R2 = 0.75).
We also examined the correlations between Δ[Ca2+]i(max) (in filled symbols) or CICW (in open symbols) with PI uptake (Fig. 3D). The results showed two types of Ca2+ responses that may be delineated by thresholds at PI uptake = 10 (a.u.) and CICW = 30 µm/s, respectively, (see the horizontal dashed lines in Fig. 3 B and D). In particular, the data points in the Sd = 50 µm group (circles) are below these thresholds (i.e., in the lower left quadrant), characterized by lower values in PI uptake, CICW, and Δ[Ca2+]i(max). In comparison, most of the data in the Sd = 30 µm group (squares) are above these thresholds (i.e., in the upper right quadrant), with higher values in PI uptake and CICW, and with high and yet seemingly saturated Δ[Ca2+]i(max). The data in the Sd = 40 µm group (triangles) fill the transition region between these two extremes. Furthermore, the value of CICW was found to scale linearly with [Δ[Ca2+]i(max)/tr]1/2 (Fig. 3D, Inset), which is consistent with previous models of ICW propagation (37, 38).
Subcellular Features of Ca2+ Signaling Dynamics.
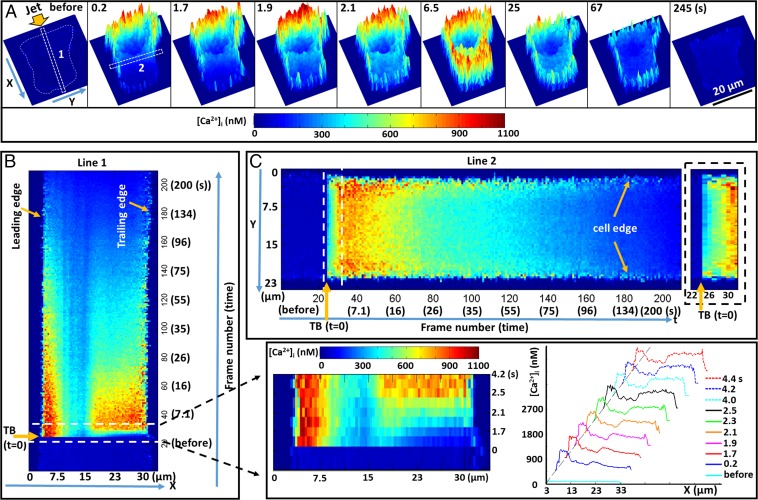
Several important features in the initiation and propagation characteristics of ICW further stood out in the time-lapse color-coded 3D images (Fig. 4A, and see also Movie S2), with both the height and color representing the Ca2+ concentration inside the cell ([Ca2+]i). First, [Ca2+]i initially elevated at the leading edge of the cell, followed by a rapid spreading of an ICW across the entire cytosol within 2.1 s. During this period, [Ca2+]i at the leading edge was sustained at a high level up to 900 nM while increasing only to ∼300 nM at the trailing edge. Thereafter, the overall [Ca2+]i in the cytosol eventually exceeded 500 nM by 6.5 s, except in the cell nucleus region. This global increase of [Ca2+]i might be caused by the temporal saturation of Ca2+ buffers by the swiping ICW, which, consequently, enhanced the CICR process (39). These dynamic features can be clearly seen in the temporal line intensity plots along the ICW propagation direction (Fig. 4B) where the x−t plot shows the [Ca2+]i profile along the length of line 1 (marked in Fig. 4A) and its variation with time (from bottom to top). An enlarged view of the initial [Ca2+]i profiles along line 1 in time sequence (the region inside the dashed box in Fig. 4B) captures the propagation of the ICW across the cytosol, with the curves showing the temporal evolution of [Ca2+]i distribution along the length of line 1 (Fig. 4, Bottom Right). Second, the elicited Ca2+ response was not uniform but rather discrete and accumulative, with Ca2+ puffs and sparks in the appearance of “spikelets” in three dimensions, as shown in Fig. 4A (or “speckles’’ in two dimensions; see Fig. 2). This observation is consistent with the understanding that there is an extensive and randomly distributed network of ER in the cytosol, which serves as the major intracellular reserve of Ca2+ (39, 40). In response to external stimuli, ICWs may be activated on the membrane and propagate inside the cell by the elementary Ca2+ release from ER, which can either operate independently or be coordinated by the local diffusion of Ca2+ or other secondary messenger molecules, such as inositol trisphosphate (InsP3), from one release site to another to form a regenerative ICW through various mechanisms, such as CICR (22, 23). More details are given in Discussion.
Fig. 4.
Dynamic features of ICW initiation and propagation. (A) A representative cell (Fig. 2B) with color-coded 3D images [both the height and color represent the Ca2+ concentration inside the cell ([Ca2+]i)]; the extracellular background is subtracted by thresholding. (B) The x−t plot of the [Ca2+]i profile along the length of rectangle 1 (in the wave propagation direction) marked in A. (Bottom Right) An enlarged view for the region inside the dashed box is shown, with the evolution of [Ca2+]i profiles along the length of rectangle 1. (C) The y−t plot of the [Ca2+]i profile along the length of rectangle 2 (perpendicular to wave propagation direction) marked in A. The [Ca2+]i values are averaged across the six-pixel width of rectangles 1 and 2, respectively. B and C share the same color bar for [Ca2+]i. Small-region analysis of wave speed along the edge and inside the cell is summarized in Fig. S4.
Third, the elevation of [Ca2+]i seemed to propagate faster along the edge than inside the cell, which is evident in the space−time (y−t) plot perpendicular to the ICW propagation direction (i.e., along line 2, Fig. 4C, with the image on the right showing the enlarged view inside the white dashed box during the initiation and early propagation). This feature was also observed in three other cells treated at Sd = 30 μm or 40 µm (Fig. S4).
Mechanistic Study of Intracellular Ca2+ Response Induced by TB Treatment.
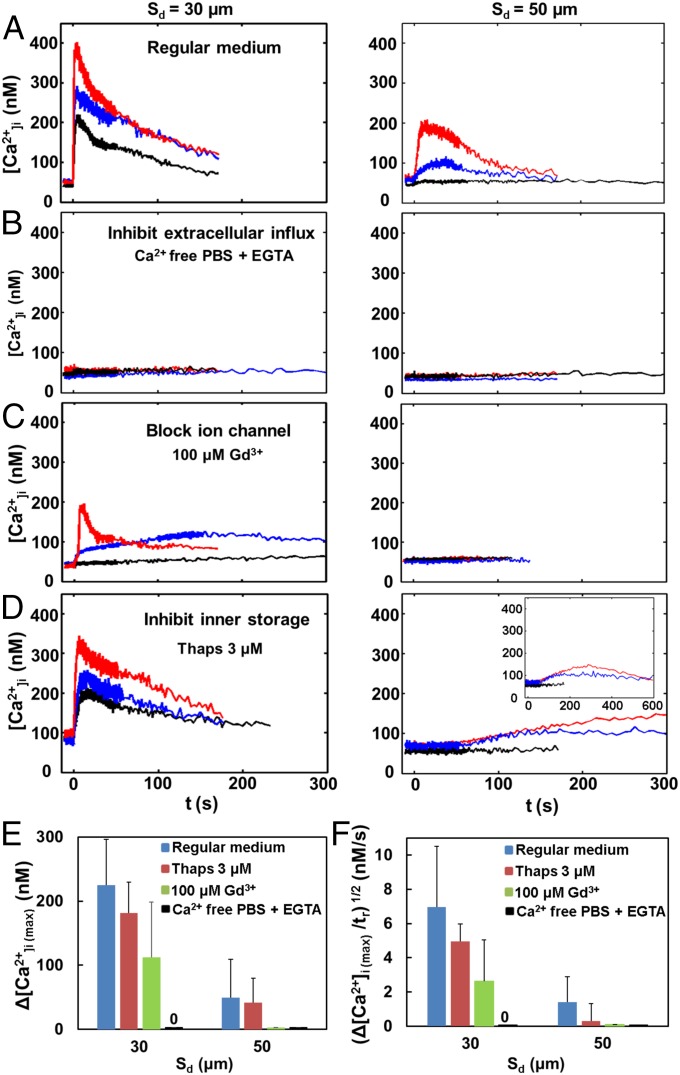
We observed two different types of Ca2+ responses: a fast ICW for cells treated at Sd = 30 µm (100% Ca2+ response with membrane poration) and a slow ICW at Sd = 50 µm (44% Ca2+ response with no membrane poration) (Fig. 3 C and D). Therefore, we conducted a mechanistic investigation to reveal the significance of these two different types of Ca2+ responses. This series of experiments was carried out at Sd = 30 and 50 µm, with three representative (high, medium, low) Ca2+ response curves shown in each plot of Fig. 5 A–D. The control experiment with regular medium is shown in Fig. 5A. When the culture medium was substituted by Ca2+ free medium (chelated with EGTA), the TB-elicited Ca2+ response could be completely abolished at both Sd (Fig. 5B), indicating that the influx of extracellular Ca2+ is essential for initiating the intracellular Ca2+ response.
Fig. 5.
Mechanistic study of intracellular Ca2+ response induced by TB treatment. Ca2+ response profiles with TB treatment at Sd = 30 and 50 µm (A) in regular culture medium, (B) in Ca2+ free culture medium (Ca2+ free 1× PBS with 0.5 mM EGTA added as Ca2+ chelator), (C) in culture medium with 100 µM Gd3+ to block nonspecific mechanosensitive ion channel, and (D) in culture medium with 3 µM Thaps to inhibit ER Ca2+-ATPases before TB treatment and thus affecting Ca2+ release. Three representative (high, medium, low) Ca2+ response curves are shown in each plot of A–D. (E and F) Comparison of Ca2+ responses from groups of individual cells treated by TB in regular culture medium (blue), with 3 µM Thaps (red), with 100 µM Gd3+ (green), and in Ca2+ free culture medium (black) for (E) Δ[Ca2+]i(max) and (F) [Δ[Ca2+]i(max)/tr)1/2] vs. Sd = 30 and 50 µm. Data are shown in mean ± SD (N = 10, 3, 4, and 3 at Sd = 30 µm, and 9, 3, 6, and 5 at Sd = 50 µm for regular culture medium, with 3 µM Thaps, with 100 µM Gd3+, and in Ca2+ free culture medium, respectively.)
The influx of extracellular Ca2+ may take two different pathways: (i) through membrane poration sites, as shown in Fig. 2 A and B, and, potentially, (ii) through the opening of stretch-sensitive (or mechanosensitive) ion channels (41, 42). The molecular expression of mechanosensitive membrane ion channels, such as transient receptor potential melastatin 7 (TRPM7), has been reported in HeLa cells (41) whose function can be blocked by Gd3+. Therefore, we added 100 µM Gd3+ to the culture medium to evaluate the effect of mechanosensitive ion channels on TB-elicited Ca2+ response (Fig. 5C). At Sd = 50 µm, where there was no detectable membrane poration, the Ca2+ response was completely abolished. This result suggests that the slow Ca2+ response elicited under such conditions is mediated by the influx of extracellular Ca2+ through the opening of mechanosensitive ion channels. The Ca2+ response at Sd = 30 µm was found to be either suppressed with reduced amplitude in Δ[Ca2+]i or, very often, converted from fast to slow Ca2+ responses. Under such conditions, it is likely that the TB-elicited fast Ca2+ response is mediated primarily by membrane poration, while also influenced by mechanosensitive ion channels.
Upon initiation, the transient Ca2+ signal inside the cytosol may spread out via the CICR mechanism through ER that has been well established in the biology literature (22, 23). Therefore, we used 3 µM thapsigargin (Thaps) to discharge intracellular Ca2+ stores before the TB treatment by inhibiting ER Ca2+-ATPases, which, in turn, will reduce the Ca2+ release from the ER during TB treatment (43, 44) (Fig. 5D). At Sd = 30 µm, the Ca2+ response was found to be suppressed in both Δ[Ca2+]i(max) and (Δ[Ca2+]i (max)/tr)1/2 with a reduced CICW in the range of 4 μm/s to 11 μm/s, compared with the regular medium; see Fig. 5 D–F. At Sd = 50 µm, significantly prolonged Ca2+ responses were observed in cells, indicating that the propagation of the Ca2+ transient in the cytosol was substantially delayed compared with those in the regular medium.
Fig. 5 E and F summarizes the variations in Δ[Ca2+]i(max) and (Δ[Ca2+]i(max)/tr)1/2 (proportional to CICW; see Fig. 3D, Inset) among the aforementioned culture media. Overall, Thaps, Gd3+, and Ca2+ free media were found to affect the amplitude and propagation speed of the Ca2+ response. The results indicate that extracellular Ca2+ influx is essential for initiating both types of ICWs in the TB-treated HeLa cells: (i) through mechanosensitive ion channels (no injury) in the slow Ca2+ response and (ii) through both membrane poration (with injury) and mechanosensitive ion channels during the fast Ca2+ response. In both cases, the initiated Ca2+ response then propagated through the cytosol via CICR from the ER.
Enhanced Intracellular Ca2+ Response with Integrin-Binding Beads on the Cell Membrane.
In our system of TB-generated jetting flow, both the Ca2+ response and membrane poration show a clear Sd dependency that is closely related to the cell membrane deformation (Fig. 2). At large standoff distances, such as Sd = 60 µm, no Ca2+ response in a target cell could be induced by the TB, because of the diminished strength of the jetting flow and resultant small cell membrane deformation (28). It was found previously that displacement by TB-induced jetting flow of a 10-μm polystyrene bead is larger compared with a small bead of 2 μm (45). Furthermore, recent studies have revealed that mechanical force-induced Ca2+ signals at the plasma membrane can be triggered by the displacement of 10-μm fibronectin-coated beads attached to cell surface with laser tweezers (46). Therefore, we hypothesized that shear flow−cell interaction and resultant membrane deformation may be enhanced by attaching micrometer-sized beads on the cell surface, which could amplify the local drag on the cell surface produced by the jetting flow.
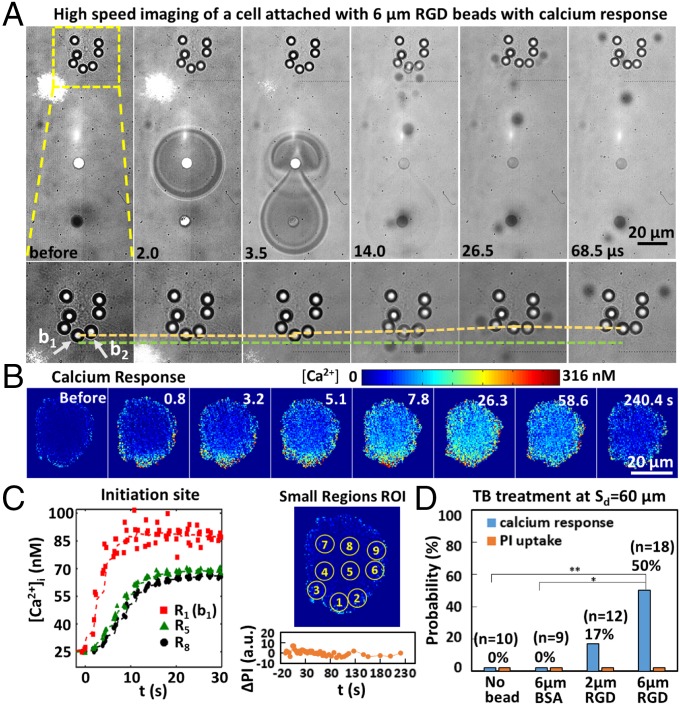
Fig. 6A shows an example of TB−cell interaction at Sd = 60 µm, in which 6-μm RGD (integrin-binding) beads were attached at the cell’s leading edge (such as b1) and found to be significantly displaced (shown by the dashed yellow line in Fig. 6A, Bottom) from their original locations (dashed green line for b1). Consequently, a mild Ca2+ response was elicited inside the cytosol (see the FL images in Fig. 6B), initiated from local regions with beads at the leading edge nearest to the jetting flow (i.e., b1 and b2), and propagated along the edge and into the cytosol (Fig. 6 B and C and Table S2). Moreover, negligible PI uptake was detected (see ΔPI vs. t profile in Fig. 6C), indicating no cellular injury. The CICW from R1 or R2 to R8 was estimated to be 3.71 μm/s or 3.35 µm/s, respectively. The low CICW, together with the slow Ca2+ response profile, is similar to the Ca2+ transient initiated by the activation of mechanosensitive ion channels observed in the Sd = 50 µm group (Fig. 2D).
Fig. 6.
Beads-enhanced Ca2+ response. (A) (Top) TB interaction with an H-patterned cell with 6-µm integrin-binding beads attached on the cell membrane. (Bottom) Enlarged region inside the dashed box in Top; the displacement of an individual bead at the cell leading edge (indicated by the yellow dashed line; the green dashed line serves as a reference). (B) FL image sequence showing Ca2+ response of the cell in A. (C) (Left) Time evolution of [Ca2+]i for small ROI on the same cell shown in A. (Right) Locations of the small ROI on the same cell. PI uptake in the cell is negligible, as shown in Bottom Right (ΔPI vs. t). ΔPI is in the same scale as shown in Fig. 2H. (D) Probability of Ca2+ response and PI uptake in cells with no beads, attached with 2- and 6-µm RGD-coated integrin-binding beads, or with 6-µm BSA-coated nonspecific binding beads treated by TB at Sd = 60 µm. **P < 0.01 and *P < 0.05 from Fisher’s exact test; the P value between the Ca2+ response of no beads and 2-µm RGD beads is not significant.
We further observed that the enhancement of Ca2+ response at Sd = 60 µm was bead-size-dependent, increasing from 17% with 2-µm RGD beads to 50% with 6-µm RGD beads (Fig. 6D). The Ca2+ response probability from 6-µm RGD beads is significantly higher compared with that from no beads (P < 0.01). In contrast, the results from 2-µm RGD beads were not statistically significant compared to that from no beads (P = 0.48). No Ca2+ response was observed in the control experiment with 6-µm BSA beads that nonspecifically bind to the cell membrane (P < 0.05 compared with RGD beads). An enhanced Ca2+ response (66.7%) without membrane poration was also observed by attaching 6-µm RGD beads to the cell membrane with single-bubble (Dmax = 51 ± 5 µm) treatment at Sd = 40 µm, where no Ca2+ response was elicited with no beads or 6-µm BSA beads (Fig. S5 A and B). Furthermore, the enhanced Ca2+ response with RGD beads could be significantly suppressed by inhibiting the mechanosensitive ion channels with Gd3+ or ruthenium red (RR) (Fig. S5C).
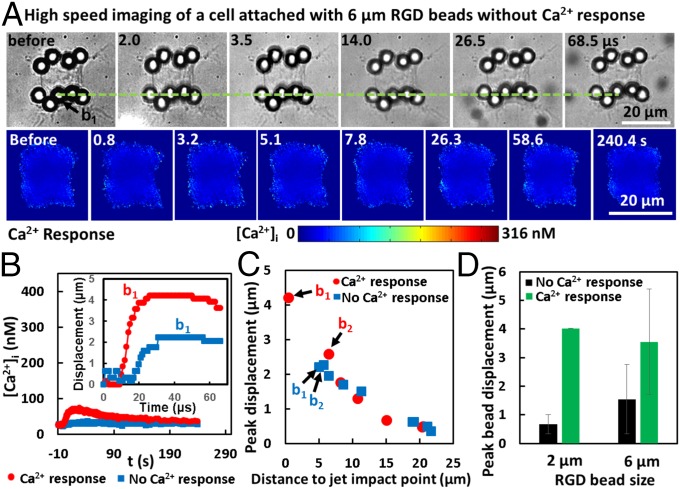
Fig. 7A shows an individual cell with beads attached at the leading edge along the jet axis with TB treatment (Fig. 7A, Top) that showed no Ca2+ response (FL images in Fig. 7A, Bottom). The maximum bead displacement (e.g., b1 in Fig. 7A) was found to be significantly less than its counterpart in the responsive cell (Fig. 6, and also Fig. 7B, Inset and Movie S3). Moreover, the peak displacements of individual beads on the cell membrane correlated inversely with their original distance to the jet impact point on the cell’s leading edge (Fig. 7C). However, except for b1, the bead’s displacement, in general, was not significantly different between the Ca2+ responsive and nonresponsive cells. More importantly, for both 2- and 6-μm beads, the maximum bead displacement (of b1) was found to be significantly larger in Ca2+ responsive cells than in nonresponsive cells (Fig. 7D). This finding again suggests that large bead displacement or cell membrane stretching is critical for the enhanced Ca2+ response at Sd = 60 µm.
Fig. 7.
Mechanisms of beads-enhanced Ca2+ response. (A) An individual cell attached with 6-µm RGD-coated integrin-binding beads were treated with TB-induced directional jetting flow at Sd = 60 µm, but with no obvious Ca2+ response. “No Ca2+ response” is defined as when Δ[Ca2+]i(max) is smaller than 10 nM. (Top) The displacement of an individual bead at the cell leading edge (the green dashed line serves as a reference). (Bottom) FL image sequence showing no Ca2+ response of the cell. (B) Time evolution of [Ca2+]i for the two cells shown in Fig. 6A and A, respectively. (Inset) The time evolution of the displacement of the beads located in the middle of the cell’s leading edge (b1). (C) Maximum displacement of each bead on cells shown in B as a function of its original distance to the jet impact point (defined by the intersection of jet axis direction with the cell leading edge before TB treatment; see Fig. 1D). (D) Summary of results showing the peak displacement of the bead with the largest displacement (b1) on the cells for each group (mean ± SD).
Altogether, we found that the Ca2+ response was enhanced by the amplified membrane stretching from flow-induced displacement of large integrin-binding beads. The force generated needs to be transmitted through integrin and the cytoskeleton (47) to activate mechanosensitive ion channels on the cell membrane (48). The results demonstrate the potential of using integrin-binding beads attached to cell membrane to stimulate mechanotransduction without inflicting cell injury under cavitation-induced shear flows.
Discussion
We have shown that the controlled directional jetting flow from TB interaction can elicit two distinct types of ICWs with or without membrane poration. The transient Ca2+ response is locally initiated, spreads out directionally, and correlates with the impulsive shear stress-induced cell membrane deformation in an Sd-dependent manner (Fig. 2). At small Sd (=30 µm, γ = 1.2), a strong Ca2+ response accompanied by significant membrane poration (or injury), highly elevated Δ[Ca2+]i (389 ± 60 nM), and fast CICW (57 ± 21 μm/s) is produced (Fig. 3). Such a Ca2+ transient is likely to occur during sonoporation (5, 12, 49), in which a strong and rapid Ca2+ influx at the poration site is primarily responsible for initiating the intracellular Ca2+ response. In our study, chelating extracellular Ca2+ via EGTA abolished the induced response (Fig. 5B, Left), consistent with previous sonoporation studies (9, 12). In comparison, at large Sd (=50 µm, γ = 2), a weak and slow Ca2+ response with negligible membrane poration (indicating no injury), mild elevation of Δ[Ca2+]i (14 ± 13 nM), and slow CICW (4 ± 3 μm/s) is often induced (Fig. 3). This noninjurious mode of Ca2+ response is triggered by Ca2+ influx through stretch-activated ion channels on the cell membrane, such as TRPM7, which can be blocked by Gd3+ (Fig. 5C, Right). This weak Ca2+ response may be important for ultrasound-stimulated mechanotransduction (13, 16). At even greater Sd (≥60 µm, γ ≥ 2.4), no appreciable Ca2+ response could be generated, because of the significantly diminished strength of TB-induced jetting flow (28) (Fig. 2F). These findings provide evidence of discrete mechanisms associated with different magnitudes of mechanical stimulations, resulting in potential pathologic or physiologic Ca2+ signaling.
As shown in Fig. 4, once initiated, the ICW will propagate and spread out in the target cell. Inhibiting Ca2+ release from the ER by Thaps both suppressed the amplitude of Δ[Ca2+]i and slowed the propagation of ICW (Fig. 5 D–F). CICW was reduced to the range of 4 μm/s to 11 µm/s for Sd =30 µm, confirming that TB-elicited ICW propagation inside the cytosol was facilitated by the CICR mechanism. It has been shown that the influx of extracellular Ca2+ (50) will activate InsP3, which further diffuses inside the cytosol to bind with its receptors (e.g., InsP3Rs) on the ER to release the stored Ca2+ rapidly (∼10 ms to 50 ms). A hierarchical sequence of events will occur, ranging from Ca2+ blips released by individual InsP3R channels to Ca2+ puffs formed by the concerted activation of a cluster of InsP3Rs, to build up an ICW when the local [Ca2+]i reaches a first threshold level of ∼0.2 μM (40). Our observation of nonuniform Ca2+ response pattern with Ca2+ puffs and sparks in the appearance of spikelets in three dimensions (Fig. 4A) or speckles in two dimensions (Fig. 2) is in accordance with these previous findings. Thereafter, if the local [Ca2+]i increases further to exceed a second threshold level of ∼0.3 μM (51), the Ca2+ channels on the ER membrane will begin to shut down slowly (1 s to 10 s) to maintain cellular homeostasis (23, 35). Ultimately, the excessive Ca2+ in the cytoplasm will be removed through a combined action of buffers, pumps, and exchanges (40). These general features in the dynamics of ICW initiation and propagation are consistent with our experimental observation of TB-induced Ca2+ response profile, which is characterized by an initial short and rapid rise of [Ca2+]i to a peak value, followed by a long and gradual decay to the resting level (Figs. 2G and 4A). The bimodal action of Ca2+ (i.e., a rapid release at low concentration coupled with a gradual shutdown at high concentration) on the InsP3Rs drives successive bursts of regenerative Ca2+ release from the ER to sustain the ICW propagation through the cytosol (22, 23).
Besides, it is interesting to note that, in four of the cells treated at Sd = 30 μm or 40 µm, we observed that the TB-induced Ca2+ transient propagated faster along the cell boundary (or edge) than inside the cytosol (Figs. 2B and 4C). The ratio of CICW at the edge to that at the interior in this subgroup of cells is about 2.4, with CICW along the cell edge in the range of ∼68 μm/s to 108 μm/s (Fig. S4). This observation may be explained by a different mechanism known as calcium-induced calcium influx (CICI) in contrast to CICR. In particular, it has been reported in a few previous studies (52) that unusually rapid Ca2+ transients with CICW in the range of ∼40 μm/s to 1,000 μm/s (at 20 °C) may be mediated by CICI, e.g., along flagella or cilia, and even some nonflagellar cells, such as neurons and cancer cells within relatively thin or flat regions (such as in the periphery of HeLa cells). Based on the theory of CICI, it is possible that a focal deformation of the cell membrane may open stretch-activated Ca2+ channels, leading to a local Ca2+ influx that will trigger the sliding of subsurface filaments past each other. Consequently, the nearby membrane will be stretched in sequence to create a traveling motion and, correspondingly, a propagating local Ca2+ influx and thus an ICW along the cell boundary (52).
The physical characteristics of ICW are highly conserved among various species with CICW typically in the range of ∼3 μm/s to 30 μm/s at 20 °C (34, 35, 38). Quantitatively, CICW can be scaled by (Jeff Deff/C0)1/2, where Jeff is the effective Ca2+ current density (e.g., 27 µM/s), Deff is the effective diffusion constant of Ca2+ (e.g., 22 µm2/s), and C0 is the [Ca2+]i threshold for Ca2+ channel activation (e.g., 0.5 μM) (37). Assuming, under physiological conditions, Deff and C0 are constants for each cell type, CICW is then determined primarily by (Jeff)1/2. This feature is consistent with our results in Fig. 3D, Inset, especially for the data in the Sd = 50 μm group and most of the Sd = 40 µm group with CICW in the range of ∼3 μm/s to 30 μm/s (at 20 °C) commonly found among eukaryotic cells (24, 34). In contrast, much higher CICW (33 μm/s to 93 μm/s) was found in the Sd = 30 µm group, where all cells showed significant membrane poration and cell injury, which may reduce the [Ca2+]i threshold for initiating an ICW (53, 54). Therefore, a high extracellular Ca2+ influx (i.e., greater Jeff) combined with a low C0 may contribute to the significantly elevated CICW observed in the Sd = 30 µm group (Fig. 3B).
Furthermore, it is worth noting that the fast ICW induced at Sd = 30 µm may reflect the injury response of the target cell, albeit sublethal, to TB-induced repairable membrane poration, which typically resealed in 30 s (Fig. 2H). The rapid Ca2+ influx induced under such conditions may accelerate the depolymerization of cytoskeleton filaments to facilitate internal vesicles (lysosomes) to migrate and patch the rupture site through exocytosis (55, 56), and, potentially, later on, even the related compensatory response of endocytosis for lesion removal (57). Although the membrane pore is resealed, such a strong Ca2+ response is associated with cytotoxicity, manifested by the loss of F-actin stress fibers, cell shrinkage, and even apoptosis (Fig. S3) observed in this and other studies (58). These collateral injuries, while facilitating the apoptosis of cancer cells in tumor therapy, will limit the range of cavitation-mediated biomedical applications such as gene delivery and neural modulation.
To address this challenge, we have developed a strategy to enhance cavitation-induced Ca2+ response without injury by attaching micrometer-sized beads to the cell membrane through the RGD–integrin link (Fig. 6). With 6-μm integrin-binding beads, we found that the Ca2+ response probability was increased to 50% without membrane poration or injury under cavitation conditions (Sd = 60 µm, γ = 2.4), which normally do not produce a Ca2+ response without the beads. This enhancement is integrin-binding-specific, since no Ca2+ response was produced with 6-μm BSA beads that were nonspecifically attached to cell plasma membrane. The specificity of this response was further confirmed by additional single-bubble−cell interaction experiments (Fig. S5 A and B), which also demonstrated that the enhanced response was significantly suppressed by Gd3+ or RR (Fig. S5C), two well-known inhibitors of mechanosensitive ion channels. Furthermore, we found that the maximum bead displacement in Ca2+ responsive cells is significantly larger than that in nonresponsive cells attached with 6-μm integrin-binding beads (Fig. 7). Altogether, our results suggest that the amplified membrane stretch is transmitted through cell surface integrins (48) to activate mechanosensitive ion channels on the cell membrane, and is critical for the enhanced Ca2+ response at Sd = 60 µm.
In conclusion, we have systematically examined the dynamics and mechanisms of TB-induced ICW at the single-cell level. The knowledge acquired in this work sheds light on how to target different biological effects such as cell injury and apoptosis, drug delivery, and mechanotransduction by tuning the magnitudes of mechanical perturbations through the high-strain-rate shear flows associated with inertial cavitation and dynamic bubble–bubble interactions that are prevalent in therapeutic ultrasound applications. Here we used cavitation-induced jetting flow to study the ICW in a highly controlled manner, and the results and insights gained in this work may also apply to single inertial cavitation and other similar mechanical perturbations. Furthermore, the mechanistic insight from this study may offer us opportunities to differentiate the dynamics and mechanisms of pathologic and physiologic Ca2+ responses as a potential diagnostic method to study diseases linked to the dysregulation of Ca2+ signals (such as cardiac disease) and/or monitor treatment at the single-cell level (51). Other future work may focus on utilizing repeated and programmed cavitation induced shear flow to stimulate cells for long-term bioeffects such as cell proliferation, differentiation, and gene expression for tissue growth and wound healing.
Materials and Methods
Fabrication of Microfluidic Chip.
The microfluidic chip was made of a polydimethylsiloxane (PDMS, mixing ratio 10:1; Sylgard184; Dow Corning) housing on a patterned glass substrate (75 × 38 × 0.19 mm). A chrome mask was designed with hundreds of repeating units, each of which consists of a pair of gold dots and an H-shaped island. The glass substrate was first patterned with the arrays of gold dots by photolithography, E-beam Evaporation, and metal lift-off. The H-shaped islands were then fabricated at various Sd to the gold dots with aligned photolithography. A passivating solution [0.5 mg/mL PLL(20)-g[3.5]-PEG(2) (SuSOS) in 10 mM Hepes buffer] was coated on the substrate surface to avoid cell adhesion except on the H-shaped regions. After lift-off of the photoresist, the H-shaped islands were covered with fibronectin to control the cell patterning. The PDMS microchannel with a cross-section of 800 × 25 μm (W × H) was produced from a silicon mold using soft lithography. After O2 plasma treatment, the patterned glass substrate and the PDMS microchannel were aligned under a stereomicroscope and bonded together. More detailed fabrication protocol can be found in ref. 32.
Cell Culture and Handling.
HeLa cells (307-CCL-2, p.148; Duke Cell Culture Facility) were routinely maintained in DMEM culture medium (11995-065; ThermoFisher Scientific) supplemented with 10% FBS in a cell culture incubator at 37 °C with 5% CO2. Cells were trypsinized and resuspended into prewarmed (37 C°) culture medium to a density of about 5 × 106 cells per milliliter before being injected into the microchannel. The introduced cells were incubated in the microchannel at 37 °C for 45 min to allow adhesion onto the fibronectin-coated H-shaped islands. After flushing out nonattached cells, the chip was put back in the incubator for 2 h with continuous perfusion of culture medium at 0.5 μL/min, allowing for the individual cells to fully spread on the fibronectin-coated regions. Thereafter, the culture medium was replaced with fura-2 AM (F1221; ThermoFisher Scientific) working solution [6 μM in Opti-MEM (11058-021; ThermoFisher Scientific)], and the chip was incubated at room temperature in the dark for 40 min with 1 μL/min perfusion rate for loading fura-2 into the cells. Subsequently, 1× PBS was used to flush out unloaded fura-2 AM solution from the microchannel before perfusion of different media under various experimental conditions. For more details, please refer to ref. 32. In mechanistic studies, several different media were used. For example, to remove extracellular Ca2+, the microchannel was filled with 1× PBS (Ca2+ free) with 0.5 mM EGTA to ensure that residual Ca2+ was completely chelated. To monitor concurrent cell membrane poration during Ca2+ response measurement, the microchannel was perfused with PI solution (100 μg/mL in Opti-MEM). To block mechanosensitive ion channel, Gd3+ solution [100 μM GdCl3 in 1× PBS (with Ca2+)] was added to the microchannel. To inhibit ER Ca2+ release, cells in the microchannel were incubated with Thaps (3 μM in Opti-MEM) for 30 min before loading fura-2 (with 3 μM Thaps), and subsequently replaced with Opti-MEM (3 μM Thaps) before experiment. In experiments to facilitate intracellular Ca2+ response at Sd = 60 μm, cells were incubated at 37 C° for 30 min with 2- or 6-μm carboxyl functionalized PS beads (activated with water-soluble carbodiimide), which were precoated with BSA (2.5% in PBS) or RGD-containing peptide (Peptite-2000; 100 μg/mL in PBS) for nonspecific binding and integrin-binding to the cell membrane, respectively. The seeding densities of 2- and 6-μm beads were about 1 × 109 and 1 × 108 beads per milliliter, corresponding to about 60 and 6 beads per cell, respectively, on the apical membrane surface. For all measurements, a constant flow rate of 0.5 μL/min was used throughout the experiment.
TB Treatment and Optical Imaging.
As shown schematically in Fig. 1, the microfluidic chip with fura-2-loaded cells was fixed on the translation stage of an inverted microscope (Axio Observer Z1; Zeiss). TB was generated next to a target cell by focusing two Q-switched Nd:YAG lasers (New Wave Research) onto a pair of gold dots through a 63× objective (LD Plan Neofluar; Zeiss). A monochromator (DeltaRAM X; PTI) with shutter was employed to create a sequence of light pulses with defined time intervals from a 75-W xenon lamp at excitation wavelengths of 340 and 380 nm and 539 nm for fura-2 and PI imaging, respectively. Since PI could also be excited at 340 and 380 nm, a custom-made narrow bandwidth filter (510 ± 40 nm) was used for the detection of fura-2 emission at 510 nm to avoid the overlap with PI emission at 610 nm. A sCMOS (scientific complementary metal–oxide–semiconductor) camera (EDGE 5.5 CL; PCO) was used to record the intracellular fura-2 and PI images at 5:1 frame ratio with an exposure time of 50 ms and an interframe time (IFT) of 0.2 s for 1 min. Thereafter, fura-2 images were recorded with an IFT at 1, 2, and 5 s for a duration of 1, 1, and 2 min, respectively, for a total recording time of 300 s, during which one PI image was recorded every five frames of fura-2 images. After the trigger of the first laser, bubble dynamics alone or together with resultant bead displacement on the cell membrane were captured by a high-speed camera (Imacon 200 or HPV-X, respectively), at 2 million frames per second with 200-ns exposure time. A BF image was taken to register the morphology of the cell immediately before and after the TB treatment; see Fig. 1C. The microscope was programmed to control the illumination shutter, dichroic mirror, and switching between two adjacent alternative positions in the rotating turret of the FL cube for fura-2 and PI imaging. A delay generator (565-8c; Berkeley Nucleonics Corporation) was used to synchronize the two lasers for TB generation and the high-speed cameras for image acquisition. We used μManager software (version1.4; Open Imaging) to communicate and coordinate the operation sequence between the microscope, monochromator, and delay generator. In particular, the FL excitation, filter cube, and recording were automatically switched between fura-2 and PI with a switching interval about 0.7 s.
Measurement of [Ca2+]i.
[Ca2+]i was determined from calibrated ratiometric imaging. The raw FL emission images at 510 nm from excitation at 340 and 380 nm were first corrected with background noise subtraction from an area free of cells. The ratiometric value (R = I340/I380) was then calculated with MATLAB at each pixel, and the averaged ratio within each cell was obtained from a manually segmented region of the cell in the image sequences. The ratio R was related to the [Ca2+]i by
where the parameters Rmin = 0.157, Rmax = 4.305, β = 7.115, and Kd = 113 nM were obtained by using a fura-2 calibration kit (Invitrogen F6774).
Analysis of CICW.
To quantify the speed of intracellular Ca2+ propagation in HeLa cells after TB and jetting flow treatment, six identical circles, with three near the leading edge and the other three close to the trailing edge of the cell, were drawn manually with the ROI manager in ImageJ. As shown in Fig. 3A, the cell leading and trailing edges were nearly perpendicular to the jetting flow direction, and thus they were divided into four equal segments on each side. The centers of the six circles (each with an area of 16.6 μm2) were located at 1/4 length and 3/4 length of the lines linking the points of quadrisection on the two boundaries (Fig. 3A). The wave propagation distances in three different paths were d14, d25, and d36. The individually averaged [Ca2+]i over the circles were obtained and curve was fitted using MATLAB, as illustrated in Fig. 3A. The risetime at 50% peak concentration change was determined for the six circles (i.e., t1, …, t6; see Fig. 1E). The Ca2+ wave speeds along the three paths were calculated by C14 = d14/(t4 − t1), C25 = d25/(t5 − t2), and C36 = d36/(t6 − t3). Thereafter, the ICW in the cell was determined by the average of these three speeds: CICW = (C14 + C25 + C36)/3. Table S1 shows a detailed example of calculating CICW for the two cells presented in Fig. 3A.
Supplementary Material
Acknowledgments
The authors would like to thank Frank Kosel of Specialised Imaging for providing the Kirana camera with SI-LUX640-400 pulsed laser used in this study. The authors also want to acknowledge the technical support of Ricky Park, Michael Kovach, George Sankin, Holly Leddy, Julia Ross, and Katelyn McCraken, and Edwin Iversen for statistical analysis. This work was supported by National Institutes of Health Grants R03-EB017886-01A1, R01-AR48182, and R37-DK052985-20, and by National Science Foundation Grant 1638442.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713905115/-/DCSupplemental.
References
- 1.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 2.Hu Z, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34. doi: 10.1186/1479-5876-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30:1217–1222. doi: 10.1016/j.ultrasmedbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Fan Z, Liu H, Mayer M, Deng CX. Spatiotemporally controlled single cell sonoporation. Proc Natl Acad Sci USA. 2012;109:16486–16491. doi: 10.1073/pnas.1208198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong P. 2013. Shock wave lithotripsy. Bubble Dynamics and Shock Waves, Shock Wave Science and Technology Reference Library, ed Delale CF (Springer, Berlin), Vol 8, pp 291–338.
- 8.Roberts WW, et al. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–738. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- 9.Fan Z, Kumon RE, Park J, Deng CX. Intracellular delivery and calcium transients generated in sonoporation facilitated by microbubbles. J Control Release. 2010;142:31–39. doi: 10.1016/j.jconrel.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijering BD, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res. 2009;104:679–687. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- 11.Kudo N, Okada K, Yamamoto K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys J. 2009;96:4866–4876. doi: 10.1016/j.bpj.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leow RS, Wan JMF, Yu ACH. Membrane blebbing as a recovery manoeuvre in site-specific sonoporation mediated by targeted microbubbles. J R Soc Interface. 2015;12:20150029. doi: 10.1098/rsif.2015.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Commun. 2015;6:8264. doi: 10.1038/ncomms9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compton JL, Luo JC, Ma H, Botvinick E, Venugopalan V. High-throughput optical screening of cellular mechanotransduction. Nat Photonics. 2014;8:710–715. doi: 10.1038/nphoton.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Godinez V, et al. Laser-induced shockwave paired with FRET: A method to study cell signaling. Microsc Res Tech. 2015;78:195–199. doi: 10.1002/jemt.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan MA, Campbell P, Kondo T. The role of Ca2+ in ultrasound-elicited bioeffects: Progress, perspectives and prospects. Drug Discov Today. 2010;15:892–906. doi: 10.1016/j.drudis.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Shi J, Cui J, Deng CX. Effects of extracellular calcium on cell membrane resealing in sonoporation. J Control Release. 2008;126:34–43. doi: 10.1016/j.jconrel.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 19.Fan Z, et al. Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility. Sci Rep. 2013;3:2176. doi: 10.1038/srep02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumann D, et al. Treatment of human mesenchymal stem cells with pulsed low intensity ultrasound enhances the chondrogenic phenotype in vitro. Biorheology. 2006;43:431–443. [PubMed] [Google Scholar]
- 21.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 23.Chun JT, Santella L. Intracellular calcium waves. In: Lennarz WJ, Lane MD, editors. The Encyclopedia of Biological Chemistry. Vol 2. Academic; Waltham, MA: 2013. pp. 640–647. [Google Scholar]
- 24.Jaffe LF. Fast calcium waves. Cell Calcium. 2010;48:102–113. doi: 10.1016/j.ceca.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1–21. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- 26.Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 27.Mohammadzadeh M, Li FF, Ohl CD. Shearing flow from transient bubble oscillations in narrow gaps. Phys Rev Fluids. 2017;2:014301. [Google Scholar]
- 28.Yuan F, Yang C, Zhong P. Cell membrane deformation and bioeffects produced by tandem bubble-induced jetting flow. Proc Natl Acad Sci USA. 2015;112:E7039–E7047. doi: 10.1073/pnas.1518679112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Chan CU, Ohl CD. Yield strength of human erythrocyte membranes to impulsive stretching. Biophys J. 2013;105:872–879. doi: 10.1016/j.bpj.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohl CD, et al. Sonoporation from jetting cavitation bubbles. Biophys J. 2006;91:4285–4295. doi: 10.1529/biophysj.105.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sankin GN, Yuan F, Zhong P. Pulsating tandem microbubble for localized and directional single-cell membrane poration. Phys Rev Lett. 2010;105:078101. doi: 10.1103/PhysRevLett.105.078101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Yuan F, Sankin G, Yang C, Zhong P. A microfluidic system with surface patterning for investigating cavitation bubble(s)–Cell interaction and the resultant bioeffects at the single-cell level. J Vis Exp. 2017;119:e55106. doi: 10.3791/55106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan F, Sankin G, Zhong P. Dynamics of tandem bubble interaction in a microfluidic channel. J Acoust Soc Am. 2011;130:3339–3346. doi: 10.1121/1.3626134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffe LF. Classes and mechanisms of calcium waves. Cell Calcium. 1993;14:736–745. doi: 10.1016/0143-4160(93)90099-r. [DOI] [PubMed] [Google Scholar]
- 35.Dawson SP, Keizer J, Pearson JE. Fire-diffuse-fire model of dynamics of intracellular calcium waves. Proc Natl Acad Sci USA. 1999;96:6060–6063. doi: 10.1073/pnas.96.11.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SS, Thompson SH. Local positive feedback by calcium in the propagation of intracellular calcium waves. Biophys J. 1995;69:1683–1697. doi: 10.1016/S0006-3495(95)80086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kupferman R, Mitra PP, Hohenberg PC, Wang SSH. Analytical calculation of intracellular calcium wave characteristics. Biophys J. 1997;72:2430–2444. doi: 10.1016/S0006-3495(97)78888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaffe LF. The path of calcium in cytosolic calcium oscillations: A unifying hypothesis. Proc Natl Acad Sci USA. 1991;88:9883–9887. doi: 10.1073/pnas.88.21.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bootman MD, Berridge MJ. Subcellular Ca2+ signals underlying waves and graded responses in HeLa cells. Curr Biol. 1996;6:855–865. doi: 10.1016/s0960-9822(02)00609-7. [DOI] [PubMed] [Google Scholar]
- 40.Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol. 1997;499:307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol. 2007;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 42.Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 43.Iwanaga S, et al. Location-dependent photogeneration of calcium waves in HeLa cells. Cell Biochem Biophys. 2006;45:167–176. doi: 10.1385/CBB:45:2:167. [DOI] [PubMed] [Google Scholar]
- 44.Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lautz J, Sankin G, Yuan F, Zhong P. Displacement of particles in microfluidics by laser-generated tandem bubbles. Appl Phys Lett. 2010;97:183701. doi: 10.1063/1.3511538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim TJ, et al. Distinct mechanisms regulating mechanical force-induced Ca2+ signals at the plasma membrane and the ER in human MSCs. eLife. 2015;4:e04876. doi: 10.7554/eLife.04876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt C, Pommerenke H, Dürr F, Nebe B, Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J Biol Chem. 1998;273:5081–5085. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- 48.Matthews BD, Thodeti CK, Ingber DE. Activation of mechanosensitive ion channels by forces transmitted through integrins and the cytoskeleton. Curr Top Membr. 2007;58:59–85. [Google Scholar]
- 49.Helfield B, Chen X, Watkins SC, Villanueva FS. Biophysical insight into mechanisms of sonoporation. Proc Natl Acad Sci USA. 2016;113:9983–9988. doi: 10.1073/pnas.1606915113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao Y, Parker I. Ca2+ influx modulation of temporal and spatial patterns of inositol trisphosphate-mediated Ca2+ liberation in Xenopus oocytes. J Physiol. 1994;476:17–28. [PMC free article] [PubMed] [Google Scholar]
- 51.Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 52.Jaffe LF. Stretch-activated calcium channels relay fast calcium waves propagated by calcium-induced calcium influx. Biol Cell. 2007;99:175–184. doi: 10.1042/BC20060031. [DOI] [PubMed] [Google Scholar]
- 53.Carbone E, Marcantoni A, Giancippoli A, Guido D, Carabelli V. T-type channels-secretion coupling: Evidence for a fast low-threshold exocytosis. Pflugers Arch. 2006;453:373–383. doi: 10.1007/s00424-006-0100-7. [DOI] [PubMed] [Google Scholar]
- 54.Weiss N, et al. A Cav3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem. 2012;287:2810–2818. doi: 10.1074/jbc.M111.290882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McNeil PL, Steinhardt RA. Plasma membrane disruption: Repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 56.Reddy A, Caler E, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Mol Biol Cell. 2001;12:266a. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 57.Idone V, et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heo J, Sachs F, Wang J, Hua SZ. Shear-induced volume decrease in MDCK cells. Cell Physiol Biochem. 2012;30:395–406. doi: 10.1159/000339033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chazotte B. Labeling cytoskeletal F-actin with rhodamine phalloidin or fluorescein phalloidin for imaging. Cold Spring Harb Protoc. 2010;2010:pdb.prot4947. doi: 10.1101/pdb.prot4947. [DOI] [PubMed] [Google Scholar]
- 60.Elosegui-Artola A, et al. Image analysis for the quantitative comparison of stress fibers and focal adhesions. PLoS One. 2014;9:e107393. doi: 10.1371/journal.pone.0107393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.